Abstract
Introduction
The ATP-binding cassette (ABC) transporters P-glycoprotein (Pgp/ABCB1), multidrug resistance-associated protein 1 (MRP1/ABCC1), and breast cancer resistance protein (BCRP/ABCG2) are known to transport a wide range of structurally diverse compounds. Their high level of expression at the blood-brain, maternal-fetal, and blood-testis barriers as well as their purported roles in oral absorption suggests that ABC transporters play important pharmacologic roles.
Methods
We have developed a method to characterize the function and inhibition of ABC transporters using an automated cell counter with fluorescence detection capability. The assay was performed using stably-transfected HEK293 cells expressing P-gp, MRP1, or ABCG2 and examining transport of fluorescent substrates in the presence or absence of known inhibitors and compared to results obtained with a flow cytometer. Fold-increase in intracellular fluorescence was then calculated for cells incubated with fluorescent substrate in the absence of inhibitor versus in the presence of inhibitor.
Results
Fold-increase values obtained either with the cell counter or flow cytometer were comparable for cells expressing either MRP1 or ABCG2; slightly higher fold-increase values were observed when cells expressing P-gp were read on a flow cytometer compared to the cell counter.
Discussion
The assay described provides an inexpensive detection method to aid in the development of novel ABC transporter inhibitors or to characterize potential drug-drug interactions.
Keywords: ABCG2, methods, MRP1, fluorescence, P-glycoprotein, ABC transporter
1. Introduction
The ATP binding-cassette (ABC) transporters P-glycoprotein (P-gp, encoded by the MDR-1 or ABCB1 gene), multidrug resistance-associated protein 1 (MRP1, encoded by the MRP1 or ABCC1 gene), and breast cancer resistance protein (BCRP/ABCG2, encoded by the ABCG2 gene) have been shown to mediate energy-dependent transport of a variety of structurally dissimilar compounds out of cells, against a concentration gradient. P-gp, the first ABC transporter discovered and by far the best characterized, has been shown to transport a wide variety of substrates including anthracyclines, vinca alkaloids, taxanes and tyrosine kinase inhibitors; HIV protease inhibitors such as nalfinavir, ritonavir and amprenavir; as well as steroids and HMG-CoA inhibitors. It has been shown to participate in oral drug absorption and is also a component of the blood-brain barrier, suggesting that P-gp plays a role in normal tissue protection. The MRP1 transporter has been shown to confer resistance to a narrower range of antineoplastics, including the anthracyclines, vinca alkaloids, etoposide and teniposide, and it has also been shown to transport glucuronide and glutathione conjugates (e.g. leukotriene C4). Expression of MRP1 at the blood-brain barrier and choroid plexus suggests that it, too, serves a protective role by preventing accumulation of drugs in these sanctuary sites. ABCG2 has also been shown to transport a growing list of substrates including mitoxantrone, the camptothecin analogs topotecan and irinotecan, and the tyrosine kinase inhibitors gefitinib and imatinib as well as antibiotics, HMG-CoA inhibitors and HIV protease inhibitors. ABCG2 is believed to form part of the blood-brain barrier, blood-testis barrier, and maternal-fetal barrier and has also been shown to modulate oral drug absorption. Therefore, ABC transporters play significant roles in the pharmacology of substrate compounds.
The purported roles for ABC transporters in drug disposition have led to increased interest in describing the interactions between ABC transporters and novel drug therapies. Recognizing the significant pharmacologic role of ABC transporters, the FDA has published several guidance documents to address the role of ABC transporters in drug development and to determine potantial drug-drug interactions. Additionally, the purported role of ABC transporters in clinical drug resistance has sparked the development of inhibitors that can block efflux of substrate compounds. Such inhibitors may also be used to improve drug penetration into sanctuary sites such as the brain or to increase oral drug bioavailability. Several animal studies have demonstrated increased brain penetration of the tyrosine kinase inhibitors gefitinib and imatinib when the drugs were coadministered with the dual P-gp and ABCG2 inhibitor elacridar (GF120918). Additionally, human clinical trials have shown that oral coadministration of elacridar with topotecan leads to significantly increased serum levels of topotecan compared to oral administration of topotecan alone
Flow cytometry-based functional assays are often used to characterize interactions between drugs and ABC transporters and usually involve the use of fluorescent transporter substrates such as rhodamine 123 and calcein AM for P-gp; calcein AM for MRP1; and BODIPY-prazosin and pheophorbide a for ABCG2. Flow cytometers have the sensitivity to provide accurate and reliable results, but they are often costly and require extensive calibration and user training. Therefore, the development of alternative and cost-effective methods would be advantageous to researchers in this field.
Herein we report a convenient method for analyzing the function of ABC transporters and characterizing drug-transporter interactions using an automated cell counter with fluorescence detection, the Cellometer® Vision (Nexcelom Bioscience, Lawrence, MA). The Vision allows for rapid detection of intracellular fluorescence of ABC transporter substrates using an LED excitation light source, optical filtering, and cooled CCD camera technology for fluorescence detection. The instrument can automatically analyze acquired cell images and measure cell concentration, viability, and cell size. A proprietary image process algorithm is utilized to analyze cell images. Furthermore, the analyzed results (i.e. cell image, size, and fluorescence distribution histogram) may be saved for research records.
In this work, we have developed a robust detection method using a cell counter with fluorescence detection for measuring intracellular fluorescence of P-gp, MRP1, and ABCG2 substrates. The assay and results are validated by comparing overlays generated with the cell counter to overlays generated when the samples were analyzed on a flow cytometer. The methods presented here have the potential to identify compounds that could mediate drug-drug interactions through ABC transporter inhibition. Additionally, these methods could identify novel inhibitors of ABC transporters that might be used to increase drug uptake in the CNS, modulate drug oral bioavailability, or alter drug uptake in tumors.
2. Materials and Methods
2.1 Reagents
Rhodamine 123 and verapamil were obtained from Sigma Chemical (St. Louis, MO). Calcein-AM and BODIPY-prazosin were purchased from Invitrogen Corporation (Carlsbad, CA). MK571 was obtained from EMD Bioscience (Gibbstown, NJ). Fumitremorgin C was isolated by Thomas McCloud, Developmental Therapeutics Program, NIH (Bethesda, MD). Valspodar (PSC 833) was a gift from Novartis Pharmaceuticals (East Hanover, NJ). Tariquidar (XR9576) was provided by Xenova Research (Slough, Berkshire, UK).
2.2 Cell lines
ABCG2-, ABCB1-, and ABCC1-transfected human embryonic kidney (HEK-293) cells were grown in Eagle's Minimum Essential Medium supplemented with 10% FCS, glutamine and antibiotic along with 2 mg/ml G418 to enforce transporter expression.
2.3 Fluorescence measurement using the fluorescent cell counter
Methods for detecting inhibition of ABC transporters were based on previous assays, with slight modifications. Briefly, trypsinized HEK293 cells expressing P-gp, MRP1, or ABCG2 were incubated with complete medium (phenol red-free IMEM with 10 % FBS) containing 2 μg/ml rhodamine 123, 500 nM BODIPY-prazosin, or 500 nM calcein AM, respectively, in the presence or absence of the desired inhibitor for 30 min at 37°C. The inhibitors valspodar (3 μg/ml), MK571 (100 μM), and FTC (10 μM) were used as positive controls for cells expressing P-gp, MRP1 or ABCG2, respectively. Cells were subsequently washed and allowed to incubate in substrate-free medium continuing with or without the corresponding inhibitor for 1h. Cells were then washed with cold phosphate buffered saline (PBS), resuspended in cold PBS, and kept on ice until analysis. Cell suspension (20 μL) was loaded into the counting chamber and cells were then analyzed using the Cellometer® Vision (Nexcelom Bioscience, Lawrence, MA) with an excitation/emission filter pair of 470 nm/525 nm. Cell concentration was adjusted so that 300-750 cells were counted on each slide (approximately 15,000 to 50,000 cells/ml). Exposure time was 5 s for rhodamine and BODIPY-praozosin and 1 s for calcein. Images were captured with the software provided by the manufacturer and results were exported as a Microsoft Excel File format for further data analysis.
2.4 Flow cytometry
Intracellular fluorescence of rhodamine, calcein and BODIPY-prazosin was also determined in an aliquot of cells treated as in the above paragraph using a FACSort flow cytometer (Becton Dickinson, San José, CA) equipped with a 488 nm argon laser and an 530 nm bandpass filter (FL-1) to detect rhodamine, calcein or BODIPY-prazosin fluorescence. For each sample, 10,000 events were collected.
3. Results and Discussion
3.1 Detection of Pgp-, MRP1- and ABCG2-mediated transport using a cell counter with fluorescence detection
Fluorescence images captured with the cell counter demonstrated that P-gp overexpressing cells uptake little rhodamine 123 in the absence of the known P-gp inhibitor valspodar (Figure 1A, upper right), due to transport out of the cell by P-gp. However, cells incubated with rhodamine in the presence of valspodar demonstrate increased intracellular rhodamine fluorescence, due to inhibition of P-pg by valspodar (Figure 1 A, lower right). Bright-field images are also shown in figure 1A with the corresponding fluorescent images (upper and lower right images in figure 1A).
Figure 1.
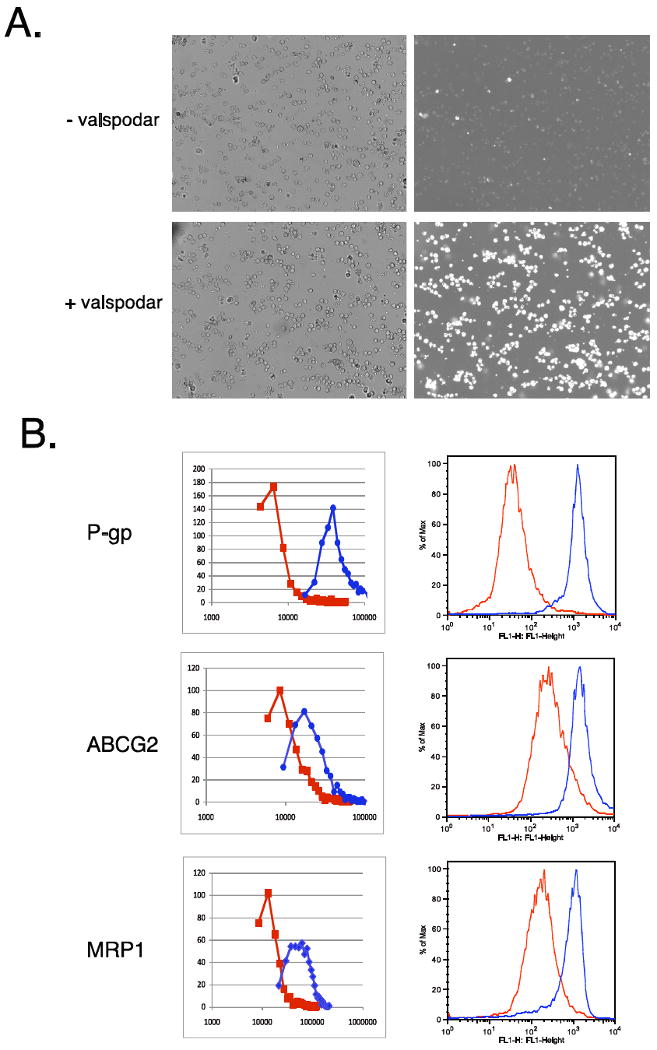
Intracellular fluorescence of fluorescent substrates measured by the Vision and by flow cytometer. A. Brightfiled images (left row) and fluorescent images (right row) of cells incubated with 2 μg/ml rhodamine 123 in the absence (top row) or presence (bottom row) of the P-gp inhibitor valspodar. B. Transfected cells were incubated with fluorescent substrate in the presence (red line) or absence (blue line) of the appropriate inhibitor and then overlays were generated with the Vision or a flow cytometer. Top row, ABCB1-transfected cells were incubated with 2 μg/ml rhodamine in the presence or absence of 3 μg/ml valspodar; center row, ABCG2-transfected cells were incubated with 500 nM BODIPY-prazosin in the presence or absence of 10 μM FTC; bottom row, ABCC1-transfected cells were incubated with 500 nM calcein AM in the presence or absence of 100 μM MK571.
The relative intracellular rhodamine fluorescence was quantified by the cell counter software, which is normalized to the cell area determined from the bright-field images. P-gp expressing HEK cells incubated with rhodamine in the presence or absence of valspodar are denoted by the red and blue lines, respectively (Figure 1B, top left). An aliquot of the same cells was analyzed by flow cytometry, and the difference in fluorescence intensity was found to mirror results from the Vision, as we again observed that cells incubated with rhodamine in the presence of valspodar exhibit increased fluorescence compared to cells incubated with rhodamine alone (Figure 1B, top right). Similar results were obtained when ABCG2 function was analyzed using the fluorescent substrate BODIPY-prazosin (Figure 1B, middle row). In ABCG2-overexpressing cells, the known inhibitor FTC (red line) was able to increase intracellular BODIPY-prazosin fluorescence compared to cells incubated in BODIPY-prazosin alone (blue line), when either the cell counter (left overlay) or a flow cytometer were used to measure intracellular fluorescence. Calcein AM was also used to examine inhibition of MRP1-mediated transport (Figure 1B, bottom row). When MRP1-transfected cells were incubated with calcein AM in the presence of the control MRP1 inhibitor MK571, increased intracellular calcein fluorescence was observed (red line) compared to cells incubated with calcein AM alone (blue line). Again, overlays obtained with the cell counter (left) were comparable to those obtained by flow cytometry (right).
Quantitation by the two instruments was also compared. Peak fluorescence values obtained with the cell counter for cells incubated with substrate and inhibitor were divided by fluorescence values in the absence of the inhibitor; mean fluorescence values were determined from data obtained with the flow cytometer. The fold-increase in intracellular fluorescence between cells incubated with fluorescent substrate in the presence of inhibitor and cells incubated with fluorescent substrate alone was then determined and values are reported in Table 1. The average fold-increase values were compared by student's t-test. For cells expressing MRP1 or ABCG2, both the cell counter and the flow cytometer yield similar increases in fluorescence. In cells expressing P-gp, a statistically significant higher average fold-increase in intracellular rhodamine fluorescence was observed with the flow cytometer, likely due to the high-powered laser in the flow cytometer.
Table 1.
Average fold-increase values for HEK293 cells expressing P-gp, MRP1 or ABCG2 as measured by flow cytometer or cell counter.
| Transporter | Fold-increase | ||
|---|---|---|---|
| Cell counter | Flow cytometer | p | |
| P-gp | 10.0±5.0 | 27.5±9.0 | 0.0085 |
| MRP1 | 3.7±1.5 | 4.1±0.9 | 0.61 |
| ABCG2 | 2.3±0.9 | 3.1±0.8 | 0.22 |
Fold-increase was determined by dividing intracellular fluorescence determined in cells incubated with substrate in the presence of inhibitor alone by intracellular fluorescence determined in the presence of substrate alone. Substrate/inhibitor pairs were rhodamine/valspodar, calcein AM/MK571, and BODIPY-prazosin/FTC for P-gp, MRP1, and ABCG2, respectively. Mean ± standard deviation is shown; p values were determined by Students t-test between average fold-increase values measured by flow cytometer or cell counter. p < 0.05 was considered significant.
3.2 Validation of assay with other Known Inhibitors of P-gp, MRP1 and ABCG2
To confirm that the cell counter has the capability to identify inhibitors of ABC transporters, we examined the effect of tariquidar and verapamil on P-gp, ABCG2 and MRP1. Tariquidar has been shown to inhibit ABCG2 as well as P-gp-mediated transport, while verapamil has been shown to inhibit P-gp and MRP1 function. We examined the effects of 0.1, 1 or 10 μM tariquidar on ABCB1-transfected HEK293 cells. As shown in the overlay generated by the cell counter in Figure 2 (top row, left overlay), tariquidar potently inhibits P-gp mediated rhodamine transport even at 0.1 μM, as intracellular fluorescence of cells incubated with rhodamine in the presence of any amount of tariquidar was nearly identical to that obtained with valspodar. Similar results were observed when the cells were examined with a flow cytometer (Figure 2, top row, right overlay). Tariqudar is known to be a less potent inhibitor of ABCG2 than P-gp, and, as observed in Figure 2 second row, tariquidar at a concentration of 1 μM was required to increase intracellular BODIPY-prazosin to levels achieved by 10 μM FTC (Figure 2, second row, left overlay). Overlays obtained by flow cytometry analysis were similar (Figure 2, second row, right overlay). Tariquidar had no effect on MRP1-mediated calcein transport (data not shown).
Figure 2.
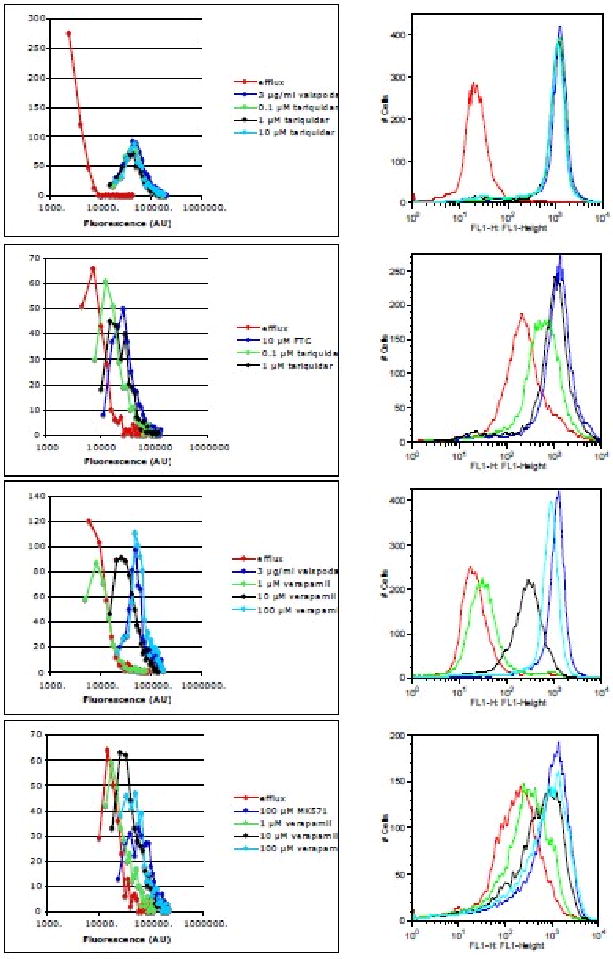
Comparison of fluorescence overlays obtained with the Vision or a flow cytometer. Top row: Pgp-overexpressing cells were incubated with 2 μg/ml rhodamine 123 alone (red line), or with rhodamine in the presence of 3 μg/ml valspodar (dark blue line), 0.1 μM (green line), 1 μM (black line) or 10 μM (light blue line) tariquidar. Second row: ABCG2-overepxressing cells were incubated with 500 nM BODIPY-prazosin alone (red line) or with prazosin in the presence of 10 μM FTC (blue line), 0.1 μM (green line) or 1 μM (black line) tariquidar. Third row: cells were incubated as in the top row except that, instead of tariquidar, cells were incubated with rhodamine 123 in the presence of 1 μM (green line), 10 μM (black line) or 100 μM (light blue line) verapamil. Bottom row: MRP1-overexpressing cells were incubated with 500 nM calcein AM alone (red line) or with calcein in the presence of 100 μM MK571 (blue line), 1 μM (green line), 10 μM (black line) or 100 μM (light blue line) verapamil. Results obtained with the Vision are in the left column while results obtained with a flow cytometer are in the right column. Results from one of at least 3 independent experiments are shown.
Experiments with verapamil yielded results consistent with previous reports. The effects of verapamil at a concentration of 1, 10, or 100 μM were determined on each of the transporters. As expected, 100 μM verapamil was able to inhibit rhodamine efflux from P-gp expressing cells as efficiently as valspodar, although it began to lose efficacy at 10 μM, and virtually no effect was observed with 1 μM (Figure 2, third row, left overlay). Again, this result was mirrored in results obtained with a flow cytometer (Figure 2, third row, right overlay). Verapamil was also able to inhibit MRP1, but was most effective only at the highest concentration (100 μM) as observed with both the cell counter (Figure 2, bottom row, left overlay) and a flow cytometer (Figure 2, bottom row, right overlay). Verapamil had no effect on ABCG2-mediated BODIPY-prazosin transport (data not shown).
As cells transfected with ABCB1 yielded the largest dynamic range in terms of fold increase, we evaluated the increase in rhodamine fluorescence when cells were incubated with tariquidar or verapamil. Results are summarized in Table 2. Results paralleled those in Table 1, showing that the fold increase in rhodamine fluorescence was still greatest with the flow cytometer.
Table 2.
Average fold-increase values for P-gp-expressing cells incubated with various concentrations of tariquidar or verapamil as measured by flow cytometer or cell counter.
| Flow cytometer | Cell Counter | ||||
|---|---|---|---|---|---|
| Tariquidar | |||||
| 0.1 μM* | 1 μM* | 10 μM* | 0.1 μM* | 1 μM* | 10 μM* |
| 28.2±8.5 | 27.7±7.7 | 28.3±7.1 | 12.9±2.3 | 13.0±4.6 | 11.8±5.7 |
| Verapamil | |||||
| 1 μM | 10 μM* | 100 μM* | 1 μM | 10 μM* | 100 μM* |
| 1.7±0.2 | 8.2±0.9 | 24.0±5.1 | 1.3±0.2 | 4.1±0.9 | 10.3±2.3 |
Fold-increase was determined by dividing intracellular fluorescence determined in cells incubated with rhodamine in the presence of inhibitor by intracellular fluorescence determined in cells incubated with rhodamine alone. Mean ± standard deviation is shown; p values were determined by Student's t-test. Average fold-increase values were compared for the various concentrations for the flow cytometer and cell counter; fold-increase values for concentrations marked with an * were statistically significantly different between the two methods. p < 0.05 was considered significant.
3.3 Applications and modifications
Using the method presented herein would aid in determining the ability of compounds to inhibit ABC transporter activity, information that would be valuable from a pharmacologic standpoint. For example, a number of drugs and natural compounds have been shown to adversely interact with digoxin, a P-gp substrate, most likely due to their ability to inhibit P-gp. Therefore, determining that a given compound is an ABC transporter inhibitor may avoid potential drug-drug interactions. Additionally, the methods described may be used for the development of ABC transporter inhibitors that could increase brain penetration of substrate drugs. Such inhibitors may have value in treating non-small cell lung cancer patients with brain metastases where increased brain levels of tyrosine kinase inhibitors such as gefitinib or erlotinib may result in an improved response. Finally, as mentioned above, coadministration of oral topotecan with elacridar increased oral bioavailability of topotecan in the absence of dose-limiting diarrhea. This may serve to decrease interpatient variability and facilitate administration of oral agents. However, further clinical trials will be needed to determine if the efficacy of IV therapies are maintained when drugs are administered orally.
While the fluorescent compounds used here are typically used for detection of ABC transporter proteins, other substrates or even fluorescent-tagged antibodies could also be used. BODIPY-labeled conjugates such as BODIPY-vinblastine and BODIPY-verapamil have been used for the detection of P-gp. Fluorescent anions such as 5(6)-carboxyfluorescein diacetate (CFDA) have been used to detect MRP1 and we have shown that BODIPY-ceramide is transported by ABCG2. Additionally, antibodies could be used to directly detect surface protein expression levels of ABC transporters. Antibodies such as UIC-2 or MRK-16 are routinely used to detect P-gp by flow cytometry as is the 5D3 antibody for ABCG2. If the primary antibodies are used with appropriate secondary antibodies or are directly conjugated to a fluorescent moiety, this would be an additional detection method for the cell counter. This could theoretically be done for any antibody used in flow cytometry to detect cellular protein expression, highlighting the fact that the uses of a fluorescent cell counter are not limited only to detection of ABC transporters.
In summary, we have demonstrated that the Cellometer® Vision, an automated cell counter with fluorescence capability, can be used to characterize ABC transporter function as well as drug/transporter interactions. The results obtained with the cell counter were comparable to those obtained with a flow cytometer, although in the case of Pgp, the dynamic range was lower with the cell counter. Nevertheless, the cell counter was useful as a rapid screen for interaction with ABC transporters. Therefore, the experiments described here provide a rapid, convenient, and less expensive method for assessment of transporter activity and may be useful in identifying compounds that inhibit ABC transporter function.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This paper is subject to the NIH Public Access Policy.
Footnotes
Conflict of Interest Statement: B. L., J. Q., and L. L-Y. C. are employed by Nexcelom Bioscience, the manufacturer of the Cellometer® Vision described in this work. The remaining authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–73. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 4.Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;453:621–641. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 5.Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A. 1999;96:3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Peng H, Zhang JT. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14:689–701. doi: 10.2174/092986707780059580. [DOI] [PubMed] [Google Scholar]
- 7.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 8.Giacomini K, Huang S, Tweedie D, Benet L, Brouwer K, Chu X, Dahlin A, Evers R, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Agarwal S, Shaik N, Chen C, Yang Z, Elmquist W. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956–963. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura K, Yamasaki T, Yui J, Hatori A, Konno F, Kumata K, Irie T, Fukumura T, et al. In vivo evaluation of P-glycoprotein and breast cancer resistance protein modulation in the brain using [(11)C]gefitinib. Nucl Med Biol. 2009;36:239–246. doi: 10.1016/j.nucmedbio.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Lagas J, van Waterschoot R, van Tilburg V, Hillebrand M, Lankheet N, Rosing H, Beijnen J, Schinkel A. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009;15:2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 12.Kruijtzer CM, Beijnen JH, Rosing H, Ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased Oral Bioavailability of Topotecan in Combination With the Breast Cancer Resistance Protein and P-Glycoprotein Inhibitor GF120918. J Clin Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 13.Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, Beijnen JH, Voest EE, Schellens JH. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–3285. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 14.Feller N, Kuiper CM, Lankelma J, Ruhdal JK, Scheper RJ, Pinedo HM, Broxterman HJ. Functional detection of MDR1/P170 and MRP/P190-mediated multidrug resistance in tumor cells by flow cytometry. Br J Cancer. 1995;72:543–549. doi: 10.1038/bjc.1995.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogan A, Legrand O, Faussat A, Perrot J, Marie J. Evaluation and comparison of MRP1 activity with three fluorescent dyes and three modulators in leukemic cell lines. Leuk Res. 2004;28:619–622. doi: 10.1016/j.leukres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T, Bates SE. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–182. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 17.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 18.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61:749–758. [PubMed] [Google Scholar]
- 20.Breuninger L, Paul S, Gaughan K, Miki T, Chan A, Aaronson S, Kruh G. Expression of multidrug resistance-associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995;55:5342–5347. [PubMed] [Google Scholar]
- 21.Robey RW, Shukla S, Finley EM, Oldham RK, Barnett D, Ambudkar SV, Fojo T, Bates SE. Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1((R)) Biochem Pharmacol. 2008;75:1302–1312. doi: 10.1016/j.bcp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 23.Kimchi-Sarfaty C, Gribar JJ, Gottesman MM. Functional characterization of coding polymorphisms in the human MDR1 gene using a vaccinia virus expression system. Mol Pharmacol. 2002;62:1–6. doi: 10.1124/mol.62.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113:2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 25.Wuchter C, Leonid K, Ruppert V, Schrappe M, Büchner T, Schoch C, Haferlach T, Harbott J, et al. Clinical significance of P-glycoprotein expression and function for response to induction chemotherapy, relapse rate and overall survival in acute leukemia. Haematologica. 2000;85:711–721. [PubMed] [Google Scholar]


