Abstract
In an extension of our previous studies showing potent antitumorigenic activity of synthetic triterpenoids of oleanolic acid against prostate cancer cell lines, we examined the efficacy of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) in preventing the development and/or progression of prostate cancer in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Data show that oral gavage with CDDO (10 μmol/kg) for 20 weeks resulted in inhibition of the progression of preneoplastic lesions in the dorsolateral prostate and ventral prostate to adenocarcinoma without toxicity. CDDO also inhibited metastasis of tumor to the distant organs. Treatment with CDDO significantly inhibited cell proliferation, reduced the density of blood vessels and promoted apoptosis in the prostatic tissue. Further, Akt, NF-κB and NF-κB regulated Bcl-2, Bcl-xL, survivin and cIAP1 appear to be the molecular targets of CDDO for inhibiting the progression of prostate cancer in TRAMP mice. Thus, these studies show for the first time the potential of CDDO for chemoprevention of human prostate cancer.
Introduction
Carcinoma of the prostate (CaP) is the most commonly diagnosed cancer and the second leading cause of cancer-related mortality in men in the USA. Epidemiological studies have shown that besides race and age, high-fat diet and lifestyle are also prominent risk factors for prostate cancer (1). These population studies have also shown that the incidence of prostate cancer in Asian men is much lower than their counterparts in North America and other high-fat diet-consuming countries of the Western Hemisphere. Low incidence of prostate cancer among Asian men has been attributed to the consumption of low-fat diet and high intake of dark green vegetables, fruits and soy products (2). The cancer-preventing effects of plant-derived foods are due to the presence of polyphenolic phytochemicals with strong antioxidant and anti-inflammatory activity (3). Indeed, the activity of plant-derived polyphenolic compounds in preventing and/or slowing the progression of cancer has been investigated in animal models and human trials (4–8). Because of the long latency period and slow progression of the preneoplastic lesions to the malignant stage, CaP is ideally suited for primary, secondary and tertiary prevention strategies. Thus, intervention with plant-derived polyphenolic compounds or their synthetic analogs represents a promising approach to preventing/delaying the incidence, progression, recurrence, morbidity and mortality associated with cancer of the prostate.
Triterpenes or triterpenoids are members of a larger family of structurally related compounds known as cyclosqualenoids that are widely distributed in the plant kingdom (9). Oleanolic acid and ursolic acid are naturally occurring triterpenoids that have been used in traditional medicine as anticancer and anti-inflammatory agents (10–12). However, recent studies have shown that the synthetic derivatives of oleanolic acid such as 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) and its methyl ester (CDDO-Me) or imidazole (CDDO-Im) derivatives exhibit greater anti-inflammatory and antitumorigenic activity than oleanolic acid (13–15). Synthetic CDDOs have shown potent antiproliferative and antitumorigenic activity against diverse types of tumor cell lines, including those of leukemia, multiple myeloma, osteosarcoma, breast, brain, prostate and lung cancer in culture (16–20). Although the mechanisms of the anticancer effects of CDDOs are not fully understood, cancer cell differentiation, induction of caspase-dependent and caspase-independent apoptosis and modulation of mitogen-activated protein kinase (extracellular signal-regulated kinase 1/2), nuclear factor-kappaB (NF-κB), transforming growth factor beta/Smad and peroxisome proliferator-activated receptor gamma signaling pathways contribute to the antitumor activity of CDDOs (19,21–25). CDDOs have also shown chemopreventive activity in animal models of liver, breast and lung cancer (26–28) but they have not been investigated for the prevention of prostate cancer. We have shown previously that CDDOs inhibit the growth of hormone-sensitive and hormone-refractory human prostate cancer cell lines in vitro and in vivo by inducing apoptosis (17,29). Furthermore, induction of apoptosis was associated with the participation of reactive oxygen species and inhibition of prosurvival Akt, NF-κB and mammalian target of rapamycin signaling. In the present study, we investigated the effect of CDDO on the development and progression of CaP and cellular and molecular targets of tumorigenesis in the transgenic adenocarinoma of the mouse prostate (TRAMP) mouse model of prostate cancer. Results showed that long-term treatment with CDDO inhibits the progression of preneoplastic lesions to adenocarcinoma and tumor metastasis to the distant organs. The tumor inhibitory effect of CDDO was associated with the inhibition of cell proliferation and angiogenesis and promotion of apoptosis. In addition, CDDO inhibited p-Akt, NF-κB and NF-κB-regulated gene products associated with apoptosis.
Materials and methods
Reagents
CDDO was obtained from the National Cancer Institute, Bethesda, MD, through the Rapid Access to Intervention Development Program. Antibodies against p-Akt (ser473), NF-κB (p65), Bcl-2, Bcl-xL, p-Bad, survivin, c-IAP-1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Ki67 antibody was from Vector Laboratories (Burlingame, CA) and anti-CD31 antibody was purchased from Abcam (Cambridge, MA). The In Situ Apoptosis Detection kit was obtained from Chemicon Internatioinal (Billerica, MA).
Mice
TRAMP mice were bred by the Jackson Laboratories (Bar Harbor, ME) through their Speed Expansion Service involving in vitro fertilization of C57BL/6 females with C57BL/6-Tg (TRAMP) 8247NG/J males. Mice were genotyped for the transgene (Tag) and delivered to us when 4 weeks old. Mice were maintained in temperature-controlled room (68–72°F) with a 12 h light/dark cycle and provided semi-purified AIN-76A mouse chow and water ad libitum. Mice were acclimated for 1 week before starting the experiment. All animal treatments were according to the protocol approved by the Institutional Animal Care and Use Committee.
Treatment protocol
At the age of 5 weeks, 100 male TRAMP mice were weighed and randomized into two groups. Mice in vehicle control group (n = 50) were administered 0.1 ml of vehicle consisting of cremophor EL:dimethyl sulfoxide:phosphate-buffered saline (1:1:8), 5 days a week for 7 or 20 weeks by oral gavage. In the CDDO treatment group, mice were administered CDDO at a dose of 10 μmol/kg in 0.1 ml of vehicle, 5 days a week for 7 weeks (n = 25) or 20 weeks (n = 25). Body weight of control and CDDO-treated mice was recorded each week and mice were observed for treatment-related stress, such as water and food withdrawal, unusual posture, ruffled fur or listlessness. At the age of 12 and 25 weeks, mice from both groups were killed 24 h after the last administration of vehicle or CDDO. After opening the abdomen, mice were visually observed for the presence of tumor mass and enlargement of seminal vesicles, prostate lobes and pelvic lymph nodes. Urogenital track, vital organs (liver, lung, kidney and small intestine) and pelvic lymph nodes were harvested. Tissue samples were processed for histological, immunohistochemical or biochemical analyses.
Pathological grading and detection of metastases
The pathologic grading of prostate cancer was according to the grading system for TRAMP described by Greenberg et al. (30,31). Following this grading system, prostate lesions in the dorsolateral prostate (DLP) and ventral prostate (VP) lobes were histologically graded as normal (ducts lined with single layer of secretory epithelial cells surrounded by two to three cell layers of fibromuscular stroma), low-grade prostatic intraepithelial neoplasia (PIN) (epithelial cells with variably elongated nuclei with condensed chromatin), high-grade PIN (epithelial stratification and tufting, presence of micropapillary and cribiform structures), well-differentiated (WD) carcinoma (epithelial cells invading fibromuscular stroma) and moderately differentiated (MD) to poorly differentiated (PD) adenocarcinoma of the prostate (sheets of neoplastic cells with little or no glandular structures). Ten randomly selected microscopic fields on hematoxylin and eosin (H&E)-stained sections of the DLP and VP were scored for the incidence and the pathological grade of the prostate cancer in control and CDDO-treated TRAMP mice. For the incidence of metastasis (percentage of mice with metastatic lesions), H&E-stained sections of liver, lung, kidney and pelvic lymph nodes were evaluated microscopically.
Western blotting
DLP tissue lysates were prepared by grinding tissue in lysis buffer [1% Triton X-100 (vol/vol), 10 mM Tris–HCl (pH 7.5), 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 10% glycerol, 2 mM sodium vanadate, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatinin and 10 μg/ml 4-2-aminoethyl-benzenesulfinyl fluoride]. Lysates were clarified by centrifugation at 14 000g for 10 min at 4°C, and protein concentrations were determined by Bradford assay. Samples (50 μg) were boiled in an equal volume of sample buffer [20% glycerol, 4% sodium dodecyl sulfate, 0.2% bromophenol blue, 125 mM Tris–HCl (pH 7.5) and 640 mM 2-mercaptoethanol] and separated on precasted Tris–glycine polyacrylamide gels using the XCell SurelockTM Mini-Cell in Tris–glycine sodium dodecyl sulfate running buffer, all from Novex (Invitrogen, Carlsbad, CA). Proteins resolved on the gels were transferred to nitrocellulose membranes. Membranes were blocked with 5% milk in 10 mM Tris–HCl (pH 8.0), 150 mM NaCl with 0.05% Tween 20 and probed using protein specific antibodies to p-Akt (ser473), Akt, NF-κB (p65), Bcl-2, Bcl-xL, survivin, c-IAP-1, p-Bad or β-actin (loading control) and horseradish peroxidase-conjugated secondary antibody. Immune complexes were visualized with enhanced chemiluminescence using the ECL Detection Kit (Amersham Biosciences). Protein bands were imaged and band densities analyzed using the National Institutes of Health/Scion image analysis software. The protein band densities were normalized to the corresponding β-actin band densities and percent change in signal strength was calculated by the formula: density of experimental protein band ÷ density of control protein bans × 100.
Immunohistochemical analysis
Tissue sections were deparaffinized, rehydrated and incubated in 0.3% H2 O2 for 5 min to block endogenous peroxidase activity. For antigen retrieval, sections were boiled in 10 mM sodium citrate buffer (pH 6.0) for 15 min followed by cooling at room temperature for 20 min. Sections were blocked in 1% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature and then incubated with anti-Ki-67 (1:200) or anti-CD31 (1:50) or anti T-antigen (1:200) primary antibodies overnight. Sections were then sequentially treated with biotinylated second antibody (1:200) and Elite ABC peroxidase complex for 30 min each at room temperature. The brown color was developed by incubating sections with 3,3-diaminobenzidine substrate followed by counterstaining with Harris hematoxylin. Sections were examined under Leica microscope for nuclear staining (Ki-67 and Tag staining) and staining of microvessels (CD31 staining) in randomly selected five microscopic fields at ×400 magnification.
TUNEL staining of apoptotic cells
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) of apoptotic cells was performed using the ApopTag Plus Peroxidase In Situ Apoptosis kit from Chemicon International (Billerica, MA). Briefly, deparaffinized and hydrated tissue sections were subjected to TUNEL staining following the manufacturer’s protocol. Sections were counterstained with methyl green and apoptosis was quantified by counting the number of TUNEL-positive cells in randomly selected five non-overlapping microscopic fields at ×400 magnification.
Histology
Tissue specimens of prostate gland, liver, lung, kidney, small intestine and pelvic lymph nodes were fixed in 10% neutral-buffered formalin for 48 h and then embedded in paraffin. Five micrometer-thick sections were cut and stained with H&E for routine histology, TUNEL assay and immunohistological analyses. Histological interpretations of tumor grade and the extent and character of the inflammatory infiltrate, cell necrosis and vascular thrombosis in liver, lung, kidney, small intestine and tumor metastasis were made in a blinded fashion.
Statistical analysis
Most outcomes for treated and untreated mice were compared by t-test. However, Wilcoxon rank-sum tests were used for histological grade which was scored from 1 (normal) to 6 (PD) as described above.
Results
Treatment with CDDO is well tolerated
Treatment with CDDO for 20 weeks was well tolerated without the evidence of noticeable toxicity with respect to animal appearance, behavior or change in the body weight. Body weight increased as a function of age at the same rate in both the vehicle control and the treatment group from 20 ± 0.8 g (control group) and 20.5 ± 0.9 g (treatment group) at 5 weeks of age to 32.7 ± 1.5 and 31.9 ± 1.9 g, respectively, at 25 weeks of age (supplementary Figure 1A is available at Carcinogenesis Online). In addition, microscopic examination of tissue sections of liver, kidney, lung and small intestine showed no discernable histopathological changes, such as the presence of mononuclear cell infiltration, cell necrosis, cellular aplasia, fibrosis etc. in any of these organs in mice treated with CDDO for 20 weeks (supplementary Figure 1B is available at Carcinogenesis Online). Thus, long-term oral treatment with CDDO is safe without any evidence of organ toxicity (e.g. liver, lung, kidney or small intestine).
CDDO slows down the progression of prostate carcinoma in TRAMP mice
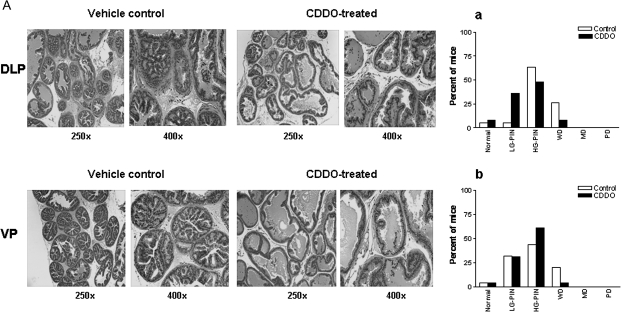
Whether treatment with CDDO interferes with the development and/or progression of CaP, TRAMP mice were orally treated with CDDO for 7 or 20 weeks starting at the age of 5 weeks. After treatment with vehicle or CDDO for 7 week, visual examination of the abdominal cavity did not reveal unusual enlargement of the seminal vesicles, prostatic lobes or pelvic lymph nodes in control or CDDO-treated mice. Microscopic evaluation of the DLP showed normal histology in one control (1/19, 5%) and two (2/25, 8%) CDDO-treated animals. Majority of the control animals showed high-grade PIN (65%) and 25% of the animals were graded as having WD CaP (Figure 1A, a). The distribution of lesions in CDDO-treated animals was 36% low-grade PIN, 48% high-grade PIN and 8% WD. None of the animals exhibited MD or PD CaP in the DLP in either group (Figure 1A, a). In the VP, the distribution of pathological lesions in control and CDDO-treated mice was as follows: low-grade PIN, 31 and 32%; high-grade-PIN, 44 and 61% and WD, 20 and 4%. None of the animals exhibited MD or PD CaP in VP in either group (Figure 1A, b). Overall, there was a significant shift toward more normal/non-cancerous pathological grade for DLP (P < 0.01); however, shift toward non-cancerous pathological grade in VP did not reach statistical significance (P > 0.05).
Fig. 1.
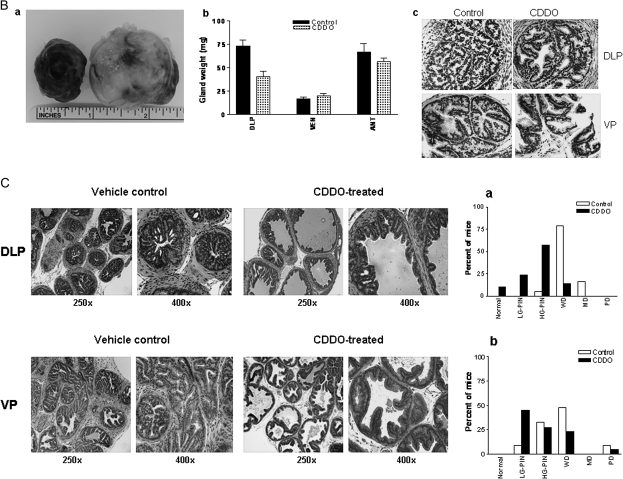
(A) H&E-stained sections of DLP and VP of a 12-week-old vehicle control or CDDO-treated TRAMP mouse. Control DLP and CDDO-treated DLP showing high-grade PIN and low-grade PIN lesions, respectively. Control VP and CDDO-treated VP showing WD carcinoma and low-grade PIN, respectively. Bar graphs (a and b) show distribution of histological grades (normal, low-grade PIN, high-grade PIN, WD, MD or PD carcinoma) in DLP (control, n = 19; CDDO treated, n = 25) and VP (control, n = 25; CDDO treated, n = 25). LG-PIN, low-grade PIN; HG-PIN, high-grade PIN. (B) (a) Large-sized tumors originating in two control mice; (b) bar graph showing weight of DLP, VP and anterior prostatic lobes of control and CDDO-treated mice (20 week); (c) expression of T-antigen in DLP and VP of control and CDDO-treated mice. *Significantly different (P < 0.05) compared with control mice. (C) H&E-stained sections of DLP and VP of a 25-week-old vehicle control or CDDO-treated TRAMP mouse. Control DLP and CDDO-treated DLP showing WD carcinoma and low-grade PIN lesions, respectively. Control VP and CDDO-treated VP showing WD and low-grade PIN, respectively. Bar graphs (a and b) show distribution of histological grades (normal, low-grade PIN, high-grade PIN, WD, MD or PD carcinoma) in DLP (control, n = 19; CDDO treated, n = 21) and VP (control, n = 21; CDDO-treated, n = 22). LG-PIN, low-grade PIN; HG-PIN, high-grade PIN.
In mice treated with CDDO for 20 weeks, two control mice (2/19, 11%) showed very large tumors (Figure 1B, a). None of the CDDO-treated mice had large-sized tumors. Tumor growth in seminal vesicles was present in 74% of control mice as opposed to 33% of CDDO-treated mice. Further, there was a significant decrease in the weight of DLP (P < 0.01) but not in the weight of ventral or anterior prostate lobes (P > 0.05) of treated mice compared with the control mice (Figure 1B, b). In addition, T-antigen expression in the DLP and VP of control and CDDO-treated (20 weeks) animals was comparable (Figure 1B, c).
Histological evaluation of the DLP of control mice showed WD adenocarcinoma in 79% of mice, 16% had MD and 1 mouse exhibited high-grade PIN (Figure 1C, a). These data indicated that 95% of the control mice had cancerous lesions in the DLP. In contrast, 9% of CDDO-treated mice had normal DLP, 14% showed WD CaP, 24% had low-grade PIN and lesions in 52% of mice were high-grade PIN. These data demonstrated that majority of the treated animals (86%) had non-cancerous lesions ranging from normal tissue to low-grade PIN and high-grade PIN compared with 95% of the control mice that showed CaP. CDDO also inhibited the progression of cancer in VP. As can be seen in Figure 1C (b), 42% of control mice had preneoplastic lesions and 58% had WD to PD adenocarcinoma. Among the CDDO-treated animals, 72% had preneoplastic lesions (45% low-grade PIN and 27% high-grade PIN); WD carcinoma was present in 23% of mice and 5% had PD. Overall, for both DLP and VP, CDDO treatment was associated with a shifting in pathological grade distribution toward normal/non-cancerous lesions (P < 0.01 and P < 0.001, respectively). Taken together, these data show that although CDDO did not prevent the development of preneoplastic lesions in majority of the animals, but it significantly inhibited their progression to CaP.
CDDO decreases metastasis of prostate cancer
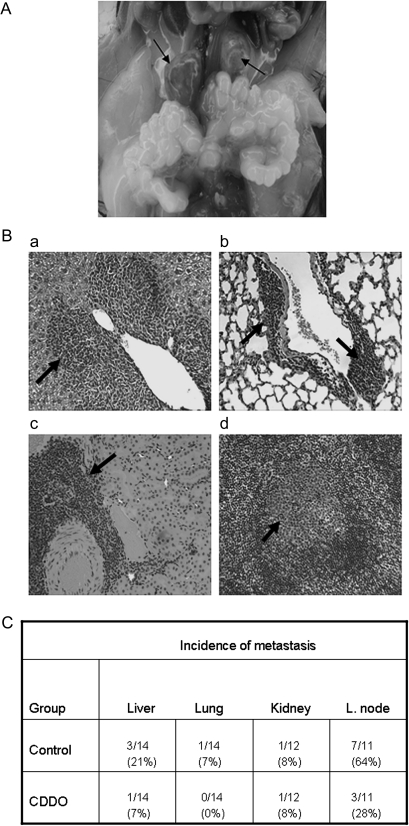
At necropsy, one control mouse was found to have enormously enlarged pelvic lymph nodes (Figure 2A) and other mice also showed enlargement of these lymph nodes compared with treated mice. No other organ in either group showed visible lesions. H&E-stained sections of lung, liver, kidney and pelvic lymph nodes were examined for the presence of microscopic metastases in these organs. Figure 2B shows the histological appearance of metastatic lesions in the liver (a), lung (b), kidney (c) and pelvic lymph node (d) in control mice. As can be seen in Figure 2C, three control mice (3/14) and one CDDO-treated mouse (1/14) showed liver metastasis; one control mouse (1/14) and none of the treated mice (0/14) had lung metastasis. One mouse in each group had a solitary lesion in the kidney (1/12). However, the most noticeable anti-metastatic effect of CDDO was observed on the spread of cancer to the pelvic lymph nodes. Seven of the 11 control mice (63%) that were examined showed multiple metastatic foci in the lymph nodes, whereas only 3 of the 11 (27%) treated mice had metastatic lesions at reduced multiplicity compared with the control mice. Overall, these data indicated that CDDO inhibits metastasis of prostate cancer to distant organs in TRAMP mice.
Fig. 2.
Effect of CDDO on metastasis of prostate cancer. (A) A 25-week-old control TRAMP mouse showing highly enlarged pelvic lymph nodes because of tumor metastasis. (B) H&E-stained sections of liver (a), lung (b), kidney (c) and pelvic lymph node (d) of 25-week-old control TRAMP mice showing metastatic lesions (×250). (C) Shows the incidence of metastasis in different organs in control and CDDO-treated 25-week-old TRAMP mice.
Effect of CDDO on cellular processes associated with tumorigenesis
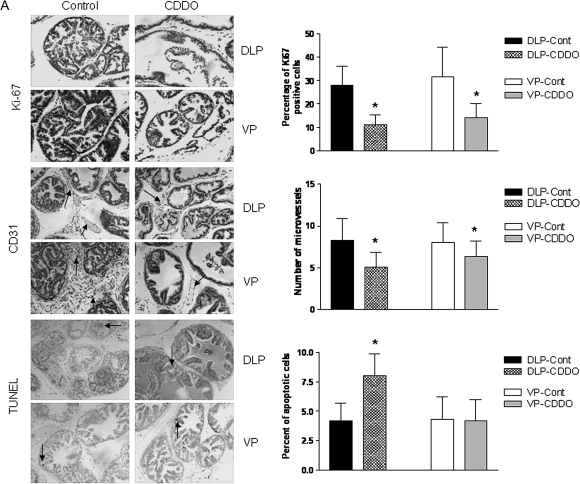
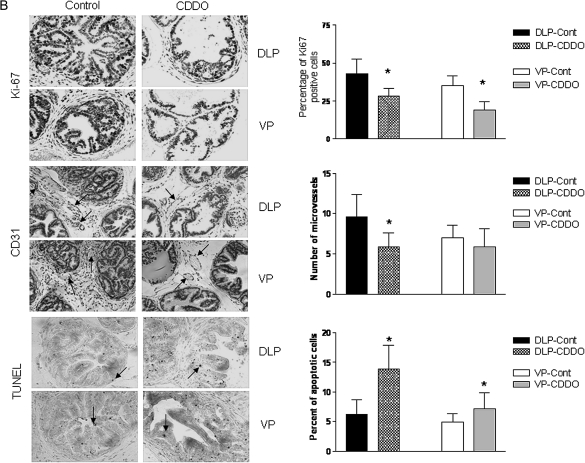
The ability of an agent to modulate cellular processes such as cell proliferation, apoptosis and angiogenesis determines in part its ability to prevent tumor development, progression and metastasis. We determined the effect of CDDO on cell proliferation and angiogenesis by immunohistochemistry (Ki-67 and CD31 staining, respectively) and on apoptosis by TUNEL in the DLP and VP of TRAMP mice killed after treatment with CDDO for 7 or 20 weeks (n = 7). As can be seen in Figure 3A, CDDO significantly reduced the number of cells stained with the proliferation marker Ki-67 both in the DLP and VP of animals killed after 7 weeks (P < 0.05). The number of blood vessels (CD31 staining) was also significantly reduced in both prostate lobes in CDDO-treated mice (P < 0.05). On the other hand, DLP but not VP showed significant increase in apoptotic cells following treatment with CDDO. Mice killed after 20 weeks also showed significant reduction in Ki-67-stained cells in both prostatic lobes (P < 0.05), but decrease was less dramatic compared with 7 weeks treatment group (Figure 3B). The number of blood vessels was significantly reduced in the DLP (P < 0.05), but reduction in blood vessels in the VP was insignificant. Both DLP and VP had significantly increased apoptotic cells in CDDO-treated animals compared with control mice (P < 0.05). Together, these results indicated that inhibition of cell proliferation and angiogenesis and induction of apoptosis contribute to the antitumorigenic activity of CDDO in TRAMP mice.
Fig. 3.
Analysis of proliferating cells, blood vessels and TUNEL-positive cells in DLP and VP after treatment with CDDO for 7 week (A) and 20 week (B). Proliferating cells were detected by staining with anti-Ki67 antibody; blood vessels (arrows) by staining endothelial cells lining the microvessels with anti-CD31 and apoptotic cells (arrows) were assessed by TUNEL assay. All IHC photographs ×250 magnification. Columns are means ± SDs (n = 7). *Significantly different (P < 0.05) compared with control mice. IHC, immunohistochemistry.
CDDO reduces p-Akt, NF-κB (P65) and NF-κB-regulated proteins in DLP
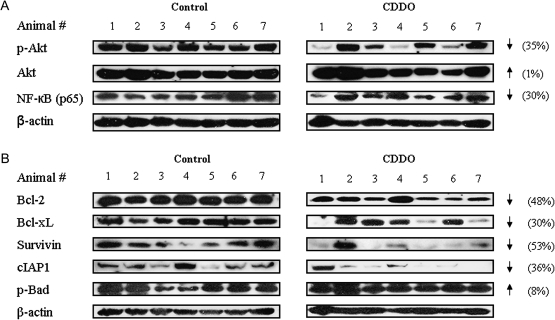
Akt and NF-κB are major antiapoptotic pathways that confer survival advantage and resistance to anticancer therapies in cancer cells. We have previously reported that triterpenoids inhibit Akt and NF-κB in TRAMP prostate cancer cell line in culture (32). Here, we investigated whether the expression of p-Akt and NF-κB is altered in the DLP of mice treated with CDDO for 20 weeks. Figure 4A shows the levels of p-Akt and NF-κB in the DLP of seven control mice and seven CDDO-treated mice. Blot clearly shows complete inhibition of p-Akt in three mice and reduction in other two of seven mice treated with CDDO compared with control mice. Overall, there was 35% reduction in p-Akt in the DLP of treated animals. The level of basal Akt was not affected by CDDO. NF-κB was also reduced in most of the CDDO-treated mice compared with control mice (30% reduction).
Fig. 4.
Effect of CDDO on p-Akt, NF-κB (p65) and NF-κB-regulated proteins in DLP. DLP lysates prepared from control and CDDO-treated mice (20 week) were fractionated on polyacrylamide gel and p-Akt, Akt, NF-κB (p65) (A) and NF-κB-regulated Bcl-2, Bcl-xL, survivin, cIAP1 and p-Bad (B) were detected by immunoblotting. Uniformity of sample loading was determined by anti-β antibody. Protein band densities were normalized to their β-actin band densities and percent change in signal strength was determined as described in Materials and Methods.
Next, we investigated whether reduction in NF-κB impacts the expression of NF-κB-regulated antiapoptotic Bcl-2, Bcl-xL, survivin, cIAP1 or p-Bad in DLP of CDDO-treated mice. As can be seen in Figure 4B, the level of Bcl-2, Bcl-xL, survivin and cIAP1 was reduced in the DLP of treated mice (48, 30, 53 and 36% reduction, respectively) without much change in p-Bad. Together, these data indicated that modulation of prosurvival Akt and NF-κB and NF-κB-regulated apoptosis-related proteins in the DLP potentially plays a role in inhibition of the progression of prostate cancer in TRAMP mice by CDDO.
Discussion
The present study builds on our earlier findings showing potent antitumorigenic activity of CDDOs against human and mouse prostate cancer cell lines in vitro and tumor xenografts in vivo (17,29,32). Oleanane-derived synthetic triterpenoids have been shown by others to have chemopreventive activity in animal models of lung, liver and breast cancer (26–28); however, chemoprotection against prostate cancer with CDDOs has not been reported. Long-term treatment with CDDO was tolerated well without toxic side effects, a prerequisite for considering new agents for human trial. Short-term (7 weeks) treatment showed weak but measurable suppressive effect on the development of prostate cancer in TRAMP mice. On the other hand, long-term treatment with CDDO for 20 weeks showed potent activity in inhibiting the progression of preneoplastic lesions in DLP and VP to invasive adenocarcinoma. Overall, treatment with CDDO for 20 weeks was associated with a significant shift in pathological grade distribution toward normal/non-cancerous lesions compared with the predominance of cancerous lesions in control animals. Thus, although CDDO did not prevent the development of preneoplastic lesions in majority of the animals, it significantly prevented their progression to the cancerous lesions. The inhibitory effect of CDDO was not due to the inhibition of T antigen (Tag) expression that drives the proliferation of prostatic epithelial cells through the inhibition of tumor suppressor gene p53. These findings are in line with the previously reported chemopreventive effects of CDDO analogs in aflatoxin-induced hepatic carcinogenesis and vinyl carbamate-induced lung cancer (26,28) and of herbal products such as sulforaphane, green tea polyphenols, garlic constituents and genistein on prostate tumorigenesis in TRAMP mice (4,6,33,34).
Tumor metastasis is the major cause of death in most cancers including cancer of the prostate (35). Although we did not explore the mechanisms of the anti-metastatic activity of CDDO in this study, metastatic lesions were detected in the lung, liver, kidney and pelvic lymph nodes. CDDO reduced the incidence of metastasis in the liver, lung and pelvic lymph nodes with the latter showing the most reduction. In TRAMP mice, prostate cancer initially metastasizes predominantly to the lung and lymph nodes between 18 and 24 weeks of age and to the bone after 30 weeks of age or later (36). In prostate cancer patients, however, bone is the most common site of the metastatic disease. Since the objective of the current study was chemoprevention of prostate cancer with CDDO, experiment was terminated at 25 weeks age, a time point when there is little evidence of metastasis of tumor to the bone in TRAMP mice. For CDDO to be relevant for prevention of metastatic spread of prostate cancer to the bone in humans, it will be necessary to first establish the ability of CDDO to prevent metastasis of prostate cancer to the bone in TRAMP mice.
Our previous in vitro studies showed that CDDOs potently inhibit cell proliferation and induce apoptosis in prostate cancer cell lines including TRAMP-C1 cell line derived from a tumor in TRAMP mouse (32). Since cell proliferation, angiogenesis and apoptosis play vital roles in the development and progression of cancers, we investigated whether modulation of these cellular processes is part of the mechanism by which CDDO inhibits the progression of prostate cancer in TRAMP mice? CDDO significantly reduced cell proliferation in prostate lobes as evidenced by decreased labeling of cells with anti-Ki-67 antibody. Treatment with CDDO also increased TUNEL-positive apoptotic cells and reduced the number of blood vessels in the DLP compared with control mice. These data suggest that inhibition of cell proliferation and angiogenesis and increase in apoptosis play an important role in CDDO-mediated inhibition of prostate tumorigenesis in TRAMP mice.
Akt and NF-κB signaling pathways play a central role in cell survival, proliferation and apoptosis. Activated p-Akt promotes cell survival by inactivating downstream substrates such as p-Bad, procaspase-9 and Forkhead transcription factors (37,38). Antiapoptotic NF-κB controls the expression of genes involved in cellular processes, such as inflammation, proliferation, oncogenesis, angiogenesis and apoptosis (39). Both of these signaling proteins are constitutively active in the DLP of the TRAMP mice and were shown to be the targets of tumor inhibition by natural polyphenolic compounds (40). We investigated whether CDDO inhibits p-Akt and NF-κB expression in the prostate of TRAMP mice. Indeed, treatment with CDDO significantly reduced the levels of both p-Akt and NF-κB (p65) in DLP in majority of the animals compared with control mice. Furthermore, CDDO also reduced the levels of NF-κB-regulated antiapoptotic proteins such as BCl-2, Bcl-xL, survivin and cIAP1, suggesting that downregulation of prosurvial Akt and NF-κB and NF-κB-regulated antiapoptotic gene products plays a role in inhibition of prostate tumorigenesis by CDDO in TRAMP mice. We have shown previously the relevance of some of these signaling proteins in mediating the growth inhibitory activity of CDDO-Me in human prostate cancer cell line PC-3 (29). Knocking down Akt or mammalian target of rapamycin with small interfering RNAs sensitized PC-3 cells, whereas overexpression of Akt rendered them resistant to CDDO-Me. These observations allow us to speculate that Akt, NF-κB and NF-κB-regulated Bcl-2 family proteins are relevant targets of CDDO for inhibition of prostate cancer progression in TRAMP mice. It should be noted, however, that CDDO-Me is a C-28 methyl ester derivative of CDDO. Whether this modification affects the target specificity of CDDO has not been determined. Thus, more studies are needed to directly prove the relevance of these molecules in mediating the antitumorigenic effect of CDDO in TRAMP mice.
Despite its appeal as a potential agent for preventing prostate tumorigenesis as shown in this study, comparison of the chemopreventive efficacy of CDDO and its analogs (CDDO-Me and CDDO-Im) deserves consideration. Several studies including our own on prostate cancer cell lines (32) have shown that the in vitro antitumor activity of CDDO is slightly weaker than that of CDDO-Me or CDDO-Im. Single-agent chemoprevention studies with CCDO-Im and CDDO-Me in the models of hepatic and lung carcinogenesis did not compare their efficacy with CDDO. Thus, although a direct comparison of the in vivo activity of three CDDO analogs remains to be made based on our studies with TRAMP-C1 cells in vitro, we speculate that long-term treatment with CDDO will be as effective as its analogs in preventing prostate tumorigenesis. In addition, structural differences between the human prostate and mouse prostate gland may also impact consideration of CDDO for the prevention or management of human prostate cancer. In TRAMP mice, prostate cancer originates both in the DLP and the VP because of Tag expression that is largely restricted to these prostatic lobes. In contrast, in human prostate which is a single glandular organ divided into poorly demarcated zones, cancer originates in the peripheral zone that is analogous to DLP in mice. Despite of these differences in the structural organization of prostate gland in two species, our findings that CDDO inhibits the progression of preneoplastic lesions both in DLP and VP and prevents tumor metastasis are relevant to managing the human prostate cancer with CDDO. On the contrary, the inability of CDDO to prevent tumor progression in DLP would have seriously questioned the significance of these findings for the prevention of human disease. In fact, inhibition of tumor progression in VP suggests that CDDO has the potential to inhibit both primary as well as extracapsular tumor growth of human prostate cancer.
In conclusion, we have shown the safety and efficacy of CDDO in inhibiting the progression of prostate cancer and its metastasis to the distant sites in TRAMP mice without affecting T-antigen expression. The inhibition of tumor progression was due, at least in part, to reduction in cell proliferation number of blood vessels and increase in apoptosis in the tumor tissue. Furthermore, antiapoptotic Akt and NF-κB and NF-κB-regulated Bcl-2, Bcl-xL, survivin and cIAP1 appear to be the potential molecular targets of CDDO for mediating antitumor activity in the TRAMP model.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/.
Funding
National Institutes of Health grants (CA130948 to S.C.G., CA122031 to A.S.A.).
Supplementary Material
Glossary
Abbreviations
- CaP
carcinoma of the prostate
- CDDO
2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid
- CDDO-Im
CDDO-imidazole
- CDDO-Me
CDDO-methyl ester
- DLP
dorsolateral prostate
- H&E
hematoxylin and eosin
- MD
moderately differentiated
- NF-κB
nuclear factor-kappaB
- PD
poorly differentiated
- PIN
prostatic intraepithelial neoplasia
- TRAMP
transgenic adenocarinoma of the mouse prostate
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- VP
ventral prostate
- WD
well differentiated
References
- 1.Fleshner NE, et al. Dietary fat and prostate cancer. J. Urol. 2004;171:S19–S24. doi: 10.1097/01.ju.0000107838.33623.19. [DOI] [PubMed] [Google Scholar]
- 2.Block G, et al. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 3.Kuo SM. Dietary flavonoids and cancer prevention: evidence and potential mechanism. Critical Rev. Oncog. 1997;1:47–69. doi: 10.1615/critrevoncog.v8.i1.30. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl Acad. Sci. U S A. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins S, et al. Chemopreventive efficacy and phramacokinetics of curcumin in the Min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- 6.Singh SV, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2010;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettuzzi S, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 8.Sartippour MR, et al. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutr. Cancer. 2004;249:59–65. doi: 10.1207/s15327914nc4901_8. [DOI] [PubMed] [Google Scholar]
- 9.Dzubak P, et al. Pharmacological activities of natural triterpenoids and therapeutic implications. Nat. Prod. Rep. 2006;23:394–411. doi: 10.1039/b515312n. [DOI] [PubMed] [Google Scholar]
- 10.Huang MT, et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–707. [PubMed] [Google Scholar]
- 11.Nishino H, et al. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-accetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988;48:5210–5215. [PubMed] [Google Scholar]
- 12.Ryu SY, et al. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta. Med. 2000;66:358–360. doi: 10.1055/s-2000-8531. [DOI] [PubMed] [Google Scholar]
- 13.Honda T, et al. Design and synthesis of 2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg. Med. Chem. Lett. 1998;8:2711–2714. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 14.Suh N, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxigenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–723. [PubMed] [Google Scholar]
- 15.Honda T, et al. Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg. Chem. Lett. 1999;9:3429–3434. doi: 10.1016/s0960-894x(99)00623-x. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, et al. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-κB and Notch1 signaling. J. Neurooncol. 2007;84:147–157. doi: 10.1007/s11060-007-9364-9. [DOI] [PubMed] [Google Scholar]
- 17.Deeb D, et al. CDDO-Me induces apoptosis and inhibits Akt, mTOR and NF-κB in prostate cancer cells. Anticancer Res. 2007;27:3035–3044. [PubMed] [Google Scholar]
- 18.Konopleva M, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–335. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 19.Shishodia S, et al. A synthetic triterpenoid, CDDO-Me, inhibits I kappa B alpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappa B-regulated gene products in human leukemic cells. Clin. Cancer Res. 2006;12:1828–1838. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda T, et al. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003;63:5551–5558. [PubMed] [Google Scholar]
- 21.Konopleva M, et al. The synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004;64:7927–7935. doi: 10.1158/0008-5472.CAN-03-2402. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, et al. The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol. Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 23.Konopleva M, et al. The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia. 2005;19:1350–1354. doi: 10.1038/sj.leu.2403828. [DOI] [PubMed] [Google Scholar]
- 24.Suh N, et al. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 2003;63:1371–1376. [PubMed] [Google Scholar]
- 25.Chintharlapalli S, et al. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol. Pharmacol. 2005;68:119–128. doi: 10.1124/mol.105.011437. [DOI] [PubMed] [Google Scholar]
- 26.Yates MS, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 27.Xiaoyang L, et al. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67:4210–4218. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 28.Liby K, et al. The synthetic triterpenoid CDDO-methyl ester and CDDO-amide prevent lung cancer induced by vinyl carbomate in A/J mice. Cancer Res. 2007;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- 29.Deeb D, et al. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro-survival Akt and mTOR. Prostate. 2009;69:851–860. doi: 10.1002/pros.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan-Lefco PJ, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 31.Gingrich JR, et al. Pathologic progression of autochthonous prostate cancer in TRAMP model. Prostate Cancer and Prostatic Dis. 1999;2:70–75. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- 32.Deeb D, et al. CDDO-Me inhibits proliferation, induces apoptosis, down-regulates Akt, mTOR, NF-κB and NF-κB-regulated antiapoptotic and proangiogenic proteins in TRAMP prostate cancer cells. J. Exp. Therapeut. Oncol. 2008;7:31–39. [PubMed] [Google Scholar]
- 33.Singh SV, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–9511. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mentor-Marcel R, et al. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–6782. [PubMed] [Google Scholar]
- 35.Nelson WG, et al. Prostate cancer. N. Engl. J. Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 36.Gingrich JR, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 37.Datta SR, et al. Akt phosphorylation of Bad couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 38.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 39.Mayo MW, et al. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochim. Biophys. Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S, et al. Constitutive activation of PI3K-Akt and NF-kB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.