Abstract
Glycation of horse heart metmyoglobin with D-ribose 5-phosphate (R5P), D-2-deoxyribose 5-phosphate (dR5P), and D-ribose with inorganic phosphate at 37 °C generates an altered protein (Myo-X) with increased SDS PAGE mobility. The novel protein product has been observed only for reactions with the protein myoglobin and it is not evident with other common sugars reacted over a one week period. Myo-X is first observed at 1-2 days at 37 °C along with a second form that is consistent in mass with that of myoglobin attached to several sugars. MALDI mass spectrometry and other techniques show no evidence of the cleavage of a peptide from the myoglobin chain. Apomyoglobin in reaction with R5P also exhibited this protein form suggesting its occurrence was not heme-related. While significant amounts of O2− and H2O2 are generated during the R5P glycation reaction, they do not appear to play roles in the formation of the new form. The modification is likely due to an internal cross-link formed during a glycation reaction involving the N-terminus and an internal amine group; most likely the neighboring Lys133. The study shows the unique nature of these common pentose sugars in spontaneous glycation reactions with proteins.
Keywords: Glycation, ribose 5-phosphate, cross-linking, protein modification, heme degradation
1. Introduction
The spontaneous glycation of proteins by cellular sugars produces various advanced glycation end-products (AGEs), some of which negatively impact protein structure and activity [1]. Hemoglobin slowly reacts with serum glucose at the N-termini of its β chains to form glycated hemoglobin HbA1c, a marker used to assess long-term hyperglycemia in diabetics. Myoglobin reacts similarly with glucose forming glycation products with solvent-exposed Lys side chains [2] and the N-terminal amine group. These heme proteins are especially prone to inactivation in the process of glycation [2]. The source of the damage is not due to sugar modification alone, but instead to H2O2 produced as a byproduct of a glyoxidation reaction. Over extended time, methemoglobin and metmyoglobin undergo irreversible damage to the heme group in reactions with glucose as evidenced by a complete disappearance of the Soret band [2-5].
Dimerization of sperm whale metmyoglobin can also occur upon exposure to H2O2 as the heme iron transitions to the ferryl form with a corresponding transfer of a second electron from Tyr103 producing a tyrosine free radical. In dimer formation, the tyrosine radical is transferred to Tyr151 and intermolecular free radicals combine to form dityrosine (mainly Tyr103-Tyr151) cross-links [6,7]. Bovine and horse myoglobins fail to show these oligomerizations as they lack the Tyr 151 residue required for homodimerization. Using free radical Tyr103, horse myoglobin can, however, form heterodimers with sperm whale myoglobin (using Tyr151) and lactoperoxidase [7,8].
In aerobic solutions, spontaneous glycation reactions between sugars and amines are known to generate reactive oxygen species such as superoxide (O2−) and hydrogen peroxide (H2O2) [9]. The rate of these and other glycation reactions depends on the nature of the sugar (high acyclic form is favorable) and the amine (low pKa is favorable). In a reaction between ribose 5-phosphate (R5P, 10 mM) and the amines contained in cytochrome c (82 μM), O2− was generated at a rate of approximately 50 μM min−1 [10]. O2− can subsequently reduce ferricytochrome c to ferrocytochrome c [10]. Similarly, glycation of myoglobin with fructose [11] or with R5P (see below) creates superoxide that can lead to reduction of metmyoglobin to oxyferromyoglobin. O2− can also spontaneously dismutate to H2O2 causing the formation of tyrosine free radicals, possible dimerization, and heme loss.
This report describes a unique reaction between horse heart myoglobin and ribose sugars with particular attention given to R5P. Along with the expected O2− and H2O2 generation leading to heme reduction and degradation, we show the reaction creates a novel protein form (Myo-X) that presents as a highly mobile form on an SDS PAGE gel. Our results suggest Myo-X contains an intramolecular glycation cross-link that is independent of heme involvement and the reactions that involve myoglobin dimerization. The reaction is only observed for ribose-containing sugars and the cross-link appears to involve the N-terminus connected to a second group of the protein. The results further demonstrate the destructive effects of glycation events on proteins and add further evidence on the unique nature of some sugars and peptide groups in the glycation process.
2. Materials and Methods
All sugars, proteins, and reagents were obtained from Sigma Chemical Co. and were of the highest purity available. The horse heart myoglobin employed was approximately 85 % ferric form as purchased. Prepared by the butanone extraction method [2], apomyoglobin concentration was determined at 280 nm using an extinction coefficient (13,940 M−1 cm−1) [12]. LC-MS analysis of the final preparation indicated a successful removal of heme.
All reactions were incubated at 37 °C using 1.0 mg/mL myoglobin and, unless indicated otherwise, 50 mM sugar. The pH of each solution was brought to 7.5; no additional buffers were used other than the phosphate contained by the sugar or added to the ribose solution.
Spectrophotometric methods were used to monitor heme loss and myoglobin reduction. The relative amounts of oxidized and reduced myoglobin were calculated based on modified Krzywicki equations [13]. SDS polyacrylamide gel electrophoresis (SDS PAGE, on 8 – 25 % gradient gels) was performed on a Phast System electrophoresis unit (GE Healthcare). Traditional SDS PAGE gels (15%) were used for the in-gel digestion study.
Liquid chromatography/mass spectrometry (LC-MS) analysis was performed using an Agilent Series 1100 LC/MSD Trap XCT Plus LC-MS with electrospray injection. Samples (5 – 25 μL) were separated using a 4.6 × 150 mm C8 Zorbex reverse phase column and typically a 50 % water/50 % isopropanol mobile phase (containing 0.1 % formic acid). Photodiode array and MS detection (in positive mode) were used to monitor the eluant flow. Ion extraction of m/z signals was used to quantify the concentrations of certain compounds (e.g., heme) and to identify (through charge state analysis) the extent of glycation on myoglobin.
LC/MS fragmentation studies were performed to locate sites of glycation. The method is based on the premise that glycated Lys and Arg sites will resist trypsin cleavage and, hence, peptides straddling this site will disappear from an extracted ion chromatogram. Myoglobin/R5P incubation samples were treated with 6 M urea for one hour at 50 °C to denature the protein and then filtered through 10,000 MWCO filters (with two washes) to remove both the urea and R5P. Proteomics grade trypsin (at 1 : 25 wt. ratio) was added to reconstituted (with 0.5 M NH4HCO3) protein and the solution was incubated at 37 °C for 15 hours to ensure complete trypsin digestion. For the in-gel digestion studies, the band of myoglobin was dissected from the 15% gel, and treated with proteomics grade trypsin (Sigma-Aldrich, product T7575) as per the instructions provided by the supplier. LC/MS analysis was performed as mentioned previously using an acetonitrile/water gradient (0 – 70 % acetonitrile, containing 0.1 % trifluoroacetic acid) over a 50 minute period with an m/z = 800 target. Tryptic peptides were identified by their mass and by peptide sequencing. Glycation sites were determined by comparing ion signal intensities (including signals for the multiple charged states) of each of the tryptic peptides versus the intensity of the signals at the zero time point. The resulting A215 and A280 chromatograms were also monitored (and integrated) to assess peptide changes.
Matrix-assisted laser desorption ionization – time of flight (MALDI TOF) analysis was performed using an Applied Biosystems Voyager-DE Pro Biospectrometry Workstation MALDI-TOF spectrometer.
3. Results and Discussion
3.1 Heme Group Reduction and Degradation
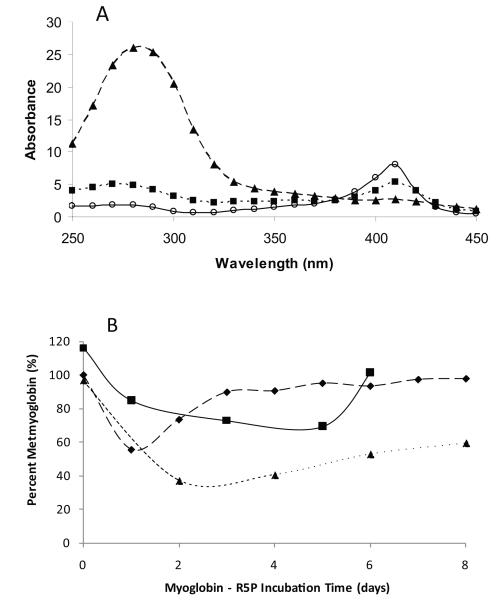
Over a period of several days at 37 °C, metmyoglobin-R5P incubation solutions showed significant changes in UV/Vis absorbance characteristics (Figure 1A) that indicated the formation of advanced glycation end-products (AGEs) (increase in absorbance in the 270 nm – 320 nm range) and the destruction of the heme group (loss of Soret band in the 400 – 420 nm range). (Heme loss was confirmed by LC-MS.) Increased absorbance in the 300 – 350 nm range is likely due to the formation of dicarbonyls from the R5P reaction. These results were expected as R5P is known to be a highly glycation-reactive sugar [10,14,15] that rapidly generates H2O2 leading to porphyrin ring degradation. Heme loss rates are correlated with R5P concentrations and heme destruction could be observed by as low as 100 μM R5P. The rate of heme loss was approximately the same for D-ribose plus equimolar phosphate. dR5P reacted significantly more slowly (approximately 10-fold) due to its inability to rearrange to a traditional Amadori product as it lacks the required C2 oxygen [15]. Comparative heme loss for D-ribose without phosphate and for other common sugars was practically unnoticeable over this time frame (< 10 % over 5 days of reaction). Interestingly, a reaction of myoglobin with arabinose 5-phosphate (A5P, 50 mM) failed to exhibit Soret band loss at a significant rate. As previously shown [10], A5P is similar in reactivity to R5P in cytochrome c systems, it is therefore curious that A5P fails to show comparable heme loss in the metmyoglobin system. The addition of the spin trap reagent DMPO (50 mM) to a R5P/metmyoglobin system slowed heme group loss to negligible levels vs. that of a myoglobin control. We interpret this as DMPO trapping the O2− prior to its ability to spontaneously dismutate to the heme-damaging H2O2 [16].
Figure 1.
A) Absorbance spectra (250 nm – 450 nm) of a reaction solution of horse heart metmyoglobin (1 mg/mL) with R5P (50 mM) at pH = 7.4 and 37 °C at 0 days (○ –– ○), 3 days (■ ···· ■), and 6 days (▲- - -▲). B) Reduction of metmyoglobin with reaction time with R5P; in exposure to air (■—■), in presence of catalase (◆- - - ◆), and in semi-anerobic conditions (▲····▲).
The R5P glycation reaction also leads to metmyoglobin reduction (see Figure 1B). Upon reaction with R5P, absorbance maxima at 540 nm and 580 nm were evident at 1 – 2 days suggesting the formation of approximately 10 – 15 % oxygenated Fe2+-myoglobin (oxyMb). Higher levels of reduction can be achieved in semi-anaerobic conditions, in the presence of catalase (0.1 mg/mL), and with addition of exogenous reactive amines (e.g., with 45 mM spermine, 30 % oxyMb produced by 1 h, data not shown). As previously reported [10], O2− is rapidly produced in R5P (or ribose/Pi) reactions and it is likely the O2− that interacts with metmyoglobin to form the reduced oxymyoglobin form. With time, oxymyoglobin returns to metmyoglobin by releasing O2−. Other sugars, including dR5P, reduced myoglobin at significantly lower rates; for example metmyoglobin reduction and heme loss was not observed at any significant level using glucose or glucose 6-phosphate.
3.2 Myoglobin Glycation
Ion trap determination (using charge state analysis) of the molecular mass of the apomyoglobin generated in the chromatographic flow yielded an initial mass of 16,951 Da appropriate for the native protein. In short time periods (i.e., 3 h) in reaction with R5P at 37 °C, ion signals corresponding to the covalent attachment of one, then two and then three R5P molecules (+ 212 Da each) to the protein were observed. At longer incubation times (>18 h), the mass spectra became un-interpretable as additional R5P molecules add to the protein and the attached sugars rearranged through phosphate cleavage, dehydration, CML formation, etc. Similar analysis of the dR5P reaction shows slower glycation rates; the primary form at 19 h was the addition of a single dR5P molecule (+196 Da, added to a small portion (estimated 30%) of the metmyoglobins); by four days the signal for the un-modified metmyoglobin had disappeared.
3.3 Myo-X Formation
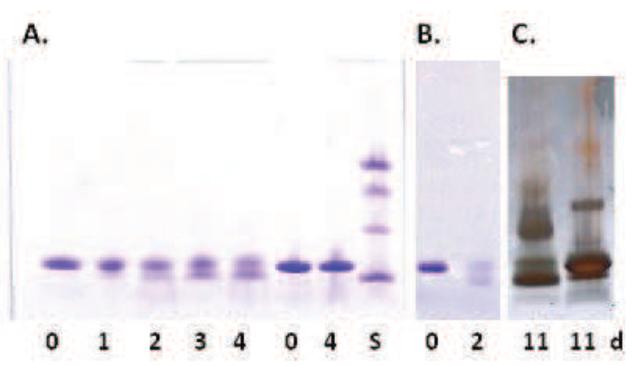
The results of SDS-PAGE analysis of R5P/metmyoglobin, R5P/apomyoglobin and dR5P/myoglobin reactions incubated over a four day period are shown in Figure 2. Under these conditions, the single myoglobin band (without R5P or dR5P) at 17 kDa (heme dissociates from metmyoglobin during the SDS treatment) splits into two bands with apparent molecular masses of approximately 18 kDa and 15 kDa. (The decrease in band intensity observed at extended times results from a lower affinity of the Coomassie stain due to the loss of positive residues[17]; silver staining did not show similar decreases in band intensity (see Figure 2C).) This “band-splitting” effect is also seen in the reaction with equimolar D-ribose/Pi, although at a slightly slower rate. The comparative reactions of myoglobin with D-ribose alone (without Pi), Pi alone (no sugar), ADP-ribose, D-glucose 6-phosphate, D-glucose, and D-glucose/Pi failed to generate these band patterns over a six day time period and only gave the single band at 17 kDa. A similar reaction using A5P produced the band in only low intensity at extended times. To date, we have not observed an analogous process leading to a highly mobile product for any other protein reacted with R5P including BSA, lactalbumin, lysozyme, ribonuclease A, ubiquitin, bovine hemoglobin, and cytochrome c.
Figure 2.
A. SDS PAGE gels of reactions of myoglobin or apomyoglobin (1 mg/mL) with R5P or dR5P (50 mM) at pH = 7.4 and 37 °C with incubation time in days listed. A. Lanes 1 – 5 Metmyoglobin reacting with R5P; Lanes 6 - 7 Metmyoglobin without sugar; Lane 8: Molecular mass standards (Lysozyme (14.4 kDa), carbonic anhydrase (29.5 kDa), ovalbumin (45.0 kDa), BSA (66 kDa).) B. Apomyoglobin reacting with R5P. C. Silver Stained Gel. Lane 1: Myoglobin reacting with dR5P; Lane 2: Myoglobin reacting with R5P (1 mM).
The rate of change of myoglobin from a single band to two bands was correlated to initial R5P concentration with noticeable band-splitting occurring for a 50 mM R5P and 1.0 mg/mL myoglobin reaction at pH = 7.4 and 37 °C typically at 1 – 2 days. Addition of superoxide dismutase (185 U/mL), catalase (95 U/mL), deferoxamine (20 μM), aminoguanidine (20 mM), DMPO (50 mM), or mannitol (10 mM) to the R5P/myoglobin reaction mixture or use of semi-anaerobic conditions did not impact the formation of the two bands. Apomyoglobin in a reaction with R5P also exhibited a similar SDS gel pattern transition over time with an initial single band for apomyoglobin at 16.9 kDa losing intensity over time in favor of a protein of apparent size of approximately 15 kDa (Figure 2B). The development of Myo-X was significantly faster (~ 2-fold) than for metmyoglobin. Unlike the metmyoglobin/R5P results, dimers and higher molecular weight products were clearly evident in the apomyoglobin system by 4 days.
In a surprise finding, dR5P was equally capable of generating Myo-X (Figure 2C). We have previously characterized the glycation rate of dR5P with amines [15] and with cytochrome c [10] and have demonstrated the extremely slow nature of dR5P to form glycation adducts and to generate O2−. At four days of incubation with metmyoglobin, dR5P gave a similar splitting pattern on the SDS PAGE gel as was observed for R5P. Thus, whatever reaction is causing the formation of the Myo-X is equally favorable for dR5P.
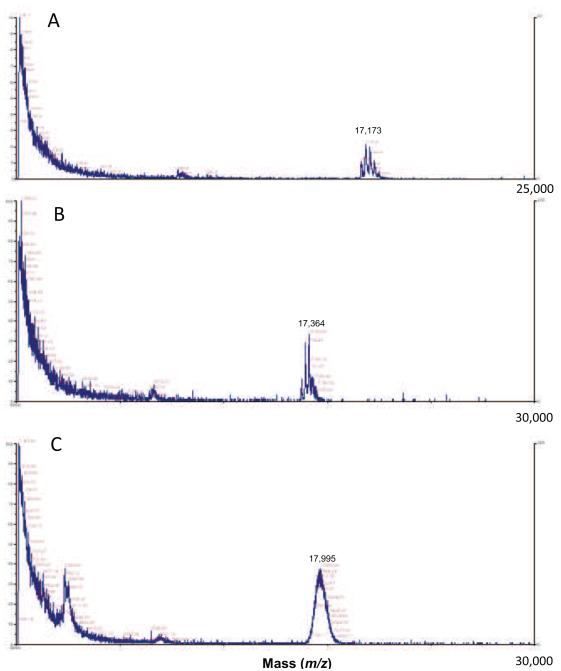
We initially suspected some type of peptide chain fragmentation, perhaps promoted by hydroxyl radical formation created by a Fenton reaction of the glycation-generated hydrogen peroxide. (Ferric ion could be released by the denaturation of myoglobin during glycation.) Peptide fragmentation caused by free radicals (•OH) created in aerobic systems exposed to radiation has been demonstrated for proteins including myoglobin [18-20]. Our gel studies employing deferoxamine, however, failed to reduce the rate of formation of Myo-X. Furthermore, MALDI TOF mass spectrometry analysis showed only progressively higher average molecular masses for the myoglobin (Figure 3) in reaction with R5P. At two hours of reaction, significant glycation (~ up to three R5P molecules added) was indicated by the presence of higher molecular mass forms of specific mass. At four days, few individual signals were observed, instead a large bell-shaped distribution of signal was evident that were centered at ~ 18 kDa. There was no evidence of a signal in the 15 kDa region that would be suggestive of a peptide cleavage process. We further confirmed that a small piece was not being hydrolyzed from the larger metmyoglobin by microfiltration studies (filtration through a 10,000 MWCO filter), gel filtration studies, and by a LC-MS separation and analysis study.
Figure 3.
MALDI TOF analysis of a reaction mixture of R5P (50 mM) and myoglobin (1.0 mg/mL) for 0 h (A), 2 h (B), and 4 d (C) at 37 °C and pH = 7.4. Mass values (m/z) given are for the largest peak in the distribution. (Note that the scan scale is 25,000 Da for A and 30,000 Da for B and C.) Other major peaks in the 0 h and 2 h spectra are typically 212 Da apart indicating the addition of R5P molecules to the 16,950 Da myoglobin molecule. The absence of a signal at approximately 15,000 Da indicates the highly mobile band on the SDS PAGE analysis is not a result of a cleavage of a piece from myoglobin.
An alternative explanation to fragmentation is the formation of an internal cross-link that allows the modified form to move more rapidly through the electrophoresis medium. Evidence of intramolecular cross-linking has been less frequently reported even though it is likely that this type of event might have higher probability due to proximity effects. Intramolecular cross-links are generally harder to assess as their occurrence is not always evident in SDS PAGE results. One possibility for such a link is a Tyr103-Tyr146 cross-link formed in a similar manner to the cross-link that has been observed in dimer formation. The formation of Myo-X in apomyoglobin and in conditions with the free radical scavenger DMPO however suggests this is not the case. The conjugation of a free radical Tyr to an available amine in a reaction promoted by superoxide [21-23] was likewise discounted due to the lack of effect of DMPO on Myo-X formation.
All evidence points to a glycation cross-link as being the cause of Myo-X. In R5P-containing systems with lactalbumin, lysozyme, RNAse A, BSA, and histones, intermolecular cross-links were rapidly generated and observed by SDS PAGE ([14] and unpublished data).
Previous studies in our laboratory have shown that R5P and ribose/Pi systems in the presence of amines generate Amadori products and dicarbonyl compounds at relatively rapid rates [15]. In the dR5P-amine reaction, an Amadori-like product is produced where the new carbonyl forms at C3 rather than at C2 [15]. α-Dicarbonyl compounds such as methylglyoxal are known to be potent protein-protein cross-linkers. In its formation subsequent to the Amadori product in the R5P- and ribose/Pi-amine systems, the dicarbonyl is released to solution where it can then link two available amines [15]. We feel however this set of reactions would likely also promote intermolecular protein cross-links in addition to intramolecular cross-links, something we fail to observe in our SDS PAGE results. Furthermore, the dR5P-amine system does not appear to release the amine following its “Amadori” product formation and instead tends to prefer second (and possibly third) reactions with additional amines [15]. Deoxyribose compounds have been demonstrated to link two amines in a glycation reaction [24].
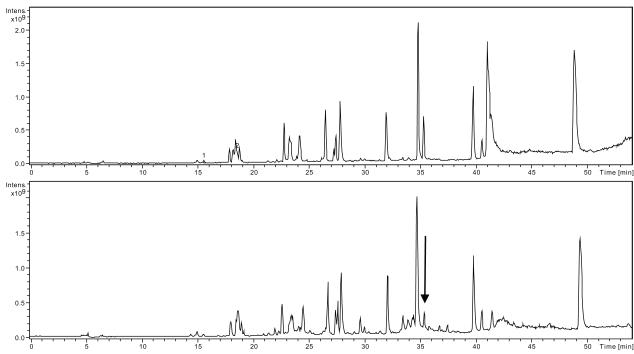
We employed trypsin fragmentation studies to determine the participants of the glycation cross-link. LC/MS fragmentation analysis of the myoglobin/R5P incubation at various times revealed a rapid loss of a peak from the total ion chromatogram (TIC) (Figure 4) which was shown by extracted ion chromatogram analysis (EIC) to be the tryptic fragment at the N-terminus of myoglobin. Within 24 h, more than 75 % reduction of the initial tryptic peptide (amino acids #1 - 16) was observed while other peptides showed less modification (generally in the range 0 – 30 %). The modification of the first segment is on the N-terminal Gly (as opposed to Lys16) as was clearly indicated by the MSn spectrum of modified forms of the initial peptide and by a lack of a corresponding change in the intensity of the second peptide. The first tryptic peptide contains two Trp residues that lend it a high 280 nm absorbance in comparison to all other peptides. The A280 chromatograms of the myoglobin/R5P with time (up to 144 h) show a decrease in the A280 signal for the first piece but no corresponding increase of a single signal at other retention times. Possible explanations are that multiple glycation forms are being produced and/or that the Trp residues are being oxidized by the generated free radicals in a manner to reduce 280 nm absorption [25].
Figure 4.
Total ion chromatograms (TIC) of trypsin digests of reactions of R5P (50 mM) and myoglobin (1.0 mg/mL) for 0 h (A) and 72 h (B) at 37 °C and pH = 7.4. Peak eluting at 35.5 min (noted by arrow) represents the first tryptic fragment (N-terminal) which decreased in intensity with time. Numerous peaks forming at the base of this signal suggests multiple modifications occur on this myoglobin fragment upon reaction with R5P.
Additionally, we performed in-gel trypsin digestion of the Myo-X band of the SDS gel (3 day reaction of R5P with myoglobin at 37 °C) and compared the relative EIC intensities of the peptide fragments contained in the digest to those of an unreacted myoglobin band digest. The two fragments that disappeared from the profile at significantly higher amounts were the N-terminal fragment (G1 through K16, decreased to ~ 15% of original) and an internal fragment (H119 through K133, decreased to ~ 5% of original). No other trypsin fragment decreased by more than 50% of original. There was no significant decrease in the fragment immediately subsequent to the H119 – K133 fragment.
We propose the internal cross-link occurs when a newly formed Amadori product of the glycation-prone N-terminus in the R5P, dR5P, or ribose system is attacked by a neighboring Lys. The most likely candidate is Lys133, a residue whose ε-amine group associates via a hydrogen bond with the carbonyl oxygen of the N-terminal glycine in the native protein. The in-gel trypsin digestion of the SDS PAGE Myo-X band identified the N-terminus and the fragment terminated by Lys133 as being the two pieces most highly impacted in this form by the glycation events. Per our proposed scenario, a reaction of Lys133 with an N-terminal Amadori product would initially produce a reversible Schiff base, followed by a rearrangement (this one being irreversible) to form the observed cross-link.
This study demonstrates the unique reactivity of ribose sugars with myoglobin leading to heme reduction, rapid heme degradation, and protein modification. Only R5P, dR5P, and ribose/Pi reactions with myoglobin generated the Myo-X band. Myoglobin appears unique among proteins in exhibiting the generation of a protein form with increased mobility. The study has implications to the status of intracellular myoglobin in the presence of R5P and R5P-related compounds which promote these protein modifying and reactive oxygen species-generating reactions.
Acknowledgements
This work was supported by a grant from the Petroleum Research Fund (PRF #44216-B4), administered by the American Chemical Society and by the Vermont Genetics Network (VGN), a five year IDeA Networks of Biomedical Research Excellence (INBRE) project sponsored by the National Institutes of Health. The authors thank the Proteomics Group at the University of Vermont for providing the MALDI TOF analyses.
Abbreviations Used
- AGEs
advanced glycation end-products
- A5P
D-arabinose 5-phosphate
- CML
carboxymethyl lysine
- dR5P
2-deoxyribose 5-phosphate
- DMPO
dimethyl-1-pyrroline N-oxide
- LC-MS
liquid chromatography – mass spectrometry
- MALDI TOF
matrix assisted laser desorption ionization – time of flight
- metMb
metmyoglobin
- R5P
D-ribose 5-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ. Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- [2].Cussimanio BL, Booth AA, Todd P, Hudson BG, Khalifah RG. Unusual susceptibility of heme proteins to damage by glucose during non-enzymatic glycation. Biophys. Chem. 2003;105:743–755. doi: 10.1016/s0301-4622(03)00100-5. [DOI] [PubMed] [Google Scholar]
- [3].Catalano CE, Choe YS, de Montellano P.R. Ortiz. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J. Biol. Chem. 1989;264:10534–10541. [PubMed] [Google Scholar]
- [4].Nagababu E, Rifkind JM. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39:12503–12511. doi: 10.1021/bi992170y. [DOI] [PubMed] [Google Scholar]
- [5].Roy A, Sen S, Chakraborti AS. In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radic. Res. 2004;38:139–146. doi: 10.1080/10715160310001638038. [DOI] [PubMed] [Google Scholar]
- [6].Wilks A, de Montellano P.R. Ortiz. Intramolecular translocation of the protein radical formed in the reaction of recombinant sperm whale myoglobin with H2O2. J. Biol. Chem. 1992;267:8827–8833. [PubMed] [Google Scholar]
- [7].Tew D, de Montellano P.R. Ortiz. The myoglobin protein radical. Coupling of Tyr-103 to Tyr-151 in the H2O2-mediated cross-linking of sperm whale myoglobin. J. Biol. Chem. 1988;263:17880–17886. [PubMed] [Google Scholar]
- [8].Lardinois OM, de Montellano PR. H2O2-mediated cross-linking between lactoperoxidase and myoglobin: elucidation of protein-protein radical transfer reactions. J. Biol. Chem. 2001;276:23186–23191. doi: 10.1074/jbc.M102084200. [DOI] [PubMed] [Google Scholar]
- [9].Smith PR, Thornalley PJ. Mechanism of the degradation of non-enzymatically glycated proteins under physiological conditions. Studies with the model fructosamine, N epsilon-(1-deoxy-D-fructos-1-yl)hippuryl-lysine. Eur. J. Biochem. 1992;210:729–739. doi: 10.1111/j.1432-1033.1992.tb17474.x. [DOI] [PubMed] [Google Scholar]
- [10].Gersten RA, Gretebeck LM, Hildick-Smith G, Sandwick RK. Maillard reaction of ribose 5-phosphate generates superoxide and glycation products for bovine heart cytochrome c reduction. Carbohydr. Res. 2010;345:2499–2506. doi: 10.1016/j.carres.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhattacherjee A, Chakraborti AS. Fructose-induced modifications of myoglobin: Change of structure from met (Fe3+) to oxy (Fe2+) form. Int. J. Biol. Macromol. 2011;48:202–209. doi: 10.1016/j.ijbiomac.2010.11.003. [DOI] [PubMed] [Google Scholar]
- [12].Gill SC, Von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- [13].Tang J, Faustman C, Hoagland TA. Krzywicki Revisited: Equations for Spectrophotometric Determination of Myoglobin Redox Forms in Aqueous Meat Extracts. J. Food Sci. 2004;69:C717–C720. [Google Scholar]
- [14].Sandwick R, Johanson M, Breuer E. Maillard reactions of ribose 5-phosphate and amino acids. Ann. N. Y. Acad. Sci. 2005;1043:85–96. doi: 10.1196/annals.1333.011. [DOI] [PubMed] [Google Scholar]
- [15].Munanairi A, O’Banion SK, Gamble R, Breuer E, Harris AW, Sandwick RK. The multiple Maillard reactions of ribose and deoxyribose sugars and sugar phosphates. Carbohydr. Res. 2007;342:2575–2592. doi: 10.1016/j.carres.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Finkelstein E, Rosen GM, Rauckman EJ, Paxton J. Spin trapping of superoxide. Mol. Pharmacol. 1979;16:676–685. [PubMed] [Google Scholar]
- [17].Tal M, Silberstein A, Nusser E. Why does Coomassie Brilliant Blue R interact differently with different proteins? A partial answer. J. Biol. Chem. 1985;260:9976–9980. [PubMed] [Google Scholar]
- [18].Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem. J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hunt JV, Simpson JA, Dean RT. Hydroperoxide-mediated fragmentation of proteins. Biochem. J. 1988;250:87–93. doi: 10.1042/bj2500087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puchala M, Schuessler H. Oxygen effect in the radiolysis of proteins. IV. Myoglobin. Int. J. Pept. Protein Res. 1995;46:326–332. doi: 10.1111/j.1399-3011.1995.tb00605.x. [DOI] [PubMed] [Google Scholar]
- [21].Mozziconacci O, Mirkowski J, Rusconi F, Pernot P, Bobrowski K, Houee-Levin C. Superoxide radical anions protect enkephalin from oxidation if the amine group is blocked. Free Radic. Biol. Med. 2007;43:229–240. doi: 10.1016/j.freeradbiomed.2007.04.006. [DOI] [PubMed] [Google Scholar]
- [22].Nagy P, Kettle AJ, Winterbourn CC. Superoxide-mediated formation of tyrosine hydroperoxides and methionine sulfoxide in peptides through radical addition and intramolecular oxygen transfer. J. Biol. Chem. 2009;284:14723–14733. doi: 10.1074/jbc.M809396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winterbourn CC, Parsons-Mair HN, Gebicki S, Gebicki JM, Davies MJ. Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem. J. 2004;381:241–248. doi: 10.1042/BJ20040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tressl R, Wondrak GT, Kruger RP, Rewicki D. New Melanoidin-like Maillard Polymers from 2-Deoxypentoses. J. Agric. Food Chem. 1998;46:104–110. doi: 10.1021/jf970657c. [DOI] [PubMed] [Google Scholar]
- [25].Finley EL, Dillon J, Crouch RK, Schey KL. Identification of tryptophan oxidation products in bovine alpha-crystallin. Protein Sci. 1998;7:2391–2397. doi: 10.1002/pro.5560071116. [DOI] [PMC free article] [PubMed] [Google Scholar]