Abstract
The role of CD4+ T helper (Th) 17 cells in malignancy is currently under debate. However, upon closer scrutiny, it becomes apparent that this discussion includes not only evaluations of Th17 cells but also IL-17+ cells from other immune populations, the cytokine interleukin (IL)-17 itself (both endogenous and exogenous) and IL-23. Further complicating the matter are occasionally conflicting results of studies in humans versus those in mice and contradictory data from immunocompetent versus immunodeficient mice. To better understand the role of Th17 cells in the tumor-bearing host, we focus first upon those studies investigating Th17 cells in patients and then those in mice, all the while keeping in mind that variables such as tumor-initiating agents, a pre-existing inflammatory environment and the immune competence of the host may have direct effects upon this T-cell subset. In this review, we will describe the phenotype of tumor-associated Th17 cells, review those studies that have examined the population directly, and finally, briefly discuss the studies involving Th17-associated signature cytokines.
Phenotype and cytokine profile of T helper 17 cells in cancer patients
Since their discovery only 5 years ago, T helper (Th) 17 cells have risen to prominence in studies of virology, autoimmune disease, inflammation, and immune responses to various parasites and fungi. Although their role in the pathogenesis of many of these conditions is rather well defined, their function(s) in the context of tumor immunology remains controversial. To begin with, it is imperative to stress that the cytokine interleukin (IL)-17 and the T-cell subset Th17 are not synonymous. If we are to arrive at a consensus on the action of Th17 cells in the tumor environment, we must make and maintain this distinction (Box 1).
Box 1. Inequalities in evaluating Th17 cells and tumor.
| Th17 ≠ IL-17+ cell |
| Th17 ≠ IL-17 |
| Th17 ≠ IL-23 |
| Exogenous IL-17 ≠ endogenous IL-17 |
| Mouse ≠ human |
| Immunodeficient organism ≠ immune competent organism |
| Chemical carcinogen-induced cancer ≠ chronic infection-associated cancer |
| Early cancer stage ≠ advanced cancer stage |
Th17 cells are defined as CD4+ T helper cells that secrete the cytokine IL-17 and whose developmental program is controlled by multiple cytokines (1) and the transcription factor retinoic acid receptor-related orphan receptor gamma T. These cells have been examined in cancer patients by a few laboratories, including our own. We have shown that human tumor-associated Th17 cells express minimal levels of human leukocyte antigen-dr, CD25 and granzyme B, suggesting that they are not a ‘conventional’ effector cell population. Moreover, these cells also do not express programmed cell death 1 or forkhead box P3, making it unlikely that they enact immune suppression through either pathway. As for cytokine products, Th17 cells in cancer patients produce high levels of granulocyte-macrophage colony stimulating factor, tumor necrosis factor alpha (TNFα), IL-2 and interferon-gamma (IFNγ), but no IL-10. This phenotype has been observed in five types of human tumor (2). In support of this, 50% of Th17 cells in patients with hepatocellular carcinoma (HCC) produced IFNγ (3). It is worth mentioning that Th17 cells expanded in vitro from tumor-infiltrating lymphocyte populations in melanoma, breast and colon cancers secrete elevated amounts of IL-8 and TNFα, but no IL-2 (4). Because this profile has been seen previously in Th17 cells isolated from healthy donors (5) and patients with autoimmune diseases (6) (Ilona Kryczek, Allen Bruce, and Weiping Zou unpublished results), it is possible that there is a difference in the phenotypes of freshly isolated Th17 cells and those expanded or induced in vitro from tumor-associated populations. Alternatively—but less likely—Th17 cell phenotypes may differ across cancers. Tumor-associated Th17 cytokine products mimic those found in some instances of viral infection (7,8); we believe that tumor-associated Th17 cells have the ability to influence immune responses through the action of these proteins.
As for homing molecules, tumor-associated Th17 highly express CXCR4 and CCR6, c-type lectin receptor CD161 and the CD49 integrin isoforms c, d and e. CCR2, CCR5 and CCR7 are not present on these cells (2). Importantly, CCR6 and CD161 have been observed on both Th17 cells from healthy donors and on lymphocytes and dendritic cells in inflammatory environments (9–11), so they may not serve as Th17-specific identification or selection molecules. Sharma et al. (12) have defined a similar Th17 phenotype in a mouse model of cancer: cells expressing high levels of IL-2 and TNFα, a subpopulation of these concurrently expressing IL-22 and a small number of cells expressing IFNγ or IL-10. It may be of interest that the cells in this study had been reprogrammed from T regulatory cells (Tregs); but as of yet, this remains the only study to directly address the cytokine profile of Th17 cells in a murine tumor environment. IL-2 and IL-2/TNFα expression in Th17 cells have also been documented in murine models of multiple sclerosis and in tumor-specific Th17-polarized cells (13,14). As might be expected, this profile has also been observed in murine virus infections (15). It seems that in both human and mouse malignancies, Th17 cells share the same effector cytokine profile. We will now discuss studies examining the role(s) of tumor-associated Th17 populations.
Th17 cells in cancer
Human
Many laboratories have studied Th17 populations in the blood and (occasionally) tissues of patients with various cancers (Table I). Our group has extensively examined Th17 distribution and function in ovarian cancer patients. We have made several key observations: firstly, that the prevalence of Th17 in the tumor-draining lymph nodes and blood of these patients is comparable with that of healthy donors. Secondly, although Th17 cells constitute a small population within the tumor microenvironment, they are found in proportionally higher numbers here in comparison with other immune cell subsets. Tumor-associated Th17 levels correlate positively with microenvironmental Th1 cells, cytotoxic CD8+ T cells and natural killer cells and inversely with Tregs (2, 23). Su et al. (4) also found significantly higher numbers of Th17 cells expanded or induced from tumor-infiltrating lymphocyte populations in cancer patients than in lymphocyte populations from non-tumor tissue. In ovarian cancer patients, Th17 cells were the sole source of IL-17 in ascites, and the level of IL-17 in this fluid correlated positively with patient survival. Even after controlling for surgical debulking and other parameters, tumor-associated IL-17 was a negative predictor of death hazard. Average survival of patients with >220 pg/ml IL-17 in ascites was 78 months, whereas that of patients with less IL-17 was 27 months. In the tumor microenvironment, IL-17 synergized with IFNγ to induce CXCL9 and CXCL10 production. These Th1-type chemokines recruit effector populations to the tumor itself: we found that ascites levels of CXCL9 and CXCL10 correlated directly with tumor-infiltrating natural killer and CD8+ T cells (2). In agreement with our finding that Th17 cells are protective, Sfanos et al. (17) found an inverse correlation between the differentiation stage of Th17 cells in prostate glands of cancer patients and their tumor progression. However, in another study examining patients with hormone-resistant prostate cancer, Derhovanessian et al. (20) demonstrated an inverse correlation between pretreatment circulating levels of Th17 cells and time to disease progression. Recall that the levels of Th17 cells are usually limited in cancer patients (2,23). A larger population of Th17 in the blood may indicate an underlying infection or inflammatory state, which would influence the efficacy of immunotherapy and speed of tumor development. It would be interesting to further evaluate these patient samples and try to determine the initial cause of the expanded blood Th17 populations. A study from Kuang et al. demonstrated that IL-17-producing cells were enriched predominantly in peritumoral stroma of HCC tissues, where their levels correlated with monocyte/macrophage density. Consistent with our observations (2), tumor-activated monocytes were better than tumor-associated macrophages in inducing in vitro expansion and proliferation of Th17 from circulating memory T cells (21). Interestingly, a very recent report examined tumor-infiltrating lymphocytes and their correlations with nasopharyngeal carcinoma patient outcomes; Zhang et al. (16) found no correlation of Th17 with patient clinicopathological characteristics or survival. Finally, Ye et al. investigated Th17 cells from 30 patients with lung adenocarcinoma or squamous cell carcinoma. Malignant pleural effusion from these patients was chemotactic for Th17 cells, and this activity was partially abrogated by CCL20 and/or CCL22 blockade. Interestingly, higher accumulation of Th17 cells in malignant pleural effusion predicted improved patient survival (18).
Table I.
Th17 cells and tumor
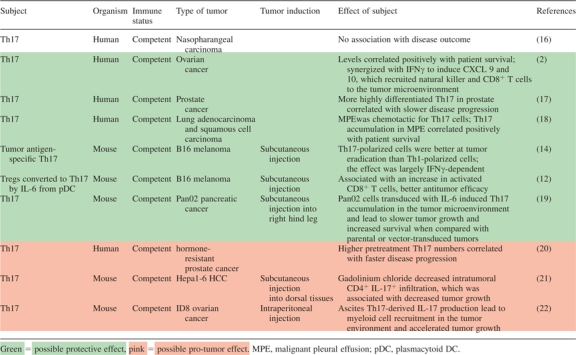 |
It is well known that patients with chronic inflammatory conditions have a greatly increased risk of cancer in the affected organs (24,25). Because inflammation resulting from viral or bacterial infections can often lead to or hasten the development of malignancy, it is vital to comprehend the kinetics and targets of inflammation in a discussion of Th17 cells and cancer. Our laboratory examined the relationship between Th1-derived IFNγ, Th17 cells and antigen-presenting cells (APCs) in humans. We found that IFNγ could rapidly induce elevated B7-H1 expression on APCs and stimulate their production of IL-1 and IL-23. B7-H1 signaling resulted in abrogation of the Th1-polarizing capacity of APC, whereas IL-1 and IL-23 directed them toward a memory Th17-expanding phenotype (26). In the course of inflammation, then, we believe that the acute Th1-mediated response is attenuated by IFNγ-induced B7-H1 on APCs and is subsequently evolved toward Th17-mediated chronic inflammation by APC-derived IL-1 and IL-23. In addition to challenging the dogma that IFNγ suppresses Th17 and enhances Th1 development, our study reinforces the notion that Th17 kinetics depend strongly on the context of the ongoing immune response and the constituents of the cytokine milieu, both of which are influenced by disease progression.
Mouse
Our laboratory has also studied Th17 cells in murine cancer. Similar to humans, Th17 populations are limited in healthy mice but relatively expanded in the blood, bone marrow and spleens of mice bearing the aggressive B16 melanoma. Perhaps not surprisingly, the largest populations of Th17 cells occurred within the tumor. We also observed expanded Th17 populations in mouse melanoma, prostate cancer, fibrosarcoma and advanced head and neck cancer (27). The laboratory of Nicholas Restifo published a study in 2008 investigating the effect of a tumor antigen-specific T-cell clone on the eradication of murine melanoma. Interestingly, Th17-polarized (via IL-6 and transforming growth factor β) T-cell clones were better than Th1-polarized clones in destroying advanced B16 tumors, although their effect seemed to depend largely on their production of IFNγ (14). As promising as this may seem, there are several complicating factors to consider. Although IFNγ has been documented as a cytokine product of human Th17 cells in vivo, secretion has been minimal in murine Th17 associated with tumor (12). Secondly, recall that in vitro-polarized cells become enriched for a particular cell type (in this case, Th17 cells) but do not constitute a pure population. Finally, the concept of plasticity needs to be taken into account in any discussion of the immune system, and we emphasize that there is a potential for Th17 cells to convert to a Th1-like phenotype in the presence of IL-12 (28,29). These issues should be carefully examined when one is interpreting instances of adoptive transfer. Soon after these experiments were published, Sharma et al. treated B16-bearing mice with an indoleamine 2,3-dioxygenase inhibitor and antitumor vaccine, which increased the frequency of IL-6 production by plasmacytoid dendritic cells. This treatment also caused a conversion of many Tregs in tumor-draining lymph nodes to Th17 cells, and the investigators observed an increase in activated CD8+ T cells along with augmented antitumor efficacy (12). Repetition of treatment in CD4+ T-cell-deficient mice led to the abrogation of this effect, suggesting (perhaps not surprisingly) that CD4+ helper cells were necessary for the antitumor response. To complete their recent study on HCC, Kuang et al. (21). demonstrated that inhibition of monocyte and macrophage inflammation in liver via treatment with gadolinium chloride (a Kupffer cell toxicant) reduced the level of murine hepatoma-infiltrating CD4+ IL-17+ cells by ∼80% and also reduced tumor growth. Charles et al. obtained similar results when they examined TNFα signaling in the ID8 mouse model of ovarian cancer. In these experiments, TNFα signaling through the TNFα receptor 1 appeared to maintain IL-17 production by ascites CD4+ T cells (but not those in the spleen), which then led to GR1+ myeloid cell recruitment into the tumor microenvironment and enhanced tumor growth (22). Studies of the myeloid compartment of the stroma once again point to the importance of an extant inflammatory environment before and during tumorigenesis; the extent to which this occurs can have profound effects on the tumor microenvironment. The most recent mouse report on intratumoral Th17 examined pancreatic cancer. Gnerlich et al. found that mice bearing IL-6-transduced (transforming growth factor β-secreting) Pan02 tumors had slower tumor growth and better overall median survival compared with mice bearing parental or empty vector-transduced Pan02 tumors. Immunohistochemistry, flow cytometry and enzyme-linked immunospot assay analysis revealed increased Th17 cells in tumors of the IL-6 Pan02 group compared with wild-type or empty vector-transduced Pan02 tumors. Thus, the addition of IL-6 to the tumor microenvironment skews the balance between Treg and Th17 cells toward Th17 in this model of pancreatic cancer. Delayed tumor growth in concert with improved survival suggests that microenvironmental induction of Th17 supports antitumor immunity (19).
IL-17 in cancer
Perhaps the most confusing data in the debate over Th17’s role in cancer arises from studies examining IL-17 itself. IL-17 is a proinflammatory cytokine, which can induce the production of other proinflammatory cytokines, chemokines and prostaglandins. It has six family members (IL-17A through F) that are expressed by a variety of innate and adaptive immune cell types, including mast cells, epithelial cells, smooth muscle cells, invariant natural killer T cells, natural killer cells, paneth cells, lymphoid-tissue inducer -like cells, neutrophils and finally, γδ and αβ T cells (both CD4+ and CD8+). The specific effects of cytokine production by the above cell types are excellently reviewed elsewhere (30). Here, we will summarize studies of this cytokine (primarily IL-17A) both in human and murine malignancies (Table II).
Table II.
IL-17 and tumor
 |
Human
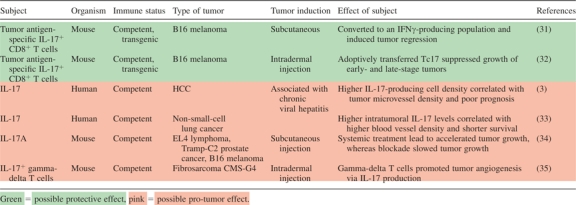
Zhang et al. have examined IL-17+ cells in patients with HCC and suggested a potential pro-tumor role for IL-17. They correlated increased IL-17-producing cell density within the tumors of HCC patients with both microvessel density and poor prognosis (3). Notably, HCC is strongly associated with chronic viral hepatitis; chronic viral infection may profoundly reshape the generation and function of Th17 cells in cancer patients. Furthermore, this study did not directly address the role of IL-17 in HCC. In non-small-cell lung cancer patients, higher levels of IL-17 within the tumor correlated with higher blood vessel density and shorter survival (33). Earlier reports have also documented pro-angiogenic roles for IL-17 (36–39); it is imperative to understand that these correlations are with the protein IL-17 and not a specific cell population.
Mouse
Several laboratories have investigated the effects of IL-17 in murine models of cancer. Hinrichs et al. adoptively transferred IL-17-producing CD8+ T cells (Tc17) into mice bearing established vascularized B16 melanoma. Once in vivo, these cells might be converted to an IFNγ-producing phenotype, induced tumor regression and persisted in the host longer than non-polarized cells (31). One year later, the Dutton group demonstrated similar findings: in vitro-generated tumor-specific IL-17-producing CD8+ T cells adoptively transferred into tumor-bearing mice controlled the growth of both early- and late-stage B16 melanoma (32). The effector cells aided in recruitment of other T cells, neutrophils and macrophages to the tumor and then in concert with newly recruited neutrophils, produced chemokines to attract Th1 cells, Tc1 cells and still more neutrophils. Interestingly, far more IL-17+ CD8+ cells were required (compared with Tc1 effectors) to exert the same level of control over tumor growth, which may suggest that IL-17-producing CD8 cells are necessary at the induction of the antitumor response to recruit other, more potent effector cells to the microenvironment. In contrast, Yang et al. [who had previously demonstrated that mast cells accumulate in the tumor microenvironment via signaling through the SCF/c-kit pathway (40)] showed this year that the mast cells mobilize myeloid-derived suppressor cell tumor infiltration and production of IL-17. This IL-17 effected increased downstream intratumoral recruitment of Treg cells, enhanced their suppressor function through upregulation of CD39 and CD73 and induced IL-9 production. Treg-derived IL-9 acted in a positive feedback loop to increase the longevity and pro-tumor effect of mast cells in the tumor microenvironment (41).
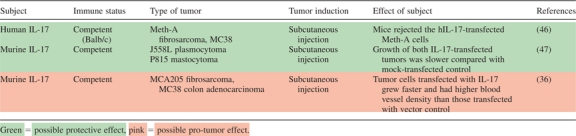
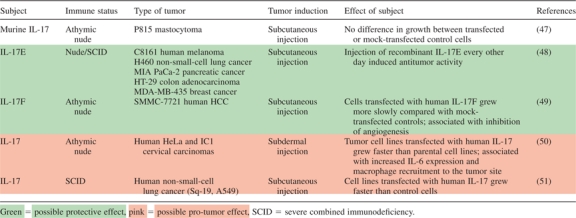
Another paradigm to consider is that of deletion (Table III) or forced ectopic expression of IL-17 in mice (Table IV). We and others have observed that IL-17A knockout (IL-17A−/−) mice have faster tumor growth and more lung metastases than wild-type mice (42–44). Contrastingly, a study from 2009 showed that two transferred tumors (B16 and bladder carcinoma MC49) grew more slowly in IL-17−/− mice (34). Additionally, He et al. recently completed experiments with IL-17R−/− mice, in which they observed slower growth of mouse tumor cell lines (EL4 lymphoma, Tramp-C2 prostate cancer and B16-F10 melanoma). Fascinatingly, systemic pretreatment with murine IL-17A lead to dramatically accelerated tumor growth in C57Bl/6 mice, whereas blockade of the same slowed tumor growth. In this study, IL-17R deficiency resulted in an increase of intratumoral CD8+ T cells and decreased numbers of myeloid-derived suppressor cells in the tumor microenvironment (45). Interestingly, transgenic expression of human or murine IL-17 in tumor cells has been shown in two studies to suppress or slow tumor progression and increase tumor-specific cytotoxic responses (46,47). In contrast, Numasaki et al. (36) transfected murine fibrosarcoma (MCA205) and colon adenocarcinoma (MC38) with mouse IL-17 and found that the transfectants experienced accelerated growth in wild-type C57Bl/6 mice when compared with non-transfected tumors.
Table III.
IL-17 signaling deficiency and tumor
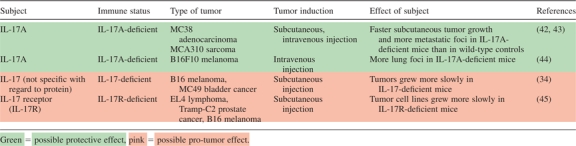 |
Table IV.
Exogenous IL-17 and tumor
 |
Several new studies investigate the antitumor capabilities of other IL-17 cytokines: Benetar et al. examined a variety of human tumor xenograft models, including melanoma, breast, lung, colon and pancreatic cancers and found that injection of recombinant IL-17E every other day resulted in induction of antitumor activity. IL-17E also synergized with chemotherapeutic or other immunotherapeutic agents to improve antitumor efficacy in human tumor xenograft models in mice. Increases in IL-17E lead to higher eosinophil numbers in blood and spleen compared with the control group; the splenic levels of eosinophils correlated with antitumor activity of IL-17E in a dose–response manner. Finally, IL-17E activated signaling pathways in B cells, which were necessary for antitumor activity (48). Xie et al. (49) have examined the efficacy of IL-17F against HCC. HCC cells (SMMC-7721) transfected with human IL-17F downregulated self-production of IL-6, IL-8 and vascular endothelial growth factor, and culture supernatant from these cells directly inhibited ECV304 vascular endothelial cell growth. When transplanted into nude mice, transfected cells grew more slowly compared with mock-transfected controls. This effect was associated with direct suppression of vascular endothelial cells in addition to downregulation of the pro-angiogenic factors listed above.
Tumor-associated roles for IL-17 have also been explored in immunodeficient mice (Table V). While instructive, it is necessary to recall that human cancers are largely initiated and develop in immune-competent hosts. Nevertheless, it is interesting to ponder the following investigations: Tartour et al. (50). injected nude mice with human cervical tumor cells transfected with human IL-17 and found that they grew more quickly than parental tumors. A few years later, Numasaki et al. (51) demonstrated that human non-small-cell lung cancer transfected with human IL-17 grew faster in severe combined immunodeficiency mice than did control non-small-cell lung cancer cells. In both of these investigations, IL-17’s pro-angiogenic capabilities seemed to contribute to accelerated tumor growth. A recent study by Wakita et al. sheds some light on the seeming duality of roles of IL-17 in the tumor environment. The investigators induced tumor foci in mouse skin and found that circulating lymphoid γδ T cells that infiltrated the lesions were the major source of IL-17 in the tumor microenvironment; they promoted angiogenesis via their production of IL-17 (35). This is a crucial piece of the IL-17 puzzle; it demonstrates that pro-angiogenic IL-17 can be produced by cells other than the ‘traditional’ (and until now more-studied) αβ T cells.
Table V.
Tumor development in immunodeficient mouse models
 |
Models of infection- or carcinogen-initiated cancer
Earlier this year, Chae et al. demonstrated that ablation of IL-17A significantly reduced tumor formation in APC+/Min mice. Interestingly, the absence of IL-17A also ameliorated immune abnormalities (splenomegaly and thymic atrophy) in the affected mice (52). Another recent study where investigators infected APCMin/+ mice with the common human commensal bacterium Bacteriodes fragilis revealed that it induced Th17-mediated colitis and then colonic tumors in the recipients (53). This cascade of events was dependent upon activation of signal transducer and activation of transcription-3 (STAT3), both an upstream mediator of the Th17 cell phenotype (54,55) and a potential downstream target of IL-17 signaling (34); it may be of interest to note that IL-22, an occasional product of Th17 cells, can signal via signal transducer and activation of transcription-3 (56). Importantly, the cellular sources of IL-17 in the B.fragilis study were determined to be both CD4+ Th17 cells and γδ T cells; however, selective STAT3 knockdown in the CD4+ T-cell compartment resulted in a decrease in colonic inflammation and hyperplasia. Interestingly, colitis, colonic hyperplasia and tumor development were inhibited by antibody-mediated blockade of IL-17A alone or in concert with the IL-23 receptor (IL-23R).
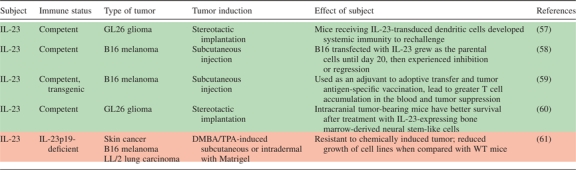
An APC-produced member of the IL-12 cytokine family, IL-23 promotes the survival and expansion of Th17 cells. It is no wonder, then, that discussions of Th17 function in tumor environments usually involve consideration for a role for IL-23 in the same setting (Table VI). Langowski et al. demonstrated in 2006 that IL-23p19-deficient (IL-23p19−/−) mice were resistant to skin tumors initiated by 9,10-dimethyl-1,2-benzanthracene and promoted by treatment with 12-O-tetradecanoyl-phorbol acetate. The investigators also observed reduced growth of murine B16-F10 melanoma and LL/2 lung carcinoma in IL-23 receptor-deficient (IL-23R−/−) mice. Functional ablation of IL-23p19 subunit or genetic deletion of the IL-23R was associated with decreased matrix metalloproteinase 9 expression and increased CD8+ T-cell infiltration into the tumor tissues (61). Despite the temptation to associate Th17 cells or their development with the pro-tumor effects of IL-23 observed in this model, there is no data linking these observations to IL-17 or a Th17 population. Furthermore, it is prudent to recall that IL-23 has many antitumor properties. Several studies have documented that overexpression of IL-23 in tumors and vaccination with IL-23-transduced dendritic cells have lead to robust intratumoral CD8+ T (and in some cases, CD4+ T) cell infiltration and subsequent inhibition of tumor growth (57–60). As in the case of IL-17, then, it is important to consider data regarding IL-23 in the context of the host and the type of tumor model used.
Table VI.
IL-23 and tumor
 |
Conclusions
As is now apparent, few studies have focused on primary Th17 cells in the human tumor microenvironment, so it is difficult to deduce the exact role(s) they may have in cancer patients. In the more exhaustive studies of patients with established epithelial cancer, Th17 presence and function have correlated with reduced tumor progression and improved patient survival. In mice with established tumors, studies have documented potent antitumor efficacy for both Th17 and Tc17 populations. However, it is possible that Th17 function may vary according to cancer cause, type and location (Table I) (62), as well as stage of disease. For example, collective evidence suggests that Th17 cells and/or IL-17, along with other factors, may induce inflammation and promote the initiation and early growth of some tumors, as we have seen in three murine models of cancer: immune-deficient mice, mice with chemical carcinogen-induced tumors and mice with pathogen-induced tumors. Nonetheless, it is important to note that most cancer patients are generally not immune-deficient, and many have clinically established tumors without known exposure to pathogens or carcinogens. Th17 cell biology has been partially examined in patients with well-established cancer. We acknowledge that human studies are technically challenging, however, we believe it is now essential to investigate the roles of Th17 cells and IL-17 in the very early phases of human tumor growth to better understand how these roles may change during disease progression.
Funding
Extramural funds from the United States National Cancer Institute (1R01CA099985, 1R01CA123088, 1R01CA0156685); the Marsha Rivkin Center for Ovarian Cancer Research; the Ovarian Cancer Research Fund (W.Z.).
Acknowledgments
We would like to thank our former and current trainees and collaborators for their intellectual input and hard work.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
antigen-presenting cell
- HCC
hepatocellular carcinoma
- IL
interleukin
- IFNγ
interferon-gamma
- STAT3
signal transducer and activator of transcription-3
- Th17
T helper 17
- TNFα
tumor necrosis factor alpha
- Treg
T regulatory cell
References
- 1.Kryczek I, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 2.Kryczek I, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JP, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Su X, et al. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, et al. Regulation of IL-17 in human CCR6+ effector memory T cells. J. Immunol. 2008;180:7948–7957. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 6.Kryczek I, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J. Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida JR, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosmi L, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinschek MA, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma MD, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arens R, et al. Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J. Immunol. 2008;180:6472–6476. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YL, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol. Cancer. 2010;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sfanos KS, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Z-J, et al. Generation and differentiation of interleukin-17-producing CD4+ T cells in malignant pleural effusion. J. Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 19.Gnerlich JL, et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J. Immunol. 2010;185:4063–4071. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derhovanessian E, et al. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int. J. Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 21.Kuang DM, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154–164. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 22.Charles KA, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.Coussens LM, et al. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 26.Kryczek I, et al. Cutting edge: IFN-gamma enables APC to promote memory Th17 and abate Th1 cell development. J. Immunol. 2008;181:5842–5846. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- 27.Kryczek I, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 28.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurieva R, et al. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J. Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cua DJ, et al. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 31.Hinrichs CS, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Hernandez Mde L, et al. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J. Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2009;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakita D, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010;40:1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 36.Numasaki M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 37.Numasaki M, et al. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol. Lett. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H, et al. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol. Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Honorati MC, et al. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage. 2006;14:345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5:e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kryczek I, et al. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei S, et al. Endogenous IL-17, tumor growth, and metastasis. Blood. 2010;115:2256–2257. [Google Scholar]
- 44.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He D, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirahara N, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 47.Benchetrit F, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 48.Benatar T, et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol. Immunother. 2010;59:805–817. doi: 10.1007/s00262-009-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie Y, et al. Interleukin-17F suppresses hepatocarcinoma cell growth via inhibition of tumor angiogenesis. Cancer Invest. 2010;28:598–607. doi: 10.3109/07357900903287030. [DOI] [PubMed] [Google Scholar]
- 50.Tartour E, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 51.Numasaki M, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J. Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 52.Chae WJ, et al. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl Acad. Sci. USA. 2010;107:5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 56.Xie MH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 57.Hu J, et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 58.Oniki S, et al. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66:6395–6404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- 59.Overwijk WW, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J. Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan X, et al. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 61.Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 62.Zou W, et al. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]


