Abstract
Three polycations, polylysine, the polyamine spermine and the polycationic protein lysozyme were used to study the formation, structure, ionic strength sensitivity and dissociation of polycation-induced actin bundles. Bundles form fast, simultaneously with the polymerization of MgATP-G-actins, upon addition of polycations to solutions of actins at low ionic strength conditions. This indicates that nuclei and/or nascent filaments bundle due to attractive, electrostatic effect of polycations and the neutralization of repulsive interactions of negative charges on actin. The attractive forces between the filaments are strong, as shown by the low (in nanomolar range) critical concentration of their bundling at low ionic strength. These bundles are sensitive to ionic strength and disassemble partially in 100mM NaCl, but both the dissociation and ionic strength sensitivity can be countered by higher polycation concentrations. Cys374 residues of actin monomers residing on neighboring filaments in the bundles can be cross-linked by the short span (5.4 Å) MTS-1 (1,1-Methanedyl Bismethanethiosulfonate) cross-linker, which indicates a tight packing of filaments in the bundles. The interfilament cross-links, which connect monomers located on oppositely oriented filaments, prevent disassembly of bundles at high ionic strength. Cofilin and the polysaccharide polyanion heparin disassemble lysozyme induced actin bundles more effectively than the polylysine-induced bundles. The actin-lysozyme bundles are pathologically significant as both proteins are found in the pulmonary airways of cystic fibrosis patients. Their bundles contribute to the formation of viscous mucus, which is the main cause of breathing difficulties and eventual death in this disorder.
Keywords: actin polymerization, actin bundling, cofilin, heparin, interfilament cross-link
Introduction
Cells contain a number of dynamic, higher order structures, including bundles of actin filaments. These structures have indispensable role in cell physiology, in the formation of cytoskeleton, cell division, motility, adhesion and signaling, etc.. Actin bundles may form via cross-linking of individual actin filaments with specific actin-bundling proteins [1] or by attracting the negatively charged filaments to the polycations which “bridge” between the filaments. The group of bivalent cross-linking proteins includes fimbrin, α-actinin, spectrin, fascin, filamin and others. These proteins [1] have two discrete F-actin binding sites and form tightly bound parallel filaments [2, 3].
Polycations, including polycationic proteins or peptides and natural and synthetic polyamines, polymerize actin and induce bundle formation via non-specific electrostatic interactions, by eliminating repulsion between individual G-actin molecules or between actin filaments [4.]. Calponin [5,6], lysozyme [7] MARCKS [8,9], ENA/VASP [10], fesselin [11,12] and myelin basic protein [13] are examples of such polycationic proteins. The natural polyamines spermine and spermidine [14, 15] and the synthetic polyamine polylysine are known to polymerize and bundle actin [16]. The bundling effect of polycations is known to decrease with increasing ionic strength [17], but the kinetics of bundle formation, cross link formation between the filaments in the bundle, interaction of polycation-induced bundles with actin binding and severing proteins remain poorly understood or uncharacterized.
Three polycations, lysozyme, spermine and polylysine were chosen in this study to investigate the various aspects of polycation-induced actin-bundling. Lysozyme is an antibacterial polycationic protein with 9 net positive charges [7]. It is abundant in the airways of cystic fibrosis patients [18], where it forms bundles with actin [19]. These bundles contribute to the accumulation of sputum, a viscous mucus, - the primary cause of bacterial infections and death in this disease [20]. The viscosity of sputum was found to be significantly decreased by the actin severing protein gelsolin [21) and by polyanions [22]. The natural polyamine spermine, which is a small tetravalent cation, is present at millimolar concentrations in proliferating cells [23], and is believed to play significant role in cell cycle, apoptosis, and signal transduction [24, 25]. The synthetic polyamine polylysine is one of the most efficient polymerizing and bundling agents and has been used as model compound in a number of studies on polycation formed actin bundles [4, 26, 27].
In this study, we examined the formation and structure of actin bundles induced by these three polycations using light scattering, low speed sedimentation and cross-linking methods. We found that bundles, which form simultaneously with actin polymerization, have a very low critical concentration at low ionic strength. The bundles are ionic strength sensitive and those induced by low millimolar concentrations of spermine or low micromolar concentrations of lysozyme or polylysine disassemble fast in the presence of 100 mM NaCl. Their ionic strength sensitivity decreases with the increase of polycation concentration. The bundles are partially disassembled by the actin filament severing protein cofilin [28], whose unbundling activity is significantly enhanced by the polysaccharide polyanion heparin. The tightly packed polycation-induced bundles contain oppositely oriented actin filaments, as indicated by the interfilament cross-links between Cys-374 residues by the short span (5.4 Å) MTS-1 cross-linking reagent.
Materials and Methods
Materials
N-(1-pyrene)maleimide was obtained from Molecular Probes (Eugene, OR). Hen lysozyme, ATP, ADP, poly-L-lysine (MW 4000), heparin (unfractionated), dithiotreitol (DTT), N-ethylmaleimide (NEM), spermine and EGTA were purchased from Sigma Chemical Co. (St Louis, MO). 1,1-methaediyl bismethanethiosulfonate (MTS-1), was obtained from Toronto Research Chemicals Inc., North York (Ontario, Canada). Bacterial transglutaminase and N-(4-azido-2-nitrophenyl) putrescine (ANP) were generous gifts from Dr. G. Hegyi (Eotvos Lorand University, Budapest, Hungary).
Preparation of actin
CaATP-G-actin was prepared from the back and leg muscles of rabbit by the method of Spudich and Watt [29] and stored in G-buffer containing 5.0 mM TrisHCl, 0.2 mM CaCl2, 0.2 mM ATP, 0.5 mM β-mercaptoethanol, pH 8.0 (CaATP-G-buffer). MgATP-G-actin was obtained by incubating CaATP-G-actin with 0.2 mM EGTA and 0.1 mM MgCl2 at room temperature for 5 min. MgATP-G-actin (≤10 µM) was used within two hours from the preparation. MgATP-G-actin was diluted for further treatments in MgATP-G-buffer containing 5 mM TrisHCl, 0.1 mM MgCl2, 0.2 mM EGTA, 0.2 mM ATP and 0.5 mM DTT, pH 8.0. Mg-F-actin was polymerized from Mg-ATP-G-actin by addition of 2 mM MgCl2. The concentration of unlabeled skeletal muscle α-G-actins was determined spectrophotometrically using the extinction coefficients E1%290 = 11.5 cm−1. (The optical density of actin was measured in the presence of 0.5 M NaOH, which shifts the maximum of absorbance from 280 nm to 290 nm). Molecular masses of skeletal actin, yeast cofilin and hen lysozyme were assumed to be 42 kDa, 15.9 kDa and 14.3 kDa, respectively.
Cofilin preparation
Yeast cofilin was prepared as previously described [30] with minor modifications. Briefly, wild-type yeast cofilin was expressed in E.coli BL21(DE3) cells under the T7-promoter (pBAT4 plasmid). Cells were grown to a density of 0.6 OD units, induced with 0.4mM IPTG, harvested by centrifugation, resuspended in 20 mM Tris-HCl, pH 7.5, and lysed by sonication. The cell lysate was clarified by centrifugation, the supernatant applied to a QAE-52 column (Pharmacia Biotech) equilibrated with 20 mM Tris-HCl, pH 7.5 and the column developed with a linear 0–0.5 M NaCl gradient. Peak fractions containing cofilin were pooled, concentrated, and applied to a Sephacryl S300 gel-filtration column (Pharmacia Biotech), equilibrated with 10 mM Tris-HCl, 50 mM NaCl, pH 7.5. The peak fractions were pooled and polished by MonoQ ion-exchange chromatography using a buffer system similar to that described for the QAE-52 chromatography.
Chemical modification
Labeling of Mg-F-actin at Cys-374 with pyrene maleimide was carried out according to Kouyama and Mihashi [31] with some modifications. CaATP-G-actin was filtered through a PD-10 column equilibrated with β-mercaptoethanol free Ca-ATP-G-buffer. After filtration, actin (1.0 mg/ml) was polymerized by 2.0 mM MgCl2 and 100 mM KCl at room temperature for 30 min, and reacted with pyrene maleimide (16 µg/ ml) on ice, for one hour. The reaction was terminated with 1.0 mM DTT. The labeled F-actin was centrifuged at 38K rpm for two hours, then the pellet was resuspended in Ca-ATP-G-buffer and depolymerized for over 36 hours at 4 °C. Finally, actin was centrifuged again at 38K rpm for two hours. The supernatant contained the purified pyrene-labeled CaATP-G-actin. The concentration of modified actin was determined by the procedure of Bradford [32] using unmodified actin as a standard. The extent of labeling, which was measured by using pyrene extinction coefficient E344 nm= 22000 cm−1M−1, was ~100%. G-actin was labeled by ANP, polymerized by 2 mM MgCl2 and photo cross-linked according to Hegyi et al. [33]. The extent of labeling was calculated by extinction coefficient E470=5400 cm−1M−1 of ANP.
Covalent cross-linking
Cross-linking of two actin monomers between Cys-374 residues at the C-terminus was carried out with the MTS-1 disulfide reagent. This reagent has a cross-linking span of 5.4 Å and has been used before for intramolecular cross-linking of actin cysteine residues [34]. Prior to the cross-linking reaction, dithiothreitol was removed from CaATP-G-actin over a PD-10 column equilibrated with β-mercaptoethanol free Ca-ATP-G-buffer. Then CaATP-G-actin was transformed to MgATP-G-actin, polymerized by 2 mM MgCl2 and finally bundled by polycations. MTS-1 was added to bundles of Mg-F-actin at 0.75:1.0 molar ratios to actin monomers. The cross-linking reaction was stopped 30–60 sec after MTS-1 addition by blocking the free SH groups with 1.0 mM NEM. The cross-linked samples were analyzed by 12 % SDS-PAGE and quantitatively evaluated by densitometry.
Fluorescence and light scattering measurements
The time course of pyrene-labeled actin polymerization was monitored by measuring fluorescence increase (with 365 nm excitation and 386 nm emission wavelengths) in a PTI spectrofluorometer (Photon Technology Industries, South Brunswick, NJ). The time course of light scattering changes was also measured in a PTI spectrofluorometer or in an Applied Photophysics stopped-flow apparatus (Leatherhead, Surrey, UK), with both excitation and emission wavelengths adjusted to 450 nm. All fluorescence and light scattering measurements were carried out at 22 C°.
Monitoring bundling by low speed sedimentation
After addition of polycations, actin samples were centrifuged at 20800 rcf for 8 min in an Eppendorf centrifuge. The supernatants were analyzed by SDS-PAGE. The measurements were carried out at 22 C°.
Analysis of the kinetics of assembly and disassembly of bundles
GraphPad Prism software was used for the analysis. The explicit forms of the equations used are the following: Two phase exponential association: Y+Ymax1*(1−exp(−K1*X))+Ymax2*(1−exp(−K2*X)); one phase exponential decay: Y=Span*(exp(−K*X)+ Plateau.
Results
Bundle formation during polymerization of MgATP-G-actin by polycations
Bundles are formed when G-actin is polymerized at low ionic strength by polycations [35]. Bundle formation can be followed either by low speed sedimentation [15] or by the increase in light scattering [4]. We compared the course of MgATP-G-actin polymerization and bundling by following these processes via increases in pyrene fluorescence and light scattering, respectively. This is feasible because pyrene fluorescence measures actins polymerization but is essentially insensitive to its bundling (fluorescence increases ~10-fold upon polymerization but only ~10% upon bundling of F-actin), while the light scattering increase is relatively small upon actin polymerization (~2.8-fold) compared to the large change (~11-fold) caused by the bundling of actin filaments. Filament bundling by polylysine, lysozyme and spermine proceeds approximately at a similar or somewhat faster rate than filament elongation. (Figure 1; only lysozyme data is shown). These results indicate that in the polycation-induced polymerization even the nascent or very short filaments assemble immediately following the nucleation step, with the elongation of the filaments continuing also after their bundling.
Figure 1. Polymerization and bundle formation of 2 µM pyrene-labeled MgATP-G-actin upon addition of 2 µM lysozyme at low ionic strength.

Polymerization and bundling was monitored by following pyrene fluorescence and light scattering changes, respectively, as described in Materials and Methods. Curve 1, bundling; curve 2, polymerization. Addition of 2 µM lysozyme is shown by an arrow.
Polycation induced bundle formation from MgF-actin
Bundle formation upon addition of polycations to preformed MgF-actin filaments (Figure 2a and b) is very fast, much faster than their formation from MgATP-G-actin (Figure 1), at low ionic strength conditions. The bundles formed at relatively low concentrations of polycations are sensitive to the ionic strength and disassemble “unbundle” very fast upon addition of 100 mM NaCl. The formation of bundles from 2 µM Mg-F-actin upon addition of 4 µM polylysine (Figure 2b) appears biphasic and could be fitted by two kinetic steps with 0.83 and 0.052 sec−1 rate constants,. The unbundling of the fresh polylysine-induced bundles by 100 mM NaCl was much faster than their formation and could be described by a single exponential decay, with a rate constant of 140 sec−1 (Figure 2c).
Figure 2. Bundle formation of Mg-F-actin by polycations and dissolution of Mg-F-actin bundles by 100 mM NaCl.



Bundling was monitored by light scattering changes. (a), Bundling of 2 µM MgF-actin by 4 µM lysozyme (1, pink), 0.5 mM spermine (2, dark blue) and 4 µM polylysine (3, green); unbundling by 100 mM NaCl. Addition of polycations and NaCl is shown by arrows. Light scattering change was followed in a PTI fluorometer. (b), Bundling of 2 µM MgF-actin by 4 µM polylysine. Green curve, fluorometer tracing; black curve, fitting to a two phase association. Light scattering was measured in a stopped flow fluorometer. (c), 100 mM NaCl dissolves bundles of 2 µM MgF-actin formed by 4 µM polylysine. Triangles, fluorometer readings; curve, fitting to a single exponential decay. Light scattering was measured in a stopped flow apparatus as described in Materials and Methods.
The extent of bundling of actin filaments by polycations, as measured by low speed sedimentation experiments, was dependent both on the nature and the concentration of the polycations, on the ionic strength of the solution (Figure 3a). At physiological ionic strength (100 mM NaCl), about one order of magnitude higher concentration of polycation was needed for the bundling than at low ionic strength. Lysozyme was the most efficient bundling agent at low while polylysine at physiological ionic strength. Half maximal bundling of 10 µM Mg-F-actin was obtained at low ionic strength at 3.1, 6.0 and 310 µM while at physiological ionic strength at 30, 20 and 3650 µM for lysozyme, polylysine and spermine, respectively. These results clearly show the competing effect of ionic strength and polycation concentration on bundling.
Figure 3. Bundling of Mg-F-actin by polycations in the presence and absence of 100 mM NaCl.

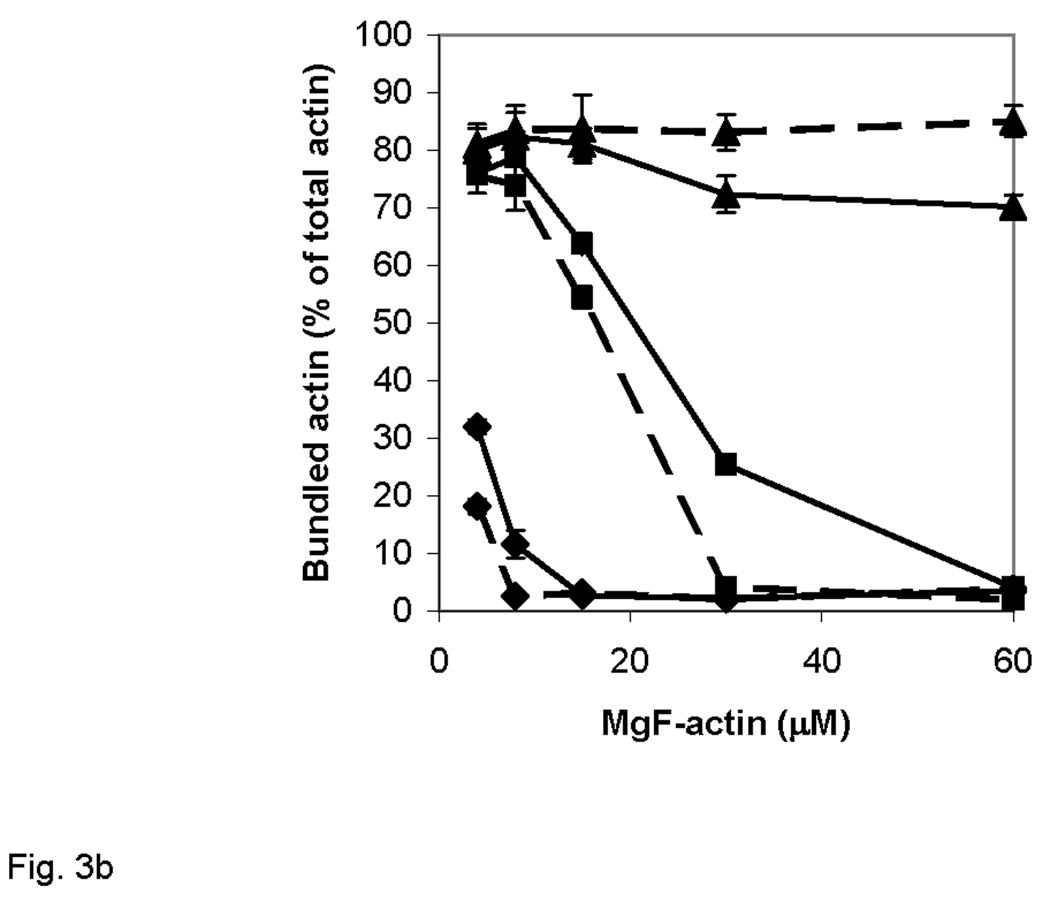
Actin bundling was monitored by low speed sedimentation as described in Materials and Methods. Error bars correspond to mean errors from three independent experiments. (a), Effects of polycation concentration and ionic strength on the bundling. Diamonds, lysozyme; squares, polylysine; full circles, spermine. Bundling in the presence and absence of 100 mM NaCl is shown in pink and dark blue, respectively. (b) Effect of F-actin concentration on its bundling. Solid line, polylysine; dashed line, lysozyme. Polycation concentration is shown by symbols: diamonds, 10 µM; squares 25 µM; triangles, 50 µM polycation.
We studied next the F-actin concentration dependence of bundling at physiological ionic strength conditions. In these experiments 10–50 µM lysozyme or polylysine were added to 8–60 µM Mg-F-actin in the presence of 100 mM NaCl and the degree of bundling was measured by low speed centrifugation (Figure 3b). The concentration of polycation needed for bundle formation increased with the concentration of MgF-actin, however, the dependence of bundling on actin concentration was less pronounced than that on the polycation concentration.
We measured the critical concentration of Mg-F-actin bundling at low ionic strength using polylysine as a model polycation. To this end, polylysine (6 µM) was added to 100 nM Mg-F-actin, and serial dilutions were carried out after maturing the bundles. Each sample was centrifuged at low speed and the protein content in the pellet was determined yielding the critical concentration for bundling of 1.8 nM actins. The extremely low critical concentration of the polylysine-bundled actin indicates that the affinity between actin filaments is very high in the presence of polycation counter-ions.
Cofilin disassembles polycation-induced actin bundles
Actin filament bundles are major contributors to the viscosity of sputum in the airways of cystic fibrosis patients. Gelsolin, an actin filament severing protein was found to significantly reduce the viscosity of sputum [21]. Here, we studied the effect of another actin filament severing protein, cofilin [28], on the stability of polycation-induced bundles. Addition of 8 µM cofilin to 8 µM lysozyme- or polylysine-induced bundles at physiological ionic strength (Fig. 4a) significantly reduced, but did not eliminate completely the bundled actin. The degree of disassembly decreased with the increase of polycation concentration used for bundling indicating competition between cofilin and the polycations. The unbundling effect of cofilin was stronger on lysozyme- than on polylysine-induced bundles. The disassembly of bundles was rather fast, it took about one min upon addition of cofilin to reach new equilibrium in light scattering measurements (Fig. 4b). Essentially the same amount of actin remained bundled after reaching equilibrium when cofilin was added to lysozyme bundled actin or lysozyme was added to F-actin, which already contained cofilin (Fig. 4b). Finally we checked the role of severing in the disassembly by inhibiting the former through ANP-cross-linking of actin bundles (Fig 4c). ANP covalently cross-links protomers along the long pitch helix of the actin filaments [33], decreasing their severing and, therefore, completely inhibiting the unbundling by cofilin.
Figure 4. Dissolution of polycation induced actin bundles by cofilin.



(a), 8 µM cofilin was added to 8 µM Mg-F-actin bundled by 16–100 µM lysozyme or polylysine in the presence of 100 mM NaCl. Bundling was monitored by low speed sedimentation as described in Materials and Methods. Diamonds, polylysine without cofilin; squares, polylysine and cofilin; triangles, lysozyme without cofilin; full circles, lysozyme and cofilin. (b) Effect of 8 µM cofilin on the light scattering of 8 µM Mg-F-actin bundled by 50 µM lysozyme (black trace) and the effect of 50 µM lysozyme on the scattering of 8 µM Mg-F-actin in the presence of 8 µM cofilin (half tone trace). Lysozyme and cofilin addition, block and simple arrows, respectively. (c) Effect of cofilin on the bundling of 4 µM ANP-Mg-F-actin (squares) and cross-linked ANP-Mg-F-actin (diamonds). Bundling was monitored by low speed sedimentation as described in Materials and Methods. Error bars correspond to mean errors from three independent experiments.
In order to enhance the disassembly of the bundles, we added heparin together with cofilin to the system (Fig 5). Heparin, which is a polysaccharide polyanion, has similar destabilizing effect on cystic fibrosis sputum [36] as the anionic polyamino acids [22]. We added increasing concentration of heparin to lysozyme-bundled actin filaments in the absence and presence of cofilin (Fig 5). Heparin also dissociates F-actin bundles, however, when cofilin and heparin were added together the extent of disassembly increased significantly. Low concentration (0.3 mg/ml) of heparin, which alone has only a slight unbundling effect, increased the cofilin induced disassembly of bundles by 50%.
Figure 5. Effect of heparin on the dissolution of the bundles of 6 µM Mg-F-actin by 6 µM cofilin.

Mg-F-actin was bundled by 50 µM lysozyme in the presence of 100 mM NaCl. Unbundling was followed by low speed sedimentation as described in Materials and Methods. Diamonds, cofilin absent; squares, cofilin present. Error bars correspond to mean errors from three independent experiments.
Polycation-induced bundles are stabilized by cross-linking “interfilament antiparallel dimers”
It has been proposed before that polycation-induced actin bundles contain antiparallel filaments (35). Such oppositely oriented filaments in the bundle should position a fraction of actin protomers in these filaments in a spatial arrangement similar to that found in antiparallel dimers, except that in the present case these would be interfilament (proximity) antiparallel dimers (IAP). The presence of such IAP was detected in polylysine-bundled Mg-F-actin by MTS-1, which cross-linked Cys-374 residues from two actin protomers, but no IAP (or AP) dimer was found in free, unbundled Mg-F-actin filaments [35]. Here, we show the presence of IAP dimers in filament bundles induced by all the three polycations used in this study (Figure 6). The amount of IAP dimers increased with the increasing polycation concentration. The highest amount of IAP dimer was found in bundles induced by polylysine (maximum 15.6% of total actin), less in spermine-induced (maximum 11.6% of total actin) and much less in lysozyme-induced bundles (maximum 2.7% of total actin). The relatively low amount of IAP dimer in lysozyme-bundled Mg-F-actin is probably due to intercalation of lysozyme between the actin filaments [19], which may increase the distance between the filaments and inhibits the formation of IAP dimers.
Figure 6. Detection of antiparallel interfilament dimers in Mg-F-actin bundles by MTS-1 cross-linking.

4µM Mg-F-actin - bundled by polycations - treated with 3 µM MTS-1 and analyzed by SDS-PAGE as described in Materials and Methods. Squares, polylysine; diamonds, lysozyme; full circles, spermine. Error bars correspond to mean errors from three independent experiments.
The effect of cross-linking AP dimers on the stability of bundles is examined in Figure 7. The bundles of 4 µM Mg-F-actin formed by lysozyme at low ionic strength were disassembled by 100 mM NaCl (as in Figure 2a). However, after cross-linking these bundles persisted in the presence of 100 mM NaCl. The reversal of cross-links with DTT leads to the dissolution of bundles by 100 mM NaCl, analogous to the disassembly of uncross-linked (by MTS-1) bundles (similar results – not shown - were obtained also with polylysine and spermine induced bundles). Both cross-linked and uncross-linked lysozyme-induced bundles contained lysozyme in addition to actin, but lysozyme disappeared from the cross-linked bundles after addition of NaCl (Figure 7). This suggests that at physiological ionic strength the affinity between actin and lysozyme is mainly electrostatic.
Figure 7. Dissolving MTS-1 cross-linked and uncross-linked Mg-F-actin bundles by DTT and NaCl.

4µM Mg-F-actin was bundled by 6 µM lysozyme at low ionic strength and cross-linked with 3 µM MTS-1 as described in Materials and Methods. NaCl (100mM) and DTT (1 mM) were added and the samples were centrifuged at 16K for 8 min. The supernatants were analyzed for the actin and lysozyme content by SDS-PAGE. Additions to 4µM Mg-F-actin and 6 µM lysozyme: (A), none; (B), 100 mM NaCl; (C), 100 mM NaCl and 1 mM DTT; (D), 3 µM MTS-1; (E), 100 mM NaCl and 3 µM MTS-1; (F), 3 µM MTS-1, 100 mM NaCl and 1 mM DTT and. Amount of actin in bundles as percentage of total actin, empty bars; amount of lysozyme in bundles as percentage of total lysozyme, full bars. Error bars correspond to mean errors from three independent experiments.
Discussion
Polycations efficiently polymerize and bundle actin. The polycation-induced bundles could be important elements of the cellular actin dynamics, participating in the formation of cytoskeletal structures. We examined the kinetics of polymerization and bundle formation following the addition of polycations to MgATP-G-actin at low ionic strength. The two processes proceed simultaneously; however, the initial rate of bundling appeared faster than that of the filament elongation. This shows that the bundling process starts during the nucleation of actin filaments, via assembly of nuclei or the initial short filaments. The strong attraction between actin molecules and filaments due to electrostatic interactions with polycations is indicated also by the low critical concentration of polylysine bundled actin at low ionic strength conditions.
As reported earlier [4, 15, 17], the extent of actin filament bundling increases with polycation concentration both at low and physiological ionic strength,. At physiological ionic strength about an order of magnitude higher polycation concentration is needed for filament bundling than at low ionic strength, as the monovalent cations (at relatively high concentrations) compete with polyvalent cations for the negatively charged actin and screen the effect of polycations. The bundling efficiency of polycations correlates with their net positive charge. However, the electrostatic interactions of positively charged proteins with actins may be enhanced by specific hydrophobic interactions, as is most likely the case for the calponin-actins system.
The very fast bundling of Mg-F–actin filaments upon addition of polycations (at low ionic strength) suggests that these bundles must contain oppositely oriented actin filaments. The bundling is too fast to allow for the randomly oriented filaments to pre-orient by diffusion into parallel filaments. This is confirmed by our findings that Cys-374 of an actin protomer can be cross-linked to Cys-374 on another actin protomer in the bundle. According to the known structure of actin filaments [37], such cross-links by MTS-1 cannot occur between Cys-374 residues within individual filaments and must involve actins protomers from neighboring filament in the bundle. The proposed arrangement is similar to that in pPDM cross-linked actin paracrystals formed by 20–50 mM MgCl2 [38].
The disassembly of bundles (Figure 2c) is even faster than their formation (Figure 2c) from actin filaments. The assembly, which is a bimolecular reaction, also involves movement and adjustment of filaments relative to each other, and thus follows biphasic kinetics. This does not happen during the disassembly, which is unimolecular. This is probably the reason for the disassembly being faster than the assembly of bundles.
The short span (5.4 Å) of the MTS-1 cross-linker of Cys-374 residues located on neighboring filaments in polycation induced actin bundles (Figure 6 and [35]) indicates a tight packing of these filaments in the bundles. The degree of cross-linking increased with the polycation concentration and depended also on the nature of the polycation. A relatively high degree (~10–15%) of cross-linking was obtained in the actin-polylysine and actin-spermine systems, but less cross-linking (~2.5%) was detected in the actin-lysozyme bundles. The low level of cross-linking in the later case is presumably caused by the intercalation of lysozyme between the actin filaments [19, 39], which increases the interfilament distance. Chemically cross-linked bundles, unlike the uncross-linked ones, do not disassemble in the presence of 100 mM NaCl (Fig. 7). This supports the assumption that these are interfilament cross-links, which connect neighboring and oppositely oriented filaments in the bundle.
Cofilin, an actin filament severing and depolymerization promoting protein [28, 40, 41] was found to disassemble lysozyme- and polylysine-induced bundles. Its effect is maximal at equimolar concentration of cofilin to actin. At physiological ionic strength, cofilin disassembles bundles fast and efficiently, especially the lysozyme-induced bundles. Unbundling by cofilin is based mostly on its severing action, since intrafilament cross-linking completely inhibits the cofilin-induced disassembly of bundles. One may speculate that the three net negative charges of yeast cofilin [42, 43] can also facilitate disassembly. Cofilin’s charges add to the net negative charge of actin protomers (over 50%) and, therefore, increase the polycation concentration required for the bundling of actin. The polysaccharide polyanion heparin, which is widely used for dissolving blood clots, also dissolves lysozyme-induced actin bundles by competing with the negatively charged actin for the polycation [36]. The unbundling effect of heparin and cofilin is cooperative since small amounts of heparin, which alone only slightly promote disassembly, facilitate strongly the unbundling by cofilin.
Bundles of actin filaments induced by two biologically relevant polycations, spermine and lysozyme, were studied in this work. Spermine bundles actin filaments only at millimolar concentration (4 mM) at physiological ionic strength. However, the presence of relatively high concentration of spermine during sperm activation and neuron repair [44, 45], which in part rely on actin bundles, raises the possibility that spermine contributes to bundle formation in vivo. The physiological or pathophysiological role of lysozyme is more likely, since both lysozyme and actin are present in high concentration in the pulmonary airways of cystic fibrosis patients [18, 19]. Actin-lysozyme bundles are formed in the airways, which are stable at physiological ionic strength, and can contribute significantly to the accumulation of viscous sputum [21], which is the primary cause of long-term bacterial infections and respiratory failure observed in this ailment. Cofilin, which is present also in extracellular fluids [46], disassembles lysozyme-actin bundles and may have the potential to decrease the viscosity of the sputum and to alleviate the symptoms of cystic fibrosis. The unbundling of actin by cofilin is significantly increased by the heparin polyanion.
Acknowledgement
This work was supported by grant from USPHS (GM 077190) to E.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Puius YA, Mahoney NM, Almo SC. The modular structure of actin-regulatory proteins. Curr. Opin. Cell Biol. 1998;10:23–34. doi: 10.1016/s0955-0674(98)80083-5. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa R, Fecheimer M. The structure, function and assembly of actin filament bundles. Int. Rev. Cytol. 1997;175:29–90. doi: 10.1016/s0074-7696(08)62125-7. [DOI] [PubMed] [Google Scholar]
- 3.Bartles JR. Parallel actin bundles and their multiple actin-binding proteins. Curr. Opin. Cell Biol. 2000;12:72–78. doi: 10.1016/s0955-0674(99)00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang JX, Janmey PA. The polyelectrolyte nature of F-actin and the mechanism of actin bundle formation. J. Biol. Chem. 1996;271:8556–8563. doi: 10.1074/jbc.271.15.8556. [DOI] [PubMed] [Google Scholar]
- 5.Tang JX, Szymanski PT, Janmey PA, Tao T. Electrostatic effects of smooth muscle calponin on actin assembly. Eur. J. Biochem. 1997;247:432–440. doi: 10.1111/j.1432-1033.1997.00432.x. [DOI] [PubMed] [Google Scholar]
- 6.Winder SJ, Walsh MP. Calponin: thin filament-linked regulation of smooth muscle contraction. Cell Signal. 1993;5:677–686. doi: 10.1016/0898-6568(93)90029-l. [DOI] [PubMed] [Google Scholar]
- 7.Sanders LK, Xian W, Guaqueta C, Strohman MJ, Vrasich CR, Luijten E, Wong GCL. Control of electrostatic interactions between F-actin and genetically modified lysozyme in aqueous media. Proc Natl. Acad. Sci. U.S.A. 2007;104:15994–15999. doi: 10.1073/pnas.0705898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubb MR, Lenox RH, Edison AS. Phosphorylation-dependent conformational changes induce a switch in the actin-binding function of MARCKS. J. Biol. Chem. 1999;274:36472–36478. doi: 10.1074/jbc.274.51.36472. [DOI] [PubMed] [Google Scholar]
- 9.Yarmola EG, Edison AS, Lenox RH, Bubb MR. Actin filament cross-linking by MARCKS: characterization of two actin-binding sites within the phosphorylation site domain. J. Biol. Chem. 2001;276:22351–22358. doi: 10.1074/jbc.M101457200. [DOI] [PubMed] [Google Scholar]
- 10.Harbeck B, Huttelmaier S, Schluter K, Joskusch BM, M B, Illenberger S. Phosphorylation of vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem. 2000;275:30817–30825. doi: 10.1074/jbc.M005066200. [DOI] [PubMed] [Google Scholar]
- 11.Beall B, Chalovich JM. Fesselin, a synaptopodin-like protein, stimulates actin nucleation and polymerization. Biochemistry. 2001;40:14252–14259. doi: 10.1021/bi011806u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeter M, Chalovich JM. Ca2+-calmodulin regulates fesselin-induced actin polymerization. Biochemistry. 2004;43:13875–13882. doi: 10.1021/bi0487490. [DOI] [PubMed] [Google Scholar]
- 13.Boggs JM. Myelin basic protein: a multifunctional protein. Cell. Mol. Life Sci. 2006;63:1945–1961. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oriol-Audit C. Polyamine-induced actin polymerization. Eur. J. Biochem. 1978;87:371–376. doi: 10.1111/j.1432-1033.1978.tb12386.x. [DOI] [PubMed] [Google Scholar]
- 15.Sowa GZ, Cannel DS, Liu AJ, Reisler E. Polyamine induced bundling of F-actin. J. Phys. Chem. B. 2006;110:22279–22284. doi: 10.1021/jp063371w. [DOI] [PubMed] [Google Scholar]
- 16.Brown SS, Spudich JA. Nucleation of polar actin filament assembly by a positively charged surface. J. Cell Biol. 1979;80:499–504. doi: 10.1083/jcb.80.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang JX, Ito T, Tao T, Traub P, Janmey PA. Opposite effects of electrostatics and steric exclusion on bundle formation by F-actin and other filamentous polyelectrolytes. Biochemistry. 1997;36:126000–126007. doi: 10.1021/bi9711386. [DOI] [PubMed] [Google Scholar]
- 18.Brogan TD, Ryley HC, Neale L, Yassa J. Soluble proteins of bronchopulmonary secretions from patients with cystic fibrosis, asthma, and bronchitis. Thorax. 1975;30:72–79. doi: 10.1136/thx.30.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guaqueta C, Sanders LK, Wong GCL, Luijten E. The effect of salt on self-assembled actin-lysozyme complexes. Biophys. J. 2001;90:4630–4638. doi: 10.1529/biophysj.105.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh MJ, Smith AE. Cystic fibrosis. Sci. Am. 1975;273:52–59. doi: 10.1038/scientificamerican1295-52. [DOI] [PubMed] [Google Scholar]
- 21.Vasconcellos CA, Allen PG, Wohl ME, Drazen JM, Janmey PA, Stossel TP. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994;263:969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- 22.Tang JX, Wen Q, Bennet A, Kim B, Sheits CA, Bucki R, Janmey PA. Anionic poly(amino acid)s dissolve F-actin and DNA bundles, enhance DNase activity and reduce the viscosity of cystic fibrosis sputum. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L599–L605. doi: 10.1152/ajplung.00061.2005. [DOI] [PubMed] [Google Scholar]
- 23.Monti MG, Pernecco L, Manfredini R, Frassineti C, Barbieri D, Marveti G, Ghiaroni S. Inhibition of cell growth by accumulated spermine is associated with transient alterations of cell cycle progression. Life Sci. 1996;58:2065–2072. doi: 10.1016/0024-3205(96)00200-7. [DOI] [PubMed] [Google Scholar]
- 24.Brooks WH. Polyamine involvement in the cell cycle, apoptosis and autoimmunity. Med. Hypothesises. 1995;44(1995):331–338. doi: 10.1016/0306-9877(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 25.Pignati C, Tantini B, Stefanelli C, Flamigni F. Signal transduction pathways linking polyamines to apoptosis. Amino Acids. 2004;27:359–365. doi: 10.1007/s00726-004-0115-3. [DOI] [PubMed] [Google Scholar]
- 26.Kwon HJ, Tanaka Y, Kakugo A, Shikinaka K, Furukawa H, Osada Y, Gong JP. Anisotropic nucleation growth of actin bundle: A model for determining the well-defined thickness of bundles. Biochemistry. 2006;45:10313–10318. doi: 10.1021/bi060721w. [DOI] [PubMed] [Google Scholar]
- 27.Shikinaka K, Kwon HJ, Kakugo A, Furukawa H, Osada Y, Gong JP, Aoyama Y, Nishioka H, Jinnai H, Okajima T. Observation of the three-dimensional structure of actin bundles formed with polycations. Biomacromolecules. 2008;9:537–542. doi: 10.1021/bm701068n. [DOI] [PubMed] [Google Scholar]
- 28.Bamburg JR. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell. Dev. Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 29.Spudich JA, Watt S. Regulation of skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4876. [PubMed] [Google Scholar]
- 30.Bobkov AA, Muhlrad A, Kokabi K, Vorobiev S, Almo SC, Reisler E. Structural Effects of Cofilin on Longitudinal Contacts in F-actin. J. Mol. Biol. 2002;323:739–750. doi: 10.1016/s0022-2836(02)01008-2. [DOI] [PubMed] [Google Scholar]
- 31.Kouyama T, Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur. J. Biochem. 1981;114:33–38. [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Hegyi G, Mak M, Kim E, Elzinga M, Muhlrad A, Reisler E. Intrastand cross-linked actin between Gln-41 and Cys-374. I. Mapping of sites cross-linked in F-actin by N-(4-azido-2-nitrophenyl) putrescine. Biochemistry. 1998;37:17784–17792. doi: 10.1021/bi981285j. [DOI] [PubMed] [Google Scholar]
- 34.Shvetsov A, Stamm JD, Phillips M, Warshaviak D, Altenbach C, Rubenstein PA, Hideg K, Hubbell WL, Reisler E. Conformational dynamics of loop 262–274 in G- and F-actin. Biochemistry. 2006;45:6541–6549. doi: 10.1021/bi052558v. [DOI] [PubMed] [Google Scholar]
- 35.Grintsevich EE, Phillips M, Pavlov D, Phan M, Reisler E, Muhlrad A. Antiparallel dimer and actin assembly. Biochemistry. 2010;49:3919–3927. doi: 10.1021/bi1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broughton-Head VJ, Shur J, Carrol MP, Smith JR, Shute JK. Unfractionated heparin reduces the elasticity of sputum from patients with cystic fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L1240–L1249. doi: 10.1152/ajplung.00206.2007. [DOI] [PubMed] [Google Scholar]
- 37.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 38.Millonig R, Salvo H, Aebi U. Probing actin polymerization by intermolecular crosslinking. J. Cell Biol. 1988;106:785–796. doi: 10.1083/jcb.106.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders LK, Guaqueta C, Angelini TE, Lee J-W, Slimmer SC, Luijten E, Wong GCL. Structure and stability of self-assembled actin-lysozyme complexes in salty water. Phys. Rev. Lett. 2005;95:108302. doi: 10.1103/PhysRevLett.95.108302. [DOI] [PubMed] [Google Scholar]
- 40.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov D, Muhlrad A, Cooper J, Wear M, Reisler E. Severing of F-actin by Yeast Cofilin is pH-independent. Cell Motility & Cytoskeleton. 2006;63:533–542. doi: 10.1002/cm.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canfield RE. The amino acid sequence of egg white lysozyme. J. Biol. Chem. 1963;238:2698–2707. [PubMed] [Google Scholar]
- 43.Pope BJ, Zierler-Gould KM, Kuhne R, Weeds AG, Ball LJ. Solution structure of human cofilin. J. Biol. Chem. 2004;279:4840–4848. doi: 10.1074/jbc.M310148200. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber RC, Boeshore KL, Laube G, Veh RW, Zigmond RE. Polyamines increase in sympathetic neurons and non-neuronal cells after axotomy and enhance neurite outgrowth in nerve growth factor-primed PC12 cells. Neuroscience. 2004;128:741–749. doi: 10.1016/j.neuroscience.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Breibart H, Rubinstein S, Lax Y. Regulatory mechanisms in acrosomal exocytosis. Rev. Reprod. 1997;2:165–174. doi: 10.1530/ror.0.0020165. [DOI] [PubMed] [Google Scholar]
- 46.Greening DW, Simpson RJ. A centrifugal ultrafiltration strategy for isolating the low-molecular weight (<or=25K) component of human plasma proteome. J. Proteomics. 2010;73:637–648. doi: 10.1016/j.jprot.2009.09.013. [DOI] [PubMed] [Google Scholar]


