Abstract
Tumor-associated macrophages and high levels of cyclooxygenase-2 (COX-2) are associated with poor prognosis in breast cancer patients, but their potential interdependence has not been evaluated. The objective of this study was to determine whether macrophages regulate COX-2 expression in breast cancer cells. For this purpose, THP-1 cells were cocultured with HCC1954 breast cancer cells. Coculture led to increased COX-2 expression in the HCC1954 cells and elevated prostaglandin E2 levels in conditioned media. Similar results were observed when THP-1 cells were incubated with HCC1937 breast cancer cells or when human monocyte-derived macrophages were cocultured with HCC1954 cells. Coculture triggered production of reactive oxygen species (ROS) in HCC1954 cells. COX-2 induction was blocked in cells preincubated with an reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor or by silencing p67PHOX, a subunit of NADPH oxidase. ROS production triggered activation of Src and mitogen-activated protein kinases (MAPKs). Blocking Src or MAPK activities or antagonizing the activator protein-1 (AP-1) transcription factor attenuated COX-2 induction in HCC1954 cells. Coculture caused rapid induction of interleukin-1β (IL-1β) in both breast cancer cells and macrophages. Increased IL-1β expression was blocked by an interleukin-1 receptor antagonist (IL-1Ra), suggesting autocrine and paracrine effects. Importantly, macrophage-induced COX-2 expression was blocked in HCC1954 cells preincubated with IL-1Ra or anti-IL-1β IgG. Together, these results indicate that macrophage-mediated induction of COX-2 in breast cancer cells is a consequence of IL-1β-mediated stimulation of ROS→Src→MAPK→AP-1 signaling. IL-1β-dependent induction of COX-2 in breast cancer cells provides a mechanism whereby macrophages contribute to tumor progression and potential therapeutic targets in breast cancer.
Introduction
Macrophages are a major component of the inflammatory infiltrate observed in many tumors including carcinoma of the breast (1,2). Evidence suggests that tumor-associated macrophages (TAMs) produce a variety of inflammatory mediators that influence angiogenesis, proliferation, integrity of the extracellular matrix, invasion and metastasis (1,3). In the breast, the presence of high numbers of TAMs is associated with a poor prognosis (1,4). Despite intense investigation, the mechanisms by which TAMs contribute to tumorigenesis and/or progression of breast cancer remains incompletely understood (5–7). In this regard, cyclooxygenase-2 (COX-2) is overexpressed in ∼40% of invasive breast cancers and is associated with increased proliferation, high histological grade, metastasis and reduced survival (8,9). Furthermore, treatment with COX-2 inhibitors or gene ablation reduced experimentally induced breast cancers (10–12), and the use of non-steroidal anti-inflammatory drugs is associated with a reduced incidence of breast cancer (13,14). Although TAMs and elevated COX-2 expression are independently associated with an aggressive tumor phenotype, the regulatory role macrophages may have on COX-2 expression in breast cancer cells is incompletely understood.
To determine whether macrophages regulate COX-2 expression in breast cancer cells, the two cell types were cocultured utilizing a transwell system. Macrophages induced COX-2 expression in cancer cells and elevated prostaglandin E2 (PGE2) levels in conditioned media (CM). Coculture triggered a rise in reactive oxygen species (ROS) levels in the breast cancer cells, which led to activation of Src kinase and subsequently mitogen-activated protein kinase (MAPK) family members. Blocking Src or MAPK activities or antagonizing the activator protein-1 (AP-1) transcription factor attenuated COX-2 induction in breast cancer cells. In addition, coculture led to a rapid rise in interleukin-1β (IL-1β) expression in both breast cancer cells and macrophages, and macrophage-mediated induction of COX-2 was blocked in breast cancer cells treated with IL-1β-neutralizing antibody or interleukin-1 receptor antagonist (IL-1Ra). Thus, macrophage-mediated induction of COX-2 in breast cancer cells is a consequence of IL-1β-dependent stimulation of ROS→Src→MAPK→AP-1 signaling. These findings provide new insights into a mechanism whereby macrophages contribute to tumor progression and suggest potential therapeutic targets for tumors containing elevated numbers of TAMs.
Materials and methods
Reagents
RPMI-1640 medium and fetal bovine serum (FBS) were obtained from American Type Culture Collection (ATCC, Manassas, VA). Dulbecco's modified Eagle's medium (DMEM) and DMEM/F-12 media were obtained from Gibco (Invitrogen Corporation, Carlsbad, CA). PP1, PP2, PD98059, SB202190, diphenyleneiodonium (DPI), N-acetylcysteine, all-trans-retinoic acid (ATRA), phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (from Escherichia coli serotype 0111:B4), p38 MAPK activity assay kit and β-actin antibody were obtained from Sigma–Aldrich (St. Louis, MO). c-Jun N-terminal kinase (JNK) inhibitor V was obtained from Calbiochem (EMD Chemicals, Gibbbstown, NJ). COX-2 and p67PHOX antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Antibodies for extracellular signal-regulated kinase (ERK), phospho-ERK, cJun, phospho-cJun (Ser73), Src and phospho-Src (Tyr416) were obtained from Cell Signaling Technology (Danvers, MA). ON-Targetplus non-targeting siRNA pool (NS siRNA) and siRNAs targeting p67PHOX, Src and cJun were obtained from Dharmacon (ThermoFischer Scientific, Lafayette, CO). IL-1Ra, IL-1β-neutralizing antibody, human recombinant interferon γ (IFNγ) and mouse IgG1 were obtained from R&D Systems (Minneapolis, MN).
Cell culture
Human breast carcinoma cell lines HCC1954, HCC1937 (15), MCF-7 (16) and SK-BR-3 (17), human monocytic cell line THP-1 (18), human urothelial carcinoma cell line RT-4 (19) and murine macrophage cell line RAW264.7 (20) were purchased from ATCC. HCC1954, HCC1937, MCF-7, SK-BR-3 and THP-1 cells were maintained in RPMI-1640 medium supplemented with FBS. RT-4 cells were maintained in McCoy's 5A medium supplemented with FBS. C57/Ras cells were grown as described previously (21). RAW264.7 macrophages were maintained in DMEM with FBS.
Isolation of human peripheral blood monocytes (PBM)
Mononuclear cells were isolated from whole human blood (22). PBMs were recovered from the mononuclear cell population utilizing the EasySep immunomagnetic system (StemCell Technologies, Vancouver, BC, Canada) according to manufacturer's instructions.
Isolation of elicited mouse peritoneal macrophage
Thioglycollate-elicited mouse peritoneal macrophages were obtained from Swiss Webster mice as described earlier (23). Mice were injected intraperitoneally with 3% Brewer Thioglycollate Medium (Difco). Four days later, peritoneal cells were harvested by lavage, recovered by centrifugation and resuspended in DMEM with FBS.
Coculture
HCC1954, HCC1937 or C57/Ras cells were plated into six-well dishes (1.5 to –2 × 106 cells per well) in their culture media described above. THP-1 cells (2 to –2.5 × 106 cells per insert), blood monocytes (1.5 to –2 × 106 cells per insert) or peritoneal macrophages (1 to 2 × 106 cells per insert) were plated directly on the transwell inserts (0.4 μm, BD Biosciences, Bedford, MA) in their culture medium. THP-1 cells were treated with PMA (10 ng/ml) overnight to differentiate them into macrophages. Blood monocytes were activated by treating with IFNγ (20 ng/ml) and lipopolysaccharide (10 ng/ml) for 4 days. Medium was replaced on the third day. Prior to coculture, breast cancer cells and macrophages were washed (×3) with either DMEM or RPMI containing 0.1% bovine serum albumin (basal medium). After the last wash, the appropriate basal medium was added to the breast cancer cells and inserts containing macrophages were placed in each well.
Western blotting
Immunoblotting for detection of COX-2, p67PHOX, ERK, phospho-ERK, cJun, phospho-cJun, Src, and phospho-Src was carried out as described previously (24).
Real-time polymerase chain reaction
Total RNA from cell lysates was isolated using the RNeasy Mini kit (QIAGEN, Valencia, CA). RNA (1 μg) was reverse transcribed using murine leukemia virus reverse transcriptase and oligo d(T)16 primer. The resulting complementary DNA was then used for amplification. Each polymerase chain reaction (PCR) reaction volume was 20 μl and contained 5 μl complementary DNA, 2× SYBR Green PCR master mix and forward and reverse primers. Real-time PCR primers for COX-2 were forward, 5′-CCCTTGGGTGTCAAAGGTAA-3′ and reverse, 5′-GCCCTCGCTTATGATCTGTC-3′. Primers for IL-1β were forward, 5′-GGACAAGCTGAGGAAGATGC-3′ and reverse, 5′-TCGTTATCCCATGTGTCGAA-3′. Primers for β-actin were forward, 5′-AGAAAATCTGGCACCACACC-3′ and reverse, 5′-AGAGGCGTACAGGGATAGCA-3′. The messenger RNA (mRNA) levels were normalized to β-actin. Relative expression was determined by ddCT (relative quantification) analysis.
ROS measurement
The effect of coculture with macrophages or coculture CM on ROS levels in breast cancer cells was determined utilizing a chloromethyl derivative of fluorescein (CM-H2DCFDA) according to a protocol provided by Invitrogen (Carlsbad, CA). CM-H2DCFDA was maintained at −20°C until immediately before use. The stock solution was prepared under low light conditions and stored in an aluminum foil-wrapped tube to protect the dye from photooxidation. Breast cancer cells were preloaded for 30 min with phosphate-buffered saline containing 10 μmol/l CM-H2DCFDA in foil-wrapped plates. Subsequently, the dye was removed and breast cancer cells were cocultured with macrophages in medium free of phenol red in foil-wrapped plates for the indicated time periods. For experiments utilizing CM, the cocultured CM were prepared utilizing medium free of phenol red. Fluorescence was measured in a CytoFluor™ 4000 multi-well plate reader (excitation: 485 nm; emission: 520 nm) and the levels of ROS were normalized by protein content in each sample. Levels of fluorescence in CM-H2DCFDA loaded breast cancer cells exposed to H2O2 and phorbol myristate acetate (PMA) were used as positive controls to optimize the conditions for measuring ROS.
Determination of PGE2 and IL-1β levels in cellular CM
PGE2 and IL-1β levels in CM were determined utilizing enzyme immunoassay kits from Cayman Chemicals (Ann Arbor, MI) and R&D Systems, respectively.
p38 Activity
p38 Activity was measured with a kit from Sigma–Aldrich according to manufacturer's instructions.
siRNA transfection
Cells were plated at 60% confluence and transfected with NS siRNA or targeting siRNA using Dharmafect4 (Dharmacon) for 48 h. The cells were then cocultured or treated with conditioned medium.
Chromatin immunoprecipitation assay
HCC1954 cells were harvested following coculture. Chromatin immunoprecipitation (ChIP) assays were conducted utilizing the ChampionChIP Kit (SABiosciences, Frederick, MD). Briefly, cells were washed and cross-linked with formaldehyde. The chromatin was sheared by sonication. Immunoprecipitation using phospho-cJun antibody was performed. Real-time PCR was performed on the eluted product using primers targeting the COX-2 promoter. DNA product before antibody immunoprecipitation was used as total input for normalization.
Statistical analysis
Mean levels of PGE2, COX-2 mRNA, ROS, IL-1β concentration or IL-1β mRNA were compared utilizing analysis of variance. If a significant difference between means was observed, individual comparisons were made utilizing the Newman–Keuls test (25).
Results
Macrophages induce COX-2 expression in breast cancer cells
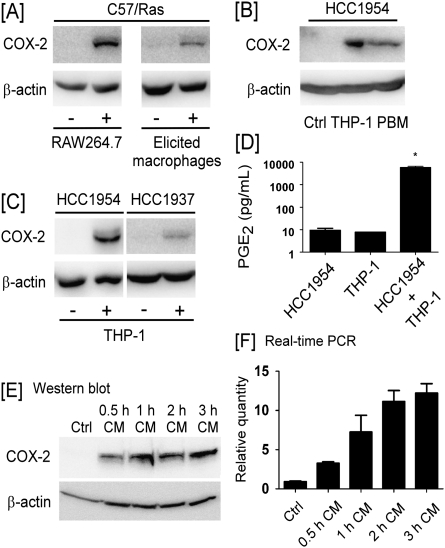
Evidence suggests that COX-2 plays a role in the formation and progression of breast cancer (8,9). To determine whether COX-2 expression in transformed mammary epithelial cells was regulated by macrophages, murine RAW264.7 macrophages or elicited peritoneal macrophages were cultured on porous tissue culture inserts and then placed into wells containing Ras-transformed mouse mammary epithelial cells (C57/Ras). As seen in Figure 1A, COX-2 expression was induced in C57/Ras cells after 24 h coculture with either RAW264.7 macrophages or elicited peritoneal macrophages. In a similar experiment, COX-2 expression was induced in human breast cancer cell line HCC1954 following coculture with either PMA-treated human THP-1 monocytes or PBM-derived macrophages (Figure 1B). Likewise, COX-2 expression was induced in human breast cancer cell line HCC1937, when cocultured with THP-1 cells (Figure 1C). Consistent with the induction of COX-2, the level of PGE2 in CM recovered from cocultured HCC1954 and THP-1 cells were significantly increased (Figure 1D).
Fig. 1.
Macrophages induce COX-2 expression in breast cancer cells. (A) C57/Ras cells were cocultured (24 h) with RAW264.7 macrophages or elicited peritoneal macrophages. (B) HCC1954 cells were cocultured (24 h) with PMA-treated THP-1 monocytes or IFNγ/lipopolysaccharide-activated PBMs. (C) HCC1954 or HCC1937 cells were cocultured (24 h) with THP-1 cells. Western blotting was utilized to determine levels of COX-2 and β-actin in cell lysates. (D) PGE2 levels in CM recovered from HCC1954 cells, THP-1 cells and cocultured cells were measured utilizing enzyme-linked immunosorbent assay. Data are mean ± standard deviation (n = 3; *P < 0.05). (E and F), CM from HCC1954 and THP-1 cells cocultured for 0.5–3 h were added to naive HCC1954 cells and incubated for 6 h. COX-2 expression was monitored utilizing (E) western blot analysis and (F) quantitative real-time–PCR. The real-time PCR values of COX-2 are normalized to β-actin, and the relative values are shown as mean ± standard deviation (n = 3).
In this coculture model, macrophages do not make direct contact with tumor cells. To demonstrate the presence of soluble factors that regulate COX-2 expression in tumor cells, naive HCC1954 cells were treated with CM recovered from HCC1954 and THP-1 cells cocultured for 0.5–3 h. As seen in Figure 1E and F, COX-2 protein and mRNA levels increased in naive HCC1954 cells incubated with coculture CM. The magnitude of CM-mediated induction of COX-2 in HCC1954 cells increased when cells were cocultured for longer periods of time (Figure 1E and F). Taken together, these data demonstrate that COX-2 was induced in breast cancer cells cocultured with macrophages.
ROS-dependent COX-2 induction in HCC1954 cells
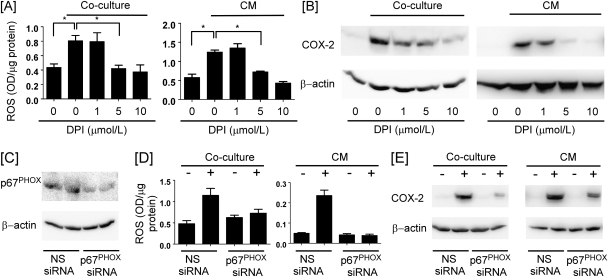
ROS-mediated signaling has been reported to regulate COX-2 gene expression in a variety of cell types (26–28). Therefore, we determined whether macrophage-induced COX-2 expression in breast cancer cells was dependent on ROS generation. For this purpose, ROS levels were examined in HCC1954 cells cocultured with THP-1 cells and naive HCC1954 cells treated with coculture CM. ROS levels increased in the breast cancer cells under either experimental setting (Figure 2A). Preincubation of HCC1954 cells with diphenyleneiodonium (DPI) DPI, a reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor, blocked the observed increase in ROS levels (Figure 2A) and COX-2 expression (Figure 2B). Preincubation (2 h) of HCC1954 breast cancer cells with 0–500 μM N-acetylcysteine led to a dose-dependent inhibition of COX-2 induction by coculture CM (data not shown). To further evaluate the role of NADPH oxidase, the p67PHOX regulatory subunit of NADPH oxidase was silenced-utilizing siRNA (Figure 2C). Silencing of p67PHOX blocked coculture and CM-mediated induction of ROS (Figure 2D) and COX-2 (Figure 2E). Thus, the induction of COX-2 in breast cancer cells cocultured with macrophages was dependent on ROS generation.
Fig. 2.
NADPH oxidase-generated ROS is required for COX-2 induction. (A) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of DPI for 1 h. ROS levels were measured in HCC1954 cells. Data are mean ± standard deviation (n = 3; *P < 0.05). (B) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of DPI for 6 h. The levels of COX-2 and β-actin in HCC1954 cell lysates were determined by western blot. (C) Levels of p67PHOX and β-actin in HCC1954 cells transfected with p67PHOX siRNA were determined by western blot. (D) ROS levels were measured in HCC1954 cells transfected with p67PHOX siRNA and cocultured with THP-1 cells or treated with 1 h coculture CM for 1 h. Data are mean ± standard deviation (n = 3). (E) Levels of COX-2 and β-actin in p67PHOX siRNA-transfected HCC1954 cells cocultured with THP-1 cells or treated with 1 h coculture CM for 6 h were determined by western blot.
ROS induces COX-2 by stimulating Src→MAPK→AP-1 pathway
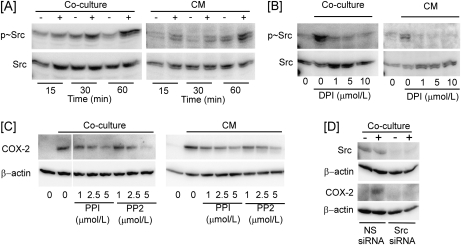
ROS initiate signaling cascades via the activation of Src kinase (29,30). Therefore, the phosphorylation of Src was examined in HCC1954 cells cocultured with THP-1 cells or treated with CM. Under either condition, Src phosphorylation increased rapidly and robustly (Figure 3A) and was blocked by preincubation with DPI (Figure 3B). Importantly, the Src kinase inhibitors PP1 and PP2 suppressed COX-2 induction in HCC1954 cells cocultured with THP-1 cells or incubated with coculture CM (Figure 3C). Similarly, silencing of Src with siRNA blocked COX-2 induction in HCC1954 cells cocultured with THP-1 cells (Figure 3D).
Fig. 3.
Macrophage induction of COX-2 in breast cancer cells is Src dependent. (A) Levels of phospho-Src (p∼Src) and Src in HCC1954 cells cocultured with THP-1 cells or treated with 1 h coculture CM were determined. (B) Levels of p∼Src and Src in HCC1954 cells cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of DPI for 1 h were determined. (C) Levels of COX-2 and β-actin in HCC1954 cells cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of PP1 and PP2 for 6 h were determined. (D) Levels of Src, COX-2 and β-actin in HCC1954 cells transfected with Src siRNA and cocultured with THP-1 cells for 6 h were determined.
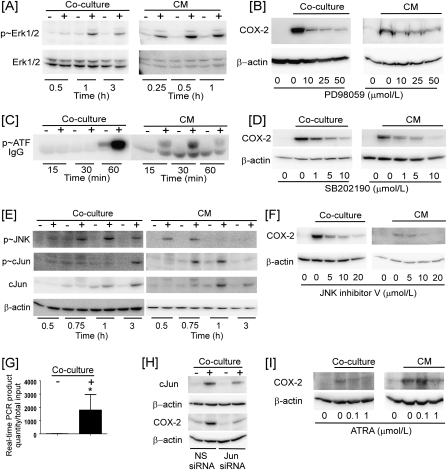
A variety of stimuli activate MAPK family members leading to COX-2 induction (24,31). Src mediates ROS-induced activation of MAPKs (32). Therefore, we determined whether macrophage-induced activation of Src in breast cancer cells triggered the activation of ERK, p38 and JNK. As seen in Figure 4A, levels of phospho-ERK in HCC1954 cells cocultured with THP-1 cells or incubated with coculture CM was strongly increased. The activation of ERK was blocked when HCC1954 cells were preincubated with PP1 or PP2 (data not shown). Furthermore, the induction of COX-2 observed in HCC1954 cocultured with THP-1 cells or incubated in coculture CM was markedly reduced when cells were preincubated with the MEK inhibitor PD98059 (Figure 4B).
Fig. 4.
Macrophage induction of COX-2 in breast cancer cells is dependent on MAPKs and cJun. (A) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM and levels of phospho-ERK (p∼ERK) and ERK in HCC1954 cell lysates were determined by western blot. (B) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h CM in the absence or presence of PD98059 for 6 h and levels of COX-2 and β-actin in HCC1954 cell lysates were determined. (C) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h CM. Cell lysates were then analyzed for p38 MAPK activity by measuring the phosphorylation of activating transcription factor (p∼ATF). (D) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of SB202190 for 6 h. (E) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM. Levels of phospho-JNK (p∼JNK), phospho-cJun (p∼cJun), cJun and β-actin in HCC1954 cell lysates were determined utilizing western blot. (F) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of JNK inhibitor for 6 h and levels of COX-2 and β-actin in HCC1954 cell lysates were determined. (G) HCC1954 cells were cocultured with THP-1 cells for 1 h. Chromatin fragments were immunoprecipitated with anti-p∼cJun, and the COX-2 promoter was amplified by real-time PCR. The COX-2 promoter was not detected when normal IgG was used (data not shown). Data are mean ± standard deviation (n = 7; *P < 0.05). (H) HCC1954 cells were transfected with siRNA-targeting cJun for 48 h and then cocultured with THP-1 cells for 6 h. Levels of cJun, COX-2 and β-actin in HCC1954 cell lysates were determined by western blot. (I) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of ATRA for 6 h, and levels of COX-2 and β-actin in HCC1954 cell lysates were determined.
Similarly, p38 activity was increased in HCC1954 cells cocultured with THP-1 cells or incubated in coculture CM (Figure 4C). Moreover, SB202190, a p38 MAPK inhibitor, suppressed both coculture and CM-mediated induction of COX-2 in breast cancer cells (Figure 4D). Coculture of HCC1954 and THP-1 cells or incubation with coculture CM also led to a rapid increase in JNK phosphorylation and increased amounts of cJun and phospho-cJun in HCC1954 cells (Figure 4E). Notably, coculture or CM-mediated induction of COX-2 in HCC1954 cells was suppressed by pretreatment with a JNK inhibitor (Figure 4F).
cJun is a component of the AP-1 transcription factor, which plays a central role in the regulation of COX-2 gene expression (24,33). Several experiments were carried out to determine whether cJun was important for macrophage-mediated induction of COX-2. First, ChIP assays demonstrated that coculture led to a rapid increase in the binding of phospho-cJun to the COX-2 promoter in HCC1954 cells (Figure 4G). Next, we demonstrated that the induction of COX-2 was markedly reduced in HCC1954 cells in which cJun expression was silenced. Finally, ATRA, an AP-1 antagonist, suppressed coculture or CM-mediated induction of COX-2 in HCC1954 cells (Figure 4I).
COX-2 induction is mediated by IL-1β
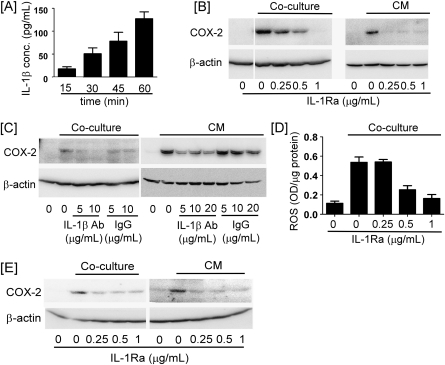
To identify the soluble mediator(s) that led to induction of COX-2 in coculture, proinflammatory cytokines, IL-1β and tumor necrosis factor-α were measured. Coculture led to a rapid and marked increase in IL-1β levels in CM (Figure 5A). In contrast, tumor necrosis factor-α levels did not increase significantly until 1 h (data not shown). Therefore, we evaluated whether macrophage-mediated induction of COX-2 was IL-1β-dependent. For this purpose, HCC1954 cells were pretreated with IL-1Ra or neutralizing anti-IL-1β IgG. Both IL-1Ra and anti-IL-1β blocked the induction of COX-2 in HCC1954 cells cocultured with THP-1 cells or incubated with coculture CM (Figure 5B and C).
Fig. 5.
IL-1β is responsible for the induction of COX-2. (A) HCC1954 and THP-1 cells were cocultured for the indicated time periods. Levels of IL-1β in CM were determined utilizing enzyme-linked immunosorbent assay. Data are mean ± standard deviation (n = 3). (B) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of IL-1Ra for 6 h, and levels of COX-2 and β-actin in HCC1954 cell lysate were determined by western blot. (C) HCC1954 cells were cocultured with THP-1 cells or treated with 1 h coculture CM in the absence or presence of IL-1β-neutralizing antibody (IL-1β Ab) or mouse IgG1 for 6 h, and levels of COX-2 and β-actin in HCC1954 cell lysates were determined. (D) HCC1954 cells were cocultured with THP-1 cells for 1 h in the absence or presence of IL-1Ra. ROS levels were measured in HCC1954 cells. Data are mean ± standard deviation (n = 3). (E) RT-4 cells were cocultured with THP-1 cells or incubated with 1 h coculture CM in the presence or absence of IL-1Ra, and levels of COX-2 and β-actin in RT-4 cell lysates were determined.
IL-1β can stimulate ROS production through the activation of NADPH oxidase complex (34,35). Since macrophage-induced expression of COX-2 in HCC1954 cells was dependent on both ROS and IL-1β (Figures 2 and 5), we determined the effect of IL-Ra on ROS levels in HCC1954 cells cocultured with THP-1 cells. As seen in Figure 5D, macrophages failed to induce ROS in breast cancer cells incubated with IL-Ra. These data indicate that IL-1β plays a causal role in macrophage induction of ROS and subsequently COX-2 expression in the breast cancer cells.
Recent studies of human urothelial cancers demonstrated a positive correlation between the degree of macrophage infiltration and the level of COX-2 expression in tumor cells (36). Given this finding, we determined whether macrophages induced COX-2 in RT-4 urothelial cancer cells. COX-2 expression was upregulated in RT-4 cells cocultured with THP-1 cells or incubated in cocultured CM (Figure 5E). Importantly, the induction of COX-2 in RT-4 cells was markedly attenuated by IL-1Ra. Thus, IL-1β plays an important role in mediating macrophage-dependent COX-2 induction in urothelial cancer cells in addition to breast cancer cells.
Autoamplification of IL-1β expression in macrophage breast cancer cell cocultures
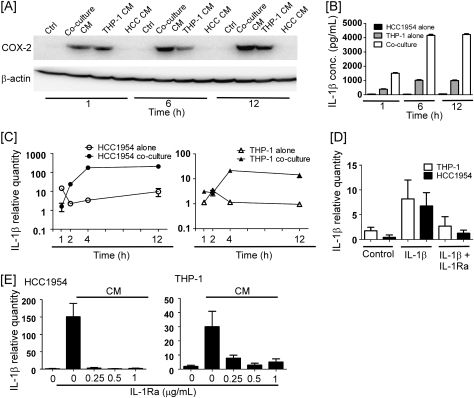
The ability of HCC1954/THP-1 coculture CM to induce COX-2 in naive HCC1954 cells increased as a function of duration of coculture and was greater than that observed utilizing CM derived from THP-1 cells alone (Figure 6A). Since COX-2 induction in HCC1954 cells was dependent on IL-1β (Figure 5B and C), we monitored IL-1β expression by THP-1 and HCC1954 cells over the 12 h experimental period. As seen in Figure 6B, HCC1954 cells secreted relatively small amounts of IL-1β. In CM recovered from THP-1 cells, IL-1β levels rose from ∼250 pg/ml at 1 h to ∼1000 pg/ml at 6 and 12 h. CM recovered from HCC1954/THP-1 cocultures at 1 h contained 1500 pg/ml IL-1β and >4000 pg/ml at 6 and 12 h. Thus, the level of IL-1β in cocultures was higher than found when HCC1954 or THP-1 cells were cultured alone, suggesting the importance of macrophage–tumor cell interactions for regulating IL-1β production.
Fig. 6.
Autoamplification led to increased production of IL-1β in macrophages cocultured with breast cancer cells. (A) HCC1954 and THP-1 cells were cultured alone or cocultured for the indicated time periods. CM collected at each time point were then used to treat naïve HCC1954 cells for 6 h. COX-2 and β-actin levels in HCC1954 lysates were determined by western blot. (B) HCC1954 and THP-1 cells were cultured alone or cocultured for the indicated time periods. IL-1β concentrations in CM were determined utilizing enzyme-linked immunosorbent assay. Data are mean ± standard deviation (n = 3). (C) HCC1954 and THP-1 cells were cultured alone or cocultured for the indicated time periods. (D) HCC1954 and THP-1 cells were treated with vehicle, IL-1β (10 ng/ml) or IL-1β and IL-1Ra (0.5 μg/ml) for 3 h. (E) Naive HCC1954 and THP-1 cells were treated with 1 h coculture CM in the absence or presence of the indicated concentrations of IL-1Ra for 3 h. In panels C–E, total RNA was isolated, and quantitative real-time PCR was performed. Levels of IL-1β mRNA were normalized to levels of β-actin mRNA. Data are mean ± standard deviation (n = 3).
When IL-1β message levels in HCC1954 cells were monitored utilizing real-time PCR, relative expression showed little change over time (Figure 6C). In contrast, there was a dramatic increase in amounts of IL-1β mRNA in HCC1954 cells cocultured with THP-1 cells. Likewise, the expression of IL-1β in THP-1 cells increased >10-fold when cocultured with HCC1954 cells. Thus, coculture stimulated IL-1β expression by both cell types.
To determine whether the observed increase in IL-1β expression resulted from autoamplification (37), the ability of exogenous IL-1β to induce IL-1β in HCC1954 and THP-1 cells was investigated. As shown in Figure 6D, exogenous IL-1β strongly induced IL-1β in both HCC1954 and THP-1 cells. This inductive effect of IL-1β was blocked by IL-1Ra. Furthermore, IL-1Ra blocked coculture CM-mediated induction of IL-β in both naive HCC1954 and THP-1 cells (Figure 6E). To corroborate and extend these observations, the ability of exogenous IL-1β to stimulate the production of IL-1β by MCF-7 and SK-BR-3 breast cancer cells was determined. Cells were treated with 10 ng/ml IL-1β for 3 h, medium removed, and fresh medium was added for another 3 h. Levels of IL-1β in media derived from control MCF-7 cells increased >20-fold following treatment with exogenous IL-1β (17 ± 3 versus 380 ± 52 pg IL-1β/μg cell protein; mean ± standard deviation; n = 6; P < 0.0001). Likewise, levels of IL-1β in media derived from SK-BR-3 cells increased >25-fold following treatment with IL-1β (29 ± 4 versus 759 ± 75 pg IL-1β/μg cell protein; mean ± standard deviation; n = 6; P < 0.0001). Collectively, these data suggest that IL-1β secreted by macrophages stimulates an autoamplification process, which results in accumulation of IL-1β and induction of COX-2 expression in breast cancer cells.
Discussion
Stromal–epithelial interactions are important in breast carcinogenesis. Both an increased number of TAMs and elevated COX-2 levels in tumor cells have been associated with poor prognosis for breast cancer patients (1,4,8,9). However, little is known about the potential of stromal cells to regulate the expression of COX-2 in tumor cells. Here, we showed that IL-1β secreted by macrophages, an important stromal component in many breast cancers, stimulated a ROS→Src→MAPK→AP-1 pathway in breast cancer cells leading to increased COX-2 levels. Several studies have yielded in vivo results, which underscore the relevance of these mechanistic studies. Levels of IL-1β are higher in invasive breast cancers than in ductal carcinoma in situ or benign lesions, and IL-1β content in breast cancer correlated with the degree of macrophage infiltration (38). IL-1 genetic polymorphisms have been associated with poor prognosis in breast cancer patients (39), and mammary tumor growth was inhibited in IL-1β knockout mice (40). Moreover, in a mouse model of mammary tumorigenesis, the activation of an inducible fibroblast growth factor receptor 1 (iFGFR1) transgene within epithelial cells resulted in induction of IL-1β and COX-2 in the mammary gland, recruitment of macrophages and the formation of hyperplastic-budding structures (41). Treatment of these mice with neutralizing antibody to IL-1β led to reduced levels of COX-2 in mammary epithelium, and a significant decrease in hyperplastic lesions present in the mammary glands (41). Similarly, treatment with a selective COX-2 inhibitor led to a decreased frequency of hyperplastic lesions. Collectively, these data demonstrate the potential of targeting inflammatory mediators as a therapeutic strategy.
Macrophage–epithelial interactions were suggested to be important in the iFGFR1-inducible model since activation of iFGFR1 in stably transfected HC-11 mammary epithelial cells (HC-11/R1) failed to induce IL-1β expression, whereas levels of IL-1β were significantly increased in the cocultures of HC-11/R1 cells and RAW264.7 macrophages (41). In monocultures, activation of iFGFR1 resulted in robust induction of COX-2 in HC11/R1 cells. In contrast, COX-2 was weakly induced by recombinant IL-1β and iFGFR1-induced COX-2 was unaffected by blocking anti-IL-β IgG. These data indicate that IL-1β and iFGFR1 induce COX-2 expression via different pathways in the HC-11/R1 cells (41).
In the current study, we present evidence that IL-1β secreted by macrophages stimulates an autoamplification loop, which results in enhanced expression of IL-1β in both macrophages and breast cancer cells. The importance of IL-1β was underscored by evidence that treatment with either an IL-1Ra or a neutralizing antibody to IL-1β blocked coculture and CM-mediated induction of COX-2 in tumor cells (Figure 5). The inductive effects of IL-1β were mediated by NADPH oxidase-dependent production of ROS. Several findings support this conclusion. Coculture or treatment of breast cancer cells with CM led to both increased ROS and COX-2 levels (Figure 2). Importantly, treatment with DPI or silencing of p67PHOX suppressed the induction of both ROS and COX-2 (Figure 2). Given the evidence that IL-1β induced ROS was causally linked to the induction of COX-2, we next attempted to elucidate the signal transduction pathway.
ROS generated by NADPH oxidase can activate Src kinase (42). This mechanism was important for IL-1β-mediated induction of COX-2, since DPI blocked both coculture and CM-mediated activation of Src and induction of COX-2. Moreover, silencing of Src or treatment with Src kinase inhibitors blocked the induction of COX-2 (Figure 3). Src can mediate ROS-dependent activation of MAPKs (32) and the activation of MAPKs plays a central role in regulating COX-2 transcription (31,43–45). Several lines of evidence suggest that IL-1β induced COX-2 via activation of ERK, p38 and JNK MAPKs. The activities of ERK, p38 and JNK were elevated in breast cancer cells following coculture or treatment with CM. Selective inhibitors of MAPK kinase, p38 and JNK suppressed the induction of COX-2. In addition, cJun, a component of the AP-1 transcription factor, plays a key role in IL-1β-mediated induction of COX-2. Western blotting showed a rapid increase in levels of cJun and phospho-cJun following coculture or treatment with CM (Figure 4E). Consistent with these findings, ChIP assays revealed a rapid increase in the binding of phospho-cJun to the COX-2 promoter (Figure 4G). Finally, silencing of cJun or treatment with ATRA, a prototypic AP-1 antagonist, blocked the induction of COX-2 (Figure 4H and I). Collectively, these findings are consistent with previous evidence that stimulation of MAPKs results in AP-1-dependent induction of COX-2 transcription (24,33,46).
Based on these intriguing findings, future studies are warranted to determine whether cross talk between macrophages and tumor cells amplifies the production of other molecules implicated in carcinogenesis. Notably, both IL-1β (47) and COX-derived PGE2 (48) were shown to mediate the accumulation of myeloid-derived suppressor cells in 4T1 mammary carcinoma, which inhibit immune surveillance and allow the proliferation of malignant cells. The results of the current study suggest that the reported immuosuppressive effects of IL-1β may be mediated, in part, by the induction of COX-2 in breast cancer cells and subsequent synthesis of PGE2. Additional studies are needed to test this possibility.
Macrophages are broadly classified as M1 or M2 depending on activating signals and ensuing functional activities (49,50). M1 macrophages express high levels of inflammatory cytokines (i.e. tumor necrosis factor-α and IL-1β), major histocompatibility complex class II molecules and ROS and reactive nitrogen intermediates. In contrast, the M2 phenotype is characterized by relatively low levels of inflammatory cytokines, ROS and reactive nitrogen intermediates and high levels of scavenger receptors, arginase, transforming growth factor-β and IL-10 (49,50). That said, the phenotype of TAMs is influenced by tumor type and stage and specific location in the tumor (51,52). Thus, it is probably that TAMs fall into subtypes between the M1 and M2 polarized phenotypes. In reference to the macrophages utilized in these coculture studies, PMA-treated THP-1 monocytes differentiate into macrophages with M2 functional properties (53), IFNγ and lipopolysaccharide activated peripheral blood-derived macrophages express the M1 phenotype (49) and based on major histocompatibility complex-class II expression and inflammatory cytokine expression, thioglycollate-elicited peritoneal macrophages are tilted toward the M1 phenotype (54,55). We have shown that all three macrophage populations stimulated COX-2 expression in species appropriate breast cancer cells. The observation that phenotypically diverse macrophages are able to induce COX-2 in breast cancer cells points to the robustness of this effect.
In summary, although increased numbers of TAMs and elevated COX-2 expression are associated with aggressive breast cancers, the role of macrophages in regulating COX-2 levels in breast cancer cells is not well understood. Here, we demonstrate that IL-1β secreted by macrophages stimulates an autoamplification loop, which results in enhanced expression of IL-1β by both macrophages and breast cancer cells. IL-1β triggers the activation of a ROS→Src→MAPK→AP-1 pathway in breast cancer cells leading to increased COX-2 levels. Finally, in so much that components of this signaling pathway have been identified as therapeutic targets in the treatment of breast cancer (56,57), our results suggest a rationale for targeting these mediators in breast cancers characterized by large numbers of macrophages.
Funding
National Institutes of Health (T32 CA062948-14 to Z.H., HL093331 to D.J.F.); Breast Cancer Research Foundation to A.J.D.; Botwinick Wolfensohn Foundation (in memory of Mr and Mrs Benjamin Botwinick) to A.J.D.
Acknowledgments
Conflict of Interest Statement: A.J.D. is a member of the Scientific Advisory Board of Tragara Pharmaceuticals.
Glossary
Abbreviations
- ATRA
all-trans-retinoic acid
- AP-1
activator protein-1
- ChIP
chromatin immunoprecipitation
- CM
conditioned media
- COX-2
cyclooxygenase-2
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- iFGFR1
inducible fibroblast growth factor receptor 1
- IL-1β
interleukin-1β
- IL-1Ra
interleukin-1 receptor antagonist
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- mRNA
messenger RNA
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- PCR
polymerase chain reaction
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- TAM
tumor-associated macrophage
References
- 1.Lewis CE, et al. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 3.Leek RD, et al. Tumor-associated macrophages in breast cancer. J. Mammary Gland Biol. Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 4.Leek RD, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 5.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemann T, et al. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 7.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 8.Singh-Ranger G, et al. The role of cyclooxygenase-2 in breast cancer: review. Breast Cancer Res.Treat. 2008;109:189–198. doi: 10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- 9.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatsugi S, et al. Chemoprevention by nimesulide, a selective cyclooxygenase-2 inhibitor, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary gland carcinogenesis in rats. Jpn. J. Cancer Res. 2000;91:886–892. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris RE, et al. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]
- 12.Howe LR, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–10119. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 13.Cotterchio M, et al. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2001;10:1213–1217. [PubMed] [Google Scholar]
- 14.Khuder SA, et al. Breast cancer and NSAID use: a meta-analysis. Br. J. Cancer. 2001;84:1188–1192. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazdar AF, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int. J. Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Soule HD, et al. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 17.Trempe GL. Human breast cancer in culture. Recent Results Cancer Res. 1976;57:33–41. doi: 10.1007/978-3-642-81043-5_5. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya S, et al. Establishment and characterization of a human acute leukemia cell line (THP-1) Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 19.Rigby CC, et al. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br. J. Cancer. 1970;24:746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raschke WC, et al. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 21.Telang NT, et al. Coordinated expression of intermediate biomarkers for tumorigenic transformation in RAS-transfected mouse mammary epithelial cells. Breast Cancer Res.Treat. 1991;18:155–163. doi: 10.1007/BF01990031. [DOI] [PubMed] [Google Scholar]
- 22.Muller WA, et al. Monocyte-selective transmigration: dissection of the binding and transmigration phases by and in vitro assay. J. Exp. Med. 1992;176:819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan KMF, et al. Extracellular matrix-induced cyclooxygenase-2 regulates macrophage proteinase expression. J. Biol. Chem. 2004;279:22039–22046. doi: 10.1074/jbc.M312735200. [DOI] [PubMed] [Google Scholar]
- 24.Subbaramaiah K, et al. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer - evidence for involvement of AP-1 and PEA3. J. Biol. Chem. 2002;277:18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 25.Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice Hall; 1984. [Google Scholar]
- 26.Kim EH, et al. 15-Deoxy-delta12,14-prostaglandin J2 induces COX-2 expression through Akt-driven AP-1 activation in human breast cancer cells: a potential role of ROS. Carcinogenesis. 2008;29:688–695. doi: 10.1093/carcin/bgm299. [DOI] [PubMed] [Google Scholar]
- 27.Grau R, et al. Inhibition of activator protein 1 activation, vascular endothelial growth factor, and cyclooxygenase-2 expression by 15-deoxy-Delta12,14-prostaglandin J2 in colon carcinoma cells: evidence for a redox-sensitive peroxisome proliferator-activated receptor-gamma-independent mechanism. Cancer Res. 2004;64:5162–5171. doi: 10.1158/0008-5472.CAN-04-0849. [DOI] [PubMed] [Google Scholar]
- 28.Chen JJ, et al. Transcriptional regulation of cyclooxygenase-2 in response to proteasome inhibitors involves reactive oxygen species-mediated signaling pathway and recruitment of CCAAT/enhancer-binding protein delta and CREB-binding protein. Mol. Biol. Cell. 2005;16:5579–5591. doi: 10.1091/mbc.E05-08-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosado JA, et al. Hydrogen peroxide generation induces pp60src activation in human platelets: evidence for the involvement of this pathway in store-mediated calcium entry. J. Biol. Chem. 2004;279:1665–1675. doi: 10.1074/jbc.M307963200. [DOI] [PubMed] [Google Scholar]
- 30.Giannoni E, et al. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuura H, et al. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells - Role of mitogen-activated protein kinases. J. Biol. Chem. 1999;274:29138–29148. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- 32.Mehdi MZ, et al. H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid. Redox Signal. 2005;7:1014–1020. doi: 10.1089/ars.2005.7.1014. [DOI] [PubMed] [Google Scholar]
- 33.Bachelor MA, et al. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin. Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Roy D, et al. Levels of IL-1 beta control stimulatory/inhibitory growth of cancer cells. Front. Biosci. 2006;11:889–898. doi: 10.2741/1845. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, et al. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen WT, et al. Overexpression of cyclooxygenase-2 in urothelial carcinoma in conjunction with tumor-associated-macrophage infiltration, hypoxia-inducible factor-1alpha expression, and tumor angiogenesis. APMIS. 2009;117:176–184. doi: 10.1111/j.1600-0463.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 37.Dinarello CA, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J. Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 38.Jin L, et al. Expression of interleukin-1beta in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(sici)1097-0142(19970801)80:3<421::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Grimm C, et al. The prognostic value of four interleukin-1 gene polymorphisms in Caucasian women with breast cancer: a multicenter study. BMC Cancer. 2009;9:78. doi: 10.1186/1471-2407-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voronov E, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl Acad. Sci. USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed JR, et al. Interleukin-1beta and fibroblast growth factor receptor 1 cooperate to induce cyclooxygenase-2 during early mammary tumourigenesis. Breast Cancer Res. 2009;11:R21. doi: 10.1186/bcr2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, et al. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem. J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subbaramaiah K, et al. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2-Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 2000;275:14838–14845. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 44.Subbaramaiah K, et al. Ceramide regulates the transcription of cyclooxygenase-2-evidence for involvement of extracellular signal-regulated kinase c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 1998;273:32943–32949. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]
- 45.Subbaramaiah K, et al. Regulation of cyclooxgenase-2 mRNA stability by taxanes - evidence for involvement of p38, MAPKAPK-2, and HuR. J. Biol. Chem. 2003;278:37637–37647. doi: 10.1074/jbc.M301481200. [DOI] [PubMed] [Google Scholar]
- 46.Subbaramaiah K, et al. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 2002;62:2522–2530. [PubMed] [Google Scholar]
- 47.Bunt SK, et al. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 48.Sinha P, et al. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 49.Mosser DM, et al. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez FO, et al. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 51.Sica A, et al. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Van Ginderachter JA, et al. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Tjiu JW, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J. Invest. Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 54.Marcinkiewicz J. In vitro cytokine release by activated murine peritoneal macrophages: role of prostaglandins in the differential regulation of tumor necrosis factor alpha, interleukin 1, and interleukin 6. Cytokine. 1991;3:327–332. doi: 10.1016/1043-4666(91)90501-4. [DOI] [PubMed] [Google Scholar]
- 55.Cook AD, et al. The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J. Immunol. 2003;171:4816–4823. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- 56.Arun B, et al. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin. Oncol. 2004;31:22–29. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Saad F, et al. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat. Rev. 2010;36:177–184. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]