SUMMARY
Background
Patients with hepatitis C viral (HCV) may perceive barriers to accessing speciality care for HCV, and these barriers may be related to depressive symptoms.
Aim
To evaluate the relationship between barriers to care, demographics, and depressive symptoms.
Methods
A cross-sectional analysis of 126 patients referred for HCV at two speciality HCV clinics. Barriers to care, depressive symptoms and sociodemographics were measured using standardized instruments. A retrospective chart review was conducted to collect clinical outcome data.
Results
Depressive symptoms were reported in 26%. Common barriers included lack of personal financial resources; lack of HCV knowledge in the community; lack of professionals competent in HCV care; stigmatization of HCV; and long distances to clinics offering care. After we controlled for sociodemographics, depression accounted for an additional 7–18% of variability in all barriers (all p values <0.01). Lower depression, marital and employment status were associated with subsequent receipt of HCV treatment in 38% (45/120) of patients; perceived barriers were not.
Conclusions
Depression is independently associated with perceived barriers to care. Higher depressive scores, but not perceived barriers, were associated with nontreatment. Healthcare providers who diagnose HCV need to be cognizant of numerous perceived barriers to accessing HCV care, and the impact that depression may have on these perceptions and receipt of treatment.
INTRODUCTION
Chronic hepatitis C viral (HCV) infection is a silent epidemic in the US with almost 4 million Americans estimated to be infected,1 which is four times the prevalence rate of HIV/AIDS.2 HCV-related cirrhosis and hepatocellular carcinoma are expected to double or triple in the next two decades.3 Fortunately, health benefits and risk reduction can be gained by undergoing the current antiviral regimen of pegylated interferon alpha and ribavirin for 24 or 48 weeks, with chances of sustained virological response in 40–80% of treated patients.4 These eradication rates will probably improve in the next few years with the advent of triple therapy.5, 6 Viral clearance of HCV may, in turn, reduce subsequent complications of advanced liver disease.7, 8
Barriers to accessing medical care for HCV have been receiving more attention in the last few years, with the realization that only a small proportion of individuals infected with HCV undergo antiviral treatment.9, 10 However, barriers to hepatitis C medical care are probably quite similar to the barriers which exist among other medically ill populations, especially persons infected with HIV/AIDS.11-13 Barriers to HCV treatment may occur at the patient, provider and healthcare system levels. Patient barriers include the ability to navigate the healthcare system; cost of health services and treatment; lack of insurance and personal financial resources; lack of access to mental health or substance abuse services; social marginalization and stigma; negative experiences with the healthcare system and providers; lack of patient knowledge; occupational concerns; fear of drug or alcohol relapse; and unstable family or social circumstances.13-20 Providers also experience barriers to delivering high quality HCV care, including patient non-adherence to clinic visits; concomitant HIV or other medical comorbidities; lack of adequate training and staffing to treat patients with co-morbid substance use or mental health disorders; and the challenging side effect profile of the antiviral regimen.13-20 Thus, HCV patients may face myriad barriers and challenges to accessing specialized HCV care required to treat this potentially life-threatening disease.
In addition to the aforementioned barriers, another oft-cited barrier to antiviral treatment for patients and providers alike are co-occurring depressive symptoms. One-fourth of patients with HCV meet DSM-IV diagnostic criteria for major depressive disorder, while 45–60% of patients endorse some level (mild-to-severe) of depressive symptomatology.21-26 As depression is often under-recognized and under-diagnosed, the co-morbid depression in many HCV patients may go untreated, and unstable depression is a contraindication to antiviral therapy.19, 27 Depression is associated with poor self-efficacy, will to function, low self-esteem, low self-worth, reduced motivation, reduced adherence, lack of or poor social support, greater perceived stigmatization, pessimism, hopelessness and reluctance to seek healthcare services.28-32 These components of depressed mood are known to disrupt a broad spectrum of health-related, proactive behaviours.32 Depression also reduces access to general medical care because depressed patients often perceive minimal benefits from most medical treatments.28 These cognitive and behavioural correlates of depression may be particularly detrimental to HCV patients if depressed mood is associated with the perception of insurmountable obstacles to accessing speciality care for HCV.
To our knowledge, no study has explored the relationship between patients’ perceptions of barriers to speciality hepatitis care and depressive symptomatology among those infected with HCV. We hypothesized that perceived barriers to speciality care for HCV are prevalent and associated with depressive symptoms among persons living with HCV, over and above relevant sociodemographic factors.
The aims of the present study were as follows:
To determine the most common perceived barriers to speciality care for HCV.
To assess the bivariate relationships between sociodemographic factors, barriers to care, and depressive symptoms.
To determine if depressive symptoms are associated with perceived barriers to care, after controlling for relevant sociodemographic factors.
To evaluate the relationship between barriers to care and depressive symptoms with subsequent clinical outcomes such as commencement and completion of antiviral treatment. (Investigation of subsequent clinical outcomes recommended by journal reviewers.)
MATERIALS AND METHODS
Overview of study design
The primary analysis for this study was based on cross-sectional survey data from 126 participants referred for speciality hepatitis care over a 2-year period from 2004 to 2006. A retrospective chart review was undertaken in 2010 to collect subsequent clinical outcome data. These studies were approved by the UNC Institutional Review Board.
Participants
A total of 247 patients were approached to participate in this cross-sectional survey study. Forty patients declined participation, and data on the variables of interest (i.e. barriers and depression) were incomplete for 81 patients. Therefore, the cross-sectional analysis was based on data collected from 126 participants. Inclusion criteria included a confirmed diagnosis of HCV and new referral to one of the two hepatology clinic sites. Exclusion criteria included a diagnosis of HIV or hepatitis B, and non-English speaking patients. All patients who met criteria for the study were screened by the hepatology clinicians and research assistant during the first or second visit to the clinic.
Setting
Patients were recruited, screened and informed consent was obtained at two speciality hepatology clinics: an out-patient clinic in an academic medical centre (n = 72), and a community-based outreach hepatology clinic (n = 54) affiliated to another healthcare system. At the time of recruitment, these sites were staffed by the same hepatology clinicians (two attending hepatologists; two mid-level providers).
Procedure
Patients were recruited, consent was obtained and they were assured confidentiality of the self-administered surveys by a research coordinator. A pen and paper literacy questionnaire was administered prior to completion of computer-based surveys to identify participants in need of reading assistance. Participants were provided instruction on the use of computer tablets with touch screen capabilities, and surveys were completed on the tablet computers during the patients’ visit.
A post hoc retrospective chart review was conducted to gather subsequent clinical outcome data on the 126 participants. At least some data were available on 120 of the 126 participants. Medical records were reviewed from the time of cross-sectional data collection until April 2010 for the following information: (i) time (in years) from HCV diagnosis to presentation in the speciality hepatology clinics; (ii) receipt of antiviral treatment; and for those who commenced therapy, (iii) treatment completion, virological response, and reasons for premature treatment discontinuation.
Measures
Depression was measured using the 10-item Center for Epidemiological Studies-Depression (CES-D) Scale.33, 34 The CES-D has been used to assess depressive symptoms in the HCV population.35, 36 Depressive symptoms are rated on a four-point Likert scale ranging from 0 (‘Rarely’) to 3 (‘Most of the Time’). Scores range from 0 to 30, with a score of 10 or greater generally considered in the depressed range.
The Sociodemographic Survey assessed gender, age, marital status, race, education level, employment status, annual household income, insurance status, travel time to clinic and means of transportation to clinic. With the exception of age, all variables were dichotomized.
The Barriers to Care Scale (BACS) is a 12-item scale originally developed for HIV patients and modified for the current study with HCV patients (16). Participants rated the extent to which each item was perceived as a barrier to speciality care for HCV. A four-point Likert scale was used: 1 (No problem at all), 2 (Very slight problem), 3 (Somewhat of a problem), and 4 (Major problem). The 12 items are collapsed into four subscales:
Geography/Distance to HCV clinic (two items): Long distances to HCV facilities and personnel; lack of transportation to access the HCV services needed.
Medical/psychological services (four items): Medical personnel who decline to provide direct care to persons infected with HCV; lack of healthcare professionals who are adequately trained and competent in HCV treatment; a shortage of psychologists, social workers, and mental health counsellors who can address mental health issues; lack of psychological support groups for person infected with HCV.
Community stigma (two items): The level of knowledge about HCV among citizens in the community; community residents’ stigma against persons infected with HCV.
Personal resources (four items): Lack of employment opportunities for people infected with HCV; lack of supportive and understanding work environments for people infected with HCV; lack of personal and financial resources; lack of adequate and affordable housing.
Statistical analyses
Means and standard deviations (s.d.) or proportions were calculated for all independent variables. Site differences were assessed using two-sample t-tests (continuous variables) or chi-square tests of association (categorical variables). Bivariate associations between the four BACS subscales (continuous variables), CES-D depressive scores (continuous variables), and sociodemographic factors were evaluated using Pearson’s correlation for continuous variables or two-sample t-tests for categorical variables. Each of the four subscales was an outcome of interest and thus analysed as a dependent variable using multiple linear regression models. For the regression models, the bivariate associations were used for model reduction purposes and to preserve degrees of freedom. Hence, only sociodemographic variables with a P ≤ 0.20 were included in the regression models. The additional amount of variability in mean BACS subscale measures, explained by the depression score after controlling for relevant sociodemographic factors and site, was assessed using the change in R2. For clinical outcomes collected via chart extraction, time from HCV diagnosis to presentation in clinic was based on data available for 76 of the original 126, and treatment outcomes were based on 45 of the available 120 who underwent antiviral treatment. Only descriptive data were presented for the subgroup of 45 treated patients as the sample size was small. All significance levels were set at P < 0.05. All analyses were conducted using SAS version 9.1 (SAS, Cary, NC, USA) and SPSS version 15.0 (SPSS, Chicago, IL, USA).
RESULTS
There were no significant differences between patients who refused to participate in the study (n = 40), and participants with missing barriers and depression data who were excluded from the current analysis (n = 81). These groups were combined (nonparticipants = 121). When compared with participants in this study (n = 126), nonparticipants did not differ on age, gender, marital status and insurance coverage. However, there were significantly more Caucasians among the participants than nonparticipants (83% vs. 58%; P < 0.01), and participants had higher educational attainment compared with nonparticipants (55% vs. 41% with more than high school; P = 0.03).
Sociodemographic characteristics
When the two clinic sites were compared, we found that participants attending the academic medical centre at the state hospital had higher rates of being uninsured (17%) compared with those who attended the community-based clinic (4%; P = 0.04), and more participants attending the academic medical centre travelled >1 h to clinic (49%), compared with patients who attended the community-based clinic (2%; P < 0.01). These were the only significant differences in sociodemographic characteristics between sites; the sociodemographic data for the combined sample (n = 126) are presented in Table 1.
Table 1.
Patient characteristics (N = 126)
| Variable | Levels | |
|---|---|---|
| Mean (s.d.) | ||
| CES-D | 6.9 (6.3) | |
| Age | 46.3 (6.7) | |
| n (%) | ||
| Gender | Male | 68 (54) |
| Female | 58 (46) | |
| Race | Caucasian | 105 (83) |
| Non-Caucasian | 21 (17) | |
| Marital status | Married | 62 (50) |
| Non-married | 62 (50) | |
| Education | High school or less | 56 (45) |
| More than high school | 68 (55) | |
| Employment status | Full-time or part-time | 86 (72) |
| Other | 34 (28) | |
| Annual income | ≤$40 000 | 66 (55) |
| >$40 000 | 53 (45) | |
| Insurance coverage | Insured | 108 (89) |
| Uninsured | 14 (11) | |
| Travel distance to clinic |
≤1 h | 90 (71) |
| >1 h | 36 (29) | |
| Transportation to clinic |
Self-transportation | 99 (80) |
| Other means | 25 (20) |
Missing data were observed for marital status, education, and transportation to clinic (N = 124); insurance coverage (N = 122); employment status (N = 120); annual income (N = 119).
Retrospective data collection revealed that 66% (68/103) of participants had current or lifetime illicit substance use, 50% (52/104) reported symptoms suggestive of alcohol abuse or dependence, and 50% (53/107) reported current or lifetime psychiatric disturbances. Of the 76 participants with available data, time from HCV diagnosis to presentation varied greatly. Participants who had undergone interferon-based therapy previously at another clinic (n = 15) presented at the speciality clinic 7.7 years (s.d. = 5.3 years) after HCV diagnosis, whereas treatment-naïve participants (n = 61) presented 3.7 years (s.d. = 5.1 years) after HCV diagnosis (P < 0.01). Barriers to care subscales, depressive symptoms and sociodemographic factors were not significantly associated with the number of years from HCV diagnosis to presentation in the speciality clinics (data not shown).
Perceived barriers to care responses
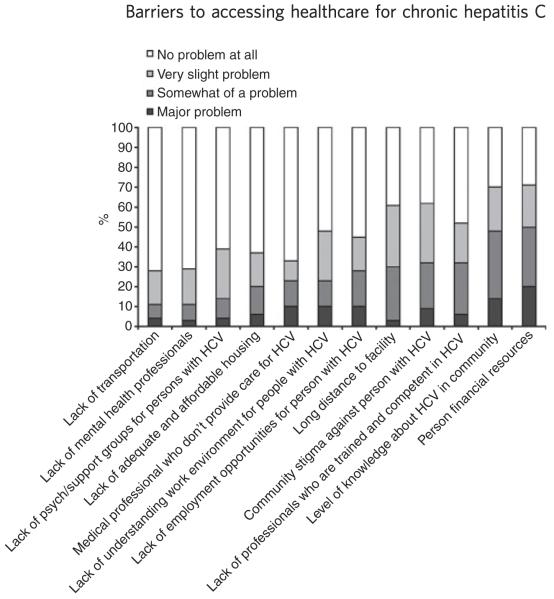
Figure 1 provides the percentage of the 12 individual barrier items perceived as ‘no problem’, ‘a very slight problem’, ‘somewhat of a problem’ and ‘a major problem’. When we combined the endorsement of an item as ‘somewhat of a problem’ or a ‘major problem’, the greatest barriers were as follows: lack of personal financial resources (50%); lack of HCV knowledge in the community (48%); lack of professionals competent in HCV care (33%); feeling stigmatized for having HCV (32%); and long distances to a treatment facility (30%). Long distances to a treatment facility was the only barrier significantly different between the two clinic sites, with 40% of patients who attended the academic medical centre endorsing this barrier as problematic, compared to 17% of patients who attended the satellite, community-based clinic (P < 0.01).
Figure 1.
Endorsement of 12 individual BACS items.
Bivariate associations between barriers, depression, and sociodemographic characteristics
As shown in Table 2, Distance to an HCV clinic was perceived as a barrier in patients who did not have self-transportation (P = 0.01). Personal and financial resources as a barrier was associated with being unmarried (P < 0.01), having an annual household income of <$40 000 (P < 0.01), being unemployed or not working (P < 0.01), being uninsured (P = 0.04) and not having self-transportation (P < 0.01). No sociodemographic factors were significantly associated with the BACS subscales Lack of Medical/Psychological Services or Community Stigma. Additionally, participants experiencing higher CES-D depression scores were more likely to be unmarried (P < 0.01), unemployed (P < 0.01) and report less income (P < 0.01).
Table 2.
Bivariate associations between BACS subscales, sociodemographic factors, and depression score
| Variable | Levels | Geography/ distance |
P-value | Medical and psychological |
P-value | Community stigma |
P-value | Personal resources |
P-value | CES-D | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | Correlation | Correlation | Correlation | ||||||||
| CES-D | 0.28 | <0.01 | 0.27 | <0.01 | 0.27 | <0.01 | 0.49 | <0.01 | |||
| Age | 0.08 | 0.39 | −0.03 | 0.78 | 0.00 | 0.99 | −0.06 | 0.52 | −0.01 | 0.95 | |
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | |||||||
| Gender | Male | 1.7 (0.7) | 0.56 | 1.6 (0.6) | 0.19 | 2.1 (0.8) | 0.10 | 1.8 (0.8) | 0.31 | 6.5 (6.5) | 0.48 |
| Female | 1.6 (0.7) | 1.7 (0.8) | 2.3 (1.0) | 2.0 (0.9) | 7.3 (6.1) | ||||||
| Race | Caucasian | 1.7 (0.7) | 0.72 | 1.6 (0.7) | 0.61 | 2.2 (0.9) | 0.40 | 2.0 (0.9) | 0.28 | 7.0 (6.4) | 0.80 |
| Non-Caucasian | 1.7 (0.8) | 1.7 (0.8) | 2.0 (0.9) | 1.7 (0.8) | 6.6 (5.7) | ||||||
| Marital status | Married | 1.6 (0.6) | 0.29 | 1.6 (0.7) | 0.85 | 2.2 (0.9) | 0.84 | 1.7 (0.7) | <0.01 | 5.0 (3.7) | <0.01 |
| Non-married | 1.8 (0.8) | 1.7 (0.7) | 2.2 (1.0) | 2.2 (1.0) | 8.6 (6.7) | ||||||
| Education | ≤High school | 1.7 (0.7) | 0.56 | 1.7 (0.8) | 0.29 | 2.1 (0.9) | 0.69 | 2.0 (0.9) | 0.36 | 6.3 (5.7) | 0.34 |
| >High school | 1.7 (0.7) | 1.6 (0.7) | 2.2 (0.9) | 1.9 (0.8) | 7.4 (6.8) | ||||||
| Employment | Full- or part-time | 1.6 (0.6) | 0.13 | 1.6 (0.7) | 0.59 | 2.1 (0.9) | 0.45 | 1.7 (0.7) | <0.01 | 5.7 (5.3) | <0.01 |
| Other | 1.8 (0.9) | 1.7 (0.8) | 2.3 (1.0) | 2.3 (1.0) | 9.7 (7.8) | ||||||
| Income | ≤$40 000 | 1.7 (0.8) | 0.14 | 1.7 (0.8) | 0.32 | 2.3 (1.0) | 0.22 | 2.2 (0.9) | <0.01 | 8.2 (6.6) | <0.01 |
| >$40 000 | 1.6 (0.5) | 1.5 (0.6) | 2.1 (0.8) | 1.5 (0.6) | 5.0 (5.6) | ||||||
| Insurance | Insured | 1.7 (0.7) | 0.19 | 1.6 (0.7) | 0.34 | 2.2 (0.9) | 0.91 | 1.9 (0.9) | 0.04 | 6.5 (6.4) | 0.14 |
| Uninsured | 1.9 (0.9) | 1.8 (0.7) | 2.2 (1.0) | 2.4 (0.7) | 9.1 (4.7) | ||||||
| Distance to clinic | ≤1 h | 1.7 (0.8) | 0.95 | 1.7 (0.8) | 0.49 | 2.1 (0.9) | 0.48 | 1.9 (0.8) | 0.88 | 6.9 (6.3) | 0.89 |
| >1 h | 1.7 (0.6) | 1.6 (0.6) | 2.3 (0.9) | 1.9 (0.9) | 6.8 (6.4) | ||||||
| Transportation | Self-transportation | 1.6 (0.6) | 0.01 | 1.6 (0.7) | 0.39 | 2.1 (0.9) | 0.23 | 1.8 (0.8) | <0.01 | 6.4 (5.2) | 0.11 |
| Other means | 2.1 (1.0) | 1.8 (0.8) | 2.4 (0.9) | 2.5 (0.9) | 8.7 (5.7) |
Associations between barriers to care and depression
Higher depression scores were positively correlated with all four barriers to care subscales (Table 2). Results from the multiple regression models using the four BACS subscales as dependent variables are presented in Table 3 and provide further support to the relationship between depression and the BACS subscales. After controlling for any important sociodemographic factors (significant at P ≤ 0.20 in bivariate analyses) and clinic site (Model 1), depression accounted for an additional 7.1% of the variance in the subscale of Distance (P < 0.01), an additional 7.0% of the variance in the subscale of Lack of Medical and Psychological Support (P < 0.01), an additional 7.0% of the variability in the Community Stigma subscale (P < 0.01), and 17.6% more of the variance in the Lack of Personal Resources subscale (P < 0.01) (Model 2).
Table 3.
Regressions of BACS subscales with sociodemographic factors and depression score
| BACS subscale | Model 1: Variability* (explained by sociodemographic factors + site) |
Model 2: Additional variability† (explained by adding depression score) |
P-value‡ (explained by depression score) |
|---|---|---|---|
| Geography/Distance§ | 10.3% | 7.1% | 0.0032 |
| Medical and Psychological¶ | 1.5% | 7.0% | 0.0027 |
| Community Stigma¶ | 2.2% | 7.0% | 0.0027 |
| Personal Resources** | 20.9% | 17.6% | <0.0001 |
Variability explained in Model 1 = R2 × 100%.
Change in R2 × 100% with addition of depression variable to Model 1.
Significance of additional variability explained by depression in Model 2, after adjusting for sociodemographic factors.
Sociodemographic factors = employment, income, transportation, insurance.
Sociodemographic factors = gender.
Sociodemographic factors = marital status, employment, insurance, income, transportation.
Post hoc sensitivity analysis of association between barriers to care and depression
During model diagnostics of the regression models, we observed that the distribution of the residuals slightly to moderately deviated from the assumption of normality. We therefore applied a Box-Cox power transformation37 to the data to ensure all assumptions of the linear model held; however, the transformation did not prove useful. Thus, to strengthen the confidence of our regression model findings, we ran a post hoc sensitivity analysis to assess the relationship between depression scores and the four BACS subscales and its strength. We re-defined each of the BACS subscales as a dichotomous variable. An average subscale score of 2 or less was defined as ‘a slight problem or less’, and an average score of >2 was defined as ‘somewhat of a problem or greater’. Logistic regression models were fit to the four new outcome measures, and the significance of the depression score was assessed after controlling for the same sociodemographic variables as in the linear regression models. Results indicated that after adjustment, depression scores were still significantly associated (P < 0.05) with all four BACS subscales. In other words, the odds of having ‘somewhat of a problem or greater’ increased with increasing CES-D score. For a clinically significant 5-point increase in CES-D scores, the odds ratio ranged from 1.47 to 2.28 for the four subscales. There was a 47–128% increase in the odds of having ‘somewhat of a problem or greater’ compared with ‘a slight problem or less’ for each additional five point on the CES-D scale.
Clinical outcomes
The retrospective analysis demonstrated that 38% of individuals (45/120) subsequently underwent antiviral treatment after participating in the cross-sectional survey study from 2004 to 2006. Compared with the 75 patients who did not initiate antiviral therapy, the 45 patients who received treatment were significantly less depressed [mean CES-D score (s.d.): 4.2 (3.6) vs. 8.9 (6.9); P < 0.01], more likely to be married (60% vs. 41%; P = 0.05) and more likely to be employed (85% vs. 64%; P = 0.02) during the cross-sectional study. No BACS scales were significantly associated with receipt of antiviral treatment, but the BACS Lack of Personal and Financial Resources subscale approached significance (P = 0.08) with untreated patients reporting greater personal resource barriers [mean (s.d.): 2.1 (0.9)] compared with patients who received treatment [mean (s.d.): 1.8 (0.8)].
Of the 45 patients who received antiviral treatment, 62% (28/45) completed a full course of the prescribed regimen. Of those who did not complete treatment (n = 17), 47% (8/17) were discontinued prematurely due to side effects, 29% (5/17) for nonresponse, 12% (2/17) for non-adherence problems, and 12% (2/17) due to patient preference. With regard to virological response, 60% (27/45) achieved a sustained virological response, 18% (8/45) were nonresponders, 18% (8/45) were relapsers at 6 months post-treatment, and 4% (2/45) had missing data. For the eight patients who discontinued treatment prematurely due to side effects, the side effects that lead to discontinuation were neuropsychiatric (n = 3), gastrointestinal (n = 3), fatigue (n = 2), lab abnormalities (n = 2) and other (n = 2). Totals do not sum to 8 as some patients had more than one side effect leading to discontinuation.
DISCUSSION
Very few individuals infected with HCV actually undergo interferon-based treatment, which is potentially curative for 40–80% of patients treated.9, 38, 39 Thus, there is growing need to identify barriers to HCV management and treatment which may be present at the patient, provider, institutional and societal levels. Barrier identification is the first step in devising solutions, such as stimulating the development of models or programmes that can overcome barriers and increase access to potentially life-saving therapy for many infected individuals.
In the present study, patients who were newly referred for a diagnosis of HCV to two hepatology clinics endorsed many perceived barriers to accessing speciality care for HCV. Consistent with other reports, sociodemographic factors such as employment, income level, insurance status and marital status were associated with perceived barriers to HCV care.17, 40 One of the most significant findings from this study was that depressive symptoms, which were prevalent in >25% of this sample, were independently associated with all perceived barriers, over and above the impact of sociodemographic factors. Finally, higher depression scores predicted lower rates of antiviral treatment utilization, and therefore represent a patient level barrier to accessing treatment, even after these patients ostensibly overcame initial barriers to being referred to speciality clinics which treat HCV.
Between 30% and 50% of patients surveyed perceived the following significant problems in accessing specialized care for hepatitis C: lack of personal financial resources, knowledge about HCV in their community, professionals who were competent to provide care for hepatitis C, feeling stigmatized for having HCV and long distances to a clinic that treats HCV. These findings are consistent with previous research with the hepatitis C population, as well as other medically ill populations, including HIV. For example, the cost of health services, lack of insurance, social stigma, and lack of adequate training and staffing to treat HCV patients with co-morbid psychiatric disorders, are all previously described barriers.13, 14, 20
In regression models, depressive symptoms were associated with the perception of multiple barriers, over and above the contribution of sociodemographic characteristics. In other medically ill populations, depression has been related to the perception of extensive barriers to accessing medical care, and to the perception of minimal benefits from medical treatment.28 For the larger HCV community who suffers from depressive symptoms, which may range from 25% to 60%,21, 22 these barriers, whether they be actual or perceived, can be detrimental if patients do not access care early in their disease stage when management and treatment may be most effective. Although we did not assess mediating variables by which depression may exert an effect on perceived barriers to care, it is well-documented that depression is associated with poor self-efficacy, reduced will to function, reduced motivation, greater perceived stigmatization, pessimism, hopelessness and reluctance to seek healthcare services.28-32 These cognitive and behavioural correlates of depression may be considered barriers at the patient level which probably affect the perception and health-promoting behaviours that may be required to access specialized care for hepatitis C. For instance, the motivation, self-efficacy, perseverance and resourcefulness needed to overcome financial, distance and transportation obstacles may be attenuated in HCV patients with co-morbid depression. The pessimism and hopelessness that accompany depression may translate into inaction and passivity about pursuing treatment for their medical condition. Lastly, as patients with both depression and HCV often perceive lower social support and greater social stigma about their conditions,28-31 these beliefs may enhance the perception that there is a lack of providers willing to treat or interested in treating HCV patients. It does not matter whether these barriers are real or imagined; patient perceptions, attitudes and beliefs are often strong predictors of intentions and behaviours.41-43
Results of the post hoc retrospective chart review need to be viewed with caution, and are primarily descriptive in nature, although they offer some preliminary insights into the relationship between demographics, depressive symptoms and barriers to care on time from diagnosis to presentation in clinic, uptake of antiviral therapy, and treatment completion rates. Although it took 61 treatment naïve patients approximately 4 years from the time of HCV diagnosis to attend an initial visit in a speciality HCV clinic, none of the variables investigated (depression, perceived barriers to care, demographics) were significantly associated with this lag time. Lack of bivariate associations could be due to small sample size, data collection time frame and methods, or other reasons not assessed (e.g. patient preference, ongoing alcohol abuse, normal liver enzymes). We also found that patients who subsequently received antiviral therapy in the future were less depressed, and more likely to be married and employed. The notion that mental health issues, such as depression, are significant barriers to treatment is consistent with other reports.44, 45 While there was a trend for the lack of personal and financial resources as a barrier to be negatively associated with receipt of antiviral therapy, it does not appear that perceptions about distance to an HCV clinic, lack of medical/psychological services, or community stigma predict subsequent receipt of antiviral therapy. Perhaps such barriers are most relevant to patients prior to referral to a speciality HCV clinic, wherein, these barriers may cease to exert a significant impact on subsequent clinical outcomes. This speculation would need validation from prospective, observational studies. Finally, 62% of patients completed a full course of the prescribed regimen. Treatment discontinuation was due to protocol-defined termination for viral nonresponse, and adverse side effects, the most problematic of which were neuropsychiatric and gastrointestinal.
Healthcare providers, including primary care and other front-line referring providers, may be able to reduce barriers and increase access to speciality HCV care through altering clinical management strategies. It is important that providers who screen for and diagnose chronic hepatitis C recognize that co-morbid depression is quite prevalent in this patient population and is associated with perceived barriers and receipt of antiviral therapy. Thus, depression should be regularly screened for, and treated if present. Referral to mental health professionals for nonpharmacological or pharmacological treatment, is another viable option, and may actually improve a patient’s chances of becoming eligible for antiviral treatment. Second, it should be recognized that the cognitive (e.g. hopelessness, pessimism) and behavioural (e.g. loss of motivation, social withdrawal) correlates of depression may inhibit the requisite proactive, help-seeking behaviours needed to pursue specialized hepatitis care. Depressed patients may require more direct, concrete assistance (e.g. reminder phone calls, encouragement to attend) to follow through with a referral to a speciality clinic. This strategy may be particularly effective in outpatient settings where HCV is prevalent among patients (e.g. methadone clinics). Feelings of guilt and shame regarding the mode of viral acquisition, self-blame, low self-worth and social stigma are common in depressed HCV patients, and may also feed into the belief that they are undeserving of medical care for HCV. Providing educational materials and encouragement about HCV in the primary care or gastroenterological setting, increasing referrals to clinics that treat a large number of HCV patients, and providing referral for comorbid mental health or substance abuse, may be ways in which healthcare providers can aid in overcoming perceived barriers and increasing access to specialized care for HCV. Finally, as personal and financial resources were cited as the most significant barrier to speciality care, there may be other simple and effective solutions to increase referral and access to medical care for HCV. For instance, public health campaigns can increase awareness among patients and front-line referring providers about clinical trials, which can offset travel and financial burden, as well as industry-sponsored patient assistance programmes, which defray the cost of treatment for individuals from impoverished backgrounds. Using telehealth and web-based strategies to assist healthcare providers practising in rural community settings to screen, manage and treat HCV is another potential vehicle that has demonstrated promise in reducing personal and financial barriers to accessing HCV care.46
There are several limitations of this study. First, inherent to all cross-sectional study designs, we examined associations between variables measured at the same time point, and cannot make inferences about causal order. As such, the relationships between the variables examined in this study may be bi-directional or mediated by a third variable. Second, our sample comprised patients who were already being evaluated in a speciality HCV clinic. Thus, they had already managed to navigate the healthcare system and obtain a referral to a speciality hepatology clinic either by their primary care provider or by a community gastroenterologist. This circumstance may have affected our findings. For instance, it is likely that we are understating the magnitude of barriers facing the larger HCV population; patients with the greatest obstacles to care (e.g. active drug users) were unlikely to be referred to speciality clinics, and therefore were unrepresented in this study. It is likely that the rate of depression and perceived obstacles to accessing care is even higher among the larger HCV population who are not involved in a healthcare system and not represented in this study. Additionally, these barriers may not affect subsequent clinical outcomes once the patient is under the care of a speciality clinic. Third, our study analysed patient perceptions of barriers to care, and perceptions may not reflect actual barriers. Fourth, our analysis of clinical outcomes was based on a post hoc retrospective chart review and small sample size, thus these data are prone to more measurement errors and need to be viewed with caution. Finally, there were some deviations from the assumptions of the linear model that could affect the robustness of the linear regression estimates. However, the same conclusions were attained through sensitivity analyses using logistic regression.
In sum, patients with HCV report several perceived barriers to accessing healthcare specific to HCV at the individual, community and societal levels. Common mood disorders, such as depression, may amplify negative perceptions about the obstacles to obtaining medical care for HCV, although it is likely that some barriers (e.g. lack of personal and financial resources) represent actual barriers to obtaining speciality HCV care and antiviral treatment. As the prevalence of depressive symptoms in this population is high, it is critical that frontline healthcare providers screen, treat, or refer for depression. It is also important to recognize that the cognitive and behavioural correlates of depression may negatively affect patient perceptions and the help-seeking behaviours needed to overcome obstacles in the way of accessing specialized care for HCV.
ACKNOWLEDGEMENTS
We thank Dr. Morris Weinberger of the UNC School of Public Health for providing a critical review and suggestions for this manuscript. Declaration of personal interests: M. W. Fried has served as a consultant and advisory board member for Roche, Tibotec, Vertex, Pharmasset, Merck, GlaxoSmithKline, Novartis; receives grant support from Roche, Merck, Human Genome Sciences, Vertex, Tibotec, Bristol Myers Squibb and Anadys’, and owns stock in Pharmasset. Other authors have nothing to disclose. Declaration of funding interests: This investigator-initiated study was funded, in part, by a research grant from Hoffman LaRoche (PEG228; Evon). The preparation, writing and data analyses of this paper were supported, in part, by NIH grants: K24 DK066144 (Fried), KL2RR025746 (Evon), and UL1RR025747 (Esserman).
Footnotes
Disclaimer: The authors of this article are solely responsible for its content. Statements in the presentation should not be construed as endorsement by the Agency for Healthcare Research and Quality, the US Department of Health and Human Services, the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1994;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Everson GT, Weinberg H. Living with Hepatitis C: A Survivor’s Guide. 4th edn. Hatherleigh Press; New York: 2006. [Google Scholar]
- 3.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–82. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 4.Seeff L, Hoofnagle JH. National institutes of health consensus conference: management of hepatitis C. Hepatology. 2002;36:S1–20. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 5.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 7.Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 8.Shiratori Y, Ito Y, Yokosuka O, et al. Tokyo-Chiba Hepatitis Research Group Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;18:142. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Volk ML. Antiviral therapy for hepatitis C: why are so few patients being treated? J Antimicrob Chemother. 2010;65:1327–9. doi: 10.1093/jac/dkq157. [DOI] [PubMed] [Google Scholar]
- 10.McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol. 2008;22:133–7. doi: 10.1155/2008/949582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reif S, Golin CE, Smith SR. Barriers to accessing HIV/AIDS care in North Carolina: rural and urban differences. AIDS Care. 2005;17:558–65. doi: 10.1080/09540120412331319750. [DOI] [PubMed] [Google Scholar]
- 12.Taylor LE, Costello T, Alt E, Yates G, Tashima K. Psychiatric illness and illicit drugs as barriers to hepatitis C treatment among HIV/hepatitis C virus coinfected individuals. AIDS. 2002;16:1700–1. doi: 10.1097/00002030-200208160-00024. [DOI] [PubMed] [Google Scholar]
- 13.Fleming CA, Tumilty S, Murray JE, Nunes D. Challenges in the treatment of patients coinfected with HIV and hepatitis C virus: need for team care. Clin Infect Dis. 2005;40(Suppl. 5):S349–54. doi: 10.1086/427452. [DOI] [PubMed] [Google Scholar]
- 14.Edlin BR, Kresina TF, Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40:S276–85. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk, and willingness to receive treatment. Drug Alcohol Depend. 2001;61:211–5. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 16.Heckman TG, Somlai AM, Peters J, et al. Barriers to care among persons living with HIV/AIDS in urban and rural areas. AIDS Care. 1998;10:365–75. doi: 10.1080/713612410. [DOI] [PubMed] [Google Scholar]
- 17.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C: patient, provider, and system factors. J Gen Intern Med. 2005;20:754–8. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylvestre DL, Litwin AH, Clements BJ, Gourevitch MN. The impact of barriers to hepatitis C virus treatment in recovering heroin users maintained on methadone. J Subst Abuse Treat. 2005;29:159–65. doi: 10.1016/j.jsat.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93:141–7. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Zacks S, Beavers K, Theodore D, et al. Social stigmatization and hepatitis C virus infection. J Clin Gastroenterol. 2006;40:220–4. doi: 10.1097/00004836-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hilsabeck RC, Malek-Ahmadi P. Neurobehavioral correlates of chronic hepatitis C. J Psychopathol Behav Assess. 2004;26:203–10. [Google Scholar]
- 22.Evon DM, Verma A, Simpson K, Galanko JA, Dougherty KA, Fried MW. Psychiatric symptoms during interferon treatment for hepatitis C: experiences from a tertiary care hepatology centre. Aliment Pharmacol Ther. 2008;27:1071–80. doi: 10.1111/j.1365-2036.2008.03640.x. [DOI] [PubMed] [Google Scholar]
- 23.Asnis GM, De La GR. Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322–35. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 24.Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49:311–7. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123:476–82. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- 26.Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen Hosp Psychiatry. 2005;27:431–8. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Davidson JR, Meltzer-Brody SE. The underrecognition and undertreatment of depression: what is the breadth and depth of the problem? J Clin Psychiatry. 1999;60(Suppl. 7):4–9. [PubMed] [Google Scholar]
- 28.Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Res Social Adm Pharm. 2005;1:508–25. doi: 10.1016/j.sapharm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Barney LJ, Griffiths KM, Jorm AF, Christensen H. Stigma about depression and its impact on help-seeking intentions. Aust N Z J Psychiatry. 2005;40:51–4. doi: 10.1080/j.1440-1614.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- 30.Robert JE, Monroe SM. A multidimensional model of self-esteem in depression. Clin Psychol Rev. 1994;14:161–81. [Google Scholar]
- 31.Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and preferences regarding treatment of depression. J Gen Intern Med. 1997;12:431–8. doi: 10.1046/j.1525-1497.1997.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kravitz RL, Ford DE. Introduction: chronic medical conditions and depression. Am J Med. 2008;121:S1–7. doi: 10.1016/j.amjmed.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 34.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiology Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 35.Clark CH, Mahoney JS, Clark DJ, Eriksen LR. Screening for depression in a hepatitis C population: the reliability and validity of the Center for Epidemiologic Studies Depression Scale (CES-D) J Adv Nurs. 2002;40:361–9. doi: 10.1046/j.1365-2648.2002.02378.x. [DOI] [PubMed] [Google Scholar]
- 36.Golub ET, Latka M, Hagan H, et al. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the Beck Depression Inventory. J Urban Health. 2004;81:278–90. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Box GEP, Cox DR. An analysis of transformation. J Royal Stat Soc, Series B. 1964;26:211–52. [Google Scholar]
- 38.Evon DM, Verma A, Dougherty KA, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007;52:3251–8. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- 39.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–92. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed SM, Lemkau JP, Nealeigh N, Mann B. Barriers to healthcare access in a non-elderly urban poor American population. Health Soc Care Community. 2001;9:445–53. doi: 10.1046/j.1365-2524.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- 41.De Wit JB, Vet R, Schutten M, van Steenbergen J. Social cognitive determinants of vaccination behavior against hepatitis B: an assessment among men who have sex with men. Prev Med. 2005;40:795–802. doi: 10.1016/j.ypmed.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 42.Iriyama S, Nakahara S, Jimba M, Ichikawa M, Wakai S. AIDS health beliefs and intention for sexual abstinence among male adolescent students in Kathmandu, Nepal: a test of perceived severity and susceptibility. Public Health. 2007;121:64–72. doi: 10.1016/j.puhe.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Sherman AC, Pennington J, Simonton S, Latif U, Arent L, Farley H. Determinants of participation in cancer support groups: the role of health beliefs. Int J Behav Med. 2008;15:92–100. doi: 10.1080/10705500801929601. [DOI] [PubMed] [Google Scholar]
- 44.Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–9. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 45.Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes among patients with psychiatric illness. Psychiatr Serv. 2006;57:570–2. doi: 10.1176/ps.2006.57.4.570. [DOI] [PubMed] [Google Scholar]
- 46.Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment-Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124–33. doi: 10.1002/hep.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]