Abstract
Objective
We investigated exercise effects on health-related quality of life (HRQOL) and exercise self-efficacy, and tested effect modification by baseline body mass index (BMI) and gender.
Methods
Middle-aged women (n=100) and men (n=102) were randomly assigned to either exercise (360 minutes/week of moderate-to-vigorous aerobic exercise) or control in Seattle, WA from 2001–2004. Demographics, anthropometrics, exercise self-efficacy (5-item self-efficacy questionnaire) and HRQOL (SF-36) were assessed at baseline and 12 months. Analysis of covariance adjusting for baseline scores was used to compare HRQOL and exercise self-efficacy scores between the exercise and control groups.
Results
At 12 months, exercisers demonstrated higher exercise self-efficacy than controls (percent change from baseline: −6.5% vs. −15.0%, p<0.01), without differences in HRQOL. Baseline BMI category and gender did not modify these effects. In exploratory analyses comparing exercisers and controls within subgroups defined by gender and BMI, 12-month HRQOL scores [(role-physical (+7.0% vs. −13.1%), vitality (+15.6% vs. −4.2%), social functioning (+10.0% vs. −3.5%), and mental health (+6.8% vs. −2.9%)] were higher only among overweight male exercisers (p<0.05, vs. control).
Conclusion
360 minutes/week of exercise, recommended for weight maintenance, did not have negative effects on exercise self-efficacy or HRQOL. This level of exercise may increase HRQOL among overweight men.
Introduction
Sixty minutes of moderate-to-vigorous-intensity exercise on most days of the week is recommended for adult weight maintenance (Physical Activity Guidelines Advisory Committee, 2008). However, little is known about the effect of this high exercise dose on health-related quality of life (HRQOL) and exercise self-efficacy.
Many studies have shown a positive relationship between exercise dose and HRQOL. In the Nurses’ Health study (N=56,436), exercise dose was positively associated with physical functioning, role-limitation, bodily pain and vitality scores (Michael et al., 1999). Two reviews found positive cross-sectional and longitudinal relationships between exercise dose and HRQOL (Bize et al., 2007) (Spirduso and Cronin, 2001).
However, it is not clear if higher doses of exercise are related to better HRQOL compared to moderate doses. A study among older adults (55–89 years old) found that moderately (150–420 minutes/week) and highly active (>420 minutes/week) vs. inactive (<150 minutes/week) individuals showed higher HRQOL (physical and mental composite scores), while there were no differences between moderately and highly active groups (Mummery et al., 2004). Similarly, a survey of European adults found that being physically active (>24 MET-h/week) is associated with better mental health compared with being less active (≤24 MET-h/week) (Abu-Omar et al., 2004), but the highest physical activity group (≥51.11 MET-h/week) did not consistently have better mental health compared with the second highest group (24.1–51.1 MET-h/week). Finally, 2001 data from the Behavioral Risk Factor Surveillance System suggested individuals who exercised >90 minutes/day or everyday had lower HRQOL compared with those exercising 30–60 minutes/day or 5–6 days/week (Brown et al., 2004). To our knowledge, no previous exercise trials have tested the effect of 360 minutes/week of moderate-to-vigorous intensity exercise on HRQOL.
Self-efficacy refers to one’s confidence in performing an activity (Bandura, 1997). Observational studies have shown positive associations between exercise self-efficacy and exercise behavior (Booth et al., 2000; Marcus et al., 1992; Sternfeld et al., 1999). However, the findings on the effects of exercise intervention on exercise self-efficacy are mixed (Castro et al., 1999; Gallagher et al., 2006; Hughes et al., 2004; McAuley et al., 2003b; McAuley et al., 2010). In a 12-month randomized controlled trial among 144 previously inactive older adults, McAuley and colleagues reported decreased exercise self-efficacy in both exercise (120 minutes/week) and flexibility-toning-balance groups (McAuley et al., 2010). Another exercise trial testing 3 times/week of walking among 174 older adults also found a decline in exercise self-efficacy during the 6-month intervention (McAuley et al., 2003b). Among 150 older adults with osteoarthritis, the exercise group (270 minutes/week) reduced exercise self-efficacy during the 8-week intervention (Hughes et al., 2004). Considering that exercise self-efficacy predicts exercise behavior (McAuley, 1993; McAuley et al., 2003a; McAuley et al., 2007) and that 360 minutes/week of exercise is recommended for weight maintenance, it is important to assess the effects of this exercise dose on exercise self-efficacy.
Increased HRQOL and self-efficacy have been shown to predict intervention adherence (Barbour and Miller, 2008; Jones et al., 2005; Keller et al., 1999). Studies have also shown lower exercise adherence in participants with higher BMI and in women (Bautista-Castano et al., 2004; Courneya et al., 2008b; Irwin et al., 2004; Jackson et al., 2005; Latka et al., 2009). It is possible that baseline BMI and gender may modify intervention-related changes in psychological predictors of adherence (exercise self-efficacy and HRQOL), leading to the differences in adherence.
Because no intervention studies have tested the effect of a high exercise dose of 360 minutes/week on HRQOL and exercise self-efficacy, the effects of the recommended amount of exercise for weight maintenance on HRQOL and exercise self-efficacy are unknown. Some studies have shown that adherence differs by BMI and gender. However, the reasons are unknown. The purposes of this study were to: 1) investigate the overall effects of a 12-month exercise intervention on exercise self-efficacy and HRQOL; and 2) determine whether BMI and gender modified the observed effects. We hypothesized that the 12-month exercise intervention would increase exercise self-efficacy and HRQOL but that female gender and higher baseline BMI would be associated with smaller changes in exercise self-efficacy and HRQOL.
Methods
Participants
The study took place at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA. Both men (n=102) and women (n=100) residing in the Seattle area were recruited to a trial that examined the effects of exercise on colon cancer biomarkers (Campbell et al., 2007; McTiernan et al., 2006). Eligibility criteria included: 40 to 75 years old; colonoscopy within the previous 3 years; engaged in < 90 minutes/ week of moderate-to-vigorous intensity exercise during the previous 3 months [or low-fitness on VO2max testing]; <two servings of alcohol/day; no history of invasive cancer or high risk for colon cancer (e.g., familial polyposis, ulcerative colitis) or other serious medical conditions; normal response to a maximal exercise tolerance test; normal complete blood count and blood chemistries; and no contraindications for colon biopsy.
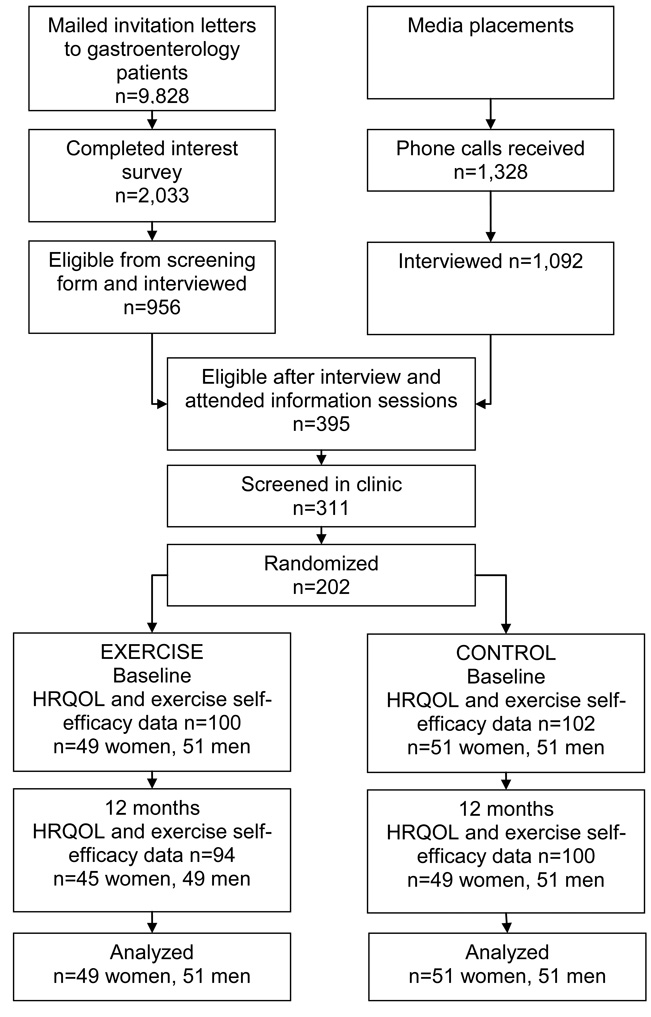
Participants were recruited though gastroenterology practices, media placement, flyers, a study web site, and referrals between 2001 and 2004 (Figure 1). Of the invitation letters sent to 9,828 gastroenterology patients, 2,033 (21%) responded, 956 were eligible and were interviewed. A total of 1,328 people responded to media, and 1,092 were interviewed. Principle reasons for ineligibility were unwilling to be randomized (n=297), too active (n=339), and insufficient time for the study (n=48). A total of 395 attended information sessions, 311 were screened in clinic, and 202 were enrolled. All participants completed the informed consent approved by the FHCRC Institutional Review Board.
Figure 1.
Recruitment and flow of participants in the study, Fred Hutchinson Cancer Research Center, Seattle, WA, 2001–2004
NOTE: HRQOL = health-related quality of life
Participants were randomized into exercise (n=100) or control (n=102) groups blocked on gender, use of non-steroidal anti-inflammatory (NSAIDs) medications (≥2 times/week vs. less often), current smoking status, and among women, menopausal status and current use of postmenopausal hormone therapy. Randomization was performed by the study coordinator using a computerized program developed by the study biostatistician.
The goal of the intervention was 60 minutes per day, 6 days per week of moderate-to-vigorous intensity aerobic exercise performed at facilities and at home, with gradual increase over the first 12 weeks. Participants were required to exercise 3 times/week at one of four facilities under the supervision of exercise specialists. Participants were provided with Polar (Polar Electro Inc., Lake Success, NY) heart rate monitors and advised to exercise at 60%–85% of their maximal heart rate on their baseline VO2max test. Participants also were asked to exercise at home or at other exercise facilities for 3 days per week with the same instructions for exercise duration and intensity. To maintain adherence, a variety of strategies were used, such as regular monitoring and feedback, monthly progress review meetings with trained exercise specialists, newsletters, incentives (e.g., water bottles), and group social events. Exercisers were asked not to change their dietary habits during the trial.
Controls were asked not to change their exercise or diet habits during the trial. After completion of all the 12-month measures, controls were offered the opportunity to participate in exercise classes and use the exercise facilities for 2 months.
Measurements
At baseline and 12 months, demographic (gender, age, ethnicity, education) and health information (NSAID use, menopausal status, current smoking habits) were collected by self-administered questionnaire. Physical activity levels were assessed at baseline, 3, 6, 9, and 12 months via an interviewer-administered Physical Activity Questionnaire that measured recreational, sports, household, work, and transportation-related physical activity over the previous 3 months (Taylor et al., 1978). At baseline and 12 months, all participants underwent VO2max testing to assess cardiopulmonary fitness (Pate et al., 1991). Exercise participants also kept logs of recreational/sports activities, daily steps assessed by pedometers, and maximal heart rate during facility sessions.
Height and weight were assessed in the clinic at baseline and 12 months to the nearest 0.1cm and 0.1 kg, respectively using a stadiometer and balance-beam scale. Each participant was measured twice, and the average was recorded. BMI was calculated as weight [kg]/height [m]2, and participants were categorized into: normal (BMI<25), overweight (25≤ BMI <30), and obese (BM ≥30) (World Health Organization, 2009).
Exercise self-efficacy was assessed using a previously validated 5-item exercise self-efficacy scale (Marcus et al., 1992). This scale has been employed in studies of both non-clinical (Folta et al., 2009; Yates et al., 2009) and clinical populations (Dutton et al., 2009). Scores ranged from 0 to 55 with higher scores indicating greater self-efficacy for exercise.
HRQOL was assessed by the SF-36 Health Status Survey Short Form (Ware, 1993). Eight subscales of the SF-36 (i.e., physical functioning, role-physical, bodily pain, vitality, general health, social functioning, role-emotional, and mental health) were calculated according to the SF-36 scoring manual (Ware, 1993). Raw scores on each subscale were converted to transformed scores ranging from 0 to 100 with higher scores indicating greater HRQOL in that domain.
Statistical analysis
Descriptive analyses were performed to understand the characteristics of the study participants. The study retention was 96% (n=194). Eight missing cases of the 12-month data were imputed using the last observation carried forward, according to intention-to-treat principles. Baseline characteristics were compared between exercise and control groups by t-test, chi-square test, and Fisher’s exact tests, as appropriate. Analyses of covariance (ANCOVA) models adjusting for baseline scores were used to compare 12-month exercise self-efficacy and HRQOL scores between exercise and control groups. In exploratory analyses, we also compared 12-month exercise self-efficacy and HRQOL scores between exercisers and controls within subgroups defined by gender and baseline BMI category. All analyses were performed by SAS program version 9.1 (SAS Institute Inc, Cary, NC).
Results
Baseline characteristics were not significantly different between exercise and control groups (Table 1). The proportion of women (p=0.05) and non-Hispanic white (p=0.18) participants was different across the 3 BMI categories. We used a p-value of 0.2 to determine potential covariates for model testing; thus, these variables were included as covariates in the subsequent analyses. Baseline pedometer count (p<0.01) and physical fitness (p<0.01) were significantly higher among participants in normal-weight compared to obese participants.
Table 1.
Baseline characteristics of study participants, Fred Hutchinson Cancer Research Center, Seattle, WA, 2001–2004
| Exercise | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| total | BMI category | total | BMI category | p-value | |||||
| BMI<25 | 25≤BMI<30 | 30≤BMI | BMI<25 | 25≤BMI<30 | 30≤BMI | ||||
| n=100 | n=23 | n=34 | n=43 | n=102 | n=20 | n=38 | n=44 | ||
| Female, n (%) | 49 (49.0) | 16 (69.6) | 14 (70.0) | 19 (41.2) | 51 (50.0) | 14 (47.4) | 18 (44.2) | 19 (43.2) | 0.89* (0.10) |
| Age (years), Mean (SD) | 55.4 (6.9) | 55.1 (6.5) | 54.2 (7.4) | 56.4 (6.7) | 55.2 (6.8) | 55.1 (7.1) | 54.8 (6.9) | 55.5 (6.8) | 0.85* (0.81) |
| Race (non-Hispanic White), n (%) | 90 (90.0) | 19 (82.6) | 33 (97.1) | 38 (88.4) | 95 (93.1) | 17 (85.0) | 37 (97.4) | 41 (93.2) | 0.42* (0.18) |
| College degree, n (%) | 61 (61.0) | 15 (65.2) | 22 (64.7) | 24 (55.8) | 62 (60.8) | 14 (70.0) | 23 (60.5) | 25 (56.8) | 0.97* (0.87) |
| Colon polyp Hx, n (%) | 57 (57.0) | 11 (47.8) | 21 (61.8) | 25 (58.1) | 58 (56.9) | 10 (50.0) | 20 (52.6) | 28 (63.6) | 0.98* (0.76) |
| NSAID use, n (%) | 37 (37.0) | 10 (43.5) | 10 (29.4) | 17 (39.5) | 41 (40.2) | 5 (25.0) | 14 (36.8) | 22 (50.0) | 0.64* (0.36) |
| Smoker, n (%) | 6 (6.0) | 1 (4.4) | 4 (11.8) | 1 (2.3) | 6 (5.9) | 0 (0.0) | 1 (2.6) | 5 (11.4) | 0.97* (0.24) |
| Post-menopause, n (%) | 30 (61.2) | 10 (62.5) | 8 (57.1) | 12 (63.2) | 33 (64.7) | 9 (64.3) | 11 (61.1) | 13 (68.4) | 0.72* (0.99) |
| BMI (kg/m2), Mean (SD) | 29.3 (4.7) | 23.7 (1.2) | 27.7 (1.4) | 33.6 (3.4) | 29.3 (4.9) | 23.1 (1.2) | 27.3 (1.4) | 33.8 (3.3) | 0.98* (<0.01) |
| Pedometer count (steps/day), Mean (SD) | 5963 (2662) | 7366 (2444) | 6309 (2772) | 4914 (2283) | 6425 (3069) | 6931 (2374) | 8117 (3410) | 4193 (1977) | 0.26* (<0.01) |
| PA (min/week), Mean (SD) | 55.5 (92) | 81 (135) | 56 (80) | 42 (69) | 58.4 (84) | 45 (53) | 82 (108) | 44 (65) | 0.81* (0.18) |
| Vo2max, Mean (SD) | 27.1 (3.3) | 30.4 (6.4) | 29.2 (6.0) | 23.5 (4.7) | 27.5 (6.3) | 29.7 (8.6) | 29.6 (5.4) | 24.8 (4.5) | 0.58* (<0.01) |
p-values for differences between exercise and control group, ( ) p-values for differences across 3 BMI categories
The results of the intervention effects on exercise adherence and body composition have been reported elsewhere (McTiernan et al., 2007) but are summarized here to aid in interpretation of the self-efficacy and HRQOL results. In brief, exercisers significantly increased physical activity levels compared with controls. Mean weekly minutes of moderate-to-vigorous physical activity over the 12-months trial period were 370 minutes/week (102.7% of goal) for male exercisers and 295 minutes/week (82% of goal) for female exercisers, respectively. Aerobic fitness (VO2max) increased by 2.5 ml/kg/min (10.5%) in female exercisers and 3.3 ml/kg/min (11%) in male exercisers, and decreased in both female and male controls (both p<0.001 comparing exercisers to controls). There were no significant changes in mean total daily caloric intake in either exercisers or controls (data not shown). The percentages of participants who had an injury were not different between exercisers (28%) and controls (27%) (Campbell et al., in press).
Overall intervention effects on HRQOL and exercise self-efficacy
There was no significant difference in the baseline exercise self-efficacy or HRQOL scores between the exercisers and controls (Table 2). Adjusting for baseline scores, ethnicity, and gender, the 12-month exercise self-efficacy score was significantly higher in exercisers compared to controls [mean (SD) 41.5 (11.5) vs. 36.6 (12.2), p<0.01]. Both exercise and control groups decreased exercise self-efficacy scores during the trial with a smaller decline in the exercise group (ΔExercise= −2.9, −6.5% vs. ΔControl= −6.5, −15.0%). There were no significant differences in 12-month HRQOL subscale scores (all p>0.05).
Table 2.
Baseline, 12 months, and change scores in HRQOL and exercise self-efficacy, Fred Hutchinson Cancer Research Center, Seattle, WA, 2001–2004
| Exercise | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) |
12 months Mean (SD) |
change | Baseline Mean (SD) |
12 months Mean (SD) |
change | p-value* | |||
| Δ | %Δ | Δ | %Δ | ||||||
| SF-36 subscales | |||||||||
| Physical functioning | 90.2 (11.6) | 91.0 (16.5) | 0.9 | 1.0 | 90.6 (10.2) | 88.9 (13.7) | −1.7 | −1.9 | 0.21 |
| Role-physical | 89.0 (21.8) | 84.5 (28.8) | −4.5 | −5.1 | 83.6 (27.6) | 79.7 (30.6) | −3.9 | −4.7 | 0.51 |
| Bodily pain | 77.4 (17.7) | 76.1 (19.5) | −1.3 | −1.7 | 80.2 (16.8) | 72.8 (21.3) | −7.4 | −9.2 | 0.14 |
| General health | 56.4 (7.2) | 56.8 (6.2) | 0.4 | 0.7 | 57.8 (6.6) | 56.4 (6.7) | −1.7 | −2.9 | 0.10 |
| Vitality | 55.8 (15.4) | 59.5 (16.6) | 3.6 | 6.5 | 55.4 (15.0) | 56.4 (16.1) | 1.0 | 1.8 | 0.11 |
| Social functioning | 91.5 (14.1) | 90.0 (19.4) | −1.5 | −1.6 | 90.2 (14.8) | 89.8 (17.8) | −0.4 | −0.4 | 0.84 |
| Role-emotional | 89.3 (23.2) | 89.0 (26.4) | −0.3 | −0.3 | 84.6 (27.6) | 90.2 (24.6) | 5.6 | 6.6 | 0.23 |
| Mental health | 79.7 (11.4) | 81.8 (12.7) | 2.1 | 2.6 | 79.7 (12.5) | 80.8 (14.8) | 1.1 | 1.4 | 0.49 |
| Exercise self-efficacy | 44.4 (9.4) | 41.5 (11.5) | −2.9 | −6.5 | 43.2 (10.0) | 36.6 (12.2) | −6.5 | −15.0 | 0.01 |
p-values for differences in 12-month scores between exercise and control group, adjusting for baseline scores.
NOTE: HRQOL = health-related quality of life; HRQOL and exercise self-efficacy scales are coded with higher scores indicating greater HRQOL or exercise self-efficacy.
Modification of intervention effects by baseline BMI and gender
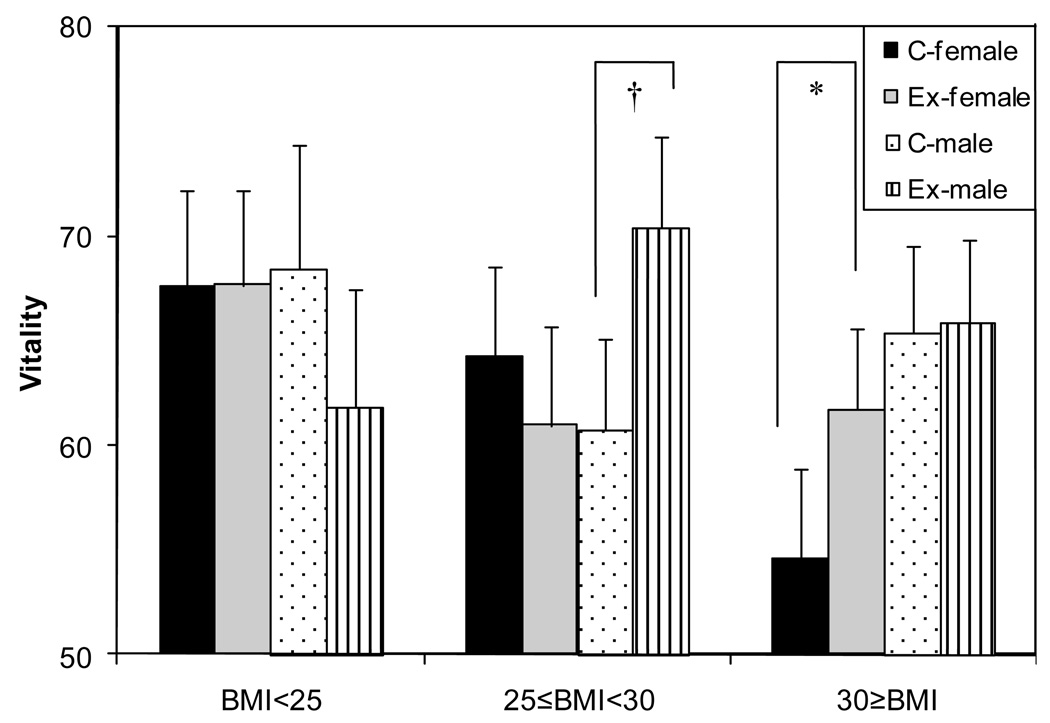
To understand whether baseline BMI and gender modified the effects of the intervention on exercise self-efficacy and HRQOL, we tested interactions between baseline BMI category or gender and study arm, controlling for baseline values, ethnicity, and gender (in the BMI interaction model only). The 2-way interactions (BMI category × study arm, gender × study arm) were not significant for any outcome variables (results not shown). We then tested the 3-way interaction of gender, BMI category, and study arm. The 3-way interaction was significant (p=0.03) for 12-month vitality score (Figure 2). Among normal weight participants, the vitality score was not significantly different between exercisers and controls (men p=0.32, women p=0.98). Among overweight participants, the 12-month vitality score was significantly higher in male exercisers (p<0.01) than in male controls, but this was not seen among women (p=0.44). Among obese participants, the 12-month vitality score was marginally higher in female exercisers (p=0.07) compared to that of controls. In obese men, the difference between exercisers and controls was not significant. The 3-way interactions (gender × BMI category × study arm) were not significant for other 7 subscales of SF-36 (p-value >0.05) or exercise self-efficacy (p=0.18, results not shown).
Figure 2.
Depiction of the 3-way interaction between baseline BMI category gender, and intervention effect on vitality, Fred Hutchinson Cancer Research Center, Seattle, WA, 2001–2004
† p<0.01, *p=0.07
p-values for differences between exercisers and controls within subgroups defined by baseline BMI and gender, adjusted for baseline scores and ethnicity
NOTE: the vitality scale is coded with higher scores indicating greater vitality.
Comparisons of intervention effects within subgroups defined by baseline BMI and gender
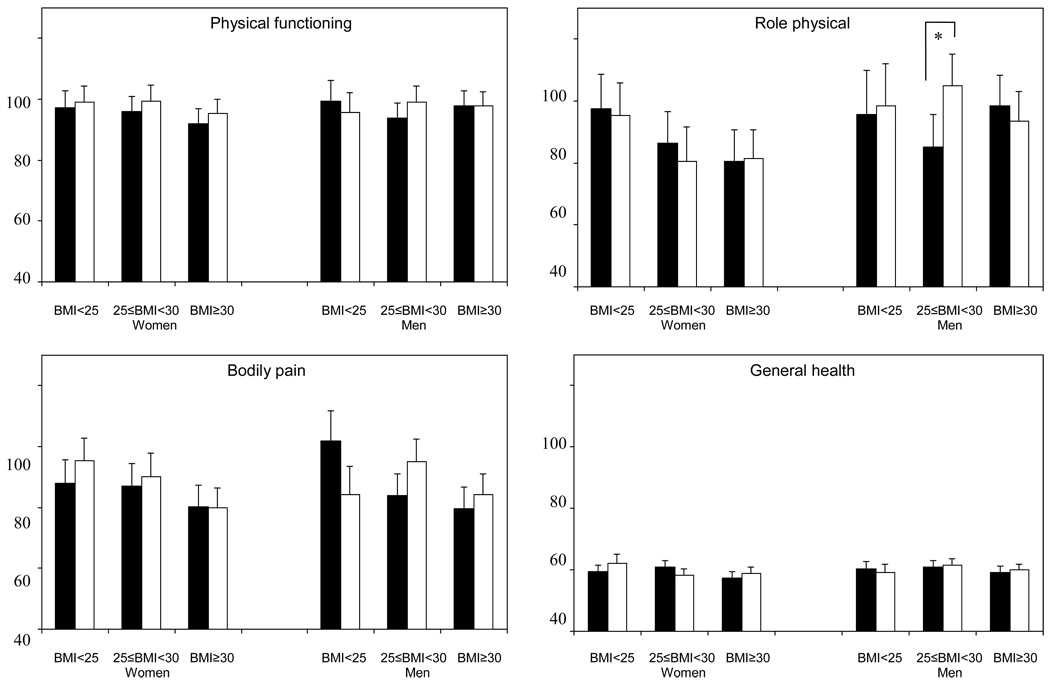
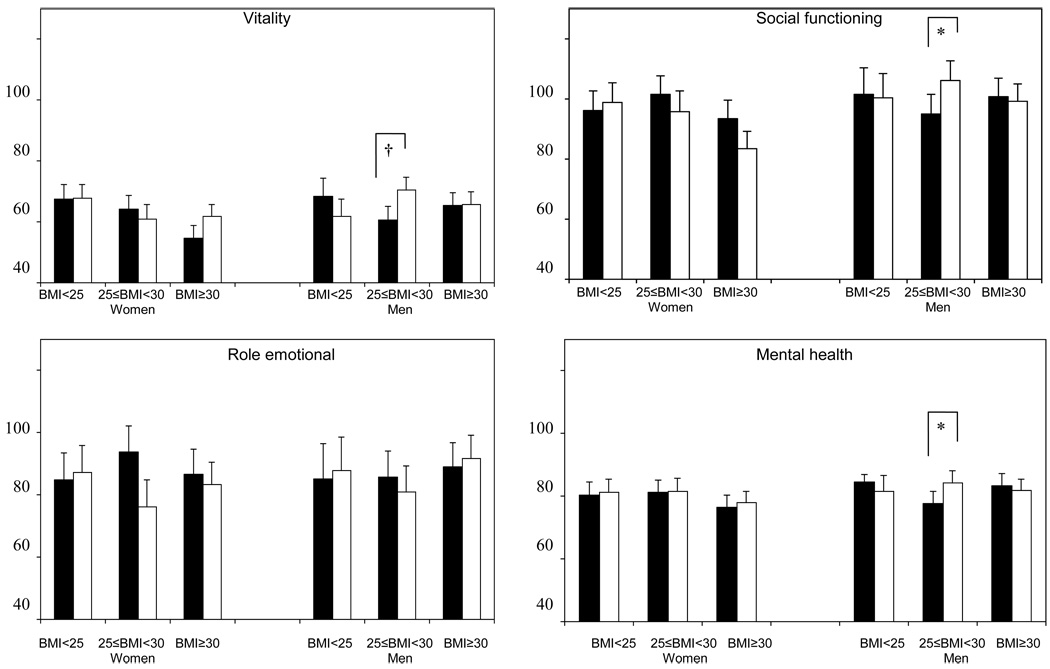
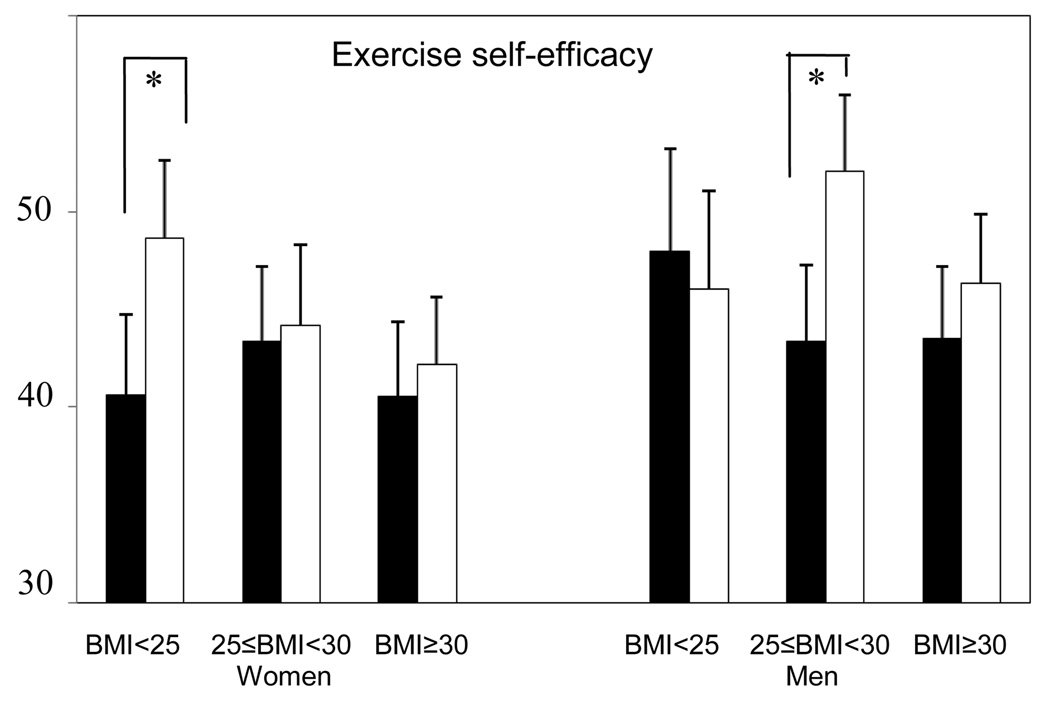
HRQOL and exercise self-efficacy scores at 12 months were compared between exercisers and controls within subgroups defined by baseline BMI and gender (Figure 3). Among overweight men at baseline, 4 subscales of SF-36 (role-physical, vitality, social functioning, and mental health) were significantly higher among exercisers compared with controls (p<0.05). There were no differences between exercisers and controls in other subgroups (p>0.05). The 12-month exercise self-efficacy scores were higher among normal weight female and overweight male exercisers compared with baseline BMI and gender-matched controls (p<0.05).
Figure 3.
Comparison of HRQOL across BMI categories and gender (Solid bars: control group, Open bars: exercise group), Fred Hutchinson Cancer Research Center, Seattle, WA, 2001–2004
*p<0.05, †p<0.01
p-values for differences between exercisers and controls within subgroups defined by baseline BMI and gender, adjusted for baseline scores and ethnicity
NOTE: HRQOL = health-related quality of life; HRQOL scales are coded with higher scores indicating greater HRQOL.
Discussion
This study examined the effects of 360 minutes/week of moderate-to-vigorous exercise intervention on exercise self-efficacy and HRQOL in middle-aged adults. We found that exercise self-efficacy was higher among the exercise group compared with controls and that 360 minutes/week of moderate-to-vigorous exercise did not have negative effects on any aspects of HRQOL among previously sedentary middle-aged adults. Our findings suggest that the 360 minutes/week of moderate-to-vigorous exercise could be safety implemented among previously sedentary adults, regardless of gender or baseline obesity status.
The 12-month exercise intervention did not increase any aspects of HRQOL. Previous studies examining the exercise effects on HRQOL among general populations have reported mixed findings (Bize et al., 2007). Several factors may be important to understand the different findings of the exercise effects on HRQOL.
The greater exercise dose tested in our trial may have resulted in our findings of non-significant changes in HRQOL. Previous trials testing up to 225 minute/week of exercise reported positive intervention effects on HRQOL. A 6-month exercise intervention testing doses of 4, 8, and12 kcal/kg/week among sedentary, overweight/obese postmenopausal women found a dose-response effect on all aspects of HRQOL except vitality (Martin et al., 2009). Our previous 12-month exercise intervention (225 minutes/week) improved physical functioning and general health scores among sedentary postmenopausal women (vs. controls) (Bowen et al., 2006). Improved HRQOL was also noted in another 12-month exercise trial (60 minutes/day, 3 times/week) among middle-aged adults (Sorensen et al., 1999). To the best of our knowledge, no previous intervention studies have tested the effect of 360 minutes/week of exercise on HRQOL; however, some cross-sectional studies have shown that the dose-response relationship between exercise dose and HRQOL may not be linear at a high exercise doses (Abu-Omar et al., 2004; Brown et al., 2004; Mummery et al., 2004). Moderate doses of exercise may be more beneficial than high doses of exercise for improving HRQOL.
Another potential reason for the non-significant effect of exercise on HRQOL may be our sample characteristics. We enrolled healthy sedentary, middle-aged adults. Rehabilitation programs have shown greater increases in overall QOL compared with non-clinical populations (Gillison et al., 2009). In addition, our samples had high levels of HRQOL at baseline (Ware, 1993) which may have caused ceiling effects, limiting our ability to detect positive changes in HRQOL.
Gender and baseline BMI did not modify the intervention effect on HRQOL, but a 3-way interaction between gender, baseline BMI, and intervention effect was significant for the vitality score. Among overweight participants, the vitality score significantly increased after intervention only in male exercisers. Further, pairwise comparisons between the exercise and control groups demonstrated that overweight male exercisers had significant improvement (p<0.05) in role-physical, vitality, social functioning, and mental health subscales of the SF-36 compared to overweight male controls.
Exercise preference has recently been assessed as a moderator of intervention effect on HRQOL. In an exercise intervention study among 242 breast cancer patients, participants who preferred resistance exercise improved HRQOL only when they were assigned to resistance exercise (Courneya et al., 2008a). In general, men are reported to prefer relatively high-intensity activities (e.g., running and weight lifting), while women are reported to prefer low-intensity activities (Myers et al., 1989; Sherwood and Jeffery, 2000). Thus, the specific type of exercise delivered in our study may have contributed to different responses by gender.
Some studies suggest gender differences in the perception of physical and psychological burden from being overweight. A national survey of Australians (n=16,314) found that being overweight was not associated with decreased physical activity among men (Atlantis et al., 2008). Another survey reported stronger associations between BMI and physical activity in women than men (Ball et al., 2001). Women tend to be more dissatisfied with their body image and weight (Tiggemann, 1994) and perceive weight as more of a barrier for exercising than men (Ball et al., 2000). It is, therefore, possible that overweight men may have preferred the type of exercise provided in our trial and perceived less burden of being overweight; hence, demonstrating improved role-physical, vitality, social functioning, and mental health scores.
Exercisers demonstrated higher exercise self-efficacy scores at 12 months compared to controls, suggesting that the intervention successfully increased exercise self-efficacy. However, closer inspection reveals that both exercisers and controls decreased exercise self-efficacy scores at 12 months, with a smaller decline in exercisers. Although the decline of exercise self-efficacy among exercisers has been reported from other intervention studies (Hughes et al., 2004; McAuley et al., 2003b; McAuley et al., 2010), only one study reported a decline in exercise self-efficacy score among controls (Hughes et al., 2004). Several hypotheses are provided for the observed decreases in exercise self-efficacy in our sample. First, changes due to inactivity over a one year period may have caused the exercise self-efficacy decline among controls. A recent exercise intervention study with cancer survivors reported benefits of exercise in preventing functional decline and enhancing HRQOL (Morey et al., 2009). This study observed a significant decline in physical functioning and HRQOL within a 12-month period among controls. Although our study sample included healthy adults, many were overweight or obese, and we observed a 7.5 point decrease in bodily pain score (indicating increased pain) among controls. It is possible that various consequences of inactivity on physical and mental health may have decreased exercise self-efficacy among controls. Second, our participants may have overestimated their exercise self-efficacy at baseline because of being enrolled in an exercise trial; whereas their exercise self-efficacy responses were more accurate at 12 months, accounting for overall declines in both study arms. Then, among exercisers, the mastery experience of exercise may have increased their exercise self-efficacy resulting in the smaller decline relative to controls. Third, it is possible that controls were concerned about exercise sessions offered after the trial. After the trial, controls were invited to use the exercise facility for 2 months. If controls were anticipating the challenges in starting an exercise program as they completed the 12-month questionnaire, they may have reported lower exercise self-efficacy scores compared to exercisers.
Study strengths include its randomized clinical trial design, inclusion of men and women at varied baseline BMI levels, and pre- and post-intervention measurement of key predictors of adherence using validated measures. There are several limitations. First, this study was a secondary analysis of the trial that aimed to examine the exercise effects on biomarkers of colon cancer; thus, the trial was not powered to assess intervention effects within small subgroups defined by both BMI and gender. However, the results of this study provide pilot data suggesting BMI and gender as moderators of intervention effects on psychological factors and potentially exercise adherence. Second, our sample consisted of carefully-selected healthy participants with relatively high HRQOL levels. Finally, the participants were mainly non-Hispanic Whites; thus, these results may not apply to other racial or ethnic groups.
Conclusion
In summary, our findings suggest that engaging in the 360 minutes per week of exercise (60 minutes/day, 6 times/week) recommended for weight maintenance (Physical Activity Guidelines Advisory Committee, 2008) does not have negative effects on HRQOL among previously sedentary adults or among obese participants. The favorable effects of the intervention observed among overweight male participants and potentially among obese women suggest the importance of gender and obesity status as moderators of intervention outcomes. Despite the need for further investigation to clarify the underlying reasons, our study suggests that future intervention studies may benefit from tailoring exercise intervention strategies by gender and baseline BMI.
Acknowledgements
This study was funded by grants from the National Cancer Institute (R01 CA77572 and U54 CA116847, Transdisciplinary Research on Energetics and Cancer). A proportion of this work was conducted at the Clinical Research Center Facility, University of Washington funded by the National Institute of Health (M01-RR-00037) and the National Institute on Aging (AG1094). KEF was supported by National Institutes of Health (5KL2RR025015-03). KLC received support from a Canadian Institutes of Health Research (CIHR) fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Contributor Information
Ikuyo Imayama, Email: iimayama@fhcrc.org.
Catherine M. Alfano, Email: alfanoc@mail.nih.gov.
Lisa A. Cadmus Bertram, Email: lcaumus@ucsd.edu.
Chiachi Wang, Email: cwan2@fhcrc.org.
Liren Xiao, Email: lxiao@fhcrc.org.
Catherine Duggan, Email: cduggan@fhcrc.org.
Kristin L. Campbell, Email: kristin.campbell@ubc.ca.
Karen E. Foster-Schubert, Email: kfoster@u.washington.edu.
Anne McTiernan, Email: amctiern@fhcrc.org.
References
- Abu-Omar K, Rutten A, Lehtinen V. Mental health and physical activity in the European Union. Sozial- und Praventivmedizin. 2004;49:301–309. doi: 10.1007/s00038-004-3109-8. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Barnes EH, Ball K. Weight status and perception barriers to healthy physical activity and diet behavior. Int J Obes (Lond) 2008;32:343–352. doi: 10.1038/sj.ijo.0803707. [DOI] [PubMed] [Google Scholar]
- Ball K, Crawford D, Owen N. Too fat to exercise? Obesity as a barrier to physical activity. Aust N Z J Public Health. 2000;24:331–333. doi: 10.1111/j.1467-842x.2000.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Ball K, Owen N, Salmon J, Bauman A, Gore CJ. Associations of physical activity with body weight and fat in men and women. Int J Obes Relat Metab Disord. 2001;25:914–919. doi: 10.1038/sj.ijo.0801622. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: the exercise of control. New York: W. H. Freeman and Company; 1997. [Google Scholar]
- Barbour KA, Miller NH. Adherence to exercise training in heart failure: a review. Heart Fail Rev. 2008;13:81–89. doi: 10.1007/s10741-007-9054-x. [DOI] [PubMed] [Google Scholar]
- Bautista-Castano I, Molina-Cabrillana J, Montoya-Alonso JA, Serra-Majem L. Variables predictive of adherence to diet and physical activity recommendations in the treatment of obesity and overweight, in a group of Spanish subjects. Int J Obes Relat Metab Disord. 2004;28:697–705. doi: 10.1038/sj.ijo.0802602. [DOI] [PubMed] [Google Scholar]
- Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med. 2007;45:401–415. doi: 10.1016/j.ypmed.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Booth ML, Owen N, Bauman A, Clavisi O, Leslie E. Social-cognitive and perceived environment influences associated with physical activity in older Australians. Prev Med. 2000;31:15–22. doi: 10.1006/pmed.2000.0661. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Fesinmeyer MD, Yasui Y, Tworoger S, Ulrich CM, Irwin ML, Rudolph RE, LaCroix KL, Schwartz RR, McTiernan A. Randomized trial of exercise in sedentary middle aged women: effects on quality of life. Int J Behav Nutr Phys Act. 2006;3:34. doi: 10.1186/1479-5868-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Brown DR, Heath GW, Balluz L, Giles WH, Ford ES, Mokdad AH. Associations between physical activity dose and health-related quality of life. Med Sci Sports Exerc. 2004;36:890–896. doi: 10.1249/01.mss.0000126778.77049.76. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Foster-Schubert KE, Alfano CM, Cadmus LA, Xiao L, Duggan CR, Irwin ML, Ulrich CM, McTiernan A. Injuries in sedentary individuals enrolled in a 1-year randomized controlled exercise trial. J Phys Act Health. doi: 10.1123/jpah.9.2.198. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, McTiernan A, Li SS, Sorensen BE, Yasui Y, Lampe JW, King IB, Ulrich CM, Rudolph RE, Irwin ML, Surawicz C, Ayub K, Potter JD, Lampe PD. Effect of a 12-month exercise intervention on the apoptotic regulating proteins Bax and Bcl-2 in colon crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16:1767–1774. doi: 10.1158/1055-9965.EPI-07-0291. [DOI] [PubMed] [Google Scholar]
- Castro CM, Sallis JF, Hickmann SA, Lee RE, Chen AH. A prospective study of psychosocial correlates of physical activity for ethnic minority women. Psychol Health. 1999;14:277–293. [Google Scholar]
- Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, Segal RJ. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer. 2008a;112:1845–1853. doi: 10.1002/cncr.23379. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, Proulx C, Lane K, Ladha AB, Vallance JK, McKenzie DC. Predictors of supervised exercise adherence during breast cancer chemotherapy. Med Sci Sports Exerc. 2008b;40:1180–1187. doi: 10.1249/MSS.0b013e318168da45. [DOI] [PubMed] [Google Scholar]
- Dutton GR, Tan F, Provost BC, Sorenson JL, Allen B, Smith D. Relationship between self-efficacy and physical activity among patients with type 2 diabetes. J Behav Med. 2009;32:270–277. doi: 10.1007/s10865-009-9200-0. [DOI] [PubMed] [Google Scholar]
- Folta SC, Lichtenstein AH, Seguin RA, Goldberg JP, Kuder JF, Nelson ME. The StrongWomen-Healthy Hearts program: reducing cardiovascular disease risk factors in rural sedentary, overweight, and obese midlife and older women. Am J Public Health. 2009;99:1271–1277. doi: 10.2105/AJPH.2008.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KI, Jakicic JM, Napolitano MA, Marcus BH. Psychosocial factors related to physical activity and weight loss in overweight women. Med Sci Sports Exerc. 2006;38:971–980. doi: 10.1249/01.mss.0000218137.25970.c6. [DOI] [PubMed] [Google Scholar]
- Gillison FB, Skevington SM, Sato A, Standage M, Evangelidou S. The effects of exercise interventions on quality of life in clinical and healthy populations; a meta-analysis. Soc Sci Med. 2009;68:1700–1710. doi: 10.1016/j.socscimed.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217–228. doi: 10.1093/geront/44.2.217. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Tworoger SS, Yasui Y, Rajan B, McVarish L, LaCroix K, Ulrich CM, Bowen D, Schwartz RS, Potter JD, McTiernan A. Influence of demographic, physiologic, and psychosocial variables on adherence to a yearlong moderate-intensity exercise trial in postmenopausal women. Prev Med. 2004;39:1080–1086. doi: 10.1016/j.ypmed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Jackson L, Leclerc J, Erskine Y, Linden W. Getting the most out of cardiac rehabilitation: a review of referral and adherence predictors. Heart. 2005;91:10–14. doi: 10.1136/hrt.2004.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F, Harris P, Waller H, Coggins A. Adherence to an exercise prescription scheme: the role of expectations, self-efficacy, stage of change and psychological well-being. Br J Health Psychol. 2005;10:359–378. doi: 10.1348/135910704X24798. [DOI] [PubMed] [Google Scholar]
- Keller C, Fleury J, Gregor-Holt N, Thompson T. Predictive ability of social cognitive theory in exercise research: an integrated literature review. Online J Knowl Synth Nurs. 1999;6:2. [PubMed] [Google Scholar]
- Latka RN, Alvarez-Reeves M, Cadmus L, Irwin ML. Adherence to a randomized controlled trial of aerobic exercise in breast cancer survivors: the Yale exercise and survivorship study. J Cancer Surviv. 2009;3:148–157. doi: 10.1007/s11764-009-0088-z. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–278. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. J Behav Med. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- McAuley E, Jerome GJ, Elavsky S, Marquez DX, Ramsey SN. Predicting long-term maintenance of physical activity in older adults. Prev Med. 2003a;37:110–118. doi: 10.1016/s0091-7435(03)00089-6. [DOI] [PubMed] [Google Scholar]
- McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self-efficacy in older adults: social, affective, and behavioral influences. Ann Behav Med. 2003b;25:1–7. doi: 10.1207/S15324796ABM2501_01. [DOI] [PubMed] [Google Scholar]
- McAuley E, Mailey EL, Mullen SP, Szabo AN, Wojcicki TR, White SM, Gothe N, Olson EA, Kramer AF. Growth trajectories of exercise self-efficacy in older adults: Influence of measures and initial status. Health Psychol. 2010 doi: 10.1037/a0021567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, Morris KS, Motl RW, Hu L, Konopack JF, Elavsky S. Long-term follow-up of physical activity behavior in older adults. Health Psychol. 2007;26:375–380. doi: 10.1037/0278-6133.26.3.375. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, Rudolph RE, Surawicz C, Lampe JW, Lampe PD, Ayub K, Potter JD. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 2007;15:1496–1512. doi: 10.1038/oby.2007.178. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Yasui Y, Sorensen B, Irwin ML, Morgan A, Rudolph RE, Surawicz C, Lampe JW, Ayub K, Potter JD, Lampe PD. Effect of a 12-month exercise intervention on patterns of cellular proliferation in colonic crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1588–1597. doi: 10.1158/1055-9965.EPI-06-0223. [DOI] [PubMed] [Google Scholar]
- Michael YL, Colditz GA, Coakley E, Kawachi I. Health behaviors, social networks, and healthy aging: cross-sectional evidence from the Nurses' Health Study. Qual Life Res. 1999;8:711–722. doi: 10.1023/a:1008949428041. [DOI] [PubMed] [Google Scholar]
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. Jama. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery K, Schofield G, Caperchione C. Physical activity dose-response effects on mental health status in older adults. Aust N Z J Public Health. 2004;28:188–192. doi: 10.1111/j.1467-842x.2004.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Myers AM, Weigel C, Holliday PJ. Sex- and age-linked determinants of physical activity in adulthood. Can J Public Health. 1989;80:256–260. [PubMed] [Google Scholar]
- Pate RR, Blair SN, Durstine JL, Eddy DL, Hanson P, Painter P, Smith LK, Wolfe LA. Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lea & Febiger; 1991. pp. 70–72. [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annu Rev Nutr. 2000;20:21–44. doi: 10.1146/annurev.nutr.20.1.21. [DOI] [PubMed] [Google Scholar]
- Sorensen M, Anderssen S, Hjerman I, Holme I, Ursin H. The effect of exercise and diet on mental health and quality of life in middle-aged individuals with elevated risk factors for cardiovascular disease. J Sports Sci. 1999;17:369–377. doi: 10.1080/026404199365885. [DOI] [PubMed] [Google Scholar]
- Spirduso WW, Cronin DL. Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33:S598–S608. doi: 10.1097/00005768-200106001-00028. discussion S609-510. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Tiggemann M. Gender differences in the interrelationships between weight dissatisfaction, restraint, and self-esteem. Sex roles. 1994;30:319–330. [Google Scholar]
- Ware JE. SF 36 health survey: manual and interpretation guide. Boston, MA: The Health Institute New England Medical Center; 1993. [Google Scholar]
- World Health Organization. BMI classification. [30 August 2009];2009 Available: http://apps.who.int/bmi/index.jsp?introPageintro3.html.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care. 2009;32:1404–1410. doi: 10.2337/dc09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]