Abstract
Objective
HIV-associated nephropathy (HIVAN) is characterized by the development of glomerulosclerosis and is associated with glomerular epithelial cell proliferation. It has recently been shown that activation of the Notch signaling pathway in podocytes results in glomerulosclerosis and podocyte proliferation. To determine whether Notch signaling is involved in renal disorder associated with HIVAN, we evaluated the expression of Notch receptors in HIVAN.
Design
We evaluated the expression of the Notch signaling pathway using an HIV-transgenic (HIV-Tg) rat model of HIVAN, and biopsy samples from HIVAN and normal controls.
Methods
Paraffin sections and kidney lysates were used for immunohistochemistry, immunofluorescence and western blot analysis.
Results
A collapsing variant of glomerulosclerosis and focal segmental sclerosis was observed in HIV-Tg rats. Glomeruli of HIV-Tg rats demonstrated activation of Notch1 and Notch4, as determined by the presence of the intracellular domains. In addition, we observed increased expression of the Notch target protein, hairy enhancer of split homolog-1 in glomeruli of these animals. The expression of the Groucho homolog transducin-like enhancer protein 4, a Notch effector protein, and the homeodomain protein cut homeobox 1 were also significantly increased in glomeruli of HIV-Tg rats, and this was associated with decreased expression of the cyclin kinase inhibitor p27. Intriguingly, renal biopsy samples from HIVAN patients also showed upregulation of cleaved Notch1 and Notch4 in the glomeruli compared with the expression in normal kidneys.
Conclusion
Our results demonstrate activation of Notch signaling pathway in HIVAN, thereby underscoring its role in disease pathogenesis.
Keywords: cut homeobox 1, glomerulosclerosis, HIV-associated nephropathy, Notch
Introduction
HIV-associated nephropathy (HIVAN) is a prominent renal complication of HIV infection, characterized by heavy proteinuria and progressive renal failure with poor prognosis. It is the single most common cause of chronic renal disease in HIV-1-seropositive patients and is the third leading cause of end-stage renal disease in African–Americans [1–3]. Although the use of antiretroviral therapy has substantially improved the survival of HIV-infected individuals, morbidity and mortality rates among patients with kidney etiology remain high [4]. The disorders mediated by HIVAN include collapsing glomerulosclerosis that is associated with severe tubulointerstitial injury, microcystic dilatation, increased apoptosis in tubules and disruption of cell cycle regulation [2]. Normal mature podocytes express the cyclin kinase inhibitor p27, consistent with a quiescent cell phenotype [5]. In HIVAN, however, expression of this protein is decreased. In addition, there is a concomitant increase in cell proliferation in podocytes within the sclerotic areas of the glomeruli [6]. HIVAN is also accompanied by downregulation of the podocyte maturation markers such as Wilms tumor 1 (WT1), synaptopodin and podocalyxin [7]. Taken together, these findings suggest that HIV-1 infection leads to podocyte proliferation and dedifferentiation, a feature unlike other glomerular diseases in which podocytes apoptose and are lost. Although both clinical and experimental studies implicate direct viral infection of renal epithelial cells [8], the mechanisms of HIV pathogenesis in the kidney remain elusive.
The Notch signaling pathway is critical for normal kidney development. In mammals, Notch signaling is activated when Notch receptors (Notch1–4) bind to their cognate ligands (delta, Jagged1 and Jagged2) resulting in the proteolytic cleavage and release of the Notch intracellular domain. Notch intracellular domain translocates to the nucleus and associates with the transcription factor recombination signal sequence-binding protein-J kappa (RBP-jκ), resulting in transcription of Notch signaling effectors such as hairy enhancer of split (Hes). These newly synthesized proteins then recruit the corepressors such as Groucho-related genes/transducin-like enhancer (Grg/TLE) [9] to subsequently repress tissue-specific genes [10,11].
We have recently demonstrated that similar to the Hes, cut homeobox 1 (Cux1) can also associate with TLE protein 4 (TLE4) during kidney development to repress expression of the cyclin kinase inhibitor, p27 [12]. In keeping with the observation that Cux1 is upregulated by Notch intracellular domain, our findings suggested that Cux1 is also an effector of the Notch signaling pathway. Notch signaling is involved in multiple cellular processes, including cell fate determination, development, differentiation, proliferation, apoptosis and regeneration. During kidney development, Notch signaling is essential for initiating differentiation of podocyte progenitors; however, its suppression may be necessary for terminal differentiation [13]. Moreover, recent studies indicate that Notch1 is activated in the patients with diabetic nephropathy and focal segmental glomerulosclerosis (FSGS)[14,15]. Furthermore, conditional expression of Notch1 intracellular domain in mature podocytes in transgenic mice leads to the development of proteinuria and glomerulosclerosis [14,15]. Given the fact that HIVAN involves several kidney disorders, including FSGS, we hypothesized that Notch signaling may play a key role in the pathogenesis of HIVAN.
In the current study, we used a noninfectious HIV-1 rat model bearing a gag/pol-deleted provirus that expresses seven of the nine HIV-1 genes [16–18]. Herein, we report that adult HIV-1-transgenic (HIV-1-Tg) rats with high proteinuria develop kidney disorder similar to that of HIVAN, and report, for the first time, that the pathogenic events in HIVAN involve Notch pathway activation, upregulation of Cux1 and the repression of p27.
Methods
Animals
HIV-Tg rats (HIV-1 Sprague–Dawley) and age-matched parental wild-type Sprague–Dawley rats were purchased from Harlan Laboratories (Indianapolis, Indiana, USA). Specific pathogen-free HIV-Tg and wild-type control rats were housed under pathogen-free conditions in microisolator cages on a high-efficiency particulate air-filtered ventilated rack. Generation of the HIV-Tg rat model, detection of the transgene and the description of its kidney disorder have been reported earlier [17,19,20]. Briefly, the HIV-Tg rat was generated using a 7.4-kb proviral DNA construct containing the 5′ and 3′ long terminal repeats, and the env, tat, nef, rev, vif, vpr and vpu genes. The proviral DNA construct also carried a deletion encompassing most of the gag and pol genes to render it noninfectious. The animal care was in accordance with the National Institutes of Health (NIH) Guide for care and use of Laboratory Animals at the Kansas University Medical Center.
Human renal tissue
Paraffin-embedded human renal tissue biopsy specimens from three HIVAN patients and three transplant kidney donors were obtained from the Pathology Department of the Long Island Jewish Medical Center, New Hyde Park, New York, USA. Use of these samples has been approved by the Institutional Review Board, North Shore Long Island Jewish Health System.
Experimental design
Four HIV-Tg rats (one male and three females) within ages of 12–15 months and with the proteinuria at end-stage renal range of approximately 3000 mg/dl (measured by Multistix 10SG; Siemens, New York, USA) comprised the study group. Age-matched wild-type rats (one male and two females) with no proteinuria were used as controls. Rats were anesthetized with isoflurane and perfused with phosphate-buffered saline (PBS) with 0.25% sucrose solution, followed by excision of the kidneys. One of the kidneys was fixed in 4% paraformaldehyde overnight, followed by incubation in 70% ethanol, and the other was snap frozen for western blot analysis.
Immunohistochemistry
The kidneys from normal and HIV-Tg rats were immersion-fixed in 4% paraformaldehyde and embedded in paraffin. Five-micrometer-thick tissue sections were deparaffinized with xylene and hydrated with graded ethanols. Sections were stained with either hematoxylin and eosin, periodic acid–Schiff reagent or Mason Trichrome stain, following standard techniques [21]. For antibody labeling, the slides from human patients as well as rats were treated with antigen unmasking solution (Vector Laboratories, Inc., Burlingame, California, USA) according to manufacturer’s protocol. To block the endogenous peroxidase activity, sections were incubated with 3% hydrogen peroxide for 30 min. Sections were then washed in PBS and blocked with 10% normal serum (in PBS from the species the secondary antibody was raised in) for 1 h. The slides were incubated for 1 h with primary antibodies in a humidified chamber. Antibodies for Cux1, TLE4, WT1 and Hes homolog-1 (Hes1) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA). Antibodies for Notch2 intracellular domain, Notch4 intracellular domain and p27kip1 were obtained from Abcam plc (Cambridgeshire, UK). Mouse antisynaptopodin was purchased from Biodesign International (Saco, Maine, USA). Antibodies against cleaved Notch3 and Notch1 (Val1744) were purchased from Millipore Corporation (Billerica, Massachusetts, USA). Rabbit anti-delta1 (DLL1) was obtained from Lifespan Biosciences (Seattle, Washington, USA). Mouse anti-proliferating cell nuclear antigen (PCNA) and rabbit anti-desmin were obtained from Sigma–Aldrich (St. Louis, Missouri, USA) and rabbit anti-Ki67 was purchased from Thermo Scientific Inc. (Fremont, California, USA). Antibody dilutions for Cux-1, TLE4, Notch2 intracellular domain, Notch3 intracellular domain, Notch4 intracellular domain, Jagged1 and delta1 were 1 : 100 each. Antibodies for activated Notch1 (Val 1744), WT1, podocin and synaptopodin, and desmin were diluted at 1 : 50. Antibody against PCNA was diluted at 1 : 3000. All primary antibodies were diluted in 2% nonimmune serum (in PBS from the species in which the secondary antibody was raised). Immunohistochemistry was performed as described previously [22]. Control slides consisted of mounted tissue sections without primary antibodies. The tissue sections were viewed on a Leica DMR microscope (Leica Microsystems GmbH, Wetzlar, Germany) equipped with an Optronics Magnafire digital camera (Goleta, California, USA).
Immunofluorescence
Slides were deparaffinized and treated as described above for antigen unmasking followed by incubation with 1 mol/l NH4Cl for 1 h to block endogenous fluorescence. Sections were blocked in 10% normal horse serum for 1 h followed by washings and incubation with rabbit anti-Notch4 intracellular domain or anti-Hes1 antibody (1 : 50) and mouse antisynaptopodin (1 : 50) overnight at 4°C. Labeling was performed as described previously [12]. Slides were counterstained with 4′-6-Diamidino-2-phenylindole (Vector Labs Inc., Burlingame, California, USA) and coverslipped. The tissue sections were viewed on a Leica DMR microscope equipped with an Optronics Magnafire digital camera.
Morphologic analyses and statistics
The first 30 glomeruli from each rat kidney section were evaluated using the predominant staining intensity for scoring. Staining intensity was scored by manual counting and expressed as positive cells per glomerulus, except for synaptopodin for which image J software from the NIH was used and results were expressed as mean percentage of glomerular area stained. Results are expressed as mean value ± SEM. The difference between the two groups was compared by Student’s t-test. A P value of less than 0.05 were considered significant. All immunolabeling experiments were repeated at least three times and the figures are a representative of three independent experiments.
Preparation of tissue lysates
Lysates were prepared as described previously [12]. Briefly, frozen kidneys were chopped and suspended in ice-cold RIPA buffer (0.01 mol/l sodium phosphate, pH 7.2; 125 mmol/l sodium chloride; 50 mmol/l sodium fluoride; 0.1% SDS; 1 mmol/l EDTA; 1% sodium deoxycholate and 1% NP-40) with protease inhibitor cocktail (Thermo Scientific Inc.). Tissues were homogenized followed by centrifugation for 20 min at 15 000 rpm in a microfuge. The lysates were removed and stored in −80°C until use. Protein was measured using the bicinchoninic protein assay (BioRad Laboratories, Inc., Hercules, California, USA). Thirty microgram protein was loaded from each group and western blotting was performed on a 4–15% gradient gel as previously described [22]. Membranes were stripped and reprobed with an antibody against β-actin (Sigma–Aldrich) to normalize for protein loading. The intensity of bands was measured using NIH image J software. The band intensity for each Notch protein was normalized with the band intensity of the β-actin and presented as relative intensity. Western blots were repeated at least three times and the figures are a representative of three independent experiments.
Results and discussion
Analysis of kidney disorder, proliferation and differentiation in HIV-transgenic rats
HIVAN is characterized by renal lesions, including collapsing glomerulosclerosis associated with severe tubulointerstitial injury, microcystic dilatation and increased apoptosis in tubules, and mononuclear infiltration [2]. In addition, HIVAN is also characterized by podocyte proliferation and dedifferentiation. The underlying molecular mechanisms resulting in the glomerular changes in HIVAN, however, remain unknown. Nevertheless, the increase in podocyte proliferation in HIVAN suggests the disruption of cell cycle regulation. To confirm the HIVAN phenotype, kidneys from HIV-Tg rats (12–15 months of age) were evaluated and compared with wild-type age-matched controls. HIV-Tg rats demonstrated a spectrum of pathological changes in the kidneys compared with the wild-type animals. Gross evaluation of the kidneys from HIV-Tg rats displayed a patchy, pitted external surface, as has been reported previously [19]. Morphological evaluation of transgenic kidneys revealed various types of glomerular lesions, including hypercellular glomeruli, sclerotic changes, as well as scarring. In addition, HIV-Tg rats also demonstrated focal segmental lesions and collapsed glomeruli (Fig. 1b and d). Kidneys from HIV-Tg rats demonstrated interstitial fibrosis with accumulation of extracellular matrix proteins in the sclerotic glomeruli (Fig. 1b) compared with the normal wild-type controls (Fig. 1a). Additionally, the dilated tubules of the kidneys of HIV-Tg rats demonstrated the presence of tubular protein casts [19]. Hence, the morphological evaluation of the adult HIV-Tg kidneys was in agreement with previous studies [19].
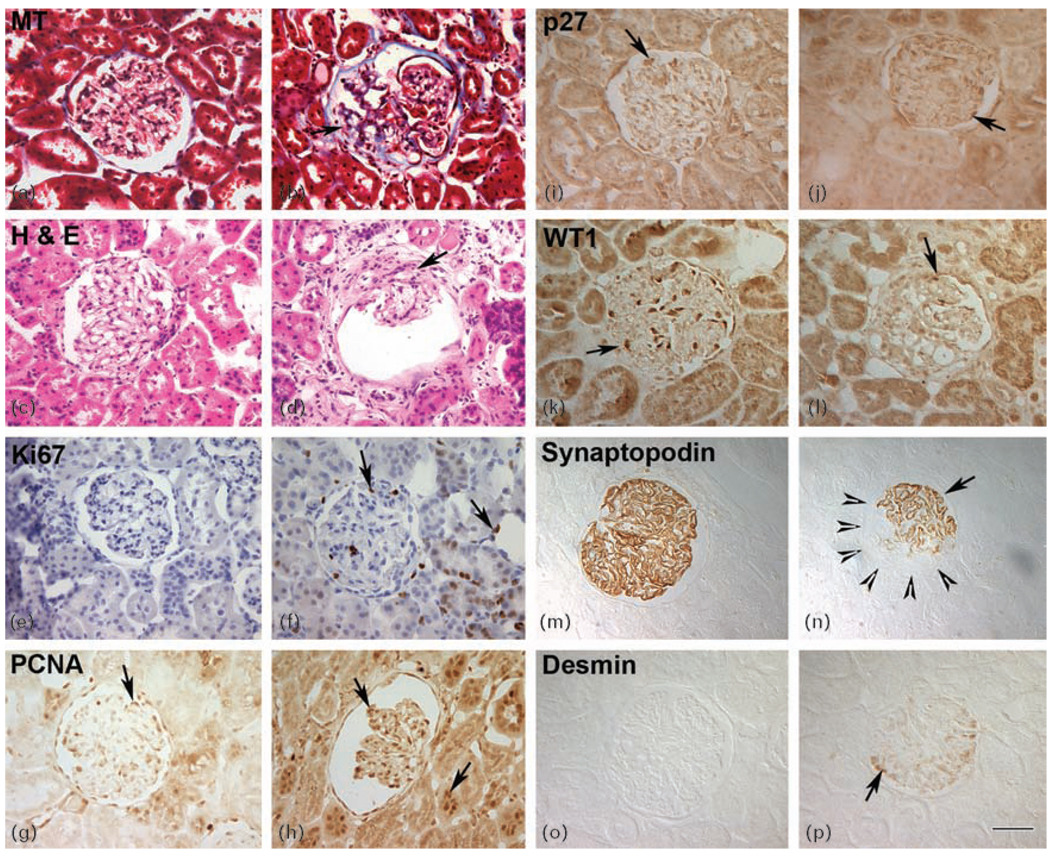
Fig. 1. HIV-transgenic rats display multiple glomerular phenotypes, disrupted cell cycle regulation and podocyte dedifferentiation and dysregulation.
Panels (a–d) represent renal histology and panels (e–p) represent paraffin kidney sections from wild-type and HIV-Tg rats labeled with antibodies against markers specific for proliferation, podocyte dedifferentiation and dysregulation. (a and b) MT-stained kidney sections from HIV-Tg (b) and wild-type age-matched controls (a). Accumulation of extracellular matrix in HIV-Tg glomeruli was revealed by blue color. (c and d) H&E-stained kidney sections from HIV-Tg (d) and wild-type (c) rats. HIV-Tg rats show signs of podocyte hyperplasia and scarring in a collapsing glomerulus (arrow) compared with wild-type sections (c). (e and f) Labeling for Ki67 revealed cellular proliferation in both the glomeruli (left arrow) and tubules (right arrow) from HIV-Tg sections (f) compared with the wild-type controls (e). (g and h) PCNA-positive cells (arrows) were observed in both wild-type (g) and HIV-Tg (h) kidney sections. (i and j) Normal expression of p27 in quiescent wild-type cells (arrow in i), whereas HIV-Tg glomeruli had decreased expression of p27 (j). (k and l) Kidney sections from both wild-type (k) and HIV-Tg (l) rats were labeled with antibody directed against WT1. The HIV-Tg podocytes exhibited a loss of WT1 expression in the sclerotic glomeruli (l). (m and n) Focal expression of synaptopodin was observed in the sclerotic glomeruli (arrow) (panel n). Arrowheads define the boundary of glomeruli that has lost synaptopodin. Normal synaptopodin expression (m) was seen in wild-type controls. (o and p) Desmin was highly upregulated in dysregulated podocytes from HIV-Tg sections (panel p, arrow), whereas wild-type glomeruli showed no desmin expression (o) (original magnification ×40). H&E, hematoxylin and eosin; HIV-Tg, HIV-transgenic; MT, Mason Trichrome; PCNA, proliferating cell nuclear antigen; WT1, Wilms tumor 1.
To determine whether the adult HIV-Tg rats also exhibited other pathological features of HIVAN, we evaluated the extent of proliferation and differentiation in kidneys from these animals. It is well recognized that normal mature podocytes express synaptopodin and WT1, as well as the cyclin kinase inhibitor p27, features that are consistent with a differentiated, quiescent phenotype [5]. In HIVAN, loss of the podocyte markers synaptopodin and WT1 occurs, resulting in podocyte dedifferentiation. Furthermore, previous studies [6,23] on HIVAN kidneys have demonstrated increased proliferation in the sclerotic areas of glomeruli, suggesting disruption of cell cycle regulation. We thus sought to quantify the extent of proliferation in HIV-Tg rats. Cells positive for Ki67, PCNA and p27 within the glomeruli of HIV-Tg (Fig. 1f, h and j, and Table 1) and wild-type kidneys (Fig. 1e, g and i, and Table 1) were scored and counted. Although PCNA was detected in both the wild-type and HIV-Tg rats, the number of PCNA-positive cells was significantly higher in the HIV-Tg rats compared with the wild-type group (Fig. 1g and h). As expected, none of the cells in the wild-type glomeruli expressed proliferation marker Ki67 (Fig. 1e and Table 1). In contrast, expression of Ki67 was significantly increased in the glomeruli and tubules of the HIV-Tg rats (Fig. 1f and Table 1). To determine whether p27 expression correlated with the findings in human and animal models of HIVAN, glomerular cells from the HIV-Tg and wild-type animals were scored for expression of p27. In glomeruli from HIV-Tg rats, expression of p27 was downregulated (Fig. 1j and Table 1) compared with the wild-type controls (Fig. 1i and Table 1).
Table 1.
Comparison of immunohistochemistry staining from the glomeruli of normal and HIV-transgenic rats.
| Normal rats | HIV-1 Tg rats | P | |
|---|---|---|---|
| PCNA | 25.1 ± 9.77 | 40.59 ± 13.7 | <0.0001** |
| Ki67 | ND | 3.26 ± 2.56 | <0.0001** |
| p27 | 50.8 ± 14.2 | 35.0 ± 10.1 | <0.0001** |
| WT1 | 16.18 ± 4.3 | 13.52 ± 5.2 | 0.017* |
| Synaptopodin | 50.0 ± 6.8 | 26.26 ± 13.5 | <0.0001** |
| Desmin | ND | 14.8 ± 19.2 | <0.0001** |
| Notch1 IC | 0.15 ± 0.87 | 2.5 ± 3.3 | <0.0001** |
| Notch2 IC | 51.4 ± 13.4 | 49.3 ± 14.4 | 0.28 |
| Notch3 IC | 40.9 ± 11.7 | 44.5 ± 14.6 | 0.15 |
| Notch4 IC | 24.00 ± 9.5 | 47.0 ± 12.4 | <0.0001** |
| Hes1 | 54.27 ± 12.22 | 59.8 ± 11.8 | 0.04* |
| Cux1 | 20.33 ± 7.2 | 42.0 ± 15.9 | <0.0001** |
| TLE4 | 8.9 ± 5.14 | 33.33 ± 9.7 | <0.0001** |
Cells showing positive staining were counted manually in the first 30 random glomeruli from the three wild-type and HIV-Tg slides each. Synaptopodin staining was measured by image J program from NIH and presented as percentage area of the glomeruli positive for the staining. Values are represented as mean ± SD. Cux1, cut-like homeobox; Hes, hairy enhancer of split; HIV-Tg, HIV-1 transgenic sections; IC, intracellular; ND, not detected; NIH, National Institute for Health; normal, wild-type kidney sections; PCNA, proliferating cell nuclear antigen; TLE, transducin-like enhancer of split; WT1, Wilms tumor 1.
P < 0.05 was considered as statistically significant.
P < 0.001 was considered highly statistically significant.
We next sought to evaluate the changes in podocyte differentiation in the HIV-Tg rats. Sections of kidneys from wild-type and HIV-Tg rats were labeled with antibodies directed against WT1 and synaptopodin. As expected, glomeruli from wild-type rats showed intense expression of both WT1 and synaptopodin in the podocytes (Fig. 1k and m). The expression of WT1 and synaptopodin in the HIV-Tg sclerotic glomeruli, however, was significantly decreased (Fig. 1l and n, and Table 1). The decrease in the expression was due to focal loss of synaptopodin expression in the HIV-Tg glomeruli (Fig. 1n, arrowheads), suggesting that podocytes within these glomeruli may be undergoing dedifferentiation. We next examined expression of desmin, which is a marker of mesangial cell differentiation and is not expressed in podocytes anytime during normal glomerular development. Increased desmin expression, however, has been observed in other models of podocyte injuries [6]. Accordingly, we observed increased expression of desmin in the glomeruli of HIV-Tg rats (Fig. 1p) compared with the wild-type controls (Fig. 1o).
Thus, in addition to various histological lesions reported previously [19], we also found other characteristic features of HIVAN such as increased cell proliferation and podocyte dedifferentiation and dysregulation in the glomeruli of HIV-Tg rats. Having characterized the HIV-Tg rat model for features of HIVAN disorder, we next used the model to explore the role of Notch signaling in the disease process.
Activation of Notch pathway in HIV-associated nephropathy
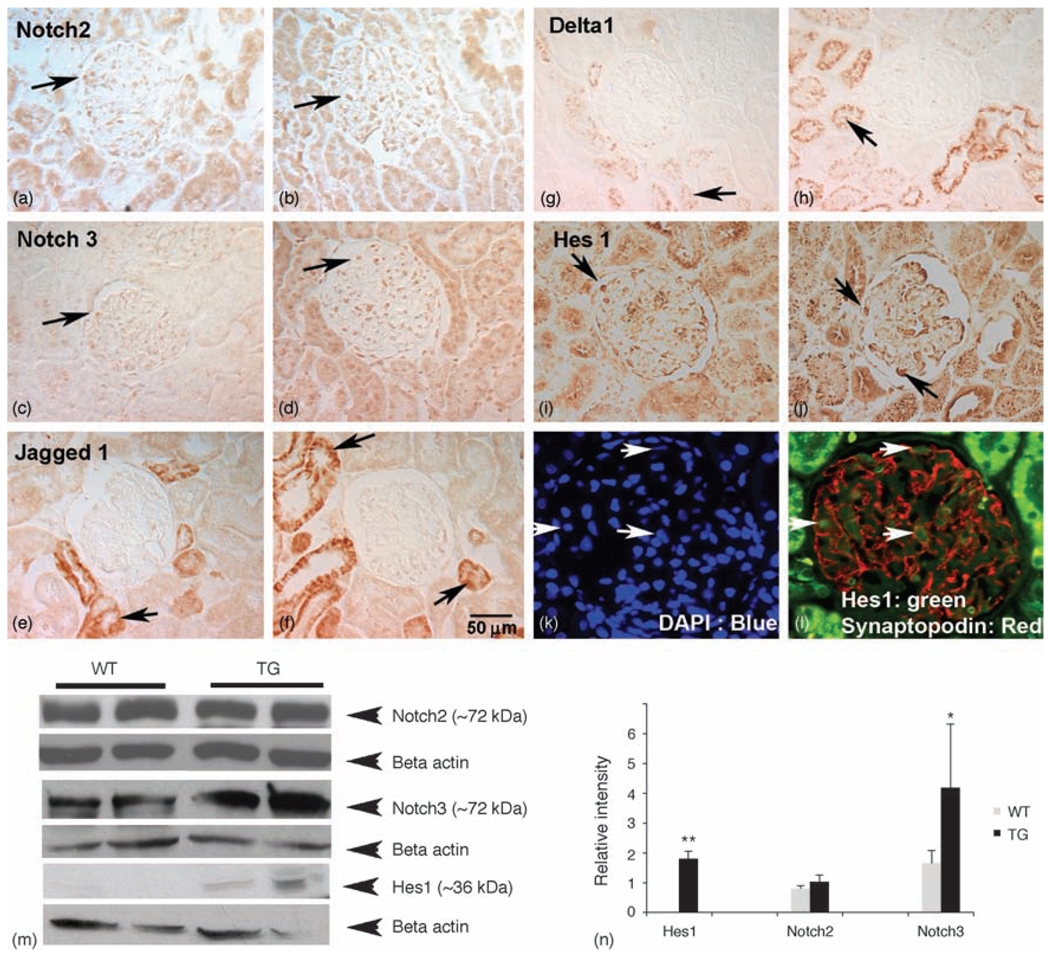
Activation of the Notch signaling pathway in glomerular epithelial cells has recently been described in both diabetic nephropathy and in glomerulosclerosis [14,15]. Upon binding to Notch ligands, the intracellular domain of the Notch receptor translocates to the nucleus and functions to activate transcription of Notch effector genes such as Hes proteins and, possibly, Cux1. We used antibodies directed against the intracellular domains of Notch2 and Notch3 to evaluate activated Notch signaling in the HIV-1-Tg rat and HIVAN patient kidneys. Using these antibodies, we observed an increased expression of Notch3 intracellular domain in both the glomeruli and tubules of HIV-Tg rat kidneys (Fig. 2c and d). Although the overall number of cells positive for Notch3 intracellular domain expression was not significantly increased in the glomeruli of HIV-Tg rats compared with wild-type rats (Table 1), western blot analysis of whole kidney lysates from wild-type and HIV-Tg rats showed increased levels of Notch3 intracellular domain in HIV-Tg rat kidneys (Fig. 2m and n). In biopsy sections from HIVAN and control patients, we did observe an increase in the number of Notch3 intracellular domain-positive cells (Table 2). In contrast, there was no change in Notch2 intracellular domain expression or distribution between the wild-type and the HIV-Tg rats, or between HIVAN patients and normal controls (Fig. 2a and b, m and n and Tables 1 and 2).
Fig. 2. Upregulation of Notch pathway members in HIV-transgenic rat kidneys.
Kidney sections were labeled for Notch2 IC, Notch3 IC, Jagged1, delta1 and Hes1 expression. (a and b) Notch2 intracellular was expressed in both glomeruli and tubules from WT (a) and HIV-Tg rat sections (b). (c and d) Notch3 IC was upregulated in the HIV-Tg kidneys in both tubular and glomerular cells (d) compared with WT kidney sections (c). (e and f) Jagged1 was not expressed in the glomeruli; however, more tubules from HIV-Tg rats (f) expressed Jagged1 compared with WT controls (e). (g and h) Similar to Jagged1, delta1 was not expressed in glomeruli; however, expression was increased in tubules (arrow in h) in HIV-Tg sections compared with the WT controls (g). (i and j) Hes1 was minimally expressed in the WT kidneys (i), but was upregulated in the glomeruli from HIV-Tg kidneys (arrows in j). (k and l) To identify whether Hes1-positive cells are podocytes, double immunoflourescence was performed using antibodies specific for synaptopodin (red) and Hes1 (green), and counterstained with DAPI (k). Arrows indicate cells double positive for synaptopodin and Hes1 (l). All figures except panels (k) and (l) are original magnification 40×. Panels (k) and (l) are magnified from 40×. (m and n) Protein lysates prepared from WT and TG kidneys were subjected to western blot analysis for quantitation of Notch2 IC, Notch3 IC and Hes1. Notch2 IC did not vary between WT and TG samples, whereas Notch3 IC and Hes1 were increased in TG kidney lysates compared with WT lysates. Densitometric analysis of western blots from at least three experiments shows an increase in Hes1 and Notch3 expression in HIV-Tg kidneys. DAPI, 4′-6-Diamidino-2-phenylindole; Hes1, hairy enhancer of split homolog-1; HIV-Tg, HIV-transgenic; IC, intracellular; TG, HIV-Tg; WT, wild-type.
Table 2.
Comparison of immunohistochemistry staining for activated Notch receptors from kidney biopsies of normal verses the HIV-associated nephropathy patients.
| Normal kidney | HIVAN | P | |
|---|---|---|---|
| Notch1 IC | 0.783 ± 1.66 | 2.885 ± 4.46 | <0.001* |
| Notch2 IC | 99.24 ± 49.9 | 95.66 ± 43.03 | 0.386 |
| Notch3 IC | 41.167 ± 15.93 | 76.33 ± 37.17 | <0.0001** |
| Notch4 IC | 0.128 ± 0.741 | 15.5 ± 13.85 | <0.0001** |
Kidney biopsy section obtained from the HIVAN patients (n = 3) or the patients with no HIVAN (normal) (n = 3) were labeled for the presence of intracellular domains of Notch1, 2, 3 and 4. Positive labeling in the nuclei was counted manually from at least 20 random fields from each section. Data are represented as mean ± SD. HIVAN, HIV-associated nephropathy; IC, intracellular.
P < 0.05 was considered statistically significant.
P < 0.001 was considered highly statistically significant.
As Notch activation is mediated by binding of Notch ligands, we evaluated the expression of the Notch ligands Jagged1 and delta1 in kidney sections from wild-type and HIV-Tg rats. Expression of both ligands was exclusively in the tubules, with no glomerular expression. Moreover, expression of both ligands was elevated in the tubules of HIV-Tg rats compared with the wild-type controls (Fig. 2 e–h).
The mechanism in which Notch signaling is activated in HIVAN remains unclear at present. The absence of the ligands Jagged1 and delta1 in the HIV-Tg rat glomeruli leads us to speculate that Notch activation in the glomerular cells is independent of these ligands. One possibility is that HIV proteins may directly bind to Notch receptors, thereby modulating their physiologic functions. This notion is supported by previous studies demonstrating that the HIV-1 Tat protein interacts with the epidermal growth factor repeats present on the extracellular domain of Notch receptors [24]. Alternatively, similar to the Epstein–Barr virus and Kaposi’s sarcoma-associated herpes virus [25,26], it is possible that HIV-1 can use one or more of its regulatory proteins to bind to RBP-jκ and mimic Notch intracellular domain.
We next determined whether the conventional target of Notch signaling pathway, Hes1, was upregulated in HIV-Tg rat kidneys. Hes1 protein was observed in the glomeruli of both wild-type and HIV-Tg rats (Fig. 2 i–l). Moreover, Hes1-positive cells in HIV-Tg rats showed a swollen appearance (Fig. 2j) compared with wild-type controls (Fig. 2i). Colocalization of Hes1 and synaptopodin identified these cells as podocytes (Fig. 2l). Quantitation of western blots showed that Hes1 was significantly upregulated in the kidneys from HIV-Tg rats compared with wild-type rats kidneys (Fig. 2).
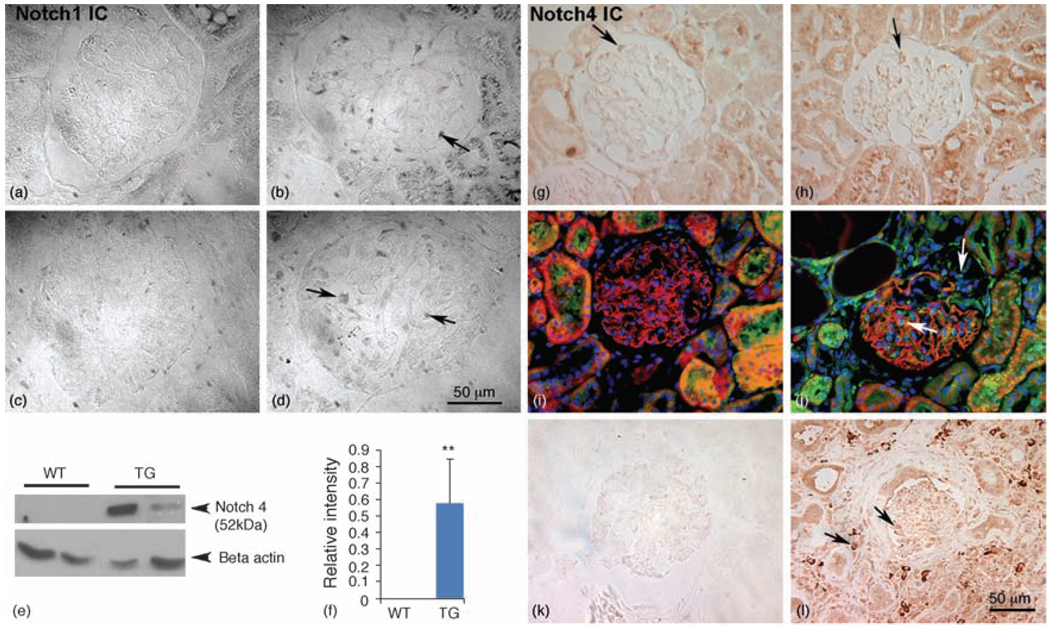
We next evaluated the role of Notch1 and Notch4 in HIVAN. Antibodies directed against Notch1 intracellular and Notch4 intracellular domains were used to evaluate activated Notch signaling in both the HIV-1-Tg rat kidneys and in kidneys from human HIVAN biopsies. As shown in Fig. 3(b), Notch1 intracellular domain was activated in the glomeruli, although weakly, from HIV-Tg rats compared with the wild-type controls (Fig. 3a). A similar increase in Notch1 intracellular domain was observed in kidney sections from human HIVAN patients (Fig. 3d, Table 2) compared with kidney sections from HIV-negative patients (Fig. 3c). However, we were unable to detect Notch1 intracellular domain expression from either wild-type or HIV-Tg kidneys by western blot analysis (not shown).
Fig. 3. Activation of Notch signaling in HIV-transgenic rats and in human HIV-associated nephropathy patients.
(a and b) Detection of Notch1 IC in HIV-Tg rat and human HIVAN tissue. Paraffin sections from WT (a) and HIV-Tg rats (b) were labeled for the presence of Notch1 IC domain. Notch1 IC was upregulated in the glomerular cells of HIV-Tg kidneys (b), whereas the WT controls had no Notch1 IC expression (a). (c and d) Kidney biopsy sections obtained from age-matched patients with no kidney disease (c) or with HIVAN (d) were evaluated for the presence of Notch1 IC. The biopsy samples from HIVAN patients (d) showed cells positive for Notch1 IC expression (arrows), whereas the age-matched normal kidney (control) biopsies did not demonstrate any Notch1 IC (original magnification ×63). (e and f) Increased expression of Notch 4 IC in HIV-Tg rat and human HIVAN tissue. (e) Western blot analysis for Notch4 IC in total kidney lysates from TG and WT rats. Notch4 IC is not detectable in WT kidneys, but is abundantly expressed in TG tissue. (f) Relative densitometry of Notch4 IC expression in kidney lysates from WT and TG rats was obtained by NIH Image J program showed a statistically significant increase (**P<0.0001). Western and densitometry data represent three independent experiments. (g and h) The expression of Notch4 IC was increased in both glomeruli and tubules of HIV-Tg rats (h) compared with WT age-matched controls (g). (i and j) Detection of synaptopodin (red) and Notch4 IC (green) in kidney sections from WT (i) and HIV-Tg (j) rats. Synaptopodin labeling in WT rat kidney sections appears very regular throughout the WT glomeruli (i) with no Notch4 IC-positive cells (green). In contrast, there is irregular labeling of synaptopodin in the sclerotic HIV-Tg glomeruli (j) with several Notch4 IC-positive cells (arrows), some of which are associated with a loss of synaptododin regions of the glomeruli (right arrow in j). (k and l) Notch 4 IC is elevated in glomeruli and tubules in kidney biopsy sections from HIVAN patients (arrows in l) compared with age-matched patients with normal kidney (k). HIVAN, HIV-associated nephropathy; HIV-Tg, HIV-transgenic; IC, intracellular; TG, HIV-Tg; WT, wild-type.
Similar to Notch1 intracellular domain, Notch4 intracellular domain expression was also increased in the HIV-Tg rat kidneys compared with the wild-type controls (Fig. 3g and h, and Table 2). Moreover, some of the Notch4 intracellular domain-positive cells from HIV-Tg glomeruli (Fig. 3j) also colabeled with synaptopodin (in red) indicating podocytes as the source of Notch 4. In contrast, glomeruli from wild-type rats exhibited very few Notch4 intracellular domain-positive cells (Fig. 3g and i). As synaptopodin expression was lost from sclerotic regions of glomeruli in HIVAN, it is likely that some of the cells positive for Notch4 intracellular domain and negative for synaptopodin are dedifferentiating podocytes. Western blot analysis confirmed the increase in the Notch4 intracellular domain protein (52 kDa) in kidneys from HIV-Tg rats. In contrast, Notch4 intracellular domain was not detected in lysates prepared from wild-type controls (Fig. 3e and f). To evaluate whether the increased activation of Notch 4 we observed in HIV-Tg rats is a common feature of HIVAN, we labeled human HIVAN kidney sections for Notch4 intracellular domain. As shown in Fig. 3l (arrows), there was a striking upregulation of Notch4 intracellular domain in the glomeruli, as well as in the tubules from patients with HIVAN. In contrast, kidney sections from controls without kidney disease lacked Notch4 intracellular domain-positive cells (Fig. 3k).
Taken together, our findings demonstrate that most of the key components of the Notch signaling pathway were elevated in both the HIV-Tg rat glomeruli and in human HIVAN biopsy samples, thereby underscoring the role of the Notch signaling pathway in HIVAN. The localization of Hes1 and Notch4 intracellular domain in the podocytes of HIV-Tg rats suggests these cells as the target cells for Notch activation in the pathogenesis of HIVAN. This is consistent with the observation that podocytes can also serve as the reservoirs for HIV replication [27]. We also observed increased levels of Notch1 intracellular domain in the Human HIVAN biopsy samples. Similarly, increased levels of Notch4 intracellular domain were observed in both glomeruli and tubules from human HIVAN biopsies, suggesting that Notch 4 signaling may play a major role in the pathogenesis of HIVAN. Notch4 transcripts have been found expressed in endothelial cells from adult mouse kidney in heart, lung and kidney [28]. Thus, it is likely that Notch 4 is being activated in endothelial cells in HIVAN glomeruli, leading to proliferation and dedifferentiation of podocytes.
Cut homeobox 1 and transducin-like enhancer protein 4 expression is increased in HIV-transgenic rat kidneys
Cux1 is a cell-cycle-dependent transcription factor that represses the cyclin kinase inhibitors p21 and p27. We have previously shown that transgenic mice constitutively expressing Cux1 develop glomerulosclerosis and interstitial fibrosis [29]. In addition, it is known that Cux1 is regulated by the Notch signaling pathway and interacts with the corepressor TLE4 to repress p27 gene expression during kidney development [12]. Recent studies have demonstrated that ectopic Notch signaling in podocytes results in the development of proteinuria and glomerulosclerosis. Moreover, Cux1 expression is induced by activated Notch signaling [22]. Therefore, we evaluated the expression of Cux1, TLE4 and p27 in HIV-Tg rats.
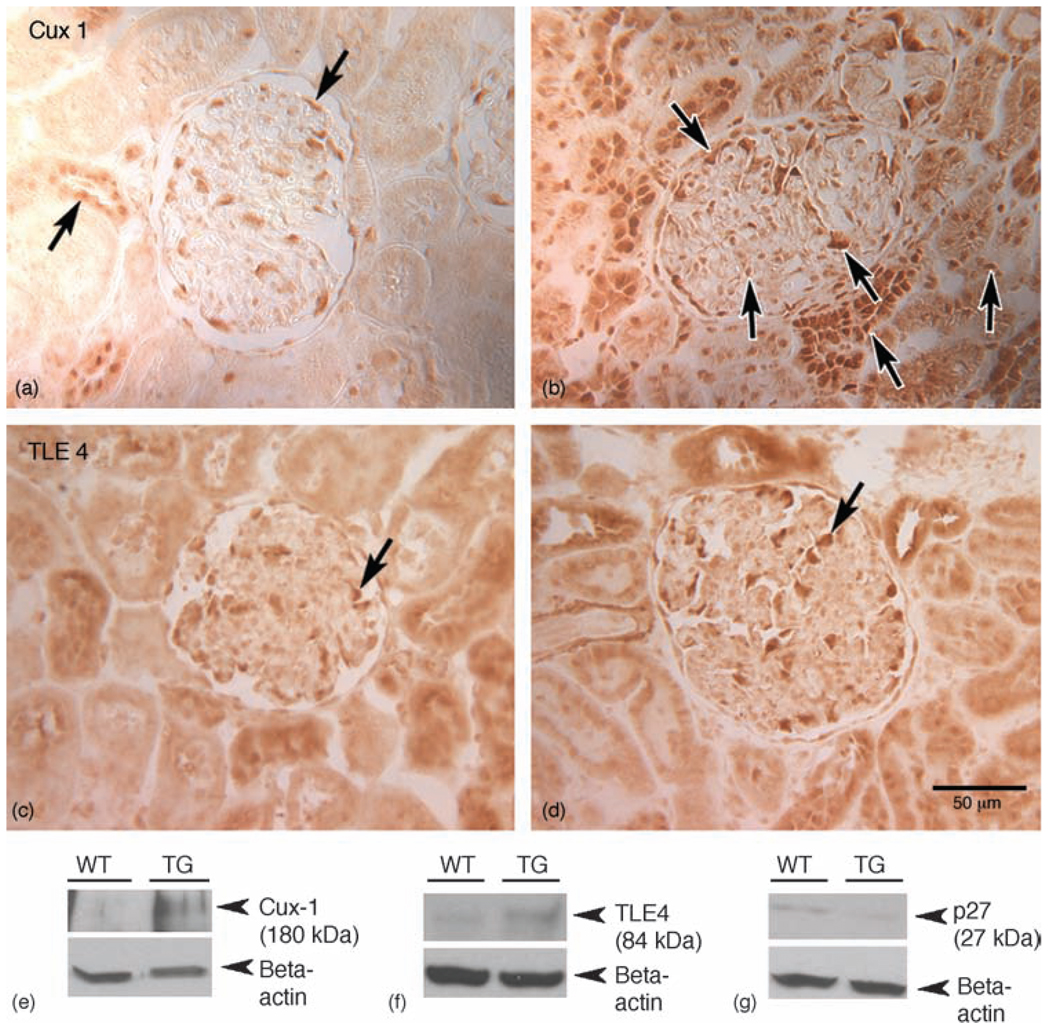
Kidney sections from wild-type and HIV-Tg rats were stained for the presence of Cux1, TLE4 and p27. Cux1 expression was markedly elevated in the glomeruli and tubules of HIV-Tg rats compared with the wild-type controls (Fig. 4a and b). Similar to Cux1, there was also an increase in TLE4 expression in the glomeruli of HIV-Tg rats (Fig. 4d). Western blot analysis confirmed the increases in Cux1 and TLE4 protein expression (Fig. 4e and f), and a decrease in p27 expression in the kidneys of HIV-Tg rats (Fig. 4g). These studies underscore the involvement of Cux1 and TLE4 in regulating p27 during the pathogenesis of HIVAN.
Fig. 4. Increased expression of cut homeobox 1 and transducin-like enhancer protein 4 in HIV-transgenic kidneys.
(a–d) Paraffin sections of wild-type (a and c) and HIV-Tg (b and d) rat kidneys were labeled with antibodies directed against Cux1 (a and b) or TLE4 (c and d). Cux1 expression was minimal in both glomeruli and tubules in the wild-type kidney sections (arrows in a), but was markedly elevated in both glomerular and tubular cells in HIV-Tg rat kidneys (b). Similarly, TLE4 expression was increased in glomeruli from HIV-Tg kidneys (d) compared with the wild-type controls (c). (e–g) Western blots were performed on the kidney lysates for quantitative expression of Cux1 (e), TLE4 (f) and p27 (g). Although the expression of both Cux1 (e) and TLE4 (f) were significantly increased in the TG kidney lysates compared with the WT control, p27 was downregulated in HIV-Tg lysates (g). Cux1, cut homeobox 1; HIV-Tg, HIV-transgenic; TG, HIV-Tg; TLE4, transducin-like enhancer protein 4; WT, wild-type.
Although Notch signaling has been implicated in Kaposi’s sarcoma and diseases associated with other viruses [25,26,30], this is the first report to our knowledge showing an involvement of Notch signaling in HIVAN pathogenesis. Moreover, it appears that viral replication is not required for the activation of Notch signaling in HIVAN and merely the expression of HIV-1 proteins is sufficient to induce activation as HIV-Tg rats are noninfectious. Our results, although associative, suggest a mechanism by which HIV-1 viral proteins can lead to the activation of the Notch signaling pathway, resulting ultimately in the upregulation of Cux1 and TLE4. Upregulation of Cux1 and TLE4 by Notch activation would result in the repression of p27 and, ultimately, cell cycle deregulation. In light of the previous reports demonstrating that Notch activation in podocytes results in glomerular disease, we conclude that Notch activation may be involved in the pathogenesis of HIVAN, and that inhibition of the Notch pathway could be an attractive target for therapeutic intervention in HIVAN.
Acknowledgements
This study was supported by grants, QV815151 to G.V.H. and MH62969, MH-068212, DA020392, DA023397 and DA024442 to S.B., from the NIH.
We thank Dr Tino Piscione and Dr Katalin Susztak for many helpful discussions and for providing the DLL1 and anti-Jagged1 antibodies, respectively. Technical assistance from Rosetta Barkley is greatly acknowledged. We thank and dedicate this paper to our late dear friend Eileen Roach for expert technical help.
Footnotes
There are no conflicts of interest.
References
- 1.Winston JA, Burns GC, Klotman PE. The human immunodeficiency virus (HIV) epidemic and HIV-associated nephropathy. Semin Nephrol. 1998;18:373–377. [PubMed] [Google Scholar]
- 2.D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18:406–421. [PubMed] [Google Scholar]
- 3.Klotman P. HIV-associated nephropathy. Kidney Int. 1999;56:1161–1167. doi: 10.1046/j.1523-1755.1999.00748.x. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt CM, Klotman PE. HIV-1 and HIV-Associated Nephropathy 25 Years Later. Clin J Am Soc Nephrol. 2007;2:S20–S24. doi: 10.2215/CJN.03561006. [DOI] [PubMed] [Google Scholar]
- 5.Nagata M, Nakayama K, Terada Y, Hoshi S, Watanabe T. Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol. 1998;153:1511–1520. doi: 10.1016/s0002-9440(10)65739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000;58:173–181. doi: 10.1046/j.1523-1755.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz EJ, Cara A, Snoeck H, Ross MD, Sunamoto M, Reiser J, et al. Human immunodeficiency virus-1 induces loss of contact inhibition in podocytes. J Am Soc Nephrol. 2001;12:1677–1684. doi: 10.1681/ASN.V1281677. [DOI] [PubMed] [Google Scholar]
- 8.Lu TC, He JC, Klotman PE. Podocytes in HIV-associated nephropathy. Nephron Clin Pract. 2007;106:c67–c71. doi: 10.1159/000101800. [DOI] [PubMed] [Google Scholar]
- 9.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Kamei CN, Layne MD, Jain MK, Liao JK, Lee M-E, et al. Regulation of myogenic terminal differentiation by the hairy-related transcription factor CHF2. J Biol Chem. 2001;276:18591–18596. doi: 10.1074/jbc.M101163200. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma M, Brantley JG, Vassmer D, Chaturvedi G, Baas J, Vanden Heuvel GB. The homeodomain protein Cux1 interacts with Grg4 to repress p27(kip1) expression during kidney development. Gene. 2009;15:87–94. doi: 10.1016/j.gene.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barisoni L. Notch signaling: a common pathway of injury in podocytopathies? J Am Soc Nephrol. 2008;19:1045–1046. doi: 10.1681/ASN.2008040351. [DOI] [PubMed] [Google Scholar]
- 14.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 16.Reid W, Abdelwahab S, Sadowska M, Huso D, Neal A, Ahearn A, et al. HIV-1 transgenic rats develop T cell abnormalities. Virology. 2004;321:111–119. doi: 10.1016/j.virol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav A, Pati S, Nyugen A, Barabitskaja O, Mondal P, Anderson M, et al. HIV-1 transgenic rat CD4+ T cells develop decreased CD28 responsiveness and suboptimal Lck tyrosine dephosphorylation following activation. Virology. 2006;353:357–365. doi: 10.1016/j.virol.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Ray PE, Liu XH, Robinson LR, Reid W, Xu L, Owens JW, et al. A novel HIV-1 transgenic rat model of childhood HIV-1-associated nephropathy. Kidney Int. 2003;63:2242–2253. doi: 10.1046/j.1523-1755.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 20.Dickie P, Felser J, Eckhaus M, Bryant J, Silver J, Marinos N, et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185:109–119. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- 21.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1985;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Fopma A, Brantley JG, Vanden Heuvel GB. Coexpression of Cux-1 and notch signaling pathway components during kidney development. Dev Dyn. 2004;231:828–838. doi: 10.1002/dvdy.20175. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai N, Kuroiwa T, Ikeuchi H, Hiramatsu N, Takeuchi S, Tomioka M, et al. Fluvastatin prevents podocyte injury in a murine model of HIV-associated nephropathy. Nephrol Dial Transplant. 2009;24:2378–2383. doi: 10.1093/ndt/gfp012. [DOI] [PubMed] [Google Scholar]
- 24.Shoham N, Cohen L, Yaniv A, Gazit A. The Tat protein of the human immunodeficiency virus type 1 (HIV-1) interacts with the EGF-like repeats of the Notch proteins and the EGF precursor. Virus Res. 2003;98:57–61. doi: 10.1016/j.virusres.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Hayward SD. Viral interactions with the Notch pathway. Semin Cancer Biol. 2004;14:387–396. doi: 10.1016/j.semcancer.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Hayward SD, Liu J, Fujimuro M. Notch and Wnt signaling: mimicry and manipulation by gamma herpesviruses. Sci STKE. 2006;335:re4. doi: 10.1126/stke.3352006re4. [DOI] [PubMed] [Google Scholar]
- 27.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2138–2140. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 28.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- 29.Brantley JG, Sharma M, Alcalay NI, VandenHeuvel GB. Cux-1 transgenic mice develop glomerulosclerosis and interstitial fibrosis. Kidney Int. 2003;63:1240–1248. doi: 10.1046/j.1523-1755.2003.00889.x. [DOI] [PubMed] [Google Scholar]
- 30.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi’s sarcoma tumor cells. Oncogene. 2005;24:6333–6344. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]