Abstract
This investigation examined the effect of footshock on responses of 283 spinal dorsal horn neurons (DHNs) to urinary bladder distension (UBD). Female rats were treated with seven daily sessions of footshock (chronic footshock, CFS), six accommodation sessions followed by one exposure to footshock (acute footshock, AFS), or handled similarly without receiving any footshock (no footshock, NFS). After the final footshock or NFS session, rats were anesthetized, a laminectomy performed and extracellular single-unit recordings of L6-S1 DHNs obtained in intact or spinalized preparations. Neurons were classified as Type I - inhibited by heterotopic noxious conditioning stimuli (HNCS) or as Type II - not inhibited by HNCS - and characterized for spontaneous activity and for neuronal discharges evoked by graded UBD. A differential effect of footshock-induced stress was noted on neuronal subgroups. In intact preparations, Type I neurons were less responsive to UBD after either chronic or acute stress, while Type II neurons demonstrated significantly augmented responses to UBD. This enhanced neuronal responsiveness to UBD was present in spinalized preparations following exposure to CFS but not AFS. Type I neurons were still less responsive to stress in spinalized preparations following CFS and AFS. This study provides further evidence that (1) at least two populations of spinal neurons exist which encode for visceral stimuli and are likely to have distinct roles in visceral nociception, and that (2) the chronic stress-induced enhancement of DHN responses to UBD involves changes in at the spinal level while the acute stress effects are dependent on a supraspinal substrate.
Keywords: visceral, urinary bladder, cystitis, stress, spinal
1. Introduction
Stress is one of the most common human experiences and one that modifies many other experiences, including pain. It is the rule rather than the exception that stressful life events, unless coupled with other major physiological events such as pregnancy, lead to an exacerbation of underlying pain disorders. A prominent role for stress in the pathophysiology and clinical expression of many pain states has been well-documented [Dancy, et al., 1998; Farber et al., 1986; Garrett et al., 1991; Thomason et al., 1992; Zautra et al., 1997]. Under normal conditions, acute stress induces transient release of stress-related hormones, which can act as pro-inflammatory mediators to produce short-term effects in peripheral tissue [e.g., Boucher et al., 2010; Theoharides et al., 1998]. However, chronic stress can result in long-term and maladaptive physiological changes. For example, repeated large increases in the release of stress hormones can subsequently sensitize central stress system responsiveness [McCormick et al., 1998].
Stress is frequently reported as an exacerbator of pain symptoms in humans [Bennett et al., 1995; Rothrock et al., 2001; Zautra et al., 1997], and indeed, stress has been suggested to play a causative role in development of some functional disorders of visceral systems, including interstitial cystitis (IC). IC is a painful bladder syndrome [Bennett et al., 1995; Macaulay et al., 1987] that primarily affects the female population and is characterized by pelvic and/or perineal pain, urinary urgency and frequency, and nocturia. A majority of IC patients report symptom exacerbation during periods of clinical stress, and acute experimental stress increases bladder pain and urgency in these individuals [Koziol et al., 1993; Lutgendorf et al., 2000]. As severity of the disease increases, the relationship between stress and symptom manifestation becomes even more evident [Rothrock et al., 2001].
Animal studies have demonstrated that acute [Schwetz et al., 2005; Bradesi et al., 2002] and chronic exposure to stressors such as restraint [Costa et al., 2005; Gamaro et al., 1998; Hirata et al., 2008; Toulouse et al., 2000], footshock [Robbins and Ness, 2008] and water avoidance [Bradesi et al., 2005; Mayer et al., 2001; Robbins et al., 2007] alters visceral nociceptive processing. Specifically, these manipulations produce a hypersensitive state reflected as augmented visceromotor responses to stimulation of the gut and urinary bladder. This hypersensitivity likely occurs via a combination of stress-induced cellular, molecular, neuroendocrine, and physiological changes [Imaki et al., 1991; Kuipers et al., 2003; Van Dijken et al., 1993]. The current study examined whether exposure to intermittent footshock activates the hypothalamic-pituitary-adrenocortical (HPA) axis, thus supporting its utility as an experimental stressor, and whether intermittent footshock produces neurophysiological changes in the spinal dorsal horn. We have previously demonstrated that bladder inflammation differentially affects two subpopulations of DHNs that can be distinguished by their responses to the application of a heterosegmental noxious conditioning stimulus (HNCS) [Ness et al., 2009]. Here, the modulatory effect of intermittent footshock was assessed by examining responses of DHNs to urinary bladder distension (UBD) following exposure to chronic and acute footshock in both intact and spinalized preparations.
2. Results
2.1 Plasma Corticosterone Levels
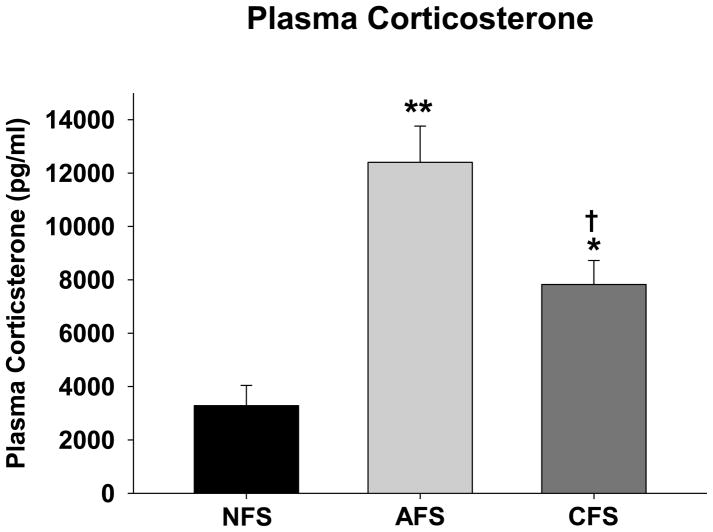
To verify that footshock exposure is a stressor, ELISA was used to quantify plasma corticosterone levels in rats exposed to the CFS, AFS and NFS conditions. A one-way ANOVA revealed a significant effect of group (F(2,20)=23.13; p<0.01). Post-hoc contrasts indicated that compared to the NFS condition (3283.38 ± 758.53 pg/ml), plasma corticosterone was significantly increased following both AFS (12399.71 ± 1359.03 pg/ml; p<0.01) and CFS (7825.57 ± 897.45 pg/ml; p=0.01) (Figure 1). Furthermore, AFS produced a significantly greater increase in circulating corticosterone than CFS (p=0.023).
Figure 1.
Plasma corticosterone concentrations measured immediately after exposure to the chronic footshock (CFS), acute footshock (AFS) or no footshock (NFS) conditions. * and ** indicate significantly different from the NFS condition with p<0.05 and p<0.01, respectively. † indicates significantly different from the AFS condition with p<0.05. N=6–12/group.
Intact Animals
2.2.1. Description of neurons responsive to UBD
UBD-evoked responses of 130 neurons were examined (Table 1). Using previously published criteria related to neurons excited by UBD [Ness and Castroman, 2001], approximately one-third (n=49) were designated as Type I neurons as a result of their observed inhibition to a HNCS. Most of these (n=20 for NFS condition; n=16 for AFS condition; n=7 for CFS condition) were of the class 2 (wide dynamic range; WDR) type, excited by both noxious and nonnoxious cutaneous stimuli. Assessment of cutaneous receptive fields demonstrated that half of the Type I neurons in the NFS condition had predominantly unilateral receptive fields, comprising 4 or 5 dermatomes, and half of the neurons had small receptive fields made up of only 1 or 2 dermatomes. Receptive fields of Type I neurons were similarly distributed in the footshock-exposed rats (62% medium, unilateral and 38% small for CFS condition; 58% medium unilateral and 42% small for AFS condition).
Table 1.
Characteristics of lumbosacral spinal dorsal horn neurons excited by urinary bladder distension (UBD) in intact, female rats
| Group | N | Class 1 | Class 2/WDR | Class 3/NS | RF Size (l:m:s) | |
|---|---|---|---|---|---|---|
| NFS | Total | 44 | ||||
| Type I | 22 | 0 | 20 | 2 | 0:11:11 | |
| Type II | 22 | 0 | 7 | 15 | 9:13:0 | |
|
| ||||||
| AFS | Total | 46 | ||||
| Type I | 19 | 0 | 16 | 3 | 0:11:8 | |
| Type II | 27 | 0 | 17 | 10 | 11:14:2 | |
|
| ||||||
| CFS | Total | 40 | ||||
| Type I | 8 | 0 | 7 | 1 | 0:3:5 | |
| Type II | 32 | 0 | 21 | 11 | 19:13:0 | |
Rats were exposed to one of three conditions: acute footshock (AFS), chronic footshock (CFS) or to no footshock (NFS). All neurons were excited by a 60 mmHg, 20 sec phasic constant pressure UBD. Neurons were further classified according to the following: (1) by counter-irritation effects: Type I neurons had spontaneous activity inhibited >20% by a non-segmental noxious cutaneous stimulus; Type II neurons were not similarly inhibited; (2) by receptive field (RF): Class 1 neurons were excited only by non-noxious stimuli; Class 2/wide dynamic range (WDR) neurons were excited by both noxious and nonnoxious cutaneous stimuli; Class 3/nociceptive specific (NS) neurons responded only to noxious cutaneous stimuli; and (3) by RF size: large (l – hind quarter or > hind quarter with contralateral input); medium (m – long axis > 4 cm, including part of hind limb but not tail); small (s – long axis < 4 cm).
Most of the total neuronal sample in the intact preparations (n=81) were not inhibited by HNCS and were designated as Type II neurons. Cutaneous classification and receptive field characteristics of these neurons differed from Type I neurons. Approximately half of the Type II neurons examined were of the class 2/WDR type and half were designated as class 3 (nociceptive specific; NS), responding only to noxious cutaneous stimuli. Receptive fields were similar in the three groups of Type II neurons, with approximately half of neurons (41% in NFS and AFS conditions; 59% in CFS condition) exhibiting large, bilateral receptive fields. The other half of the Type II neurons examined had medium-sized, predominantly unilateral receptive fields (59%, 52% and 41% for NFS, AFS and CFS conditions, respectively). Seven percent of neurons in the AFS condition had small receptive field sizes.
2.2.2. Effect of footshock
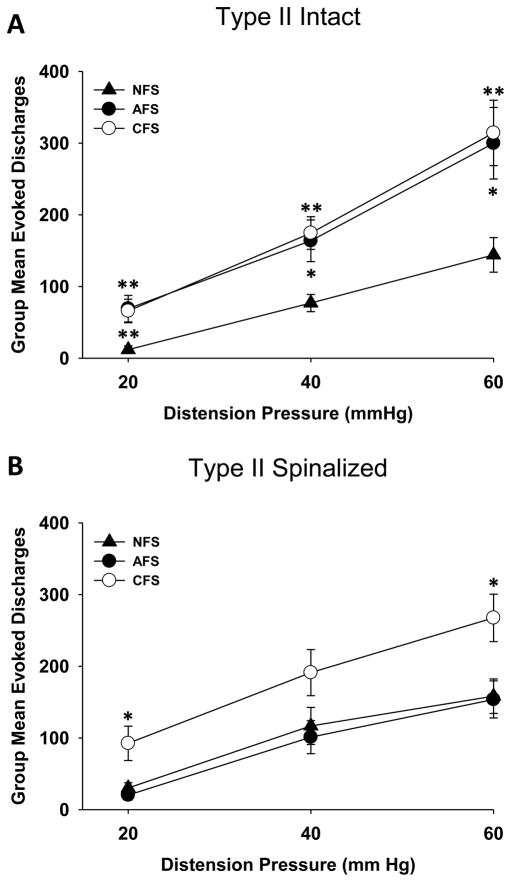
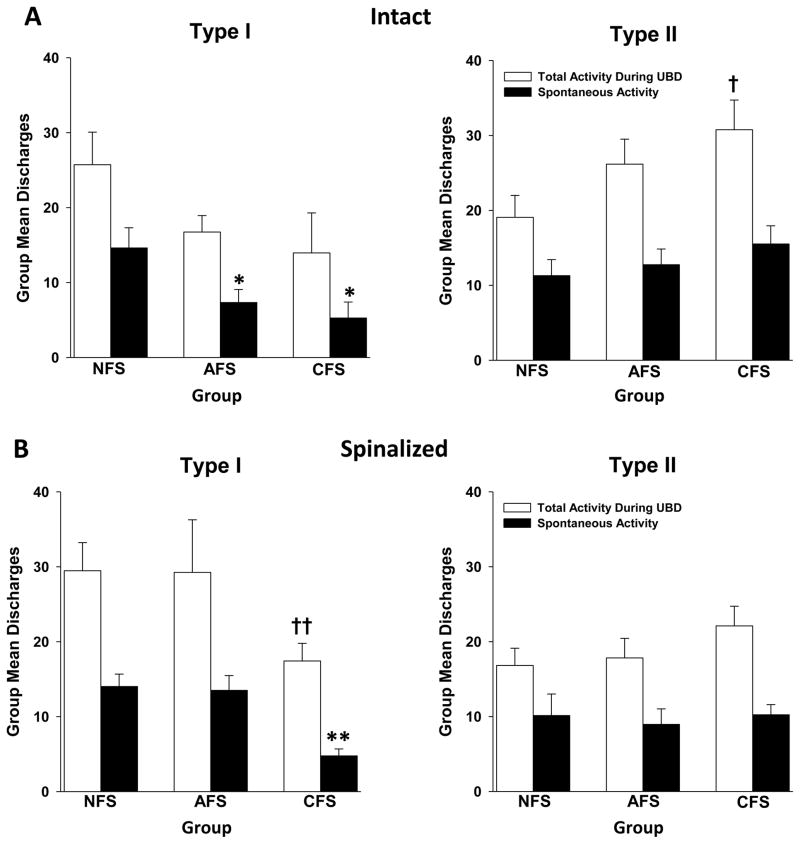
One noteworthy discovery of this study is that exposure to footshock stress differentially affected UBD-evoked discharges of Type I versus Type II neurons. A repeated measures ANOVA revealed a significant effect of footshock on Type II neurons (F(2,76)=6.341; p<0.01). Post-hoc tests indicated that UBD-evoked responses of Type II neurons were significantly augmented after either AFS or CFS at all distension pressures tested (Figure 2A). In contrast, no alterations in UBD-evoked responses of Type I neurons were evident after footshock (F(2,46)=0.026; p=0.975). Total activity of Type II neurons to a 60 mmHg UBD stimulus also increased following footshock (Figure 3A), and this was significant only after CFS exposure (t=2.382; p=0.021). Examination of spontaneous activity revealed that Type I neurons demonstrated a significant decrease in spontaneous activity after either AFS exposure (t=2.263; p=0.03) or CFS exposure (t=2.708; p=0.012) compared to the NFS condition (Figure 3A). Spontaneous activity of Type II neurons was unaffected by either footshock paradigm. These data indicate that exposure to either acute or chronic footshock stress increases excitability of Type II spinal DHNs to distension of the urinary bladder. In contrast, Type I neurons are actually inhibited by footshock stress, as evidenced by the observed decrease in their spontaneous activity.
Figure 2.
Stimulus-response functions relating group mean evoked discharges (± SEM) of L6-S1 Type II spinal dorsal horn neurons (DHNs) excited by urinary bladder distension (UBD) in rats that were exposed to acute footshock (AFS; closed circles), chronic footshock (CFS; open circles) or no footshock (NFS; closed triangles). UBD-evoked discharges were calculated as the difference between the total activity measured for the 20 sec period of UBD and the ongoing spontaneous activity. (A) In intact preparations, both AFS and CFS enhanced UBD-evoked discharges of Type II spinal DHNs compared to the NFS condition. (B) After spinalization, responses of Type II neurons were only enhanced after CFS. * and ** indicate significantly different from the NFS condition with p<0.05 and p<0.01, respectively. N=22–32/group.
Figure 3.
Histograms depict group mean (± SEM) total (open bars) and spontaneous (closed bars) activity of Type I and Type II L6-S1 spinal DHNs in Hz. Spontaneous activity was determined as the average rate of neuronal discharges per second in the 10 sec period prior to the onset of UBD. Total activity was determined as the rate of neuronal discharges in the 20 sec period during the UBD stimulus. The data represent the mean of the average response of each cell in a particular group. (A) In the intact preparations, spontaneous activity of Type I neurons was significantly decreased compared to NFS after either AFS or CFS exposure (left), while spontaneous activity of Type II neurons was unaffected by stress (right). A significant change in total activity was only observed in Type II neurons and only after CFS (right). (B) In spinalized preparations, Type I neurons demonstrated an inhibition of spontaneous activity and total activity after CFS (left). Spontaneous and total activity of Type II neurons was unchanged by stress (right). * and † indicate significantly different compared to the NFS condition with p<0.05. ** and †† indicate significantly different compared to the NFS condition with p<0.01. N=8–32/group.
2.2 Spinalized Animals
2.3.1. Description of neurons responsive to UBD
Responses of 153 neurons to UBD were examined in spinalized preparations following exposure to a footshock stressor or to the NFS condition (Table 2). Of these, approximately half (n=82) demonstrated inhibition to a HNCS and were designated as Type I neurons. Most of these (n=21 for NFS condition; n=24 for AFS condition; n=26 for CFS condition) were of the class 2/WDR type, excited by both noxious and nonnoxious stimuli. Assessment of cutaneous receptive fields demonstrated that 68% of the Type I neurons in the NFS condition had predominantly unilateral receptive fields, comprising 4 or 5 dermatomes, whereas 32% of the neurons had small receptive fields made up of only 1 or 2 dermatomes. Examination of Type I neurons in the footshock-exposed rats revealed a similar distribution of receptive fields (AFS condition: 72% medium, unilateral; 28% small; CFS condition: 64% medium, unilateral; 36% small).
Table 2.
Characteristics of lumbosacral spinal dorsal horn neurons excited by urinary bladder distension (UBD) in cervical spinal cord-transected, decerebrate female rats
| Group | N | Class 1 | Class 2/WDR | Class 3/NS | RF Size (l:m:s) | |
|---|---|---|---|---|---|---|
| NFS | Total | 47 | ||||
| Type I | 25 | 0 | 21 | 4 | 0:17:8 | |
| Type II | 22 | 0 | 11 | 11 | 17:3:2 | |
|
| ||||||
| AFS | Total | 52 | ||||
| Type I | 29 | 0 | 24 | 5 | 0:21:8 | |
| Type II | 23 | 0 | 12 | 9 | 7:16:0 | |
|
| ||||||
| CFS | Total | 54 | ||||
| Type I | 28 | 0 | 26 | 2 | 0:18:10 | |
| Type II | 26 | 1 | 12 | 13 | 16:6:4 | |
Rats were exposed to one of three conditions: acute footshock (AFS), chronic footshock (CFS) or to no footshock (NFS). All neurons were excited by a 60 mmHg, 20 sec phasic constant pressure UBD. Neurons were further classified by counter-irritation effects (Type I or Type II), by RF (Class 1, Class 2/WDR or Class 3/NS) and by RF size (large, medium or small) as described in Table 1.
Approximately half of the total neuronal sample (n=71) were not inhibited by HNCS and were designated as Type II neurons. Although cutaneous classification and receptive field characteristics of these neurons differed from Type I neurons, they were similar between the CFS and NFS conditions. Half of the Type II neurons in both conditions were of the class 2/WDR type and half were designated as class 3/NS, responding only to noxious stimuli. One neuron in the footshock condition was excited only by non-noxious stimuli. Receptive fields were also similar in the two groups of Type II neurons, with the majority of neurons (77% and 62% in NFS and CFS conditions, respectively) exhibiting large, bilateral receptive fields. 14% and 23% of neurons in the NFS and footshock conditions, respectively, had medium-sized, predominantly unilateral receptive fields, and a few neurons (9% and 15% in NFS and CFS conditions, respectively) had small receptive field sizes. Characteristics of Type II neurons assessed in the AFS condition differed slightly. Most of these neurons were of the class 2 type (61%), and the rest (39%) were designated class 3. The majority of these neurons (70%) had medium-sized receptive fields, with 30% of the neurons exhibiting large receptive field sizes.
2.3.2. Effect of footshock
Similar to the phenomena observed in the intact preparation, exposure to footshock stress differentially affected UBD-evoked discharges of Type I versus Type II neurons in spinalized preparations. A repeated measures ANOVA again revealed a significant effect of footshock on responses of Type II neurons (F(2,68)=5.788; p<0.01). Further analyses revealed that CFS, but not AFS, augmented UBD-evoked discharges (Figure 2B). In contrast, footshock exposure actually decreased evoked responses of Type I neurons (F(2,79)=3.271; p=0.043). These differences achieved statistical significance only for the AFS and not the CFS condition when compared to the NFS condition. A statistically significant decrease in spontaneous activity (t=4.510; p<0.01) and total activity at a 60 mmHg distension pressure (t=2.724; p<0.01) was also seen in Type I neurons but only after CFS exposure (Figure 3B). Spontaneous and total activity of Type II neurons was unchanged by footshock stress (Figure 3B).
These data again demonstrate that neuronal excitability of Type I spinal DHNs is decreased after exposure to a footshock stressor (either chronic or acute). Responses of Type II neurons are enhanced by chronic stress, and these changes occur without any influences from supraspinal sites. In contrast, acute stress-induced changes in neuronal excitability depend on descending modulatory influences.
3. Discussion
3.1. Footshock Activates the HPA Axis
Many studies in the field of stress biology utilize plasma ACTH and cortisol/corticosterone concentrations as indices of stressor-induced physiological activation of the HPA axis. Evidence shows that plasma levels of these hormones and CRF mRNA in the hypothalamus are enhanced following exposure to footshock [Imaki et al., 1991; Iwasaki-Sekino et al., 2009; Zelena et al., 2009]. The presence of elevated plasma corticosterone concentration following both acute and chronic footshock exposure in the present set of experiments provides validation of these models as physiological stress paradigms.
3.2. Chronic Footshock Stress Produces Changes in Visceral Nociceptive Processing at the Level of the Spinal Cord
Both acute and chronic footshock stress augmented UBD-evoked responses of Type II neurons in the intact preparation. However, following spinalization, this increase in neuronal activity only occurred after exposure to the CFS paradigm; responses of Type II neurons to UBD were similar after AFS and NFS exposure. This indicates that the enhanced responsiveness in DHNs to chronic stress occurred in the absence of any immediate descending modulatory influence arising from a supraspinal source. These findings imply that the enhanced responsiveness is representative of either sustained neurochemical and/or neurophysiological changes in sensory processing at the level of the spinal cord, or that some sustained or more permanent spinal effect derived from a supraspinal source was in place prior to transection. Previous investigations related to chronic stress effects on urinary bladder sensation have suggested a role for urocortins acting through corticotrophin releasing factor 2 (CRF2) receptors [Robbins and Ness, 2008]. One would predict from the present findings that alterations in responsiveness of spinal DHNs to the application of urocortins to the spinal cord would be present in rats which had experienced CFS stress. Future experiments will assess this potential neurochemical alteration.
That UBD-evoked responses of Type II neurons were unchanged by AFS exposure following spinalization suggests that acute stress-induced augmentation of DHNs does involve a supraspinal substrate. This finding is not surprising since a key component of the physiological stress response is activation of the HPA axis, a feedback loop by which signals from the brain trigger release of hormones necessary to respond to stress [Smith and Vale, 2006]. More specifically, stress stimulates release of CRF from the paraventricular nucleus of the hypothalamus. CRF then causes the pituitary gland to release adrenocorticotrophic hormone that stimulates synthesis and release of cortisol, epinephrine, norephinephrine, and endorphins from the adrenal cortex. The HPA axis exerts effects on the autonomic nervous system and glandular systems and communicates with a number of different brain regions that control body temperature, appetite, and pain perception. Two of these supraspinal areas are the amygdala and thalamus. Basic science studies have demonstrated a role for the amygdala in visceral nociceptive facilitation in several animal models [Greenwood Van-Meerveld et al., 2001; Han and Neugebauer, 2004; Ikeda et al., 2007; Myers and Greenwood Van-Meerveld, 2007; Qin et al., 2003a, 2003b; Suarez-Roca et al., 2008]. Pain-related plasticity within the amygdala has been observed in rats following acute onset of colitis [Han and Neugebauer, 2004] and in a cyclophosphamide-induced cystitis pain model in mice [Nishii et al., 2007]. Studies in the gastrointestinal system have demonstrated that chemical stimulation of the central nucleus of the amygdala (CeA) produces behavioral [Qin et al., 2003a, 2003b] and spinal neuronal [Greenwood Van-Meerveld et al., 2001] hypersensitivity in response to colorectal distension. To date, two similar studies have addressed the involvement of the amygdala in nociceptive modulation related to the urinary bladder. Neuroendocrine peptide expression is upregulated in the CeA after cyclophosphamide-induced cystitis of the urinary bladder [Nishii et al., 2007], and chemical stimulation of the CeA via stereotaxic application of corticosteroids results in facilitation of spinal dorsal horn neuronal responses to UBD [Qin et al., 2003b]. The CeA also is the area within the amygdala from which the principal neurons that project to the basal forebrain, hypothalamus, and brainstem structures arise [Gauriau and Bernard, 2002], and it has been shown to exert regulatory influences over the HPA axis [Feldman and Weidenfeld, 1998; Shepard et al., 2003]. The thalamus has long been considered to be the crucial cerebral structure for receiving, processing, and transferring nociceptive information, including noxious visceral sensation [Willis and Westlund, 1997]. Several studies have also reported stress activation of various thalamic nuclei [Bubser and Deutch, 1999; Paleček et al., 2003; Van de Kar et al., 1991], suggesting that the thalamus may be involved in HPA axis regulation. It is likely that at least one of these sites is involved in chronic stress-induced excitability of Type II spinal DHNs.
3.2. Responses of Type I and Type II DHNs Are Differentially Affected by Footshock Stress
The present study revealed a differential effect of exposure to stress on responses of two subpopulations of DHNs that are normally both excited by UBD. In contrast to the enhanced activity observed in Type II neurons following stress, responses of Type I neurons were actually inhibited by stress. In intact preparations, UBD-evoked responses of Type I neurons was essentially unchanged, but a robust decrease in spontaneous activity was evident after exposure to either acute or chronic stress. After spinalization, evoked responses were significantly lower in AFS-exposed animals compared to those in the NFS condition, and both spontaneous and total activity were significantly reduced by CFS exposure. Both CFS stress and bladder inflammation produce a similar augmentation of visceromotor reflexes [Randich et al., 2006; Robbins and Ness, 2008], and it is notable that in spinalized preparations, bladder inflammation also has a differential effect on neuronal subgroups [Randich et al., 2006] similar to what was observed in the present study. In both sets of studies, Type II neurons exhibited a significantly increased vigor of response to UBD in the experimental groups (inflamed or chronically stressed), while Type I neurons had a decreased vigor of response. However, the changes that occurred following inflammation versus chronic stress were not identical. Whereas bladder inflammation produces a robust increase in the spontaneous activity of Type II neurons, CFS-induced stress did not significantly affect spontaneous activity of these neurons. This may reflect differences in primary afferent input which is likely increased with bladder inflammation, but unaffected by footshock stress. Both bladder inflammation and footshock stress increased the intrinsic responsiveness of Type II neurons to bladder afferent inputs, but in some manner engaged an inhibitory system that suppressed activity of Type I neurons. Similar changes in responses of Type I and Type II neurons have been observed following inflammation of the colon/rectum [Ness et al., 2009]. In aggregate, these findings suggest that spinal mechanisms of various nociceptive modulatory systems may have common submechanisms involving both excitation and inhibition of spinal DHNs that serve to alter the balance or ratio of activity within neuronal subgroups that transmit information to supraspinal sites.
3.3. Footshock Stress Does Not Alter Cutaneous Receptive Field Properties
The characteristics of the cutaneous responses and receptive fields of the Type I and Type II neurons in the present study (Tables 1 and 2) are similar to those of a previous report [Ness et al., 2009] that also used UBD as the stimulus. Unlike bladder inflammation, however, stress did not cause any obvious expansion of cutaneous receptive fields or alteration in the response characteristics of DHNs examined in the CFS, AFS, and NFS groups: the numbers of neurons designated as class 2/WDR DHNs and class 3/NS DHNs were comparable and the receptive field sizes were grossly similar. This suggests that while stress and bladder inflammation both produce visceral hypersensitivity, the modulatory pathways, receptors and neurotransmitters, and supraspinal sites activated by these distinctive sensory modifiers are not identical. Future experiments utilizing pharmacological manipulation of neuronal subtypes and lesions of supraspinal sites may allow for a more precise distinction between the mechanisms of nociceptive processing underlying these two very different sensitizing paradigms.
In summary, the present study quantitatively examined the effect of exposure to chronic and acute stress on responses of two types of DHNs. Under normal conditions, these neurons are both excited by UBD, but can be distinguished on basis of differential responses to the application of a HNCS. Our study showed that Type I neurons (inhibited by HNCS) were less responsive to UBD after either chronic or acute stress, while Type II neurons (not inhibited by HNCS) demonstrated significantly augmented responses to UBD. This study provides further evidence that (1) at least two populations of spinal neurons exist which encode for visceral stimuli and are likely to have distinct roles in visceral nociception, and that (2) the chronic stress-induced enhancement of DHN responses to UBD involves changes in at the spinal level while the acute stress effects are dependent on a supraspinal substrate.
4. Experimental Procedure
4.1 Animal Subjects
Female Sprague Dawley rats 11–12 weeks of age (Harlan, Prattville, AL) were used in experiments. Female rats were chosen since disorders of the urinary bladder that are associated with pain primarily affect and are prevalent in the female population. Estrous cycle was not controlled for in these experiments. There was at least one week between the time of the animals’ arrival and the start of any experimental procedures. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and adhered to the guidelines of the National Institutes of Health.
4.2 Plasma Corticosterone ELISA
Immediately following exposure to the CFS, AFS, or NFS paradigm, blood was collected from anesthetized rats via cardiac puncture into heparinized vials and centrifuged at 14,000 × g for 15 min. Plasma was extracted and plasma samples (50 μl) were aliquotted onto a 96-well plate in duplicate. Corticosterone acetylcholinesterase tracer (50 μl) and corticosterone antiserum (50 μl) were added to each well. The plate was covered and incubated for two h at room temperature on an orbital shaker, then emptied and rinsed five times with wash buffer. Ellman’s reagent (200 μl) was added to each well and corticosterone acetylcholinesterase tracer (5 μl) was added to the Total Activity wells. The plate was covered and developed in the dark on an orbital shaker for 60–90 min. Optical densities (OD) at 412 nm were obtained using a plate reader (BioRad Laboratories, Hercules, CA). A standard curve was plotted and the concentration of corticosterone in each sample was calculated from the curve based on its OD value.
4.3 Footshock Paradigm
Electrical footshock is an established and readily controlled stressor [Kant et al., 1983; Rivier and Vale, 1987] that has been used to produce behavioral and neurochemical changes in a variety of experiments. Factors such as timing, predictability, frequency, intensity and duration of exposure to footshock determine the characteristics of the resultant stress response. We chose to use a CFS paradigm described by Imaki et al. [1991], which produces activation of the hypothalamic-pituitary-adrenal axis as evidenced by upregulation of CRF mRNA in the brain. This chronic stress paradigm has also been demonstrated to produce bladder hypersensitivity as measured by reflex visceromotor responses to UBD [Robbins and Ness, 2008]. Rats in the CFS group were placed in operant conditioning chambers enclosed in sound-attenuating cubicles and received daily intermittent footshock (15 min/day, 1.0 mA, 1s duration, total of 30 shocks each day) administered via a parallel rod floor under a variable-interval schedule for 7 days. This CFS paradigm was modified to examine effects of acute stress exposure. Rats in the AFS group were placed in the operant conditioning chambers for 15 min/day for 6 days followed by a single exposure to intermittent footshock on day 7. Rats in the NFS group were treated in an identical manner except they did not receive any footshocks while in the operant conditioning chambers.
4.4 Electrophysiology
4.4.1 Intact preparation
Immediately after the final CFS, AFS, or NFS session, rats were anesthetized with mask isoflurane (5% induction; 1–2% maintenance). Jugular venous and tracheal cannulae were placed, and the rats were artificially ventilated. Paralysis was established with pancuronium bromide (0.2 mg/h, i.v.), and a lumbar laminectomy was performed to expose the L6-S2 spinal cord segments. The rats were suspended by vertebral clamps, the dura mater was cut and removed, and the exposed spinal cord was covered with a protective layer of mineral oil.
4.4.2 Spinalized preparation
As in the intact preparation, rats were anesthetized immediately after exposure to the final CFS, AFS, or NFS session, instrumented with venous and tracheal cannulae, and artificially ventilated. The upper cervical spinal cord was exposed at the level of the atlanto-occipital joint, infiltrated with 50 μl of 1% lidocaine, and subsequently transected. The brain was mechanically pithed and the isoflurane anesthesia discontinued. Normal saline was administered as needed to prevent hypovolemia. The animals were kept warm with heating pads and allowed to recover for four or more hours, at which time they demonstrated brisk flexion reflex responses to tail and hindlimb pinch. Paralysis was then established with pancuronium bromide (0.2 mg/h, i.v.), and a lumbar laminectomy was performed as described above.
4.4.3. Spinal electrophysiology
Constant-pressure UBD (20–60 mmHg, 20 s) was the noxious visceral test stimulus employed and was produced by inflating the urinary bladder with air using a 22 gauge angiocatheter placed via the urethra and tightly sutured around the distal urethral orifice. UBD was administered as a phasic stimulus (rapid onset, rapid offset) as previously described [Ness et al., 2009]. Intravesical distending pressure was monitored via an in-line, low-volume pressure transducer.
Tungsten microelectrodes (Micro Probe Inc. Gaithersburg, MD; 1.2–1.5 MΩ) were used for single-unit extracellular recordings of neurons in spinal segments L6-S2 0–1.0 mm lateral to midline, 0.1–1.0 mm ventral to the spinal cord dorsum. To quantify neuronal responses, units were displayed on an oscilloscope for continuous monitoring, discriminated conventionally from background, converted into uniform pulses and saved digitally by computer. Spontaneous activity was determined as the average rate of neuronal discharges per second in the 10 sec period prior to the onset of UBD. Total activity was determined as the rate of neuronal discharges in the 20 sec period during the UBD stimulus. UBD-evoked discharges were calculated as the difference between the total activity measure and the spontaneous activity for the 20 sec period of UBD. Responses (excitatory/inhibitory) to cutaneous inputs were determined following the presentation of multiple UBD trials (20, 40, 60 mmHg) using the following stimuli: brush with a cotton-tipped applicator (nonnoxious mechanical) and pinch with a rat-tooth forceps (12 cm) at sufficient intensity to produce pain in the investigator (noxious mechanical). Definitions of cutaneous receptive field (RF) sizes were adapted from a previous study [Ness and Gebhart, 1989]. Sizes were defined as small (long axis <4 cm), medium (long axis > 4 cm, including part of hind limb but not tail) or large (hind quarter or > hind quarter with contralateral input). The effect on spontaneous activity of a 5 sec duration application of the HNCS, a noxious mechanical stimulus to cervical or upper thoracic dermatomes, was determined in all units. If a DHN was inhibited >20%, then it was defined as a Type I neuron. If a DHN was excited, unaffected or inhibited < 20%, then it was defined as a Type II neuron. This 20% criterion was based on previous quantitative studies which have observed random changes in spontaneous activity up to 20% [Ness and Castroman, 2001; Ness et al., 2009].
4.5. Statistics
Descriptive statistics are reported as group mean ± SEM. For comparisons of plasma corticosterone levels, a standard curve was plotted and the concentration of corticosterone in each sample was calculated from the curve based on its OD value. Group mean comparisons of sample concentrations among the three groups were performed using ANOVA followed by t-tests. Quantitative comparisons of group mean abdominal EMG responses were performed using repeated-measures ANOVA, and post-hoc effects were assessed by t-tests). Statistical significance for all analyses was set at p≤0.05, and Holm’s correction [Holm, 1979] was used to maintain family-wise α for each set of individual group comparisons.
Acknowledgments
Supported by DK51413, DK078655, and DK073218.
Abbreviations
- AFS
acute footshock
- CeA
central nucleus of the amygdala
- CFS
chronic footshock
- CRF2
corticotrophin releasing factor 2
- DHN
dorsal horn neuron
- HNCS
heterotopic noxious conditioning stimuli
- HPA
hypothalamic-pituitary-adrenocortical
- NFS
no footshock
- NS
nociceptive-specific
- OD
optical density
- UBD
urinary bladder distension
- WDR
wide dynamic range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–61. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher W, Kempuraj D, Michaelian M, Theoharides TC. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010;106:1394–1399. doi: 10.1111/j.1464-410X.2010.09237.x. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Stress induces fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G. Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett. 2005;383:7–11. doi: 10.1016/j.neulet.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Dancey CP, Taghavi M, Fox RJ. The relationship between daily stress and symptoms of irritable bowel: a timesseries approach. J Psychosom Res. 1998;441:537–545. doi: 10.1016/s0022-3999(97)00255-9. [DOI] [PubMed] [Google Scholar]
- Farber EM, Nickoloff BJ, Recht B, Fraki JE. Stress, symmetry and psoriasis: possible role of neuropeptides. Am J Acad Dermatol. 1986;2:305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- Gamaro GD, Xavier MH, Denardin JD, Pilger JA, Ely DR, Ferriera MB, Dlamaz C. The effects of acute and repeated restraint stress on the nociceptive response in rats. Physiol Behav. 1998;63:693–697. doi: 10.1016/s0031-9384(97)00520-9. [DOI] [PubMed] [Google Scholar]
- Garrett VD, Brantley PJ, Jones GN, McKnight GT. The relation between daily stress and Crohn’s disease. J Behav Med. 1991;14:87–96. doi: 10.1007/BF00844770. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathyways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Greenwood Van-Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 2001;893:135–142. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci Lett. 2004;361:254–257. doi: 10.1016/j.neulet.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: a comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterol Motil. 2008;20:557–65. doi: 10.1111/j.1365-2982.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stats. 1979;6:65–70. [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C. Differential regulation of corticotrophin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinol. 2009;34:226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Mougey EH, Pennington LL, Meyerhoff JL. Graded footshock stress elevates pituitary cyclic AMP and plasma β – endorphin, β-LPH, corticosterone and prolactin. Life Sci. 1983;33:2657–2663. doi: 10.1016/0024-3205(83)90350-8. [DOI] [PubMed] [Google Scholar]
- Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465–469. doi: 10.1016/s0022-5347(17)36120-7. [DOI] [PubMed] [Google Scholar]
- Kuipers SD, Trentani A, Den Boer JA, Ter Horst GJ. Molecular correlates of impaired prefrontal plasticity in response to chronic stress. J Neurochem. 2003;85:1312–23. doi: 10.1046/j.1471-4159.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol. 2000;164:1265–1269. [PubMed] [Google Scholar]
- Macaulay AJ, Stern RS, Holmes DM, Santon SL. Micturition and the mind: psychological factors in the etiology and treatment of urinary symptoms in women. Br Med J. 1987;294:540–543. doi: 10.1136/bmj.294.6571.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. Stress and irritable bowel syndrome, Am. J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Kovacs S. Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. Int J Dev Neurosci. 1998;16:175–185. doi: 10.1016/s0736-5748(98)00026-4. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1622–G1629. doi: 10.1152/ajpgi.00080.2007. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of superficial T13-L2 dorsal horn neurons encoding for colorectal distension in the rat: comparison with neurons in deep laminae. Brain Res. 1989;486:301–309. doi: 10.1016/0006-8993(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Castroman P. Evidence for two populations of rat spinal dorsal horn neurons excited by urinary bladder distension. Brain Res. 2001;923:147–156. doi: 10.1016/s0006-8993(01)03216-4. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Castroman P, Randich A. Acute bladder inflammation differentially affects rat spinal visceral nociceptive neurons. Neurosci Lett. 2009;467:150–154. doi: 10.1016/j.neulet.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishii H, Nomura M, Aono H, Fujimoto N, Matsumoto T. Up-regulation of galanin and corticotropin-releasing hormone mRNAs in the key hypothalamic and amygdaloid nuclei in a mouse model of visceral pain. Regul Pept. 2007;141:105–112. doi: 10.1016/j.regpep.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Paleček J, Paleckova V, Willis WD. Fos expression in spinothalamic and postsynaptic dorsal column neurons following noxious visceral and cutaneous stimuli. Pain. 2003;104:249–257. doi: 10.1016/s0304-3959(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Qin C, Greenwood Van-Meerveld B, Foreman RD. Visceromotor and spinal neuronal responses to colorectal distension in rats with aldosterone onto the amygdala. J Neurophysiol. 2003a;90:3–11. doi: 10.1152/jn.00023.2003. [DOI] [PubMed] [Google Scholar]
- Qin C, Greenwood Van-Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol. 2003b;90:2180–2189. doi: 10.1152/jn.00298.2003. [DOI] [PubMed] [Google Scholar]
- Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6:2–8. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Vale WW. Diminished responsiveness of the hypothalamic-pituitary-adrenal axis of the rat during exposure to prolonged stress: a pituitary-mediated mechanism. Endocrinol. 1987;121:1320–1328. doi: 10.1210/endo-121-4-1320. [DOI] [PubMed] [Google Scholar]
- Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav. 2007;91:544–550. doi: 10.1016/j.physbeh.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotropin-releasing factor receptors. J Pain. 2008;9:991–998. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff TL, Zimmerman B. Daily stress and symptom exacerbation in interstitial cystitis patients: a life stress model. Urology. 2001;57:422–427. doi: 10.1016/s0090-4295(01)01075-5. [DOI] [PubMed] [Google Scholar]
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Taché Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G704–712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Shepard J, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamic-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Roca H, Leal L, Silva JA, Pinerua-Shuhaibar L, Quintero L. Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress. Behav Brain Res. 2008;48:469–483. doi: 10.1016/j.bbr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinol. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- Thomason BT, Brantley PJ, Jones GN, Dyer HR, Morris JL. The relation between stress and disease activity in rheumatoid arthritis. J Behav Med. 1992;15:215–220. doi: 10.1007/BF00848326. [DOI] [PubMed] [Google Scholar]
- Toulouse M, Coelho AM, Fioramonti J, Lecci A, Maggi C, Buéno L. Role of tachykinin NK2 receptors in normal and altered rectal sensitivity in rats. Br J Pharmacol. 2000;129:193–199. doi: 10.1038/sj.bjp.0703040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and rennin secretion. Neuroendocrinol. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- Van Dijken HH, de Goeij DC, Sutano W, Mos J, de Kloet ER, Tilders FJ. Short inescapable stress produces long-lasting changes in the brain-pituitary-adrenal axis of adult male rats. Neuroendocrinol. 1993;58:57–64. doi: 10.1159/000126512. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Hoffman J, Potter P, Matt KS, Yocum D, Castro L. Examination of changes in interpersonal stress as a factor in disease exacerbations among women with rheumatoid arthritis, Ann. Behav Med. 1997;19:279–286. doi: 10.1007/BF02892292. [DOI] [PubMed] [Google Scholar]
- Zelena D, Domokos A, Jain SK, Jankord R, Filaretova L. The stimuli-specific role of vasopressin in the hypothalamus-pituitary-adrenal axis response to stress. J Endocrinol. 2009;202:263–78. doi: 10.1677/JOE-09-0096. [DOI] [PubMed] [Google Scholar]