Abstract
In addition to mitochondria, NADPH oxidase (NOX) is a source of oxidative stress, which can induce oxidative damage in Alzheimer’s disease (AD). For this reason, several groups have investigated the effect of its inhibition. In AD mice, NADPH oxidase 2 (NOX2) deficiency improved behavior and cerebrovascular function, and reduced oxidative stress. In our study, we administered the NOX inhibitor apocynin to Tg19959 mice, and found that it did not improve cognitive and synaptic deficits, and did not decrease amyloid deposition, microgliosis and hyperphosphorylated tau. However, apocynin reduced carbonyl levels in the cerebral cortex but not the hippocampus, which may have not been sufficient to ameliorate symptoms. Also, the reduction of NOX-mediated oxidative stress may not be sufficient to prevent AD, since other sources of reactive oxygen species such as mitochondria may be more important.
Keywords: Alzheimer’s disease, Apocynin, NADPH oxidase, Amyloid plaques, Oxidative stress
Introduction
Increased oxidative stress occurs early in Alzheimer’s disease (AD) pathogenesis, in addition to mitochondrial dysfunction. NADPH oxidase (NOX) is another source of oxidative stress via superoxide production inside and outside the cell [1, 13, 16]. For its activation, NOX requires the presence of several cytoplasmic factors, such as p47phox, p67phox, and Rac at the cell membrane. In human AD brains, levels of the cytoplasmic factors in the membrane fraction were elevated compared to control brains [11]. In addition, NOX2 is involved in Aβ-induced cerebrovascular dysregulation [9]. Its deficiency reduced oxidative stress, and improved cerebrovascular function and behavior in transgenic AD mice [10].
The NOX inhibitor apocynin [12, 14] also had neuroprotective effects in in vivo mouse models of ischemia [15], and amyotrophic lateral sclerosis [7]. Therefore, we studied the effects of apocynin in a transgenic mouse model of AD, the Tg19959 mice. Tg19959 mice, which overexpress the human APP with two mutations, develop amyloid plaques in the cortex, the hippocampus and the amygdala at 2–3 months of age, together with cognitive deficits at 4–5 months of age [5]. We have also previously reported that Tg19959 mice had increased synaptic deficits and oxidative stress, measured by synaptophysin [5] and carbonyl levels respectively [6]. In our study, Tg19959 mice and their wild-type littermates were given apocynin at 300mg/kg of body weight in the drinking water. Administration started at 1 month of age until 5 months of age, age when Tg19959 mice shown amyloid pathology.
Material and methods
Transgenic animals and treatment
Tg19959 mice were obtained from Dr. George Carlson (McLaughlin Research Institute, Great Falls, MT, USA). Tg19959 mice were constructed by injecting FVB x 129S6 F1 embryos with a cosmid insert containing human APP695 with two familial AD mutations (KM670/671NL and V717F), under the control of the hamster PrP promoter.
Tg19959 mice and their wild-type littermates were randomly treated with vehicle (water) or apocynin (Sigma, St. Louis, MO, USA) in the drinking water from 1 to 5 months of age. Apocynin was given at 300mg/kg of body weight and dissolved in heated water. This dose was chosen according to the study performed in ALS mice, in which apocynin was neuroprotective [7]. Behavioral analyses were performed at 4 months of age, and brain histopathology and biochemistry were then assessed on the same animals.
All experiments were approved by the Institutional Animal Care and Use Committee.
Behavioral study
Spatial learning and memory were assessed in the Morris water maze. An opaque basin (diameter: 120 cm; height of the wall: 51 cm) was filled with opacified water (23°C). During the acquisition period, extra-maze visual cues, such as light fixtures and wall posters, were arranged in the room. The hidden platform was located in the middle of the northwest (NW) quadrant, 1 cm beneath water level. Each day, mice were placed next to and facing the wall of the basin in 4 different starting positions: north, east, south, and west, corresponding to 4 successive trials. Distances before reaching the platform were recorded for 5 days with a video tracking system (Ethovision 3.0, Noldus Technology, Attleborough, MA, USA). After each trial, animals were placed in a plastic holding cage filled with paper towels to keep them dry and warm, with an inter-trial interval of 30 min. Whenever the mouse failed to reach the platform within the maximally allowed time of 60 sec, it was placed on the platform by the experimenter for 5 sec. A probe trial was assessed 24 h after the acquisition period, removing the platform from the pool. The mice were released on the north side, and the percentage of time spent in each quadrant was measured. To ensure that any differences were not due to visual deficits, the visible platform version of the water maze was performed two hours after the probe trial. A pole (13 cm) was added on the platform. Animals were tested during 2 sessions of 4 trials where the platform location was moved after each trial. In the first session, mice were released from the north side of the pool, and in the second session mice were released from the south side of the pool. The duration of a single trial was 60 sec with an inter-trial interval of 20 min. Distances before reaching the platform were recorded and averaged.
Immunohistochemistry for Aβ42 deposits and microgliosis
After anesthesia by intraperitoneal injection of sodium pentobarbital, half of the mice were transcardially perfused with ice cold 0.9% sodium chloride and with 4% paraformaldehyde (PFA) in 1 mM phosphate buffer pH 7.4 for 24 hr. Brains were removed, post-fixed in 4% PFA, and stored in cryoprotectant solution (30 % glycerol, 30% ethylene glycol in 20 mM phosphate buffer pH 7.4) until further processing. Regions analyzed included the retrosplenial/motor cortex and CA1/dentate region of the hippocampus. The retrosplenial/motor cortex was analyzed in five sections (350 μm apart) per mouse beginning at the level of bregma –1.06 to bregma –1.94. The CA1/dentate region was analyzed in five sections (350 μm apart) per mouse beginning at the level of bregma –1.34 to bregma –2.7.
For Aβ42 deposits, sections were pretreated with 50% formic acid for 5 min before labeling with anti-Aβ42 rabbit polyclonal antibody AB5078P (1:1,000) (Chemicon, Temecula, CA, USA). To examine microgliosis, adjacent sections were labeled with rat monoclonal anti-CD-11b (1:1,000, AbD Serotec, Raleigh, NC, USA). Immunolabeling was detected by the avidin-biotin complex peroxidase method and visualized with diaminobenzidine incubation for 5 min (Vector, Burlingame, CA, USA). Sections were viewed with the 10X objective on a Nikon Eclipse E600 microscope, and digital images were captured using Stereo Investigator 9.12 (Microbrightfield, Burlington, VT, USA). Quantitative analysis was performed using Scion Image 4.0.2 (Scion Corp., Frederick, MD, USA). Percent area occupied by amyloid plaques or by reactive microglia was calculated as well as amyloid plaque numbers (number per 0.75 mm2).
ELISA of Aβ42 and Aβ40 peptides
The other half of the mice were euthanized by decapitation and brains were removed, dissected, snap frozen in liquid nitrogen and stored at −80 C for biochemistry. Snap frozen brain tissues were homogenized in 6% sodium dodecyl sulfate (SDS) with protease inhibitor cocktail (Complete Protease Inhibitor Cocktail tablet, Roche Diagnostics, Mannheim, Germany), sonicated for 1 min, and centrifuged at 14,000 rpm for 15 min at 12 °C. Protein concentration was measured (DC Protein Measurement Kit, Bio-Rad, Hercules, CA, USA). SDS-soluble human Aβ42 and Aβ40 ELISAs were performed using commercial kits (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Western blotting
The same brain lysates used for ELISA were re-used for western blotting. Protein concentration was measured (DC Protein Measurement Kit, Bio-Rad, Hercules, CA, USA), and equal protein amounts of the homogenates were electrophoresed through 4-12% Tri-Bis NuPage (Invitrogen, Carlsbad, CA, USA). After transfer to polyvinylidene fluoride (PVDF), membranes were blocked in 5% non-fat dry milk in phosphate buffer saline with 0.05% Tween 20 (PBST) and exposed overnight to primary antibody at 4 °C. Horseradish peroxidase-conjugated (HRP) secondary antibody binding was visualized with enhanced chemiluminescence.
Primary antibodies and concentrations used for western blotting were: mouse monoclonal anti-synaptophysin (1:1,000, Millipore, Billerica, MA, USA); mouse monoclonal anti-human PHF-tau (1:1,000, Pierce, Rockford, IL, USA); rabbit monoclonal anti-Rac1 (1:500, Cell Bioloabs, San Diego, Ca, USA); mouse monoclonal anti-α-tubulin (1:10,000) and mouse anti-β-actin (1:10,000) (Sigma, St. Louis, MO, USA). Films were scanned at 600 dpi, and densitometry was quantified with Scion Image 4.0.2 (Scion Corp., Frederick, MD, USA). Ratios were calculated using densitometric values of the protein of interest divided by densitometric values of α-tubulin or β-actin.
Measurement of oxidized proteins
Brain protein carbonyl levels were measured using the Oxyblot Protein Oxidation Detection Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol, with the following modifications: 5% non-fat dry milk/PBST was used as blocking solution and antibody diluent; the membrane was blocked for 1 hour; and the primary antibody incubation was overnight. Bands were visualized by enhanced chemiluminescence. Films were scanned at 600 dpi, and Scion Image 4.0.2 (Scion Corp., Frederick, MD, USA) was used for densitometry.
Statistical analysis
ANOVA was used to compare the 4 groups: Tg19959 mice fed vehicle, Tg19959 mice fed apocynin, wild-type mice fed vehicle, and wild-type mice fed apocynin. Post-hoc Fisher PLSD (Fisher) tests were used for further analyses between groups. When only two groups were involved, two-tailed t-tests were used to compare Tg19959 mice fed vehicle and Tg19959 mice fed apocynin (Statview 5.0.1, SAS Institute Inc., Cary, NC, USA).
3. Results
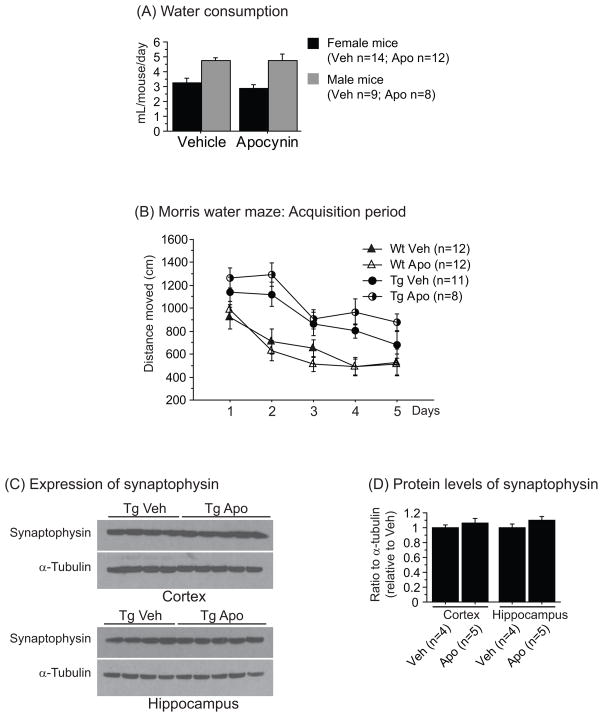
In order to determine whether apocynin affected water intake, we measured water consumption and did not found any differences between groups (Figure 1A).
Figure 1.
(A) Water consumption of both vehicle and apocynin in female and male mice. No significant differences were found between groups. (B) Distance moved during the acquisition period of the Morris water maze in Tg19959 mice (Tg) and their wild-type (Wt) littermates with (Apo) or without (Veh) apocynin. Tg19959 mice swam significantly longer than wild-type mice (Fisher; p<0.05). Apocynin treatment did not improve their navigational and learning performances. (C) Western blotting of synaptophysin and α-tubulin in the cortex and the hippocampus of Tg19959 mice (Tg) treated with (Apo) or without (Veh) apocynin. (D) Ratio of synaptophysin to α-tubulin normalized by Tg19959 mice fed vehicle. No significant differences were found between groups. All data were expressed as means ± standard errors.
Behavioral analysis was performed at 4 months of age, using the Morris water maze. During the acquisition period of the Morris water maze, apocynin administration did not improve spatial learning deficit in Tg19959 mice (Figure 1B). Since we did not observe significant memory impairment in Tg19959 mice compared to wild-type mice in the probe trial, we cannot rule out the effect of apocynin on memory retention. Indeed, there was no significant difference in the time spent in the target quadrant nor in the number of platform crossings in all 4 groups (data not shown). We also measured levels of synaptophysin, and consistent with our behavioral data on spatial learning, these levels were not affected by apocynin treatment (Figure 1C-D).
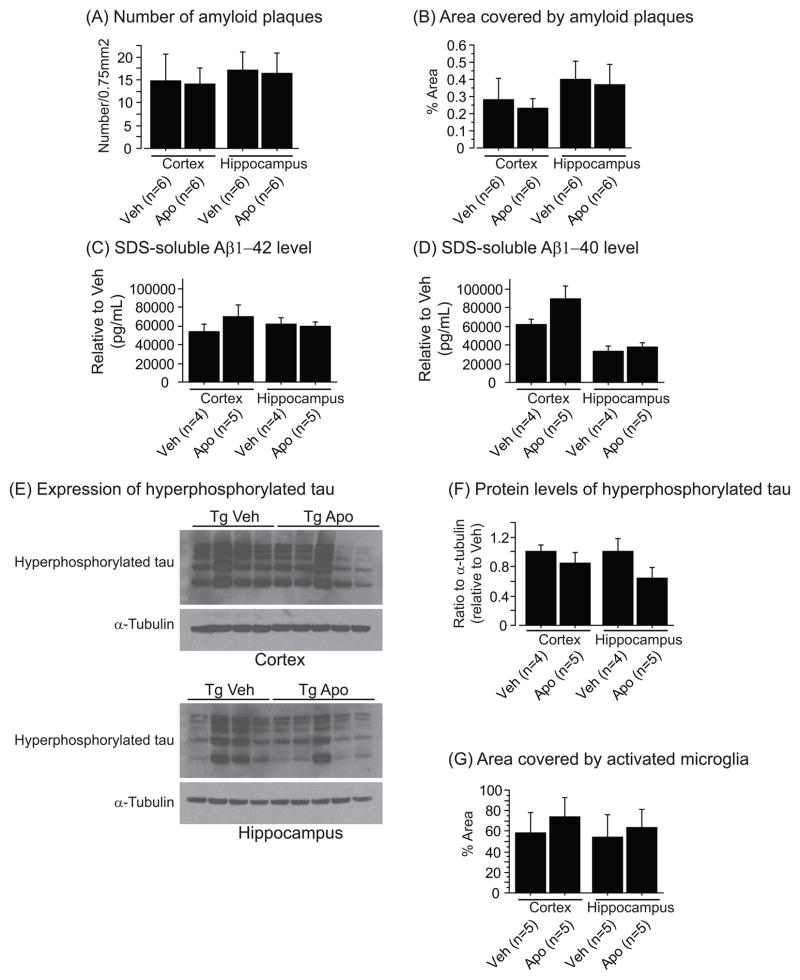
In addition, we studied the effect of apocynin on amyloid deposition, microglial activation, levels of soluble Aβ and hyperphosphorylated tau. We found that the drug did not change any of these parameters (Figure 2). However, there was a trend towards an increase of soluble Aβ40 levels in the cerebral cortex (Figure 2D) and a decrease of hyperphosphorylated tau levels in the hippocampus (Figure 2F).
Figure 2.
(A) Number per 0.75mm2 and (B) percent area covered by amyloid plaques in the cortex and the hippocampus of Tg19959 mice (Tg) treated with (Apo) or without (Veh) apocynin. Apocynin administration did not reduce amyloid deposition in Tg19959 mice. Cortical and hippocampal levels of 6% SDS-soluble Aβ42 (C) and Aβ40 (D) assessed by ELISA (data normalized by Tg19959 mice fed vehicle). There was a trend towards an increase of Aβ40 level in the cortex of Tg19959 mice treated with apocynin compared to vehicle (Fisher; p=0.12). (E) Western blotting of hyperphosphorylated tau and α-tubulin in the cortex and the hippocampus of Tg19959 mice (Tg) treated with (Apo) or without (Veh) apocynin. (F) Ratio of hyperphosphorylated tau to α-tubulin normalized by Tg19959 mice fed vehicle. There was a trend towards a decrease of hyperphosphorylated tau level in the hippocampus of Tg19959 mice treated with apocynin compared to vehicle (Fisher; p=0.15). (G) Percent area covered by activated microglia in the cortex and the hippocampus of Tg19959 mice (Tg) treated with (Apo) or without (Veh) apocynin. Apocynin administration did not change microglial activation in Tg19959 mice. All data were expressed as means ± standard errors.
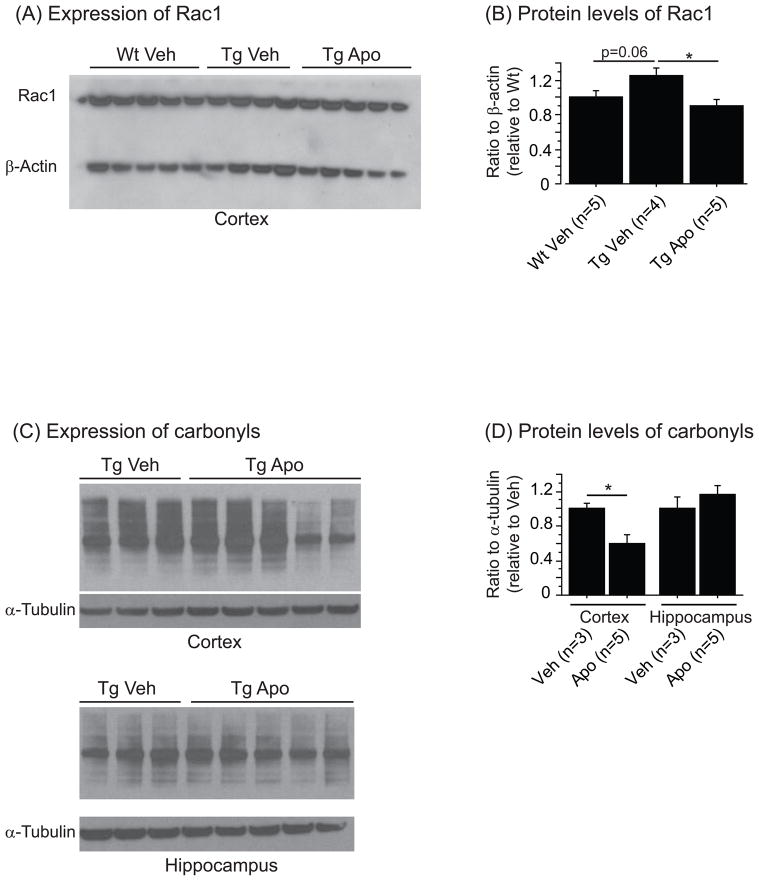
Finally, we investigated possible reasons why apocynin treatment was not protective. We found an increased Rac1 level in the membrane enriched fraction of the cortex in Tg19959 mice as compared to wild-type littermates. After apocynin treatment, the Rac1 levels were diminished in Tg19959 mice (Figure 3A-B). We also measured NADPH oxidase activity which was below detection level in all groups with or without apocynin (data not shown).
Figure 3.
(A) Western blotting of Rac1 and β-actin in the membrane enriched fraction of the wild-type (Wt) and Tg19959 cortex (Tg) treated with (Apo) or without (Veh) apocynin. (B) Ratio of Rac1 to β-actin normalized by wild-type mice fed vehicle. There was a trend towards an increase in Rac1 level in Tg19959 mice compared to wild-type littermates (Fisher; p=0.06). Apocynin reduced significantly Rac1 level in Tg19959 mice (Fisher; p<0.05). (C) Western blotting of carbonyls and α-tubulin in the cortex and the hippocampus of Tg19959 mice (Tg) treated with (Apo) or without (Veh) apocynin. (D) Ratio of carbonyls to α-tubulin normalized by Tg19959 mice fed vehicle. Apocynin decreased significantly carbonyl level in the cortex of Tg19959 mice treated with apocynin compared to vehicle (Fisher; p<0.05). All data were expressed as means ± standard errors.
Apocynin administration reduced significantly protein carbonyl levels in the cerebral cortex of Tg19959 mice, mainly in two Tg19959 treated mice, but not in the hippocampus (Figure 3C-D).
Discussion
NOX is a source of oxidative stress that can play a role in AD. In this work, we studied the effect of apocynin in Tg19959 mice and their wild-type littermates at 300mg/kg of body weight. This dose was chosen according to the study conducted by Harraz and colleagues in a mouse model of ALS, where apocynin was neuroprotective [7]. We found that after 4 months of treatment, apocynin given at 300mg/kg did not improve AD-like symptoms either cognitively or pathologically. There was no effect on spatial learning, amyloid plaques or microglial activation. We cannot not rule out the effect of apocynin on memory retention due to the lack of memory deficit in Tg19959 mice relative to wild-type mice. This may be explained by the fact that Tg19959 mice were able to perform as well as their wild-type littermates by the end of the training period (on day 5). A study at later and more severe stages and with increasing apocynin concentration could be complementary to fully address the role of apocynin in AD.
We also investigated whether apocynin affected oxidative stress and NOX pathway, and we observed that it reduced carbonyl levels in the cortex but not in the hippocampus. However, we found some variability in the level of protein carbonyl among Tg19959 treated mice. Therefore, the effect of apocynin on protein carbonyls in this transgenic mouse model is still unclear. It is also possible that apocynin may target other oxidative stress markers more specifically than protein carbonyls. Moreover, NOX expressing neurons in the cortex are more abundant than in the hippocampus [8].
Rac1 is a major trigger to NOX activation and ROS generation [4]. After translocation to the cell membrane, Rac1 and other cytoplasmic factors, such as p47phox, p67phox activates NOX. Apocynin is known to inhibit the translocation of these elements to the membrane, which prevents NOX activation [14]. Thus in our study we examined Rac1 expression at the membrane. We found that Rac1 levels, which were primarily elevated in Tg19959 cortex, were reduced after apocynin treatment, suggesting that apocynin may been partially functional. However, we cannot rule out the effect of apocynin on NOX activity since it was negligible in Tg19959 mice and their wild-type littermates at 5 months of age. Our finding is consistent with recent data showing that NOX activity is increased in MCI but not in AD patients [3]. It is possible that NOX activation in the brain is not a chronic event in AD, but only occurs intermittently.
Our data demonstrated that administration of apocynin at 300mg/kg of body weight did not improve AD-like phenotype in 5-month old Tg19959 mice. Additional investigations are required to further understand the effect of apocynin in AD models. However, it is possible that by targeting NOX-mediated oxidative stress, apocynin may not be sufficient to prevent AD. Other sources of ROS such as mitochondria may be critical in disease progression [2].
Acknowledgments
This work was supported by the National Institute of Health (NIH) grant 5P01 AG14930-08. We thank Dr. Anatoly Starkov for his help with the NADPH activity assay. We also thank Dr. Davide Tampellini for its review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Block ML. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonda DJ, Wang X, Perry G, Smith MA, Zhu X. Mitochondrial dynamics in Alzheimer's disease: opportunities for future treatment strategies. Drugs Aging. 2010;27:181–192. doi: 10.2165/11532140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H, 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 5.Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, Liby K, Sporn M, Nathan C, Flint Beal M, Lin MT. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. Faseb J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Shin KS, Chung YB, Jung KW, Cha CI, Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 9.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer's disease brains. Biochem Biophys Res Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 12.Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–258. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- 13.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 14.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]