Summary
Background
Voltage-gated potassium channels are thought to be the target of antibodies associated with limbic encephalitis. However, antibody testing using cells expressing voltage-gated potassium channels is negative; hence, we aimed to identify the real autoantigen associated with limbic encephalitis.
Methods
We analysed sera and CSF of 57 patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels and 148 control individuals who had other disorders with or without antibodies against voltage-gated potassium channels. Immunohistochemistry, immunoprecipitation, and mass spectrometry were used to characterise the antigen. An assay with HEK293 cells transfected with leucine-rich, glioma-inactivated 1 (LGI1) and disintegrin and metalloproteinase domain-containing protein 22 (ADAM22) or ADAM23 was used as a serological test. The identity of the autoantigen was confirmed by immunoabsorption studies and immunostaining of Lgi1-null mice.
Findings
Immunoprecipitation and mass spectrometry analyses showed that antibodies from patients with limbic encephalitis previously attributed to voltage-gated potassium channels recognise LGI1, a neuronal secreted protein that interacts with presynaptic ADAM23 and postsynaptic ADAM22. Immunostaining of HEK293 cells transfected with LGI1 showed that sera or CSF from patients, but not those from control individuals, recognised LGI1. Co-transfection of LGI1 with its receptors, ADAM22 or ADAM23, changed the pattern of reactivity and improved detection. LGI1 was confirmed as the autoantigen by specific abrogation of reactivity of sera and CSF from patients after immunoabsorption with LGI1-expressing cells and by comparative immunostaining of wild-type and Lgi1-null mice, which showed selective lack of reactivity in brains of Lgi1-null mice. One patient with limbic encephalitis and antibodies against LGI1 also had antibodies against CASPR2, an autoantigen we identified in some patients with encephalitis and seizures, Morvan’s syndrome, and neuromyotonia.
Interpretation
LGI1 is the autoantigen associated with limbic encephalitis previously attributed to voltage-gated potassium channels. The term limbic encephalitis associated with antibodies against voltage-gated potassium channels should be changed to limbic encephalitis associated with LGI1 antibodies, and this disorder should be classed as an autoimmune synaptic encephalopathy.
Funding
National Institutes of Health, National Cancer Institute, and Euroimmun.
Introduction
Autoimmune synaptic encephalopathies are disorders in which patients develop antibodies against synaptic proteins, including the excitatory glutamate NMDA1 and AMPA receptors,2 and the inhibitory GABAB receptor.3 Because these receptors have crucial functions in synaptic transmission and plasticity, the autoimmunity usually causes seizures and neuropsychiatric symptoms, ranging from alterations in memory, behaviour, and cognition, to psychosis. Several features characterise these disorders: the target epitopes are extracellular, the antibody–receptor binding is detectable in transfected cells expressing the receptors, the antibodies alter the function or structure of the receptors,2,4 the resulting syndromes are severe but treatable, and the clinical presentation is similar to symptoms seen in animal models of pharmacological or genetic dysfunction of the related receptor.
Some of these immune responses define distinct disorders and could be classified as a single clinical-immunological entity (eg, anti-NMDA receptor encephalitis).5–7 Other immune responses, caused by antibodies against AMPA and GABAB receptors, result in psychiatric or seizure disorders that can develop alone or as part of limbic encephalitis.3,8 The classical clinical features of these two types of synaptic disorder are similar to those of limbic encephalitis attributed to antibodies against voltage-gated potassium channels.9,10
Synaptic autoantigens can be isolated by use of immunoprecipitation with antibodies from patients with the suspected autoimmune synaptic encephalopathy.1–3 However, antibodies against voltage-gated potassium channels from the sera or CSF of patients with limbic encephalitis were initially characterised by use of immunohistochemistry in rodent tissue and immunoprecipitation of nervous tissue lysates containing voltage-gated potassium channels labelled with 125-I-α-dendrotoxin (125-I-α-dendrotoxin radioimmunoassay).11,12 Cells transfected with combinations of several Kv subunits of voltage-gated potassium channels showed reactivity with sera from 17 of 17 patients with neuromyotonia or limbic encephalitis, although only about 20–38% of successfully transfected cells were recognised by patients’ antibodies.13 In another study, the authors suggested that voltage-gated potassium channels were not the target antigen and that some patients with neuromyotonia and Morvan’s syndrome had antibodies against contactin-associated protein-like 2 (CASPR2), but the target antigen of antibodies from patients with limbic encephalitis was not studied.14 We have been unsuccessful in reproducing the reactivity of antibodies from patients with neuromyotonia, Morvan’s syndrome, and limbic encephalitis in cells ectopically expressing voltage-gated potassium channels (unpublished). On the basis of these negative findings, we postulated that the antibodies of these patients might be directed against other neuronal cell-surface proteins and we aimed to identify the real autoantigen associated with limbic encephalitis.
Methods
Study population
Patients with limbic encephalitis and control individuals with other disorders were tested for the presence of antibodies against voltage-gated potassium channels in their sera or CSF. Sera or CSF were deemed positive for antibodies against voltage-gated potassium channels if they fulfilled the first two of the following criteria or the third criteria, or both: showed a previously defined pattern of immunostaining in the neuropil of adult rat brain,11,15 reacted with the cell surface of non-permeabilised rat hippocampal neurons, or were positive in a standard 125-I-α-dendrotoxin test used routinely in clinical laboratories.
Serum or CSF from 57 patients with symptoms of limbic encephalitis16 and antibodies against voltage-gated potassium channels and from 148 control individuals were analysed. Control individuals included five patients with syndromes other than limbic encephalitis but who had antibodies against voltage-gated potassium channels (three had neuromyotonia, one Morvan’s syndrome, and one severe encephalitis and seizures), and 143 patients without antibodies against voltage-gated potassium channels: 40 who had acute encephalopathies that were suspected to be autoimmune (five with clinical and MRI features of classic limbic encephalitis), 27 anti-NMDA receptor encephalitis, 17 viral encephalitis, ten limbic encephalitis with AMPA receptor antibodies, eight Rasmussen’s encephalitis, 35 acquired neuromyotonia (all confirmed electrophysiologically), one Morvan’s syndrome, and five subacute neuropathies. Clinical information was obtained from the study investigators and referring physicians.
This study was approved by the University of Pennsylvania Institutional Review Board; ethical approval was included in the Review Board assessment. Patients or family members provided written informed consent before assessment by the investigators or referring physicians.
Procedures
We used the same experimental approaches used previously to characterise other synaptic autoimmunities in patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels.2,3,8 Specific details are given below.
We did immunohistochemistry on rat brains and immunocytochemistry on neuronal cultures. Sagittal sections were taken from the brains of female Wistar rats, immersed in 4% paraformaldehyde (PFA) at 4°C for 1 h, cryoprotected with 40% sucrose for 24 h, and snap frozen in chilled isopentane. Immunohistochemistry was done by use of a standard avidin-biotin peroxidase method, in which serum (diluted 1:200) or CSF (1:5) from patients was applied, followed by the appropriate secondary antibody, as reported previously.17
Rat hippocampal neuronal cultures were prepared as reported.18 In vitro neurons grown for 14 days on coverslips were treated for 1 h at 37°C with serum (final dilution 1:750) or CSF (1:30) from patients or controls. After removing the media and washing with phosphate-buffered saline (PBS), neurons were fixed with 4% PFA and incubated with anti-human IgG Alexa Fluor secondary antibody diluted 1:1000 (Molecular Probes, Invitrogen, Eugene, OR, USA). Results were captured by a fluorescence microscope using Zeiss Axiovision software (Zeiss, Thornwood, NY, USA).2
To identify the target autoantigen, sera of two patients with limbic encephalitis and antibodies against voltage-gated potassium channels (selected because of their intense immunohistochemical reactivity with rodent brain tissue sections and cultured hippocampal neurons) were used in experiments of immunoprecipitation. To further characterise the antigen, immunoprecipitates were analysed by mass spectrometry, and immunoblots were done using techniques previously reported3 and that are described in detail in the webappendix p 1. Both patients had antibodies against leucine-rich, glioma-inactivated 1 (LGI1), a secreted protein that has been proposed to connect presynaptic and postsynaptic protein complexes for finely tuned synaptic transmission.19
To assess whether LGI1 was recognised by antibodies from all of the 57 patients, we used immunocytochemistry on HEK293 cells. HEK293 cells were transfected using lipofectamine 2000 (Invitrogen) with plasmids containing LGI1 (human sequence; sc116925, Origene, Rockville, MD, USA). Given that LGI1 is a secreted protein, we also investigated whether blocking the secretion of the protein would increase the pattern of reactivity.20 Therefore, in experiments using LGI1-transfected cells, 100 ng/mL Brefeldin A (Cell Signaling Technology, Boston, MA, USA) was added to the media 2 h before incubation with samples from patients. Brefeldin A is a fungal metabolite that inhibits transport from the endoplasmic reticulum to the Golgi apparatus and prevents protein secretion. Cells transfected with carrier vectors and non-transfected cells were used as controls.
Since LGI1 interacts with the synaptic proteins disintegrin and metalloproteinase domain-containing protein 22 (ADAM22) and ADAM23, we investigated whether patients’ antibodies reacted with ADAM22 or ADAM23 and whether patients’ antibody reactivity changed in localisation or intensity after co-expressing LGI1 with ADAM22 or ADAM23. We randomly selected 20 patient samples (10 sera and 10 CSF) by use of an online random integer generator. HEK293 cells were transfected with plasmids containing ADAM22 or ADAM23 (both constructs containing an HA-tag), or co-transfected with LGI1 and ADAM22 or LGI1 and ADAM23. We also assessed the reactivity of patients’ antibodies with Kv1.1 by co-transfection of this subunit with Kv1.4 because this strategy has been reported to increase cell surface expression of the Kv1.1.13
For all the immunocytochemistry experiments, cells were grown for 24 h after transfection, fixed with 4% PFA, permeabilised with 0·2% Triton X-100 (Sigma-Aldrich, St Louis, MO, USA), and incubated for 1 h at 37°C with serum (final dilution 1:200) or CSF (1:10) from patients or control individuals and commercial antibodies to LGI1 (polyclonal, dilution 1:1000; Ab30868, Abcam, Cambridge, MA, USA), HA-tag (polyclonal, dilution 1:100; 70-CH-18, Fitzgerald Industries, Acton, MA, USA), Kv1.1 (monoclonal, dilution 1:50; Clone 20/78, NeuroMab, Davis, CA, USA), or Kv1.4 (polyclonal, dilution 1:50, Alomone Labs, Jerusalem, Israel). Double immunolabelling was done using the appropriate Alexa Fluor secondary antibodies diluted 1:1000 (Molecular Probes). Serum or CSF samples were deemed positive when they partially or totally co-localised with the reactivity of a commercial antibody against the protein that was being expressed.
We examined whether patients’ antibodies recognised the secreted form of LGI1 using randomly selected samples from three patients and three controls. HEK293 cells transfected with ADAM22 or ADAM23 were washed and their media replaced for 1 h at 37°C with LGI1-enriched media collected from HEK293 cells expressing LGI1. Cells were then washed, fixed, permeabilised with 0·2% Triton X-100, and incubated with serum or CSF (at dilutions as before) from patients or control individuals, and a polyclonal antibody against HA-tag (as before), for 1 h at 37°C. After washing, the reactivity of patients’ antibodies with LGI1 bound to ADAM22 or ADAM23 was assessed by double immunolabelling with the appropriate Alexa Fluor secondary antibodies.
To confirm that LGI1 was the main target antigen, sera from two randomly selected patients were immuno-absorbed with cells transfected with LGI1 or the control plasmid, and the reactivity of the sera was assessed by use of rat brain immunohistochemistry. Serum (dilution 1:200) was serially incubated with six wells containing fixed, permeabilised HEK293 cells expressing LGI1 or cells transfected with control plasmids without an insert. After sequential passes of 1 h each, the serum was applied to sections of rat brain and the reactivity was detected by use of an avidin-biotin-peroxidase method.
To further confirm that LGI1 is the autoantigen of limbic encephalitis, we did comparative brain immunohistochemistry in tissue samples from Lgi1-null mice and wild-type littermates. A mouse chromosome engineering strategy was used to create a null mutation for the ortholog gene encoding LGI1 in mice.21 The Lgi1-null mutant mice show no developmental abnormalities in routine histopathological analysis;21 these mice and similar models of genetic deletion of LGI1 do show brain expression of Kv1 subunits of voltage-gated potassium channels (webappendix p 2).19 The brains were dissected, sagittally sectioned, fixed for 1 h in 4% PFA, cryoprotected with 40% sucrose for 24 h, and snap frozen in chilled isopentane. The brain sections were then immunostained with samples of 14 patients (7 sera and 7 CSF) randomly selected from 35 patients with limbic encephalitis and antibodies against voltage-gated potassium channels according to a 125-I-α-dendrotoxin radioimmunoassay. Immunohistochemistry using a standard avidin-biotin peroxidase method was done using serum (dilution 1:200) or CSF (1:5) from patients followed by the secondary goat anti-human IgG (dilution 1:2000; Sigma).
To identify the autoantigen from the five control individuals who had antibodies against voltage-gated potassium channels, we did immunoprecipitation (as described earlier) with serum from one of them, who had encephalitis and seizures. We isolated several peptide sequences that matched rat peptides from CASPR2 (score 65; RTNSPLQVKT; MLYSDTGRN; RHDLQHAVVARY). Subsequently, we used HEK293 cells transfected with a plasmid overexpressing human CASPR2 in an immunocyto chemical assay, which confirmed that the patient’s serum had antibodies against CASPR2 (webappendix p 3). We then tested the four other control individuals for the presence of antibodies against CASPR2. To assess whether CASPR2 was recognised by serum or CSF of patients with limbic encephalitis or neuromyotonia, we examined the serum (n=27) or CSF (n=30) from all 57 patients with limbic encephalitis and LGI1 antibodies and the sera from 35 patients with neuromyotonia (negative for antibodies against voltage-gated potassium channels). HEK293 cells overexpressing CASPR2 were tested as before with serum (1:200) or CSF (1:10) from patients or control individuals and a commercial polyclonal antibody to CASPR2 (dilution 1:1000; Ab33994, Abcam).
Role of the funding source
The study sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
Demographic information, clinical features, treatments, and outcomes of the 57 patients, all of whom had antibodies attributed to voltage-gated potassium channels, are summarised in the table. All patients had clinical or radiological features of limbic encephalitis; 42 patients had seizures (which often involved the temporal lobes), and 43 had typical increased T2 signal involving one or both medial temporal lobes on brain MRI. 18 of 45 patients had myoclonus, 28 of 47 patients had hyponatraemia (defined as serum sodium concentration of less than 135 mM), and the CSF was abnormal in 19 of 46 patients. Six of 53 patients who had tumour screening including CT of the chest, abdomen, and pelvis or 18F-fluoro-deoxyglucose-PET had systemic tumours and received antitumoural therapy. 48 of 50 patients received intravenous immunoglobulin, glucocorticoids, plasma exchange, or a combination thereof, six of whom were also treated with other drugs. 39 of 50 patients had good clinical outcomes (full recovery or mild residual memory impairment that prevented full return to work), eight had moderate disability, and three patients died (one had lung cancer, one pneumonia and sepsis, and one of unclear cause). Relapses occurred in six of 33 patients for whom follow-up information was available.
Table.
Demographic and clinical characteristics of patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels
| Patients (n=57) | |
|---|---|
| Men | 37 (65%) |
|
| |
| Age (years) | 60 (30–80) |
|
| |
| Tumours present* | 6 (11%)† |
|
| |
| Clinical diagnosis of limbic encephalitis | 57 (100%) |
|
| |
| Memory loss | 57 (100%) |
|
| |
| Myoclonus‡ | 18 (40%) |
|
| |
| Hyponatraemia§ | 28 (60%) |
| Serum sodium (mM) | 128 (118–132) |
|
| |
| Seizures¶ | 42 (82%)|| |
|
| |
| MRI¶ | |
| Increased T2 signal involving medial temporal lobe(s) | 43 (84%) |
|
| |
| EEG** | |
| Any abnormality | 26 (76%) |
| Seizures | 11 (32%) |
| Epileptiform discharges | 4 (12%) |
| Diffuse or focal slowing | 11 (32%) |
|
| |
| CSF analyses†† | |
| Any abnormality | 19 (41%) |
| Elevated protein | 13 (28%) |
| Lymphocytic pleocytosis | 8 (17%) |
|
| |
| Treatments‡‡ | |
| Any treatment | 48 (96%) |
| Steroids | 42 (84%) |
| Intravenous immunoglobulin | 31 (62%) |
| Plasma exchange | 3 (6%) |
| Other treatments | 6 (12%)§§ |
|
| |
| Clinical outcomes‡‡ | |
| Full recovery | 12 (24%) |
| Mild disability | 27 (54%) |
| Moderate disability | 8 (16%) |
| Death | 3 (6%) |
|
| |
| Follow-up visits|||| | |
| Duration after initial treatment (months) | 18 (2–60) |
| Relapse reported | 6 (18%) |
|
| |
| 125I-α-dendrotoxin radioimmunoassay*** | |
| Tested positive | 35 (100%) |
| Titre (pM) | 1054 (105–7600) |
Data are number (%) or median (range).
Data available for 53 patients.
Tumours: 1 lung, 2 thyroid, 1 renal cell, 1 ovarian teratoma, 1 thymoma.
Data available for 45 patients who had detailed neurological exam.
Data available for 47 patients.
Data available for 51 patients.
Onset was focal in 95% of 38 patients for whom localisation was established; 11% of patients with seizures had convulsive or non-convulsive status epilepticus.
Data available for 34 patients.
Data available for 46 patients.
Data available for 50 patients.
Other treatments include rituximab (3), azathioprine (2), and cyclosporine (1).
Data available for 33 patients.
Data available for 35 patients.
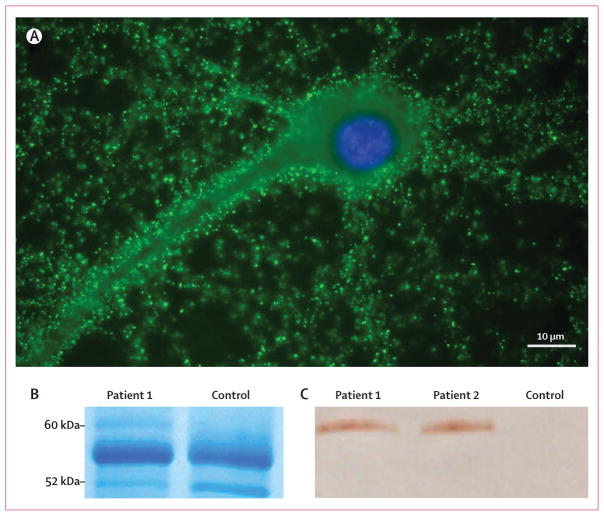
LGI1 was identified as the target antigen by immunoprecipitation using serum samples from two patients (figure 1). A distinct visible band that precipitated with one patient’s serum (the other patient’s serum showed a barely visible band; data not shown) was excised from the gel and analysed by mass spectrometry, with a cutoff score for confident protein identification of at least 70 (figure 1; webappendix p 1). This resulted in a score of 261 for the following peptide sequences: KAGFTTIYKW, KIQDIEVLKI, KFQELNVQAPRS, KGLDSLTNVDLRG, and KWGGSSFQDIQRM. Results were confirmed by immunoblotting the protein precipitated by two patients’ sera with a commercial antibody specific for LGI1 (figure 1).
Figure 1. Immunocytochemistry and immunoprecipitation of LGI1 with sera from patients with limbic encephalitis previously attributed to voltage-gated potassium channels.
(A) Immunolabelling of a rat hippocampal neuron with serum of a patient (patient 1) with antibodies previously attributed to voltage-gated potassium channels. The nucleus of the neuron is visualised with DAPI.
(B) Immunoprecipitates obtained using serum from a patient and a control individual were separated by gel electrophoresis and the gel stained with coomassie blue. A band of about 60 kDa was detected in the sample from the patient and, by mass spectrometry, was identified as LGI1. This band was not present in the sample from the control individual. The protein bands at 55 kDa and 52 kDa correspond to fragments of human IgG. (C) Immunoblot of the precipitates obtained with the sera from patient 1, another patient (patient 2), and a control individual (control). LGI1 was present in the neuronal immunoprecipitates obtained using sera from both patients but not the control individual. The antibody used in this analysis was a polyclonal LGI1 antibody that is commercially available.
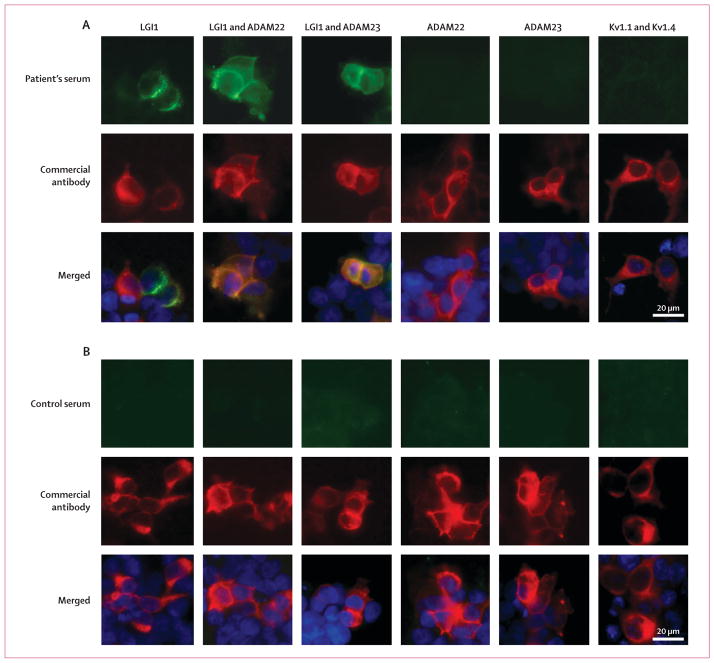
By use of immunocytochemistry on HEK293 cells transfected with LGI1 alone or with its receptors ADAM22 and ADAM23, we found that all 57 patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels had serum or CSF antibodies against LGI1. Antibody reactivity was often clustered in the cytoplasm, without uniform distribution on the cell surface, and showed poor co-localisation with the reactivity of a polyclonal antibody against LGI1 (figure 2). There was a substantial increase of reactivity with samples from all 57 patients when LGI1-transfected cells were pretreated with Brefeldin A (data not shown). Coexpression of LGI1 and ADAM22 or ADAM23 changed the localisation of LGI1 reactivity to the cell membrane and improved its detection. By contrast, antibodies from patients did not bind cells transfected with ADAM22 or ADAM23 alone.
Figure 2. Reactivity of a patient’s serum with HEK293 cells expressing LGI1 alone or coexpressing its receptors, ADAM22 or ADAM23.
Patient’s antibodies were detected with a secondary antibody labelled with Alexa Fluor 488 (in green). Antibodies specific for the indicated protein were detected with a secondary antibody labelled with Alexa Fluor 594 (in red). Nuclei were stained with DAPI. For clarity, nuclei staining (blue) is only shown in the merged images. (A) HEK293 cells expressing proteins indicated at the top of the panels immunostained with serum from a patient (top row) and commercial antibodies against each protein (second row). Merged reactivities are shown in the bottom row. When LGI1 is expressed alone, the reactivity with the patient’s antibodies is usually clustered in the cytoplasm, without uniform distribution on the cell surface, and showing partial co-localisation with the reactivity of a commercial antibody. When LGI1 is coexpressed with ADAM22 or ADAM23 (columns 2 and 3), the epitopes targeted by the patient’s antibodies might be better exposed on the cell membrane and co-localisation improves. By contrast, the patient’s antibodies did not bind ADAM22 or ADAM23 when expressed alone, nor did they react with Kv1.1 and Kv1.4 subunits. (B) HEK293 cells expressing proteins as before but incubated with serum from a control individual (top row). Serum from the control individual did not react with any of the proteins.
We found no reactivity with Kv1.1 or Kv1.4 subunits (figure 2) even though the tested samples were positive according to the 125-I-α-dendrotoxin radioimmunoassay. None of the 143 control individuals who were antibody negative on 125-I-α-dendrotoxin radioimmunoassay and none of the five patients who were antibody positive but had syndromes different from limbic encephalitis had LGI1 antibodies, as assessed in these tests.
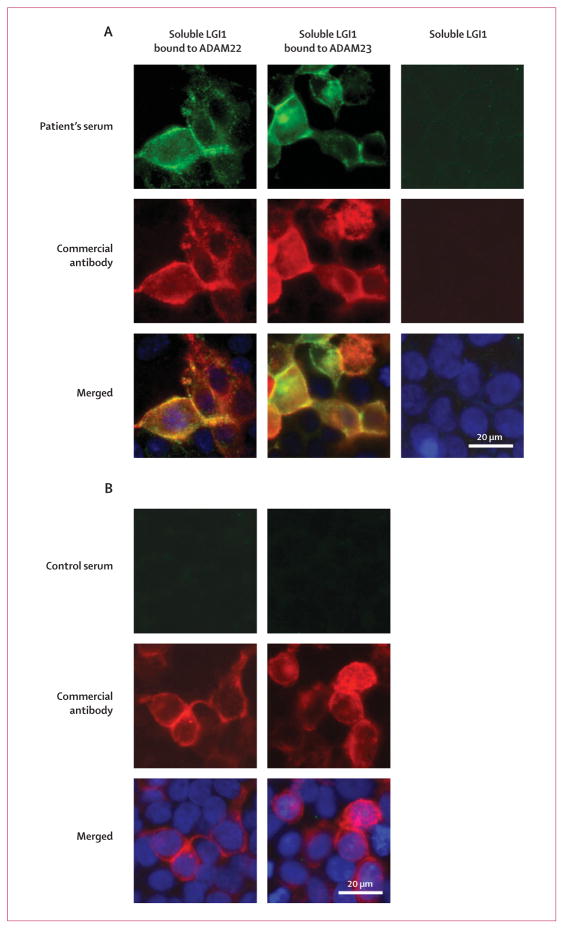
After incubating cells expressing ADAM22 or ADAM23 with media from LGI1-transfected cells, samples from patients, but not from control individuals, showed distinctive reactivity (figure 3). Because patients’ sera do not react with ADAM22 or ADAM23 expressed in isolation, these findings suggest that the antibodies specifically recognise secreted LGI1 bound on the cell surface to ADAM22 or ADAM23.
Figure 3. A patient’s serum reacts with soluble LGI1 bound to ADAM22 or ADAM23.
Patient’s antibodies were detected with a secondary antibody labelled with Alexa Fluor 488 (in green). Antibodies specific for the indicated protein were detected with a secondary antibody labelled with Alexa Fluor 594 (in red). Nuclei were stained with DAPI. For clarity, nuclei staining (blue) is only shown in the merged images. LGI1-containing media was applied to HEK293 cells expressing ADAM22 (first column) or ADAM23 (second column). (A) Patient’s antibodies recognised LGI1 bound to ADAM22 or ADAM23, but not HEK293 cells without either receptor (third row). (B) Absence of reactivity of serum from a control individual.
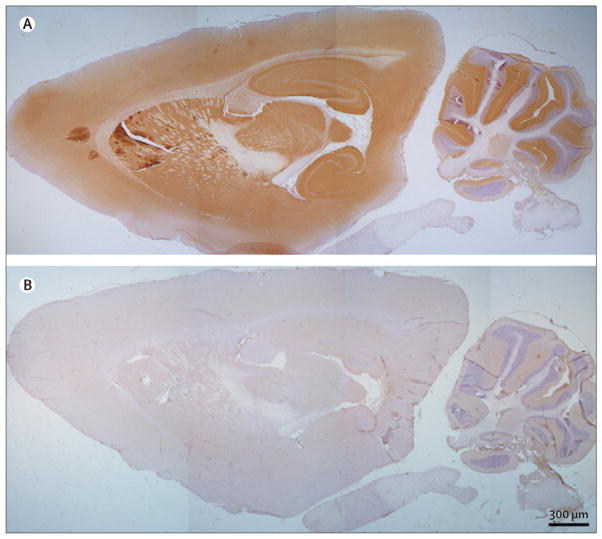
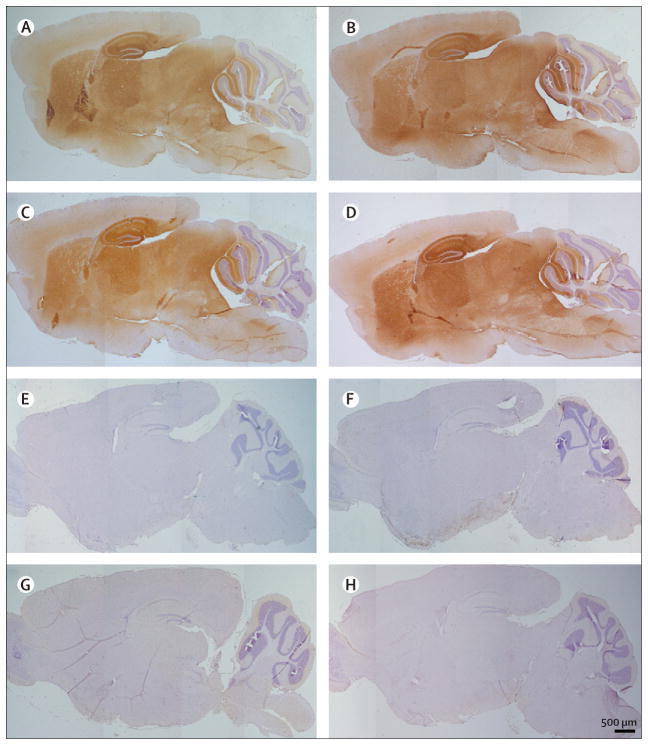
Immunoabsorption with LGI1 abrogated the reactivity of patients’ sera with rat brain (figure 4), thus confirming that LGI1 was the main target antigen. In addition, immunohistochemistry with brain tissue from Lgi1-null mice and wild-type littermates showed that patients’ sera produced the characteristic pattern of neuropil immunostaining in the wild-type mice, whereas all reactivity was abrogated in the Lgi1-null mice (figure 5).
Figure 4. Immunoabsorption confirms that LGI1 is the autoantigen in patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels.
A rat brain section immunostained with serum from a patient with limbic encephalitis and antibodies attributed to voltage-gated potassium channels before (A) and after (B) immunoabsorption with LGI1. All the patients’ serum reactivity (A, brown staining) disappeared in B, indicating that all the patients’ antibodies are directed against LGI1.
Figure 5. Reactivity of patients’ antibodies in wild-type mouse brain is abrogated in Lgi3-null mice.
Sections of brain of wild-type (A–D) and Lgi3-null mice (E–H) incubated with serum from four patients with limbic encephalitis and antibodies attributed to voltage-gated potassium channels. The difference of reactivity between the anterior and posterior cerebellar cortex in the wild-type mice is due to a fixation artifact. Note that the reactivity of patients’ sera (brown staining in A–D) is abrogated in the Lgi3-null mice (E–H), indicating that patients’ antibodies are specifically directed against LGI1.
Of the five control individuals who had antibodies against voltage-gated potassium channels, the patient with Morvan’s syndrome and the patient with encephalitis and seizures had CASPR2 antibodies (webappendix p 3). Of the 57 patients with limbic encephalitis and LGI1 antibodies and the 35 patients with neuromyotonia, only one patient from each group had antibodies against CASPR2 (webappendix p 3). No tumour was identified in any of the four individuals with CASPR2 antibodies (after follow-up at 6, 19, 48, and 84 months). Overall, CASPR2 antibodies were present in one of 38 patients with neuromyotonia (all without LGI1 antibodies) and one of 57 patients with limbic encephalitis and antibodies against LGI1.
Discussion
This study shows that the target antigen of antibodies in patients with limbic encephalitis previously attributed to voltage-gated potassium channels is in fact LGI1, a secreted neuronal protein that functions as a ligand for two epilepsy-related proteins, ADAM22 and ADAM23.19,22 Four different sets of experiments established LGI1 as the autoantigen of this disorder: immunoprecipitation of LGI1 with patients’ antibodies; immunostaining of HEK293 cells expressing LGI1 with sera and CSF from patients; specific abrogation of patients’ sera and CSF brain reactivity after immunoabsorption with LGI1-expressing cells; and comparative brain immunostaining of wild-type and Lgi1-null mutant mice, showing a lack of reactivity in LgI1-null mice.
Clinical seizures, mostly involving the temporal lobes, were identified in 82% of 51 patients, and 40% had myoclonus, a frequent feature noted also in mice lacking Lgi1.19,21 Hyponatraemia, often attributed to the syndrome of inappropriate antidiuretic hormone secretion, occurred in 60% of patients and might be related to the expression of LGI1 in the hypothalamus and the kidney.23 Most patients received immunotherapy, and 78% of 50 patients achieved substantial clinical recovery.
The LGI1 gene was first isolated by positional cloning using a glioblastoma cell line24 and has been implicated in tumour invasion and as a potential metastasis suppressor gene.25,26 Other studies using linkage analysis showed that mutations in LGI1 cause autosomal dominant partial epilepsy with auditory features,27–29 also known as autosomal dominant lateral temporal lobe epilepsy,30 which is an inherited epileptic syndrome associated with partial seizures and auditory or visual hallucinations. The LGI1 gene encodes a 63 kDa protein that contains a signal peptide and three leucine-rich repeats flanked by two cysteine-rich regions in the N-terminal region, whereas the C-terminal region consists of seven tandem repeats of 50 amino acids, named EPTP repeats31 or EAR.32 These repeats probably form a β-propeller structure that might be involved in protein–protein binding;33 a mechanism for LGI1 to bridge the synapse. The bridging may promote the interaction of secreted LGI1 with presynaptic ADAM23 and postsynaptic ADAM22, organising a trans-synaptic protein complex that includes presynaptic Kv1.1 potassium channels and postsynaptic AMPA receptor scaffolds.19
Although most hereditary epilepsy genes encode structural components of ion channels, LGI1 does not possess this function.21 Several truncating and missense mutations seem to prevent secretion of mutant LGI1 in animal models, all of which result in similar human phenotypes.34 At age 12–18 days, Lgi1-null mice present a lethal epileptic phenotype, which is characterised by myoclonic seizures.21 Targeted disruption of ADAM22,35 ADAM23,36 or Kv137 channels causes similar epileptic phenotypes and premature death, which suggests that these proteins and LGI1 are functionally related. A transgenic mouse model expressing a truncated mutant LGI1 found in human autosomal dominant lateral temporal lobe epilepsy shows inhibition of dendritic pruning and increased spine density, and mice have a marked increase of excitatory synaptic transmission compared with wild-type mice.38 This model also suggests that LGI1 decreases presynaptic release probability by upregulating presynaptic Kv1 channel activity in vivo.
The assessment of the reactivity of sera and CSF of patients is complicated by the fact that LGI1 is a secreted protein. For example, when HEK293 cells were transfected to express LGI1, the reactivity of patients’ sera was often weak, irregularly clustered in the cytoplasm, and showed partial co-localisation with the reactivity of a commercial polyclonal antibody. This suggests that the conformational epitopes targeted by patients’ antibodies (eg, not reactive by immunoblot; data not shown) are exposed differently from those recognised by the commercial antibody (linear epitopes which are detectable by immunoblot). The detection substantially improved after treating the cells with Brefeldin A, and further improvement was noted when LGI1 was co-transfected with ADAM22 or ADAM23, regardless of the use of Brefeldin A. These co-transfections resulted in a uniform distribution of LGI1 in the cytoplasm and the membrane that co-localised with that of ADAM22 or ADAM23. Patients’ antibodies not only reacted with LGI1 expressed in the cells, but also with extracellulary secreted LGI1 bound to ADAM22 or ADAM23. These data are in agreement with studies suggesting that ADAM22 and ADAM23 interact with LGI1.19,39,40 Moreover, the findings reveal a human disorder associated with antibodies to cell surface and secreted LGI1, providing the basis for an unambiguous diagnostic test. The immunocytochemical cell-based assay described here is cheaper, faster, and easier to use than the 125-I-α-dendrotoxin radioimmunoassay, and does not require a radioactive reagent.
In contrast to previous studies in which antibodies attributed to voltage-gated potassium channels were identified in a subgroup of patients with neuromyotonia,41,42 we did not find LGI1 antibodies in 38 patients with this disorder, even though three had antibodies against voltage-gated potassium channels as assessed by 125-I-α-dendrotoxin radioimmunoassay. Given that α-dendrotoxin binds to Kv1.1, Kv1.2, and Kv1.6 subunits of the voltage-gated potassium channels, the commercial test for voltage-gated potassium channel antibodies, which is based on serum immunoprecipitation of protein complexes containing these subunits, does not necessarily indicate that patients’ antibodies recognise the voltage-gated potassium channels, as our data show. Studies examining the LGI1 protein complex show that the presynaptic Kv1 potassium channels and a variety of presynaptic and postsynaptic scaffolding proteins can co-precipitate with LGI1 in addition to ADAM22 and ADAM23.19,39,40
The phenotype of patients with limbic encephalitis and LGI1 antibodies is different from that of patients with autosomal dominant partial epilepsy with auditory features or autosomal dominant lateral temporal lobe epilepsy. This is not surprising given that some mutations alter the postnatal maturation of presynaptic and postsynaptic functions, including glutamatergic circuits in an animal model.38 By contrast, antibodies to LGI1 develop as part of a subacute immune response in patients without clinical or family history of autosomal dominant partial epilepsy with auditory features or autosomal dominant lateral temporal lobe epilepsy, and therefore these patients have normal glutamatergic circuits. We speculate that antibody-mediated disruption of LGI1 function causes increased excitability, which results in seizures and other symptoms of limbic encephalopathy. These autoantibodies might also alter the function of proteins associated with LGI1, such as ADAM22 and ADAM23, which leads to a phenotype different from that caused by mutations in LGI1. In Lgi1-null mice, the increase in neuronal excitability has been attributed to a decrease in AMPA receptor function in inhibitory neurons,19 and to an increase in glutamate release.21 In this animal model, assessment of learning and memory is limited because animals die at 2–3 weeks of age, but future studies with heterozygous littermates might enable assessment of learning and memory deficits. Also, because genetic deletion and mutations of LGI1 alter glutamatergic transmission and circuitry, future studies should investigate whether glutamatergic transmission is affected in patients with LGI1 antibodies.
Our findings, and those of others,43 modify several terms and concepts and should lead to a reclassification of autoimmune disorders related to voltage-gated potassium channels. First, the term limbic encephalitis associated with antibodies against voltage-gated potassium channels should be changed to limbic encephalitis associated with LGI1 antibodies. Second, the concept of so-called autoimmune channelopathy needs to be reconsidered, given that LGI1 is not an ion channel but a secreted protein. We propose that this disorder should be included among autoimmune synaptic encephalopathies such as those associated with NMDA or AMPA receptor antibodies. Third, whether there is any disorder associated with antibodies against voltage-gated potassium channels remains unclear: a recent study implied that the antibodies of patients with Morvan’s syndrome or neuromyotonia are instead directed against CASPR2,14 a protein member of the neurexin superfamily. In myelinated axons, CASPR2 co-localises with Kv1.1, Kv1.2, and ADAM22,44 and forms part of a scaffold that is necessary to maintain voltage-gated potassium channels at the juxtaparanodal region.45 CASPR2 is also expressed in hippocampal neurons,46 and homozygous mutations have been found in Amish children with intractable seizures, hyperactivity, and abnormal behaviour.47 This phenotype resembles that of the patient whose serum we used to precipitate CASPR2 (manuscript in preparation). We did not identify CASPR2 antibodies in most patients with neuromyotonia or in patients with limbic encephalitis and LGI1 antibodies. Moreover, in contrast to a report that suggested that most patients with CASPR2 antibodies have an underlying associated tumour,14 we did not find any tumours in the four patients with CASPR2 antibodies. In another study, three additional patients with CASPR2 antibodies had Morvan’s syndrome without tumour association (unpublished). A study on one of these patients was previously reported and the patient has now been followed up for 5 years.48
This study shows that under the term “syndromes associated with antibodies against voltage-gated potassium channels” lies a broad spectrum of clinical and immunological disorders that have started to be exposed. In patients with limbic encephalitis, LGI1 is the autoantigen, but an expansion of the spectrum of anti-LGI1-associated symptoms might occur as more patients are identified. Since LGI1 is an epilepsy-related gene, future studies should assess the frequency of antibodies to LGI1 and other components of the trans-synaptic LGI1 protein complex in epileptic disorders that are suspected to be autoimmune. Identifying the antigens and repertoire of overlapping immunities in other syndromes such as Morvan’s syndrome or neuromyotonia should be the next step.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health and National Cancer Institute (RO1CA107192, 1RC1NS068204-01 [JD and RB-G], NS046706 [JC]) and by a research grant from Euroimmun, Luebeck, Germany (JD). Thanks to Yuko Fukata and Masaki Fukata (National Institute for Physiological Sciences, National Institutes of Natural Sciences, Okazaki, Japan) for kindly providing plasmids containing ADAM22 or ADAM23, to Steven Scherer (University of Pennsylvania, PA, USA) for providing plasmids containing Kv1.1 and Kv1.4, and to Elior Peles (The Weizmann Institute of Science, Israel) for providing the plasmid containing CASPR2. We thank Eugenia M Martínez Hernández (Laboratory of Neuro-Oncology, Department of Neurology, University of Pennsylvania, PA, USA) for assisting in the immunoblot studies; Michael Geschwind (Department of Neurology, Memory and Aging Center, University of California, San Francisco Medical Center, San Francisco, CA, USA), Takahiro Iizuka (Department of Neurology, School of Medicine, Kitasato University, Sagamihara, Japan), Yoko Takiyama (Department of Medicine, School of Medicine, Kitasato University, Tokyo, Japan), Vanda A Lennon (Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine, Rochester, MN, USA) and Sabrina Matà (Department of Neurological and Psychiatric Sciences, University of Florence, Florence, Italy) for providing samples and information from patients. We thank the physicians who provided clinical information. We also thank the patients and their families.
Footnotes
Contributors
ML, MGMH, EL, RB-G, JKC, and JD were involved in study design, data analysis, and writing of the report. ML and MGMH did the laboratory studies and prepared the figures. JC developed the Lgi1-null mouse and provided tissues for analysis. EL, FG, LB, and JD collected the clinical data and clinically assessed the patients.
Conflicts of interest
A patent application for the use of LGI1 antibody determination in patients’ sera and CSF as a diagnostic test has been filed in the USA by JD. None of the other authors have any conflicts of interest.
References
- 1.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–75. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–98. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gable MS, Gavali S, Radner A, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis. 2009;28:1421–29. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebire G. In search of lost time from “demonic possession” to anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2010;67:141–42. doi: 10.1002/ana.21928. [DOI] [PubMed] [Google Scholar]
- 8.Graus F, Boronat A, Xifro X, et al. The expanding clinical profile of anti-AMPA receptor encephalitis. Neurology. 2010;74:857–59. doi: 10.1212/WNL.0b013e3181d3e404. [DOI] [PubMed] [Google Scholar]
- 9.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 10.Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70:1883–90. doi: 10.1212/01.wnl.0000312275.04260.a0. [DOI] [PubMed] [Google Scholar]
- 11.Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–78. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- 12.Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62:1177–82. doi: 10.1212/01.wnl.0000122648.19196.02. [DOI] [PubMed] [Google Scholar]
- 13.Kleopa KA, Elman LB, Lang B, Vincent A, Scherer SS. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129:1570–84. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 14.Vincent A. Antibodies to contactin-associated protein 2 (CASPR2) in thymoma and Morvan’s syndrome. Ann Neurol. 2009;66:3. (abstract) [Google Scholar]
- 15.Diaz-Manera J, Rojas-Garcia R, Gallardo E, et al. Antibodies to AChR, MuSK and voltage-gated potassium channels in a patient with myasthenia gravis and Morvan’s syndrome. Nat Clin Pract Neurol. 2007;3:405–10. doi: 10.1038/ncpneuro0526. [DOI] [PubMed] [Google Scholar]
- 16.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 17.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–38. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 19.Fukata Y, Lovero KL, Iwanaga T, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci USA. 2010;107:3799–804. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986;261:11398–403. [PubMed] [Google Scholar]
- 21.Yu YE, Wen L, Silva J, et al. Lgi1 null mutant mice exhibit myoclonic seizures and CA1 neuronal hyperexcitability. Hum Mol Genet. 2010;19:1702–11. doi: 10.1093/hmg/ddq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313:1792–95. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- 23.Head K, Gong S, Joseph S, et al. Defining the expression pattern of the LGI1 gene in BAC transgenic mice. Mamm Genome. 2007;18:328–37. doi: 10.1007/s00335-007-9024-6. [DOI] [PubMed] [Google Scholar]
- 24.Chernova OB, Somerville RP, Cowell JK. A novel gene, LGI1, from 10q24 is rearranged and downregulated in malignant brain tumors. Oncogene. 1998;17:2873–81. doi: 10.1038/sj.onc.1202481. [DOI] [PubMed] [Google Scholar]
- 25.Kunapuli P, Chitta KS, Cowell JK. Suppression of the cell proliferation and invasion phenotypes in glioma cells by the LGI1 gene. Oncogene. 2003;22:3985–91. doi: 10.1038/sj.onc.1206584. [DOI] [PubMed] [Google Scholar]
- 26.Kunapuli P, Kasyapa CS, Hawthorn L, Cowell JK. LGI1, a putative tumor metastasis suppressor gene, controls in vitro invasiveness and expression of matrix metalloproteinases in glioma cells through the ERK1/2 pathway. J Biol Chem. 2004;279:23151–57. doi: 10.1074/jbc.M314192200. [DOI] [PubMed] [Google Scholar]
- 27.Gu W, Brodtkorb E, Steinlein OK. LGI1 is mutated in familial temporal lobe epilepsy characterized by aphasic seizures. Ann Neurol. 2002;52:364–67. doi: 10.1002/ana.10280. [DOI] [PubMed] [Google Scholar]
- 28.Kalachikov S, Evgrafov O, Ross B, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–41. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11:1119–28. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- 30.Poza JJ, Saenz A, Martinez-Gil A, et al. Autosomal dominant lateral temporal epilepsy: clinical and genetic study of a large Basque pedigree linked to chromosome 10q. Ann Neurol. 1999;45:182–88. doi: 10.1002/1531-8249(199902)45:2<182::aid-ana8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Staub E, Perez-Tur J, Siebert R, et al. The novel EPTP repeat defines a superfamily of proteins implicated in epileptic disorders. Trends Biochem Sci. 2002;27:441–44. doi: 10.1016/s0968-0004(02)02163-1. [DOI] [PubMed] [Google Scholar]
- 32.Scheel H, Tomiuk S, Hofmann K. A common protein interaction domain links two recently identified epilepsy genes. Hum Mol Genet. 2002;11:1757–62. doi: 10.1093/hmg/11.15.1757. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan SG, Gay NJ. Structural and functional diversity in the leucine-rich repeat family of proteins. Prog Biophys Mol Biol. 1996;65:1–44. doi: 10.1016/s0079-6107(96)00003-x. [DOI] [PubMed] [Google Scholar]
- 34.Nobile C, Michelucci R, Andreazza S, Pasini E, Tosatto SC, Striano P. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat. 2009;30:530–36. doi: 10.1002/humu.20925. [DOI] [PubMed] [Google Scholar]
- 35.Sagane K, Hayakawa K, Kai J, et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owuor K, Harel NY, Englot DJ, Hisama F, Blumenfeld H, Strittmatter SM. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci. 2009;42:448–57. doi: 10.1016/j.mcn.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smart SL, Lopantsev V, Zhang CL, et al. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhou YD, Lee S, Jin Z, Wright M, Smith SE, Anderson MP. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–14. doi: 10.1038/nm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunapuli P, Jang GF, Kazim L, Cowell JK. Mass spectrometry identifies LGI1-interacting proteins that are involved in synaptic vesicle function in the human brain. J Mol Neurosci. 2009;39:137–43. doi: 10.1007/s12031-009-9202-y. [DOI] [PubMed] [Google Scholar]
- 40.Sagane K, Ishihama Y, Sugimoto H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int J Biol Sci. 2008;4:387–96. doi: 10.7150/ijbs.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart IK, Maddison P, Newsom-Davis J, Vincent A, Mills KR. Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain. 2002;125:1887–95. doi: 10.1093/brain/awf178. [DOI] [PubMed] [Google Scholar]
- 42.Newsom-Davis J, Buckley C, Clover L, et al. Autoimmune disorders of neuronal potassium channels. Ann NY Acad Sci. 2003;998:202–10. doi: 10.1196/annals.1254.022. [DOI] [PubMed] [Google Scholar]
- 43.Irani SR, Waters P, Kleopa KA, Lang B, Vincent A. Antibodies to components of the voltage-gated potassium channel-associated complex: LGI1 and CASPR2 as antigenic targets in limbic encephalitis, Morvan’s and neuromyotonia. 2010 American Academy of Neurology Annual Meeting; Toronto, Canada. April 9–16, 2010; Abstract number 295. [Google Scholar]
- 44.Ogawa Y, Oses-Prieto J, Kim MY, et al. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci. 2010;30:1038–48. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poliak S, Salomon D, Elhanany H, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–60. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bel C, Oguievetskaia K, Pitaval C, et al. Axonal targeting of Caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J Cell Sci. 2009;122:3403–13. doi: 10.1242/jcs.050526. [DOI] [PubMed] [Google Scholar]
- 47.Strauss KA, Puffenberger EG, Huentelman MJ, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–77. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 48.Díaz-Manera J, Rojas-García R, Gallardo E, et al. Antibodies to AChR, MuSK and VGKC in a patient with myasthenia gravis and Morvan’s syndrome. Nat Clin Pract Neurol. 2007;3:405–10. doi: 10.1038/ncpneuro0526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.