Abstract
The effect of the brain on the morphology of the face has long been recognized in both evolutionary biology and clinical medicine. In this paper we describe factors that are active between development of the brain and face and how these might impact craniofacial variation. First, there is the physical influence of the brain, which contributes to overall growth and morphology of the face through direct structural interactions. Second, there is the molecular influence of the brain, which signals to facial tissues to establish signaling centers that regulate patterned growth. Importantly, subtle alterations to these physical or molecular interactions may contribute to both normal and abnormal variation. These interactions are therefore critical to our understanding of how a diversity of facial morphologies can be generated both within species and across evolutionary time.
Keywords: Shh, Fgf8, Bmp, neural crest, FEZ, evolution, disease, craniofacial
Introduction
Development of the vertebrate face occurs through the growth and fusion of distinct primordia into an integrated structure. Facial primordia are comprised of a mesodermal core surrounded by neural crest mesenchyme and encased in epithelia derived from ectoderm or ectoderm and endoderm. Each primordium contributes to a distinct region of the adult face: the lower jaw is derived primarily from the paired mandibular processes, while the lateral part of the upper jaw is formed from the paired maxillary and lateral nasal processes, and the middle part of the upper jaw and mid-face are derived from the median nasal processes and the frontonasal process (FNP). Morphogenetic events within each region are governed by signaling interactions among adjacent tissues. For instance, in the mandibular processes, and in the other pharyngeal arches, endoderm, neural crest mesenchyme, paraxial mesoderm, and overlying surface ectoderm interact to control the patterned growth of the arch derivatives (Reviewed in: (Chai and Maxson, 2006; Graham et al., 2005; Richman and Lee, 2003; Szabo-Rogers et al., 2009b)). Similarly, in the maxillary, lateral nasal, median nasal, and frontonasal processes interactions among the composite tissues produce the distinct morphologies of the upper jaw and midface and are likely to contribute to morphologic differences observed among and within populations of vertebrates. In this article we review the current understanding of the role of the forebrain, neural crest mesenchyme, and surface cephalic ectoderm during patterning and growth of the upper jaw and mid-face and their contribution to variation in morphology by focusing primarily on our own work defining the role of one important signaling center known as the frontonasal ectodermal zone (FEZ).
The Brain Physically Shapes the Face
Physical interactions between the brain and face begin during the initial formation of the face and continue throughout the fetal and early post-natal periods (Fig. 1). Initially, the prominences are situated on a base formed by the anterior neural tube and outgrowth and fusion of the facial prominences occur in close association with the rapidly developing brain. During early face formation the cranial neural tube is large relative to the face, and as development proceeds, the neural tube also grows. Hence the rate of neural tube expansion relative to facial prominence outgrowth can have important effects. Based on analyses of 3D reconstructions and medial plane sections of human embryos, Diewert et al. (Diewert and Lozanoff, 1993; Diewert et al., 1993) have shown that brain development impacts the positioning of the facial prominences relative to each other, the brain, and the eyes. We have further examined the effect of brain growth on facial morphogenesis using a wide range of genetically varied strains of mice that differ in rates of brain and face growth. Using 3D morphometrics to analyze the embryonic shape of one of these strains, Crf4, we discovered that reduction in brain growth produces an earlier developing and more prognathic (longer) face (Boughner et al., 2008). These results indicate that when the brain is smaller, the face forms on a smaller platform, and thus the same amount of facial outgrowth will produce a more prognathic and developmentally advanced face (Figure 1B).
Figure 1. Physical interactions between the brain and the face.
(A) Schematic showing the facial shape changes that occur with variation in brain size during face formation. (B) The actual shape changes that occur in Crf mice in association with reduced brain growth shown as wireframes.
While this work clearly demonstrates the effect of brain growth on early facial development, how this physical interaction influences facial morphology at later development time points is more poorly understood. For instance, despite having a long face at embryonic time points, the Crf4 mice have surprisingly shorter faces compared to controls as adults, suggesting other factors may come into play that are not necessarily driven by brain-face interactions. Diewert suggests that once cartilaginous elements of the face are formed, the brain-face interaction is less important, because morphogenesis in the skull is being driven primarily by intrinsic cartilaginous growth (Diewert, 1983; Diewert, 1985). This issue has not been explored experimentally, but there is morphometric evidence that throughout the course of brain growth, correlated variation is produced between the neurocranium and surrounding bony structures, including the face (Hallgrimsson et al., 2007a) (Aldridge et al., 2005; Richtsmeier et al., 2006; Richtsmeier and Deleon, 2009). In humans, there is correlated brain and facial variation in dysmorphologies such as cleft lip and palate (Weinberg et al, 2009). However, there is no definitive answer as to when during the course of fetal and postnatal growth, correlated morphological variation between the brain and face appears. Clearly though, understanding these continued interactions will be essential for a complete understanding of mechanisms that produce the ultimate adult facial form.
The Role of Epithelial-Mesenchymal Interactions in Facial Development
Epithelial-mesenchymal interactions are a hallmark of vertebrate development and regulate a wide array of histo- and morphogenetic processes. In the face, a series of iterative signaling interactions between the surface cephalic ectoderm and the underlying neural crest cells control facial morphogenesis. These types of interactions have been proposed to co-ordinate development of the upper and lower jaw (Depew and Compagnucci, 2008). Our work has defined an important signaling center located in the surface cephalic ectoderm of the upper jaw in mammals and avians (Hu et al., 2003; Hu and Marcucio, 2009b) that may contribute to this co-ordinated growth by regulating morphogenesis of the upper jaw. This signaling center, which we named the Frontonasal Ectodermal Zone (FEZ; (Hu et al., 2003)), is defined by the presence of a boundary between Sonic hedgehog (Shh) and Fibroblast growth factor 8 (Fgf8) expressing cells located within the ectoderm covering the FNP of stage 20 (Hamburger, 1951) chick embryos (Fig. 2). When transplanted to ectopic regions of the upper or lower jaw, the FEZ induces duplications of the underlying skeleton. Additionally, when the tissue is simply rotated rather than transplanted ectopically, the dorso-ventral polarity of the upper jaw is altered due to the presence of multiple Shh/Fgf8 boundaries. From these results we concluded that the FEZ not only induces skeletogenesis, but also specifies the pattern of the underlying mesenchymal cells and controls morphogenesis of the upper jaw. Subsequently we have determined that the FEZ is present in mouse embryos (Hu and Marcucio, 2009b), and Shh expression in human embryos suggests these embryos also have a FEZ (Odent et al., 1999). Thus, the FEZ appears to be a universal signaling center that controls growth and patterning of the distal portion of the upper jaw in vertebrates. Interestingly, when the FEZ was transplanted to the hyoid arch, which is filled with cells that express Homeobox genes, no effect on the underlying mesenchyme was observed. These results indicate that the underlying mesenchyme must be capable of responding to signals from the FEZ and the HOX code may help restrict this potential to anterior regions of the skull.
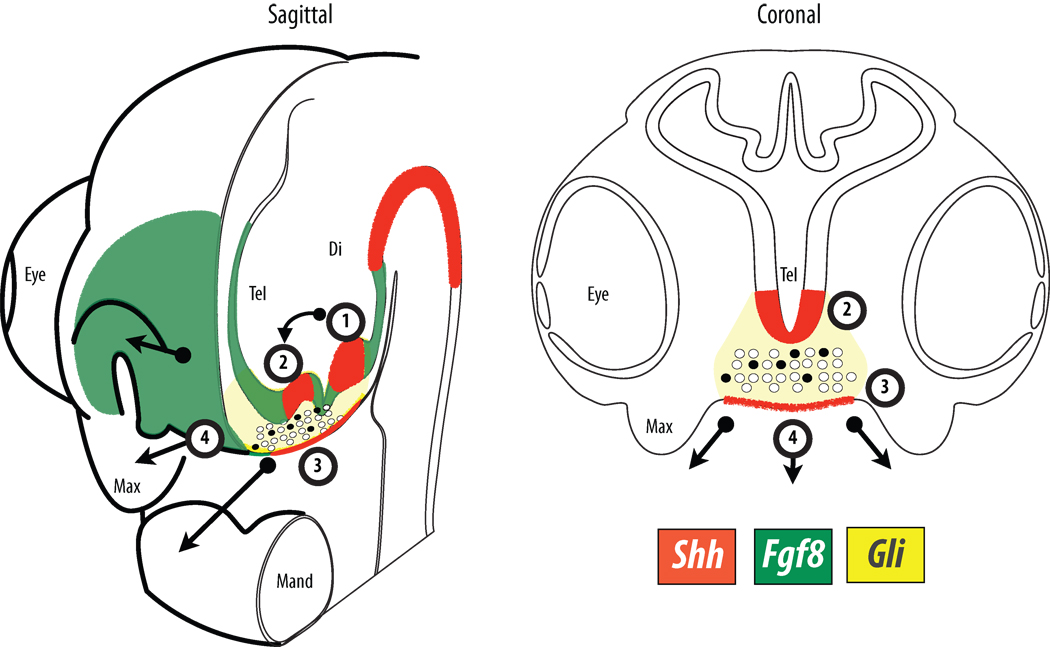
Figure 2. Ontogeny of the FEZ and the role of signaling molecules.
Shh expression (red) in the neuroectoderm of the diencephalon prior to stage 17 (1) turns on a similar zone just anterior to the optic recess in the telencephalon (2) at HH 17 that in turn establishes competency of the facial ectoderm to express Shh. Emigrating neural crest cells (circles) are required to initiate Shh expression in the facial ectoderm (3) which in turn establishes growth zones in the facial mesenchyme (4). Dorso-ventral polarity and outgrowth of the face is established at the boundary between Fgf8 (green) and Shh expression in the facial ectoderm. Activity of Shh-signaling in the mesenchyme, illustrated by Gli (yellow) activity, drives upregulation of cell-cycle related genes causing enhanced proliferation in affected neural crest cells (black) and outgrowth of the FNP.
Defining the phenomenon associated with FEZ activity has been relatively straightforward, but identifying the molecular mechanisms that underlie the ability of the FEZ to regulate patterned growth has been more challenging. In our initial experiments we showed that the FEZ induces expression of Ptc and Gli1, two downstream targets of Shh signaling (Dahmane et al., 1997; Goodrich and Scott, 1998; Ruiz i Altaba, 1997), in the mesenchyme, suggesting that Shh signals from the FEZ to the mesenchyme may be important for the patterning activity of the FEZ. Mechanistic understanding of the importance of Shh signaling within neural crest cells of the jaw have come from a number of different studies. Removing the ability of neural crest cells to transduce a Hh signal by conditional deletion of Smoothened (Smo) in developing neural crest cells reduces proliferation of the mesenchymal cells and produces severe malformations of the upper jaw (Jeong et al., 2004). Additionally, Shh from the ruggae of the epithelium covering the secondary palate appears to direct growth and may produce variation within the secondary palate (Welsh and O'Brien, 2009). In the lower jaw ectopic Shh from the pharyngeal endoderm is required for survival of neural crest cells and mandibular morphogenesis (Ahlgren and Bronner-Fraser, 1999; Brito et al., 2006). Further, activation of Shh signaling induces mirror image duplications of the mandible by regulating the expression patterns of various signaling molecules that act to control growth and patterning of the mandibular process (Brito et al., 2008). Together, these data illustrate the importance of Shh signaling during facial development, and that Shh from the FEZ acts to control growth centers that control morphogenesis of the facial structures. More recently, we have shown that transplantation of the FEZ induces expression of Bmp2, Bmp4, and Bmp7 in the underlying mesenchyme (Hu and Marcucio, 2009b). Additionally, by using retroviral mediated gene transfer, Azbhanov et al experimentally created multiple Shh/Fgf8 boundaries in the head of chick embryos, and determined that each boundary was associated with ectopic expression of Bmp4 and induction of chondrogenesis in the underlying mesenchyme (Abzhanov and Tabin, 2004). Hence, regulating the spatial pattern1 of expression of various Bmps in the mesenchyme may be a key component underlying FEZ function, because Bmps regulate growth zones that distinguish the faces of various avian species (Abzhanov et al., 2004; Wu et al., 2006; Wu et al., 2004) and are key regulators of chondrogenesis (Rosen, 2006). In the head blockade of Bmp signaling inhibits chondrogenesis and osteogenesis (Abzhanov et al., 2007; Ashique et al., 2002b; Hu et al., 2008), while activation of the Bmp pathway leads to ectopic cartilage formation and converts what would normally be dermal bone into cartilage (Abzhanov et al., 2007; Hu et al., 2008).
While our work has focused on the role of Shh and Fgf8 in mediating FEZ activity, other molecules are also expressed by the FEZ and are likely to participate in FEZ function (Ashique et al., 2002a; Foppiano et al., 2007; Francis-West et al., 1998; Geetha-Loganathan et al., 2009). Bmp2, Bmp4, and Bmp7 all have unique spatial expression patterns within the FEZ (Foppiano et al., 2007; Francis-West et al., 1994). Blocking Bmp signaling within the developing upper jaw decreases cell proliferation, alters gene expression patterns, creates defects in growth of the upper jaw anlagen, and leads to cleft lip and palate in chick embryos (Ashique et al., 2002a; Foppiano et al., 2007). Ectopic activation of the Bmp pathway alters morphology of the developing jaw (Barlow and Francis-West, 1997). Changes in gene expression in response to ectopic Bmp2 or Bmp4 were correlated with bifurcations of the palatine bone in the upper jaw, which suggests that Bmp signaling may participate in the patterning activity of the FEZ by helping to regulate bone growth. Additionally, genes encoding canonical Wnt ligands are expressed in various regions of the surface ectoderm and may signal within the plane of the epithelium (Geetha-Loganathan et al., 2009; Hu and Marcucio, 2009a) and/or to the mesenchyme (Geetha-Loganathan et al., 2009) to control facial morphogenesis.
In addition to the FEZ, another signaling center that participates in facial morphogenesis is present in the facial ectoderm. Szabo-Rogers et al. have determined that molecular signals including Fgf8 from the nasal pit are necessary for patterning the proximal and lateral portion of the upper jaw (Szabo-Rogers et al., 2008; Szabo-Rogers et al., 2009a). The nasal pit appears to work in a fashion similar to the FEZ by regulating expression of key molecules in the mesenchyme and establishing gene expression domains in the ectoderm (Firnberg and Neubuser, 2002; Szabo-Rogers et al., 2009a). Blocking Fgf signaling from the nasal pit creates malformations of the upper jaw due to decreased cell survival and proliferation and altered patterns of gene expression (Hu and Marcucio, 2009a; Szabo-Rogers et al., 2008).
Ontogeny of Gene Expression in the FEZ Suggests Multiple Modes of Regulation
Our initial description of the FEZ was based upon the anatomical boundary between Fgf8 and Shh expressing cells in the surface cephalic ectoderm beginning at stage 20 in chick embryos. However, prior to this stage the boundary between these genes is not apparent. Fgf8 transcripts are expressed in this ectoderm from very early stages of development. Before the anterior neuropore closes at stage 10, the Fgf8 is expressed in a continuous domain that spans from within the anterior forebrain through the closing neuropore to the surface cephalic ectoderm. When the neuropore closes, the Fgf8 domain becomes segregated into distinct forebrain and surface ectodermal domains (Ohkubo et al., 2002). Similarly, Bmp2, Bmp4, Bmp7 are expressed in the surface cephalic ectoderm from early stages of development. In contrast, Shh expression does not begin until the neural crest cells arrive in the FNP beginning around stage 19–20. At this time, Shh expression is induced in the ectoderm and the boundary between Fgf8 and Shh-expressing cells is apparent (Marcucio et al., 2005). Given the importance of Shh signaling from the FEZ for morphogenesis of the upper jaw (Jeong et al., 2004), understanding how Shh expression is induced in the FEZ is essential for understanding morphogenesis in this region of the head.
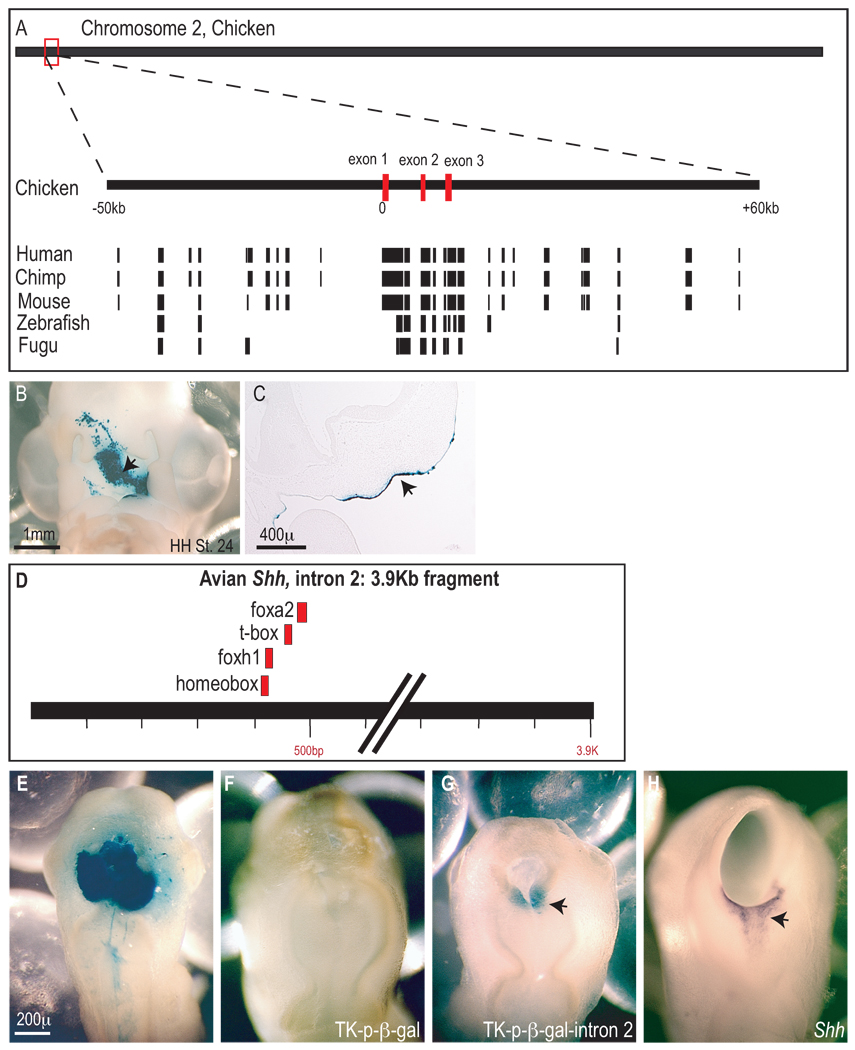
The molecular network that controls the establishment of the FEZ, and in particular Shh expression, is not known. Studies of the murine Shh locus have revealed a complex regulatory network involving multiple tissue-specific enhancers and repressors that appear to control Shh expression (Epstein et al., 2000; Epstein et al., 1999; Geng et al., 2008; Jeong et al., 2006; Jeong and Epstein, 2003; Jeong et al., 2008). However, none of this work has yet identified a FEZ-specific enhancer region. In our laboratory, we screened approximately 110kB of genomic DNA surrounding the avian Shh locus for a FEZ-specific enhancer region, because within this region a series of DNA elements that are highly conserved among a variety of vertebrates are present (Fig. 3A) and may be indicative of regions that regulate gene expression (Ahituv et al., 2004). 5kB intervals of genomic DNA that spanned 55kb upstream and downstream of the Shh start site were cloned into a vector containing a minimal thymidine kinase promoter, or the avian Shh promoter, that expresses β-galactosidase in the presence of novel enhancers (Tk-p-β-Gal). This vector was then electroporated into the FEZ of chick embryos just prior to the onset of Shh expression (Fig. 3B,C), and chicks were collected 24 hours later. In no case did we observe expression of β-galacotsidase in these embryos. As a positive control DNA containing a set of forebrain enhancer elements that are located in the second intron of the Shh gene (Fig. 3D) was cloned into the Tk-p-β-Gal vector and electroporated into the brain. Beta-galactosdiase activity appeared nearly indistinguishable from Shh expression (Fig. 3E–H). Thus, these data suggest that FEZ-specific enhancers reside farther than 55kB up- or downstream of the Shh start site, but further work is required to define the FEZ-specific enhancer region within the genome and to identify the mechanisms underlying transcriptional regulation of Shh in this tissue.
Figure 3. Enhancer analysis within the Shh locus.
(A) The avian Shh gene is located on the end of chromosome 2. A red box indicates the position of the Shh locus on the chromosome and two dotted lines are used to indicate the magnified area of the genome. The coding region of the avian Shh gene is comprised of three exons (red boxes) and two introns. This same organization is true for other species as well. Within 110 kilobases surrounding the transcription start site located in exon 1, a number of conserved regions among chick, human, chimp, mouse, zebrafish, and fugu are present. The location of each of these conserved regions are indicated as black boxes beneath the diagram of the chicken Shh locus. (B) Twenty-four hours after electroporation into the stomodeal ectoderm at HH St. 20 b-gal activity (arrow) is observed throughout the Shh expression domain in the stomodeal ectoderm. Expression of b-gal from the HSP-LacZ construct does not require additional enhancer elements and can be used to monitor gene transfer in control embryos (n=6/6, arrow). (C) Sections through the embryo shown in B illustrates that transfer of the transgene is restricted to the ectoderm (arrow). (D) Diagram of a 3.9Kb fragment corresponding to part of intron 2 of the avian Shh gene. The location of conserved consensus sequences for foxa2, t-box, foxh1, and homeodomain proteins between mouse, zebrafish and chick are shown (red boxes). (E) Electroporation of HSP-LacZ was used to optimize and visualize the extent of electroporation within the neural tube of HH St. 12 embryos. Widespread β-gal expression is observed throughout the brain (n=11/11). (F) Embryos electroporated with TK-p-β-gal demonstrate that this reporter construct exhibits no basal transcriptional activity (n=6/6). (G) After electroporation of TK-p-β-gal-intron 2 into the neural tube, β-gal activity was restricted to the ventral forebrain (n=8/9, arrow). This pattern confirms the presence of enhancers that direct gene expression to the ventral neural tube as previously described for mouse and zebrafish embryos. (H) Whole mount in situ hybridization shows that expression of Shh in the ventral neural tube (arrow) corresponds to the location of enhancer activity observed in embryo in G.
While the exact molecular mechanisms that regulate Shh expression are not known, the tissue interactions that induce Shh in the FEZ are better elucidated. The onset of Shh expression in the FEZ occurs concomitantly with the arrival of neural crest cells into the FNP. This observation suggests that neural crest cells may be involved in inducing Shh expression in the FEZ, and indeed there is ample evidence that the neural crest cells are key regulators of FEZ formation. In birds, transplantation of neural crest cells from quail embryos into ducks changes facial morphology. In part this change is associated with altered Shh expression. Quail are a faster developing species than duck, and in the presence of the quail neural crest cells, the duck ectoderm expresses Shh prematurely in response to the quail neural crest (Schneider and Helms, 2003). Likewise, in zebrafish Shh expression in the roof of the stomodeum, which may likely be homologous to the FEZ, requires the presence of neural crest cells (Eberhart et al., 2008).
The data in these two papers clearly demonstrate that the neural crest cells are required for the onset of Shh expression in the FEZ, but the molecular signals from the neural crest that induce Shh expression in the FEZ are not known. One set of signals, the Bmps, are likely to be involved in activation or maintenance of Shh expression in the FEZ. Bmp2, Bmp4, and Bmp7 are expressed in unique domains in the ingressing neural crest cells and Bmp receptors are present on the neural crest, the neuroepithelium, and the FEZ (Ashique et al., 2002a; Bennett et al., 1995; Foppiano et al., 2007; Francis-West et al., 1994). To test the involvement of Bmp signaling in FEZ formation, we blocked Bmp signaling in the FNP by exogenous expression of Noggin. These embryos exhibited severe facial malformations and had significant reductions in Shh expression in the FEZ (Foppiano et al., 2007). In support of these data, previous investigations have shown that application of Bmp-soaked beads to the developing maxillary process led to an extension of Shh expression in the surface cephalic ectoderm (Barlow and Francis-West, 1997). However, whether Bmps act directly on the ectoderm to stimulate Shh expression or operate within the mesenchyme to control neural crest gene expression patterns is not known.
Wnt signaling may also be intimately involved in regulating Shh expression in the FEZ. Conditional ablation of the beta-catenin gene from the surface ectoderm of mouse embryos reduces Shh expression in the FEZ and creates severe facial malformations, while activating the Wnt pathway in the ectoderm expands the Shh expression domain, induces Fgf4 and Fgf8 expression, and leads to hypertrophy of the median and lateral nasal processes (Reid, In press).
The brain also produces molecular signals that direct facial development. Activation of Bmp signaling in the forebrain of chick embryos leads to massive apoptosis in the basal portion of the brain and creates facial defects suggesting that signals from the brain participate in facial development (Golden et al., 1999). Within the ventral forebrain Shh exhibits a dynamic expression pattern. Initially, Shh is expressed in the basal diencephalon and then is induced in the ventral telencephalon. Blockade of Shh in the brain inhibits this induction sequence, dorsalizes the forebrain, and creates severe facial malformations (Marcucio et al., 2005). In particular, the upper jaw does not undergo mediolateral or proximodistal extension due to decreased cell proliferation. In these, and other studies (Cordero et al., 2004), we did not observe significant amounts of apoptosis in the FNP mesenchyme. This is in contrast to apoptosis that is observed in the mandible after blocking Shh signaling (Ahlgren and Bronner-Fraser, 1999; Brito et al., 2006; Cordero et al., 2004) and suggests that the role of Shh in the FNP may be slightly different from that in the pharyngeal arches. With this in mind, we have shown that restoring Shh to the FNP after blocking Shh in the brain is able to restore more normal growth of the facial complex by stimulating Shh expression in the FEZ. In experimental studies on zebrafish embryos, an early Shh signal from the brain to the stomodeal ectoderm (zebrafish homologue of the FEZ) was shown to be required for gene expression within the stomodeal ectoderm and for condensation of neural crest cells on the roof of the mouth (Eberhart et al., 2006). Collectively, these results indicate that Shh signaling from the brain to the ectoderm is required for establishing the signaling properties of the FEZ, which then patterns outgrowth of the upper jaw (Fig. 2).
Mechanisms underlying production of unique morphologies
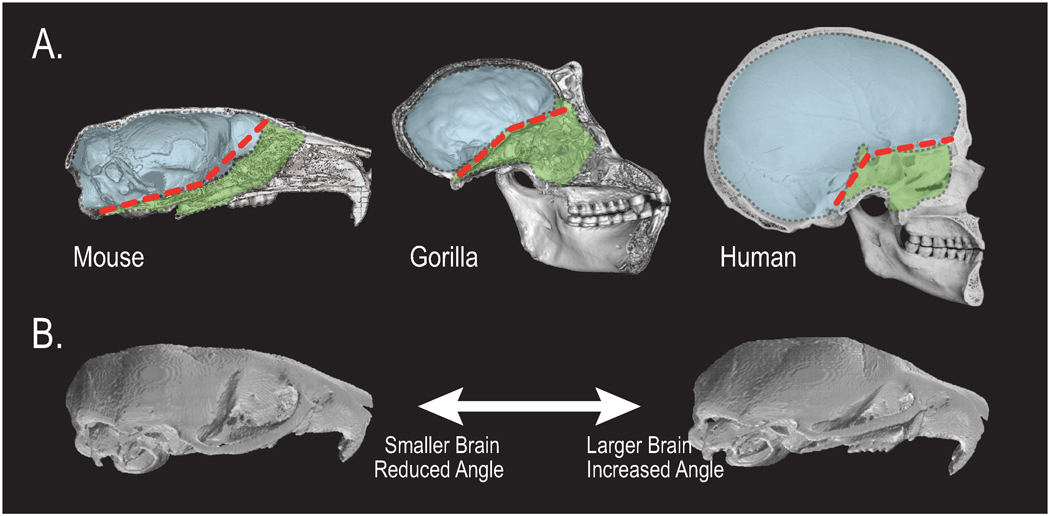
The physical influence of the growth of the brain on the shape of the surrounding cranial structures including those of the face has been the subject of much speculation in evolutionary biology. DeBeer (DeBeer, 1937), for example, speculated that variation in the size of the brain relative to the rest of the skull is a key contributor to large scale trends in the changing morphology of the vertebrate skull. In the context of human evolution, Biegert (Biegert, 1963) formalized this idea into the “spatial packing” hypothesis, which holds that increasing brain size causes a predictable series of morphological changes in the skull. The most important of these is flexion of the cranial base, which occurs as the enlarged brain is accommodated on the cranial base (Fig. 4A). Importantly, the cranial base angle describes the relative positioning of the face and braincase. Thus Biegert was arguing that the human face, which is unusual in that it is positioned below instead of anterior to the frontal lobes of the brain, is primarily a by-product of expansion of the brain. The spatial packing model has been tested on comparative data in primates, which reveal that the ratio of brain size to basicranial length explains a significant proportion of the interspecific variation in the cranial base angle (Ross and Henneberg, 1995; Ross and Ravosa, 1993). We have shown that mutations in mice that increase brain size or brain size relative to basicranial size similarly produce changes consistent with Biegert’s model (Hallgrimsson and Lieberman, 2008; Hallgrimsson et al., 2007a; Lieberman et al., 2008) (Fig. 4B). While much remains poorly understood about how the brain and face interact physically during development, these studies demonstrate that the structural relationship between development of the brain and face is important for producing the ultimate craniofacial morphology.
Figure 4. Brain Size Contributes to Variation in Facial Morphology.
(A) The angle (red dashed line) between the cranial base (green) and brain (blue) is altered by brain size (e.g., between species with varying relative brain volume), which in turn affects facial shape. (B). Comparisons among mouse strains reveals that almost all variation (87%) in the cranial base angle can be explained by three-dimensional neural and facial packing, illustrated here by mice at extremes of brain size. This suggests that physical interactions between brain, face and cranium influence the final shape of the skull in ways that are unpredictable by genetics alone.
Similarly, important molecular interactions between the forebrain, neural crest and facial ectoderm (i.e., the FEZ) that regulate development of the upper jaw have been discussed above (Abzhanov and Tabin, 2004; Hu et al., 2003; Marcucio et al., 2005; Schneider et al., 2001), and may occur in many, if not all, vertebrates (Hu and Marcucio, 2009b). Alterations to the molecular interactions that control formation of the FEZ therefore are likely to contribute to differences in facial morphology, but how might these variations originate, and what interactions are most important for population and species level variation?
Work by Schneider and Helms (Schneider and Helms, 2003) demonstrated that neural crest cells regulate species-specific facial form in part by regulating the onset of Shh expression in the FEZ, and our unpublished data suggest that neural crest cells participate along with the forebrain in establishing the spatial organization of the FEZ. This evidence strongly suggests that the brain and neural crest cells act together to establish unique FEZ organization within the developing upper jaw of diverse species. Changes in the organization of the brain, or signaling from neural crest cells may therefore lead to alterations in these centers, and thereby generate morphological variation that is relevant to evolutionary differences. Neural crest cells may also directly influence development of the brain. A recent body of work has revealed a series of signaling interactions between the neural crest and the brain that appear to regulate size and growth of the anterior part of the neural tube (Creuzet, 2009a; Creuzet, 2009b; Creuzet et al., 2006; Le Douarin et al., 2007). Thus, co-ordinated development of these two structures would be predicted to contribute to the variation produced in both the brain and the face via evolutionary processes. This has not been examined in great detail as of yet.
As discussed above, Shh plays an essential role in the epithelial-mesenchymal interactions that control proximo-distal extension and dorso-ventral polarity of the vertebrate upper jaw. Our own work shows that despite the divergent facial morphologies that characterize birds and mammals, both have a functional FEZ, albeit slightly different in size and organization. In chicks the FEZ is a single continuous band of expression, but in mice the FEZ is broken into two regions associated with the median nasal prominences (Hu and Marcucio, 2009b). Interestingly, when Shh signaling is over-activated in the chicken, the mouse FEZ condition is phenocopied. This suggests that the FEZ may be important to macroevolutionary differences, but is there any evidence to suggest that these interactions contribute to finer scaled differences, such as at the population level?
We recently performed a series of Shh gain- and loss-of-function experiments in chickens to test this question. We found that reducing Shh-signaling in the brain caused a continuous structural narrowing of the FNP, progressive hypotelorism, and medial maxillary rotation, while increasing Shh-signaling in the brain caused midfacial widening, frontonasal hypoplasia/bifurcation, and lateral divergence of the maxillaries (Young et al., 2010). These changes in shape were further associated with gene expression changes in the facial epithelia (e.g., the size of the FEZ was directly related to the treatment dosage in the brain), mesenchymal mitotic activity, and Shh expression in the brain as measured by qRT-PCR. Furthermore, at 13 days post treatment phenotypic outcomes ranged from a progressive narrowing and shortening of the midfacial skeleton to progressively wider midfaces with median clefts, consistent with the direction of midfacial growth in the embryonic shape analysis. Together these results demonstrated that altering Shh activity in the brain has a predictable effect on variation in midfacial growth, shape, and size, particularly on the width of the presumptive avian midface.
Given the broad conservation of FEZ function in both avians and mammals, we speculate that variation in Shh ligand-associated concentration parameters might play an important role in both normal and abnormal population level variation in facial shape, and thus could be a target of selection. For example, variation might exist in the concentration of Shh ligand in the facial mesenchyme, or in the sensitivity of mesenchymal cells to this concentration. In the former, gradient formation may be affected by mutations that alter the ability of Shh to signal to adjacent cells by affecting the ligand or any of the multitude of factors that are required for Shh activation, secretion, transport, or accumulation (e.g., Shh, Disp1, Cdo, Boc, Gas1) (Allen et al., 2007; Etheridge et al., 2010; Saha and Schaffer, 2006; Seppala et al., 2007; Tenzen et al., 2006; Tian et al., 2005; Tian et al., 2004; Zhang et al., 2006). Alternatively, mutations in Ptc, Smo, or genes involved in primary cilia formation or function may alter the response of a cell responding to Shh due to changes in the ability to sense and transduce the signal (Ingham and McMahon, 2001; Tobin et al., 2008). Variation in any of these parameters would be predicted to have a similar phenotypic effect by altering the location and activity of midfacial growth zones. Supporting this idea, allelic variation in Shh pathway genes is known to yield a range of midfacial phenotypes in mice that qualitatively parallel those we found in avians, and the relative effect appears to be proportional to the gene’s function within the pathway. Thus, while removing Cdo has a small effect on phentoype due to its affect on gradient formation, Shh heterozygosity contributes to midfacial variation by reducing ligand production (Tenzen et al., 2006). While this experimental outcome shows the potential of variation in the Shh pathway to contribute to variation in midfacial shape, is there evidence that this pathway outside of the lab is relevant or important to normal (i.e., intraspecific) and/or evolutionary (i.e., interspecific) variation?
Perhaps the clearest example of the contribution of Shh to evolutionary variation is in comparisons of surface to blind cavefish where variation in the regulation of Shh has been shown to cause changes in jaw shape as well as the eyes (Menuet et al., 2007; Yamamoto et al., 2009). More indirectly, evolutionary variation in midline structures is consistent with predictions of how selection on variation in Shh signaling spatial organization or activity would affect the brain and the skeletal derivatives of the FNP: i.e., the frontal, nasal, premaxilla, and nasal septum. For example, analyses of adult craniofacial variation in both humans and mutant and inbred strains of mice indicate that the width of the neurocranium and face tend to covary to the exclusion of other measures (e.g., length or depth) (Hallgrimsson et al., 2007b; Martinez-Abadias et al., 2009) while our preliminary analyses of adult craniofacial variation in both primates and domesticated pigeons suggest that a significant proportion of total variation is associated with midline structures, particularly interorbital width and spacing. Further evidence suggests that early embryonic events associated with Shh signaling may contribute to adult midfacial differences. For example, comparative analyses of the ontogeny of the derivatives of the FNP in three avian species (chicken, duck, and quail) indicate that while early FNP shape is similar in all three species, differences in width substantially increase in concert with the rate and direction of species-specific growth patterns. In particular, ducks appear to exhibit enhanced Shh activity in the FEZ and maintain a wide FNP consistent with the wider bill of the adult phenotype. These differences in growth are consistent with the spatial distribution of growth centers in these species (Wu et al., 2006) which may be a direct result of slight changes in the organization of the FEZ. Hence, growth patterns driven by Shh activity in the brain and face may contribute to differences in the shape of facial structures among avian species and may be a central force that shapes evolutionary change in this region of the vertebrate head. Although not causal, together this evidence is strong circumstantial evidence implicating variation in FEZ interactions to evolutionary variation in the midface (Fig. 5).
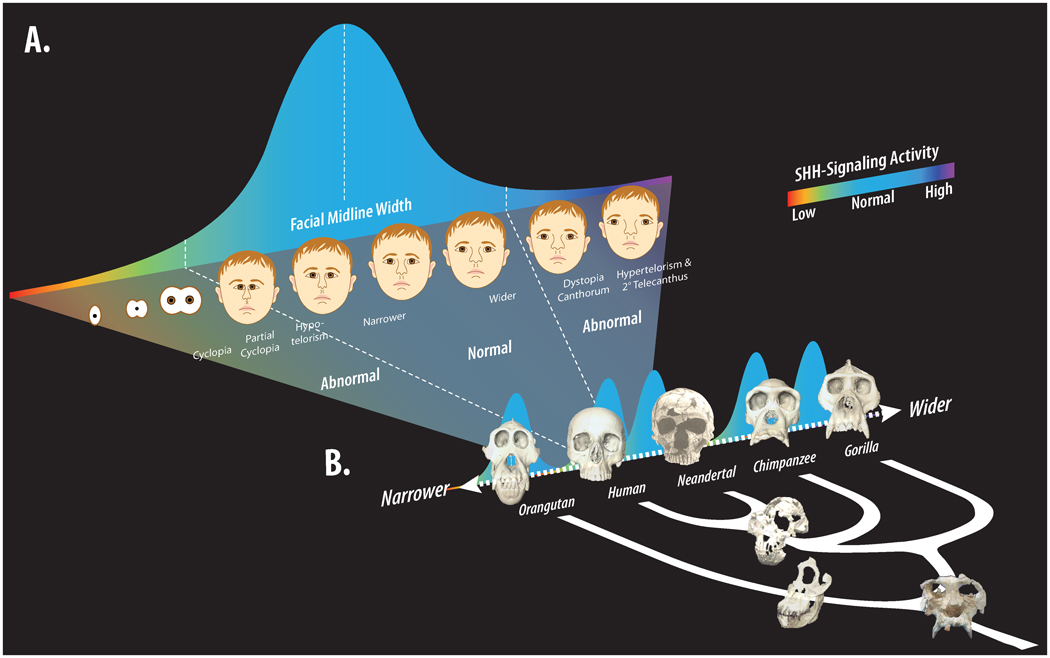
Figure 5. Hypothetical relationship of Shh signaling activity in the brain and FEZ to both population and evolutionary level variation in midfacial shape.
(A) Normal levels of Shh signaling activity in the brain establish the FEZ, which establishes growth zones that help to regulate the shape and size of midfacial structures such as the frontal, premaxilla and nasal septum. Extremes of signaling due to mutational effects, environmental factors, or a combination of both, can cause progressive loss of midline structures when signaling is abnormally low (e.g., in Holoprosencephaly), or expansion of midline structures when signaling is abnormally high. More subtle differences in signaling introduce variation in the midline facial structures of normal individuals that can serve as a target of natural selection. (B) Individual species exhibit a range of intraspecific variation in midfacial shape, as well as interspecific or evolutionary variation between species. In the case of hominoid apes, interorbital width varies from narrower in the orangutan to wider in gorillas (tree illustrates phylogenetic relationships of living and recent species as well as facial skeletons of potential ancestral species). Variation in Shh signaling, in combination with variation in associated craniofacial structures, is hypothesized to have contributed to the evolution of the midface in living apes and humans, as well as other vertebrate species.
Interestingly, comparative analyses of Shh evolution in a broad range of taxa suggest the gene itself is a target of selection, a finding that is consistent with it playing a role in evolutionary variation. For example, population-level variation in primates suggests Shh has undergone positive selection and accelerated molecular evolution relative to other mammals, and changes are most prominent in the lineage leading to humans. Importantly, sequence level changes affect Shh at the protein level, and suggest that apes and humans have evolved more complex post-translational regulation that may be related to the dramatic evolution of the brain, the skeleton of the face, or both, in this lineage (Dorus et al., 2006). Similarly, recent analysis of the Neandertal genome suggests that DISP1, a key component of Shh signaling, also experienced positive selection in modern humans that may reflect similar selection on the brain and/or midface (Green et al.). Further analyses are needed to determine how these lineage and species-specific changes affect developmental parameters associated with the brain and face.
Diseases of the brain-face complex
Just as interactions between the brain and face may serve as a source of normal or evolutionary variation, when these same processes are disrupted they may also lead to disease phenotypes. Often facial malformations are accompanied by underlying brain defects (DeMyer, 1964). We have described two fundamental ways by which development of the brain and face are interrelated. First, the brain serves as an architectural foundation upon which the face develops. Thus, as the foundation expands the face accommodates and develops accordingly, and perturbations to the frame can lead to facial defects. Second, molecular signaling between the brain and face regulates morphogenesis. Hence, disrupting this molecular dialogue can create malformations in both tissues. This is exemplified in humans where extremes of Shh signaling produce disease phenotypes (Muenke and Cohen, 2000). In Holoprosencephaly (HPE), Shh signaling is reduced, the brain can be severely malformed, and facial malformations range from hypotelorism and midfacial hypoplasia to complete cyclopia (Muenke and Beachy, 2000), while in Greig Cephalopolysyndactyly (GCPS) and Gorlin syndrome, mutations in GLI3 or PTC increase Shh signaling activity and phenotypes range from hypertelorism to medial clefts of the face (Balk and Biesecker, 2008) (Fig. 5). Importantly, in each of these diseases, family members of affected individuals may manifest less-severe midfacial phenotypes such as relatively narrower or wider faces (Balk and Biesecker, 2008; Muenke and Beachy, 2000), suggesting that variation in Shh signaling between the brain and face contributes to normal variation in midfacial shape and size.
Conclusions
In this paper, we focused on the physical interactions between the brain and face, the molecular level epithelial-mesenchymal interactions among the brain, the FEZ, and the neural crest mesenchyme, and their contribution to facial variation. However, it is important to remember that these comprise only a small number of potential factors contributing to facial morphogenesis, and that craniofacial morphology is the result of a series of overlapping developmental events, each contributing to the final outcome (i.e., the adult phenotype) (Atchley and Hall, 1991; Hallgrímsson, 2009). For example, the shape of the bones and cartilages of the jaw, which are unique in that they are neural crest derivatives (Donoghue et al., 2008; Evans and Noden, 2006), are the result of multiple inputs from neural crest formation and migration to general skeletal growth (e.g., (Atchley and Hall, 1991)), each with a relative contribution that may differ in both magnitude and direction. Early events like neural crest migration may be masked by variation in later events such as somatic growth, or early events may have a large effect that constrains later events to particular outcomes. This model of development suggests that the adult phenotype, much like a Medieval "palimpsest" which holds the entire contents of its history in opaque terms, makes simple genotype-phenotype correlations for complex traits unlikely (Hallgrímsson, 2009). Ultimately, to understand how adult phenotypic diversity is generated, we must better understand how genetic variation impacts individual developmental events and how these contribute to adult phenotypic outcomes, overall population variation, and ultimately evolutionary and disease variation.
Acknowledgements
We thank the members of the Marcucio and Hallgrimsson laboratories, as well as, the many colleagues who have discussed this work with us. This research was supported by grants from the NIH (F32DE018596 (N.M.Y.), by NIH/NIDCR R01DE018234 (R.S.M.) and R01DE019638 (R.S.M., B.H., N.M.Y.).
REFERENCES
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273:134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Ahituv N, Rubin EM, Nobrega MA. Exploiting human--fish genome comparisons for deciphering gene regulation. Hum Mol Genet. 2004;13(Spec No 2):R261–R266. doi: 10.1093/hmg/ddh229. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Current Biology. 1999;9:1304–1314. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- Aldridge K, Kane AA, Marsh JL, Yan P, Govier D, Richtsmeier JT. Relationship of brain and skull in pre- and postoperative sagittal synostosis. J Anat. 2005;206:373–385. doi: 10.1111/j.1469-7580.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002a;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002b;46:243–253. [PubMed] [Google Scholar]
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biological Reviews of the Cambridge Philosophical Society. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Balk K, Biesecker LG. The clinical atlas of Greig cephalopolysyndactyly syndrome. Am J Med Genet A. 2008;146A:548–557. doi: 10.1002/ajmg.a.32167. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Bennett JH, Hunt P, Thorogood P. Bone morphogenetic protein-2 and -4 expression during murine orofacial development. Archives of Oral Biology. 1995;40:847–854. doi: 10.1016/0003-9969(95)00047-s. [DOI] [PubMed] [Google Scholar]
- Biegert J. The evaluation of characteristics of the skull, hands and feet for primate taxonomy. In: Washburn SL, editor. Classification and human evolution. Chicago: Aldine; 1963. pp. 116–145. [Google Scholar]
- Boughner JC, Wat S, Diewert VM, Young NM, Browder LW, Hallgrimsson B. Short-faced mice and developmental interactions between the brain and the face. Journal of Anatomy. 2008;213:646–662. doi: 10.1111/j.1469-7580.2008.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito JM, Teillet MA, Le Douarin NM. An early role for sonic hedgehog from foregut endoderm in jaw development: ensuring neural crest cell survival. Proc Natl Acad Sci U S A. 2006;103:11607–11612. doi: 10.1073/pnas.0604751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito JM, Teillet MA, Le Douarin NM. Induction of mirror-image supernumerary jaws in chicken mandibular mesenchyme by Sonic Hedgehog-producing cells. Development. 2008;135:2311–2319. doi: 10.1242/dev.019125. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J. Clin. Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet SE. Neural crest contribution to forebrain development. Semin Cell Dev Biol. 2009a;20:751–759. doi: 10.1016/j.semcdb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Creuzet SE. Regulation of pre-otic brain development by the cephalic neural crest. Proc Natl Acad Sci U S A. 2009b;106:15774–15779. doi: 10.1073/pnas.0906072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet SE, Martinez S, Le Douarin NM. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci U S A. 2006;103:14033–14038. doi: 10.1073/pnas.0605899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz I, Altaba A. Activation of the transcription factor Gli 1 and the Sonic hedgehog signalling pathway in skin tumors. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- DeBeer G. The Development of the vertebrate skull. London: Oxford University Press; 1937. [Google Scholar]
- DeMyer W. The face predicts the brain: diagnostic significance of median facial anomialies for holoprosencephaly (arhinencephay) Pediatrics. 1964 August;:256–263. [PubMed] [Google Scholar]
- Depew MJ, Compagnucci C. Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zoolog B Mol Dev Evol. 2008;310:315–335. doi: 10.1002/jez.b.21205. [DOI] [PubMed] [Google Scholar]
- Diewert VM. A morphometric analysis of craniofacial growth and changes in spatial relations during secondary palatal development in human embryos and fetuses. Am J Anat. 1983;167:495–522. doi: 10.1002/aja.1001670407. [DOI] [PubMed] [Google Scholar]
- Diewert VM. Growth movements during prenatal development of human facial morphology. Prog Clin Biol Res. 1985;187:57–66. [PubMed] [Google Scholar]
- Diewert VM, Lozanoff S. A morphometric analysis of human embryonic craniofacial growth in the median plane during primary palate formation. Journal of Craniofacial Genetics and Developmental Biology. 1993;13:147–161. [PubMed] [Google Scholar]
- Diewert VM, Lozanoff S, Choy V. Computer reconstructions of human embryonic craniofacial morphology showing changes in relations between the face and brain during primary palate formation. Journal of Craniofacial Genetics and Developmental Biology. 1993;13:193–201. [PubMed] [Google Scholar]
- Donoghue PC, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30:530–541. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorus S, Anderson JR, Vallender EJ, Gilbert SL, Zhang L, Chemnick LG, Ryder OA, Li W, Lahn BT. Sonic Hedgehog, a key development gene, experienced intensified molecular evolution in primates. Hum Mol Genet. 2006;15:2031–2037. doi: 10.1093/hmg/ddl123. [DOI] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008;40:290–298. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Martinu L, Michaud JL, Losos KM, Fan C, Joyner AL. Members of the bHLH-PAS family regulate Shh transcription in forebrain regions of the mouse CNS. Development. 2000;127:4701–4709. doi: 10.1242/dev.127.21.4701. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, McMahon AP, Joyner AL. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999;126:281–292. doi: 10.1242/dev.126.2.281. [DOI] [PubMed] [Google Scholar]
- Etheridge LA, Crawford TQ, Zhang S, Roelink H. Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development. 2010;137:133–140. doi: 10.1242/dev.043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. 2006 doi: 10.1002/dvdy.20663. [DOI] [PubMed] [Google Scholar]
- Firnberg N, Neubuser A. FGF Signaling Regulates Expression of Tbx2, Erm, Pea3, and Pax3 in the Early Nasal Region. Developmental Biology. 2002;247:237–250. doi: 10.1006/dbio.2002.0696. [DOI] [PubMed] [Google Scholar]
- Foppiano S, Hu D, Marcucio RS. Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev Biol. 2007;312:103–114. doi: 10.1016/j.ydbio.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A, Graveson A. Signalling interactions during facial development. Mechanisms of Development. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Tatla T, Brickell PM. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Dev Dyn. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Antoni L, Fu K, Whiting CJ, Francis-West P, Richman JM. Expression of WNT signalling pathway genes during chicken craniofacial development. Dev Dyn. 2009;238:1150–1165. doi: 10.1002/dvdy.21934. [DOI] [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15:236–247. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JA, Bracilovic A, McFadden KA, Beesley JS, Rubenstein JL, Grinspan JB. Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly. Proc Natl Acad Sci U S A. 1999;96:2439–2444. doi: 10.1073/pnas.96.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–1257. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. Journal of Anatomy. 2005;207:479–487. doi: 10.1111/j.1469-7580.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prufer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Hober B, Hoffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Z, Gusic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, de la Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PL, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Paabo S. A draft sequence of the Neandertal genome. Science. 328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS. Deciphering the palimpsest: Studying the relationship between morphological integration and phenotypic covariation. Evolutionary Biology. 2009;36:355–376. doi: 10.1007/s11692-009-9076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson B, Lieberman DE. Mouse models and the evolutionary developmental biology of the skull. Integrative and Comparative Biology. 2008;48:373–384. doi: 10.1093/icb/icn076. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B, Lieberman DE, Liu W, Ford-Hutchinson AF, Jirik FR. Epigenetic interactions and the structure of phenotypic variation in the cranium. Evolution and Development. 2007a;9:76–91. doi: 10.1111/j.1525-142X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B, Lieberman DE, Young NM, Parsons T, Wat S. Evolution of covariance in the mammalian skull. Novartis Found Symp. 2007b;284:164–185. doi: 10.1002/9780470319390.ch12. discussion 185-90. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of developmental stages in development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hu D, Colnot C, Marcucio RS. Effect of bone morphogenetic protein signaling on development of the jaw skeleton. Dev Dyn. 2008;237:3727–3737. doi: 10.1002/dvdy.21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio R, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009a;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 2009b;325:200–210. doi: 10.1016/j.ydbio.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, El-Jaick K, Roessler E, Muenke M, Epstein DJ. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development. 2006;133:761–772. doi: 10.1242/dev.02239. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Epstein DJ. Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development. 2003;130:3891–3902. doi: 10.1242/dev.00590. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Leskow FC, El-Jaick K, Roessler E, Muenke M, Yocum A, Dubourg C, Li X, Geng X, Oliver G, Epstein DJ. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet. 2008;40:1348–1353. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Brito JM, Creuzet S. Role of the neural crest in face and brain development. Brain Res Rev. 2007;55:237–247. doi: 10.1016/j.brainresrev.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Hallgrimsson B, Liu W, Parsons TE, Jamniczky HA. Spatial packing, cranial base angulation, and craniofacial shape variation in the mammalian skull: testing a new model using mice. Journal of Anatomy. 2008;212:720–735. doi: 10.1111/j.1469-7580.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Martinez-Abadias N, Esparza M, Sjovold T, Gonzalez-Jose R, Santos M, Hernandez M. Heritability of human cranial dimensions: comparing the evolvability of different cranial regions. J Anat. 2009;214:19–35. doi: 10.1111/j.1469-7580.2008.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Alunni A, Joly JS, Jeffery WR, Retaux S. Expanded expression of Sonic Hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development. 2007;134:845–855. doi: 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Current Opinion in Genetics and Development. 2000;10:262–269. doi: 10.1016/s0959-437x(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Muenke M, Cohen MM., Jr Genetic approaches to understanding brain development: holoprosencephaly as a model. Ment Retard Dev Disabil Res Rev. 2000;6:15–21. doi: 10.1002/(SICI)1098-2779(2000)6:1<15::AID-MRDD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Odent S, Atti-Bitach T, Blayau M, Mathieu M, Aug J, Delezo de AL, Gall JY, Le Marec B, Munnich A, David V, Vekemans M. Expression of the Sonic hedgehog (SHH ) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum Mol Genet. 1999;8:1683–1689. doi: 10.1093/hmg/8.9.1683. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- Reid B, Yang H, Melvin VS, Teketo MM, Williams T. Ectodermal Wnt/beta-catenin signaling shapes the mouse face. Developmental Biology. doi: 10.1016/j.ydbio.2010.11.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JM, Lee SH. About face: signals and genes controlling jaw patterning and identity in vertebrates. Bioessays. 2003;25:554–568. doi: 10.1002/bies.10288. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Aldridge K, DeLeon VB, Panchal J, Kane AA, Marsh JL, Yan P, Cole TM., 3rd Phenotypic integration of neurocranium and brain. J Exp Zool B Mol Dev Evol. 2006;306:360–378. doi: 10.1002/jez.b.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtsmeier JT, Deleon VB. Morphological integration of the skull in craniofacial anomalies. Orthod Craniofac Res. 2009;12:149–158. doi: 10.1111/j.1601-6343.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- Ross C, Henneberg M. Basicranial flexion, relative brain size, and facial kyphosis in Homo sapiens and some fossil hominids. Am J Phys Anthropol. 1995;98:575–593. doi: 10.1002/ajpa.1330980413. [DOI] [PubMed] [Google Scholar]
- Ross CF, Ravosa MJ. Basicranial flexion, relative brain size, and facial kyphosis in nonhuman primates. Am. J. Phys. Anthropol. 1993;91:305–324. doi: 10.1002/ajpa.1330910306. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Catching a Gli-mpse of Hedgehog. Cell. 1997;90:193–196. doi: 10.1016/s0092-8674(00)80325-6. [DOI] [PubMed] [Google Scholar]
- Saha K, Schaffer DV. Signal dynamics in Sonic hedgehog tissue patterning. Development. 2006;133:889–900. doi: 10.1242/dev.02254. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest. 2007;117:1575–1584. doi: 10.1172/JCI32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Whiting CJ, Nimmagadda S, Fu K, Richman JM. Novel skeletogenic patterning roles for the olfactory pit. Development. 2009a;136:219–229. doi: 10.1242/dev.023978. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Smithers LE, Yakob W, Liu KJ. New directions in craniofacial morphogenesis. Dev Biol. 2009b doi: 10.1016/j.ydbio.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10:647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Tian H, Jeong J, Harfe BD, Tabin CJ, McMahon AP. Mouse Disp1 is required in sonic hedgehog-expressing cells for paracrine activity of the cholesterol-modified ligand. Development. 2005;132:133–142. doi: 10.1242/dev.01563. [DOI] [PubMed] [Google Scholar]
- Tian H, Tenzen T, McMahon AP. Dose dependency of Disp1 and genetic interaction between Disp1 and other hedgehog signaling components in the mouse. Development. 2004;131:4021–4033. doi: 10.1242/dev.01257. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, Tada M, Mayor R, Burns AJ, Lupski JR, Hammond P, Beales PL. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung's disease in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh IC, O'Brien TP. Signaling integration in the rugae growth zone directs sequential SHH signaling center formation during the rostral outgrowth of the palate. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong CM. Morphoregulation of avian beaks: comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–1412. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev Biol. 2009;330:200–211. doi: 10.1016/j.ydbio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development. 2010;137:3405–3409. doi: 10.1242/dev.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Andreasen NC, Nopoulos P. J Anat. 2009;214(6):926–936. doi: 10.1111/j.1469-7580.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]