Abstract
Delivering therapeutics to mucosal tissues such as the nasal and gastrointestinal tracts, is highly desirable due to ease of access and dense vasculature. However, the mucus layer effectively captures and removes most therapeutic macromolecules and devices. In previous work, we have shown that nanoengineered microparticles (NEMPs) adhere through the mucus layer, exhibiting up to 1000 times the pull-off force of an unmodified microsphere, and showing greater adhesion than some chemical targeting means. In this paper, we demonstrate that nanotopography improves device adhesion in vivo, increasing retention time up to ten-fold over unmodified devices. Moreover, we observe considerable adhesion in several cell lines using an in vitro shear flow model, indicating that this approach is promising for numerous tissues. We then demonstrate that nanowire-mediated adhesion is highly robust to variation in nanowire surface charge and cellular structure and function, and we characterize particle loading and elution. We present a form of cytoadhesion that utilizes the physical interaction of nanoengineered surfaces with subcellular structures to produce a robust and versatile cytoadhesive for drug delivery. These nanoscale adhesive mechanisms are also relevant to fields such as tissue engineering and wound healing because they likely affect stem cell differentiation, cell remodeling, migration, etc.
Introduction
Mucosal tissues are preferred drug delivery pathways because they form the primary absorptive interfaces for the uptake of therapeutics[1–3]. However, to prevent entry of unwanted substances and organisms, these tissues have evolved numerous chemical and physical defensive barriers, such as degradative enzymes, harsh pH conditions, tight junctions, and the mucus layer, which effectively clears all objects that are not anchored to the cells[4]. Although microparticles and microspheres allow relatively large volumes of therapeutics to circumvent the degradative chemical environment with minimal toxicity, and permeation enhancers can improve paracellular transport, the mucus layer remains largely impenetrable to micro-scale devices[5–7].
Because of their nanoscale dimensions, nanoparticles can penetrate the mucus layer (pore size of 100 nm[8]) and interact with cellular structures that exist on the nanoscale, such as microvilli. Furthermore, the hundreds-fold increase in surface area created by nanostructured surfaces allows for significantly increased adhesion due to geometry alone (without chemical adhesives), as seen in gecko-inspired adhesion[9–11]. However, nanoparticles suffer from significantly lower loading capacity compared to microparticles[12, 13] and potentially toxic accumulations in the liver, kidneys, and spleen.
By engineering nanostructures on the surface of microparticles, devices benefit from both the microscale and nanoscale: Nano-Engineered MicroParticles (NEMPs) have the loading capacity and biocompatibility of microparticles combined with the mucus penetration and enhanced adhesion of nanoparticles[14].
Materials and Methods
Device Fabrication
Devices were fabricated after Fischer[14]. Because silicon-based devices are not visible under x-ray, we grew nanowires on stainless steel spheres (McMaster-Carr, Elmhurst, IL). The stainless steel devices appear opaque under x-ray. Scanning electron microscopy allowed us to find their approximate diameter (Supplementary Figure 1).
Cell Culture
Cell lines were cultured according to standard protocols available from ATCC. For flow testing, cells were seeded onto Type I Rat Tail Collagen-coated glass slides to improve confluency according to the following protocol: Slides were cleaned in oxygen plasma for 30 seconds. 1 mL of 1:67 dilution of collagen: 0.02 N acetic acid was added to each slide. After a 1 hour incubation at room temperature, slides were washed two times with sterile PBS and cells were added.
Caco-2 cells grow to confluency within a week and continue to mature and differentiate spontaneously up to 3 weeks after seeding[15]. This differentiation process includes the formation of tight junctions and apical microvilli. While initial microvilli-like structures appear on the surface of a few cells by day 3 (see Supplementary Figure 2), the majority of cells express few if any microvilli, making them a possible control for Caco-2 surface nanotopography.
Surface Modification of Nanowire-Coated Devices
Surface modifications were done following the protocol described in [14] with the following modifications made to attach FITC. After hydroxylation using a five minute incubation in 1:1:5 solution of ammonia, hydrogen peroxide, and water at 80°C and a five minute incubation in 1:1:6 solution of hydrochloric acid: hydrogen peroxide: water at 80°C, samples were resuspended in 5 mL of isopropanol and 0.1 mL of 3-aminopropyltriethoxysilane (APTES) for 90 minutes. After vacuum filtration drying, devices were incubated in a solution of fluorescein isothiocyanate (FITC) – roughly 50 μg in 4 mL water – overnight at room temperature. By varying the amount of FITC added, we were able to obtain several different surface charges. Modifications with polyethylene glycol (PEG) followed [16], exposing plasma-cleaned devices to an 1.5% solution of PEG-silane (2-[Methoxy(polyethyleneoxy)propyl]trimethoxysilane, Gelest) in toluene for 2 hours, then rinsing in toluene, ethanol, and water prior to filtration and drying. Due to the very small amounts of FITC added to the solution (on the order of 25–75 μg), there was some variation between batches in coating efficiency, and thus in the zeta potential. For the charge-related adhesion studies, nanowires from each modification batch used in shear flow studies were retained and measured, so that they could be correlated with adhesion.
Zeta potential
Modified and unmodified nanowires were suspended in deionized water and their zeta potential was measured at varying pH (with the addition of hydrochloric acid and potassium hydroxide to alter pH) using a Malvern Zetasizer Nano. Zeta potential measurements can be found in the Supplementary Information. Because the measurement of zeta potential assumes that objects are suspended and spherical. Particles as large and dense as the ones used for this project (greater than 10 μm, etc) fall out of solution within 30 seconds, making accurate zeta potential measurements of an entire device impossible. Nanowires may be removed from the devices using sonication for 30 minutes, and would provide an accurate image of the surface with which the cells interact. However, because of their elongated shape, nanowires may be measured inaccurately. With these considerations in mind, along with the batch variation in surface modifications, we used several different techniques to characterize surface potential of the nanowires. Initially, we tried modifying silica nanoparticles (diameter 900 nm) with the same chemistry as the nanowire-coated devices. However, due to concerns about nanoscale geometry affecting the geometry and measurement of surface chemistry, we ultimately chose to measure nanowires directly (see Supplementary figure 3).
Confocal Microscopy
For actin staining, cells were fixed using 3.7% paraformaldehyde in PBS for 20 minutes. After a PBS wash, 0.5% Triton X-100 in PBS was incubated with cells for 10–15 minutes. Cells were washed, then incubated with 1% BSA in PBS for 30–60 minutes to block non-specific adsorption of stains. After a PBS wash, a 1:200 AlexaFluor 488 Phalloidin (Invitrogen): PBS solution was incubated on cells for 45–60 minutes at room temperature and covered with parafilm to prevent evaporation. Cells were washed and a 1:1000 dilution of Propidium Iodide (Invitrogen) in PBS was added for 5 minutes.
The confocal microscope images presented in the body of this work show side planes of cells (the x–y plane) with devices presented schematically in blue and the apical surface of the cells outlined in white. The images in the Supplementary Data section (Supplementary Figures 4–7) are included as references, so that all planes, with and without devices can be seen. The devices were imaged in brightfield then artificially colored blue, with the primary intention of locating the devices with respect to the cells. Because the brightfield images have considerable aberration on the z axis, they are shown schematically in the paper.
Scanning Electron Microscopy
Cells were fixed with 3% gluteraldehyde (Polysciences) in a 0.1 M sodium cacodylate (Polysciences), 0.1 M sucrose in HBSS buffer for 2–3 days at room temperature. The fixative was replaced with the sodium cacodylate-sucrose buffer and incubated twice for 5 minutes. Samples were then dehydrated using a graded series of ethanol solutions, each for 10 minutes in the following order: 35%, 50%, 70%, 95%, 100%, 100%. The last ethanol solution was replaced with hexamethyldisilazane (HMDS, Polysciences) for 10 minutes and then left to dry. Samples were stored in a desiccator until imaged using a NovelX MySEM. Device SEMs were taken devices sputter-coated with gold prior to imaging with a JEOL JSM-6500F field emission SEM.
Shear Flow
Shear flow experiments were done after [14], with minor modifications. Briefly, cells were grown to confluency on a glass slide as described under cell culture. Devices were added to the cells immediately before the assembly of the parallel plate flow chamber (Glycotech). After chamber assembly, 2% mucin (Porcine Gastric Mucin, Type II, Sigma) in water (pH 3.7) was flowed through the chamber and shear was increased in a step-wise fashion, every 10 minutes. Top-to-bottom and left-to-right slices of each slide were imaged and devices counted using Adobe Photoshop CS4. The detachment of the devices was quantified by dividing the number of devices remaining at a given shear by the total devices originally exposed to the cells. Mucin viscosity was measured at 37°C and at 4°C using an Ubbelohde viscometer.
In vivo experiments
Gelatin capsules (~21.7 x 7.5) were filled with roughly 1 gram of devices. The capsules were administered to healthy, female beagles (11–14 kg weight, after an overnight fast of 14 hours). Imaging was done using fluoroscopy, which takes x-rays at a frame rate of 30 frames/seconds, allowing real-time positioning. Images were taken at 15 and 30 minute post administration and then at 30 min intervals for the remainder of the experiment using a GE OEC 9800 Plus C-Arm fluoroscope. After 180 minutes, the experiment concluded and the dogs were fed. Throughout this time, the animals were observed for acute reactions and were allowed to maintain normal activity.
In the video of Dog 4 (200 μm uncoated control particles) at 15 minutes, uncoated devices are flowing through the intestine without any adhesion. However, in the supplementary videos of Dogs 1, 2, and 5 (with 300, 200, and 1000 μm particles, respectively), taken at 15 minutes, we observe nanowire-coated devices adhering to the stomach initially, and in Dog 1 continuing to adhere at 180 minutes, despite strong gastroduodenal contractile activity. In Dogs 1, 4, and 5, stomach and intestinal contractions can be seen at 15 minutes as particles are moving or expelled. Dog 2 does not display contractions at 15 minutes.
Internalization Assay
For internalization assays, cell monolayers were imaged live in PBS at 37°C. FITC – labeled devices were added to the cells, and after a 5 minute to 1 hour incubation, 100–200 microliters of 0.4% Trypan Blue solution was added. Confocal imaging was conducted 1 to 2 minutes afterwards, and brightfield imaging of the trypan blue stain was used to confirm that the cell monolayer was not otherwise compromised. A Nikon TE Spinning Disk inverted confocal microscope was used to take the images, and NIS Elements and ImageJ were used to reconstruct the images.
Particle Loading
Controlled pore glass particles (CPG, 30–70 μm width, 200 nm pore size, Sigma Aldrich) with and without nanowires were loaded by placing 30–100 μL of particles in 500 μL of concentrated loading solutions of BSA (Sigma), bovine pancreatic insulin (Sigma), and bovine immunoglobulin G (Biomeda) at 35°C for 24 hours (until dry). Dry particles were rinsed with phosphate buffered saline (PBS) in a filter flask to remove residual surface protein crystals. Loaded particles were placed in PBS on a shaker plate; the PBS solution was sampled and analyzed using a micro and/or regular BCA assay and a spectrometer.
Results
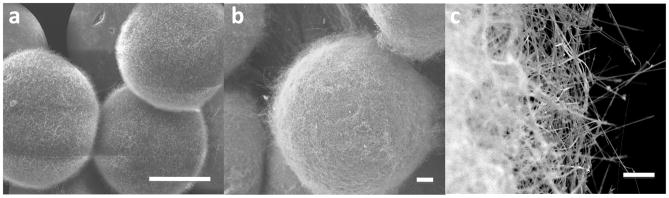
To fabricate NEMPs, silicon nanowires were grown on glass microspheres for use as a model drug delivery system, and on stainless steel microspheres to facilitate imaging, using chemical vapor deposition (Figure 1)[14, 17]. Silicon nanowires have been shown to provoke equivalent or lesser inflammatory response and reactive oxygen species when compared to glass surfaces, suggesting that these devices will not provoke a heightened immune response[18]. The nanowires on the glass spheres (30–50 μm diameter) and controlled pore glass particles (50–70 μm width, 200 nm pore size) were approximately 40 nm in diameter and 2–3 μm in length, while those on the stainless steel spheres (200–220 μm diameter) were approximately 70 nm in diameter and 11 μm in length. The glass spheres and particles show medium density, homogeneous, conformal coatings, while the stainless steel spheres have a high density, homogeneous, conformal coating of nanowires.
Figure 1.
Devices and in vivo adhesion. a) 30–50 μm diameter silica spheres with nanowires of 1–3 μm in length, 40 nm diameter. Scale bar indicates 2 μm. b and c) 200–220 μm diameter stainless steel spheres with nanowires of 11 μm in length, 70 nm diameter. Scale bar indicates 2 μm (b) and 20 μm (c).
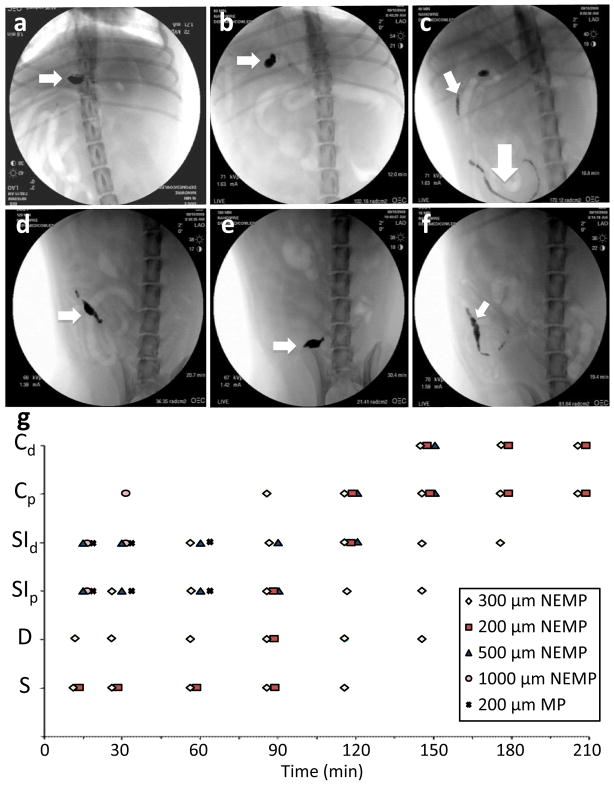
To determine the effectiveness of nanowire-mediated adhesion in vivo, we used a fluoroscope to track the stainless steel NEMPs and unmodified stainless steel microparticles (MPs). Devices were placed inside of a gelatin capsule (Capsugel, Peapack, NJ) and given to fasted, female beagle dogs (for NEMPs: n=2 for 200–300 μm, n=1 for 500 μm, and n=1 for 1000 μm; for 200 μm MPs, n=1). The dogs were imaged at 30 minute intervals (Figure 2, a–f, videos available in Supplementary Videos). The gelatin capsules dissolve in less than 10 minutes, so the initial imaging at 15 minutes mainly showed a distribution of devices in the stomach. Almost immediately, unmodified MPs were seen to transit into the intestine. In contrast, 200–300 μm NEMPs were retained in the stomach up through 120 minutes, and slowly spread to the duodenum and upper small intestine. By 180 minutes, the NEMPs had accumulated mainly in the large intestine. Larger NEMPs of 500 and 1000 μm transited more quickly. Thus, in 200–300 μm NEMPs, the nanowire coatings alone were responsible for a significant increase in gastrointestinal adhesion over uncoated devices, increasing the time that devices stayed in contact with the stomach by up to ten times.
Figure 2.
In vivo images. a–f) 200 μm, nanowire-coated, stainless steel devices at 15, 60, 90, 120, and 150 minutes, respectively. a, b) Arrow indicates particles in stomach. c) small arrow indicates particles in proximal small intestine, large arrow indicates particles in distal small intestine. d, e) Arrow indicates particles in large intestine. f) corresponding position of uncoated, stainless steel particles at 15 minutes. Arrow indicates particles in distal small intestine. g) Comparison of positions of each group of devices with time. Note 200 μm- 300 μm nanowire-coated particles remain in the stomach, duodenum, and proximal small intestine to at least 150 minutes. Vertical axis is position in the gastrointestinal tract: Cd – distal colon; Cp – proximal colon; SId – distal small intestine; SIp – proximal small intestine; D – duodenum; S – stomach.
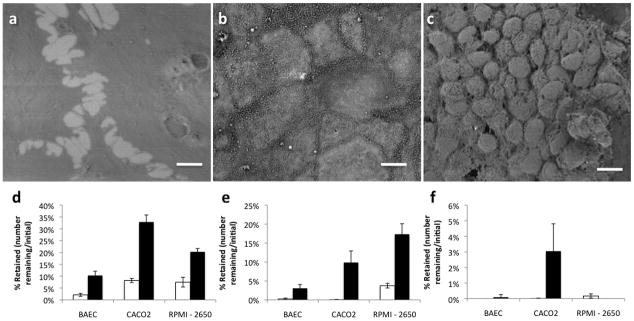
To determine if nanowire-mediated adhesion is applicable to other mucosal tissues, we conducted shear tests on a variety of cell types in vitro. The RPMI-2650 nasal cell line[19], forms large clusters on the microscale, but few active extrusions on the nanoscale (Figure 3c). Caco-2 intestinal cells form a flat monolayer on the microscale, but have numerous apical microvilli (Figure 3b)[15]. Adult bovine aortic endothelial cells (BAEC, or ABAE) form a monolayer that is flat both on the micro- and nanoscale, and since they are endothelial cells, they do not function as an absorptive interface with the external environment (Figure 3a)[20]. To measure adhesion, we added devices to a slide with the cells of interest, and then subjected it to flow of a model mucus layer consisting of 2% porcine gastric mucin (Sigma) in deionized water (pH 3.7).
Figure 3.
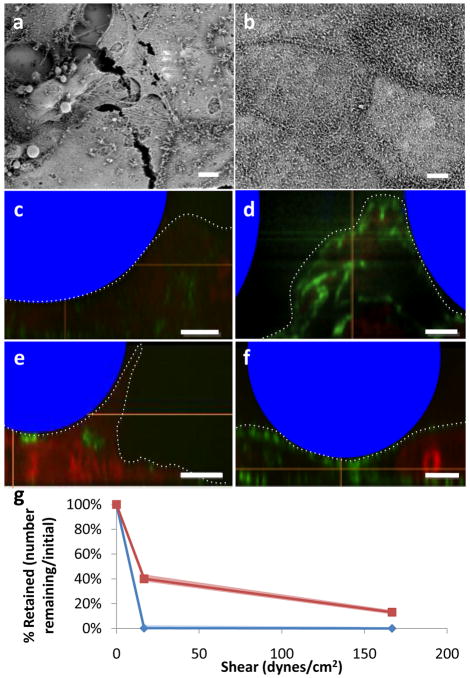
Cell-related adhesion. a–c) Scanning electron micrographs of BAEC, CACO-2, and RPMI cells, respectively. Scale bars are 10 μm. d–f) Retention of control beads (□) and nanowire-coated beads (■) at 8.33 dynes/cm2, 16.6 dynes/cm2, and 166.6 dynes/cm2, respectively. Error bars indicate 95% confidence intervals.
When NEMPs were exposed to these three cell lines, there was significant improvement in retention at low and medium shears (Figure 3d–f). Though the nanowire coating increases relative adhesion for all three cell types (4.9-fold for BAECs, 4.0-fold for Caco-2 cells, and 2.7-fold for RPMI-2650 cells over unmodified devices at a shear of 8.3 dynes/cm2), the absolute adhesion of nanowires is highest in the micro- and nano-structured Caco-2 and RPMI-2650 cells. This data suggests that nanowires can improve adhesion to many cell types regardless of cell-specific attributes, though they may be most useful in cells with micro- and nanotopographical features.
Although NEMPs adhere strongly in vivo and to several cell types, nanoscale bioadhesive mechanisms remain poorly characterized. In dry environments, nanowires and other related nanostructures adhere primarily via van der Waals forces[9, 21], demonstrating the strong effect of charge-related forces at the nanoscale. Longer nanowires improve bioadhesion over shorter wires, indicating a related effect due to nanowire geometry[14]. Particle shape affects cellular internalization[22], and nanoparticles of similar end shape are endocytosed by macrophages[23], suggesting an effect due to cytoskeletal restructuring. In this study, we consider the role of nanowire charge as well as that of cellular morphology and remodeling.
Native silicon nanowires are negatively charged both at physiological pH and at the low pHs found in the GI tract (−16.5 mV at pH 7.5 +/− 0.1, and −11.2 mV at pH 3.6 +/−0.1, respectively). To test the effects of charge on adhesion, we modified the nanowires to have different charges at low pHs using silane chemistry with amine modifications and FITC labeling. This chemistry resulted in two groups with zeta potentials of 10.6 +/− 1.7 and −8.7 +/− 4.4 mV at pH 3.6 +/−0.1.
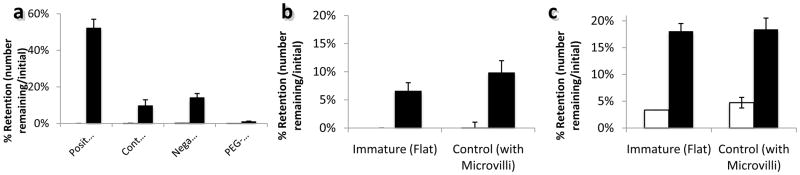
When exposed to Caco-2 intestinal cells under mucin, positively charged NEMPs adhered significantly better than negatively charged NEMPs at 16.6 dynes/cm2 (the upper limits of a healthy intestinal shear) and better than negatively charged NEMPs, though not significantly, at 166.6 dynes/cm2 (Figure 4a). Thus, positive charge improves nanowire adhesion to Caco-2 cells, expanding previous reports of mechanisms of nanoparticle adhesion and internalization to include nanowires[24]. Neutral nanowire charge, from hydrophilic polyethylene glycol (PEG) modifications, reduces adhesion to roughly the same as uncoated MPs, demonstrating that charge is essential to nanowire adhesion. Similar to findings with nanoparticles, nanowire adhesion requires some charge, and is optimal when that charge is positive[25].
Figure 4.
Chemistry and topography-mediated changes in adhesion. a) Retention of devices with different surface charges at 16.6 dynes/cm2. From left to right, charges are +10 mV, −11 mV (unmodified silica), −23 mV, assumed neutral (modified with polyethylene glycol). b) With normal restructuring activity (ie: at 37°C), at 10–20 dynes/cm2, the microvilliated, control cells show improved adhesion over immature cells which express minimal microvilli. c) When cell remodeling activity is reduced by cooling to 4°C, and microvilli are static, there is no difference between microvilliated, control cells and flat, immature cells at 10–20 dynes/cm2. In all plots, black indicates nanowire-coated beads and white indicates uncoated beads. Error bars indicate 95% confidence intervals.
Nanotopographical cytoadhesion may also be modulated by microvilli covering the apical surface of mature gastrointestinal epithelial cells – either actively, via a remodeling in response to nanowire stimuli, or passively via a “Velcro-like,” physical interdigitation. The actin cytoskeleton may be critical to these interactions, either by modulating cell structure underneath the microvilli or the structure of the microvilli themselves[26, 27].
Adhesion to immature Caco-2 cells, which do not express significant quantities of microvilli, and on Caco-2 cells at 4 °C, which slows actin polymerization, effectively immobilizing the microvilli and preventing active internalization processes can be studied to determine the importance of microvilli in adhesion. In the immobilized state (Figure 4b), both “flat” immature and microvilliated control cells exhibit similar retention of devices, suggesting that the microvilli are not acting in a “Velcro-like” fashion, despite greater adhesion by nanowire-coated beads. When the cells are maintained at an active temperature, microvilliated control cells do retain nanowires in greater quantities, though not significantly. Thus, while microvilli may be involved in adhesion to some degree, nanowires continue to adhere strongly even when microvilli interactions, both active and passive, are minimal.
Although microvilli-specific interactions are minimal, the actin cytoskeleton may be involved with adhesion at a more general level. To knock out the cytoskeleton, cells were incubated for two hours with cytochalasin D, a fungal mycotoxin which transiently prevents actin polymerization in Caco-2 cells (see Figure 5a–b)[28, 29]. A significant proportion of NEMPs were retained under flow in comparison to the rapid loss of MPs (Figure 5g). To determine the nature of cytoskeletal changes underneath the NEMPs and MPs, confocal images were taken and reconstructed to view the x–z plane. Cytochalasin D-treated cells deformed under devices regardless of nanowires, suggesting a passive, weight-related deformation process (Figure 5c and e). Control cells showed actin at their apical surface and only deformed under the influence of NEMPs, suggesting an active, actin-related deformation (Figure 5d and f, additional confocal images available in Supplementary Figures). Overall, deformation of cells increased the surface area in contact with devices, and if the devices had nanowires, this deformation increased adhesion. Thus, even in the most extreme cytoskeletal conditions, nanowires increase adhesion significantly.
Figure 5.
Effects of reducing actin cytoskeletal remodeling. a) Scanning electron micrograph of cytochalasin D treated cells. b) Scanning electron micrograph of control cells. c) Vertical confocal microscopy slice of Caco-2 cells with nanowire-coated beads after treatment with cytochalasin D. d) Vertical confocal microscopy slice of control Caco-2 cells showing modest remodeling around nanowire-coated devices and considerably more actin formation than with cytochalasin D. e) Vertical confocal microscopy slice of Caco-2 cells with uncoated beads after treatment with cytochalasin D, showing considerable height attained, but still minimal actin formation. f) Vertical confocal microscopy slice of Caco-2 cells with uncoated beads, showing normal actin structures, but minimal deformation. In c–f, beads are indicated schematically in blue, F-actin is stained in green, and nucleic acids are stained in red. Apical surface of cells is indicated by the dotted line. All scale bars (a–f) are 5 μm. g) Retention in cells with actin cytoskeleton knocked out by 2 hour incubation with cytochalasin D. n indicates nanowire-coated beads, u indicates uncoated control beads. The shaded areas correspond to 95 % confidence intervals using Greenwood’s formula for survival curves.
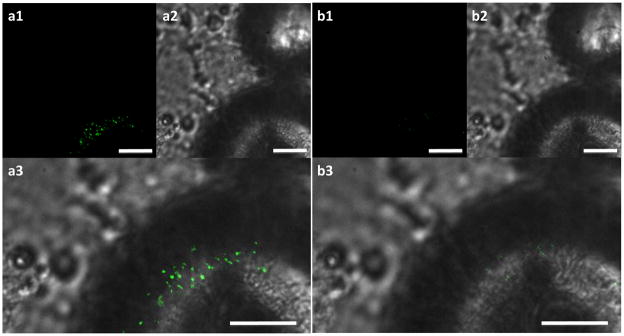
Particle internalization is another major active cell process that affects nano-scale cytoadhesion, suggesting that it may play a role in mediating nanowire adhesion. To determine if the nanowires were being internalized, cells were exposed to FITC-modified devices for 30 minutes then imaged prior to and after fluorescence quenching by Trypan Blue (Figure 6). Because minimal fluorescence is observed after the quench, and Trypan Blue does not enter healthy cells, it is concluded that the nanowires are not being internalized.
Figure 6.
Nanowire internalization is not observed. a) FITC-modified nanowire-coated bead on top of cells prior to Trypan Blue quench. b) The same bead shows no fluorescence after Trypan Blue quench, indicating no internalization. All fluorescence images (a1, b1) taken for 500 ms. Brightfield images (a2, b2) were combined with fluorescent images to obtain a3 and b3. Scale bars indicate 10 μm.
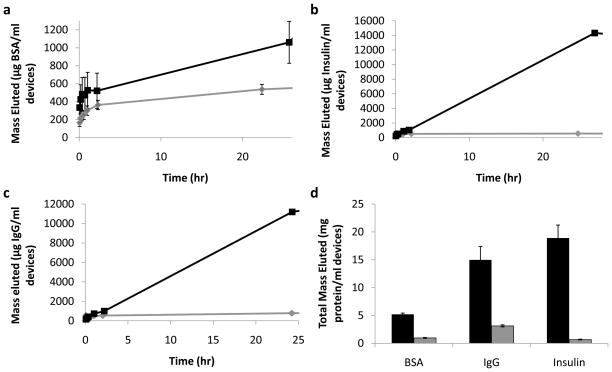
Because the nanowires increase the surface area and can create a reservoir where connected to microparticles, they have a significant capacity for loading. Using surface tension, we loaded bovine serum albumin (BSA), insulin, and Immunoglobulin G (IgG) as model therapeutics into porous NEMPs and MPs. When immersed in solution, NEMPs eluted significantly more of each therapeutic than the control porous MPs within minutes, continuing past 24 hours (Figure 7). Overall, NEMPs had a 5–10 fold higher loading capacity for therapeutics than the MPs.
Figure 7.
Elution characteristics for controlled pore glass particles loaded with bovine serum albumin, insulin, and immunoglobulin G. a–c) Mass of BSA (a), insulin (b), and IgG (c) eluted per mL devices incubated over 24 hours. In each plot, elution from NEMPs is shown by the black square line, and MPs by the gray diamond line. d) Total elution of different molecules from loaded controlled pore glass particles. NEMPs – black, MPs – gray. Error bars for all plots indicate standard error of the mean.
Discussion
Both cell-related and nanowire-related effects are involved in nanowire-mediated adhesion. Nanowires may be optimized either with chemistry, as in modification with positive charge, or geometry, as with longer nanowires[14]. The characteristics of the underlying cell substrate affect adhesion as well, with cell type, contact surface area, and the actin cytoskeleton each affecting adhesion. As long as they are charged, nanowires significantly improve adhesion when compared to control devices. PEG-coated nanowires do not increase adhesion, likely because PEG is not charged and does not interact with cell surface proteins, thereby negating the charge and surface area enhancement by nanowires.
Nanowires dramatically improve adhesion to a wide range of cells, with the relative improvement roughly independent of the cell type. However, the absolute adhesion depends strongly on the underlying cell: it is most notable in Caco-2 intestinal cells, which have a flat microstructure but sizable nanostructures, and RPMI-2650 nasal cells, which exhibit microstructural variation but fewer nanostructures.
Pinpointing the particular roles played by various cytoskeletal structures in nanowire-mediated adhesion is a complicated matter, as comparing the adhesion across experiments is difficult. Functional, active actin cytoskeletons may increase surface area, and thus adhesion, by mediating deformation. However, even under extreme circumstances such as inactivating the actin cytoskeleton or immobilizing microvilli, NEMPs continue to show significantly higher retention than control MPs.
While adhesive properties may be studied and optimized using cell lines, in vitro studies are only relevant if they can predict the outcome of devices used in vivo. Thus, it is significant that in healthy, fasted dogs, with functional and non-cancerous gastrointestinal tracts, the nanowire coating alone increased device gastroretention to 180 minutes, roughly ten times the residence time of control devices. Additional chemical or geometric optimization of the stainless steel NEMPs following the results of the in vitro studies may allow for considerable improvement in cytoadhesion.
Conclusions
Despite the impressive accomplishments of nanoscience, one of the major hurdles to widespread use is robust integration of nanoscale components into larger systems. This work establishes the robustness of hierarchical nano-microscale integration and geometry-based adhesion under numerous harsh conditions, including the full biological complement in vivo. Furthermore, the nanoengineered surface creates an additional reservoir for loading and delivery of therapeutic molecules, effectively adding multiple structure-mediated functionalities to the microparticles. Nanowire coatings show great potential for improving bioadhesion by penetrating the mucus layer and adhering to cells directly, without the need for additional chemistry, as demonstrated both in vitro and in vivo. They have the ability to be used for drug delivery to numerous types of tissue, making nanowire-coatings especially attractive for delivery of macromolecules to desirable targets in harsh physiological environments, such as the gastrointestinal, nasal, pulmonary, vaginal, and ocular mucosa. Furthermore, it is likely that similar nanotopographies will likewise increase cytoadhesion, allowing for integration into numerous other applications, from biosensing to tissue engineering to surgery.
Supplementary Material
Acknowledgments
Confocal microscopy and the shear flow imaging were done at the UCSF Nikon Imaging Center with the help of Kurt Thorn. Zeta potential measurements were done in the Habelitz laboratory at UCSF with the help of Roselyn M. Odsinada and Vuk Uskokovic. The viscosity of mucin was determined with the help of Bill Harries at UCSF. KF wishes to thank Adam Mendelsohn and Rachel Lowe for cell culture maintenance and Ryan Olf for manuscript editing. KF was supported by an NSF Graduate Research Fellowship. This research was conducted with funding from NIH grant EB01166401, a Rogers Foundation Grant and from a University of California Discovery Grant, with partnership from Nanosys, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ponchel G, Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Del Rev. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 2.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery - a promising option for orally less efficient drugs. J Controlled Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. J Pharm Sci. 2000;89(7):850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Del Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal caco-2 cell monolayers. Pharm Res. 2003;20(1):110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 6.Salonen J, Kaukonen AM, Hirvonen J, Lehto V-P. Mesoporous silicon in drug delivery applications. J Pharm Sci. 2008;97(2):632–653. doi: 10.1002/jps.20999. [DOI] [PubMed] [Google Scholar]
- 7.Mitragotri S. Recent developments in needle-free drug delivery. The Bridge. 2008;38(4):5–12. [Google Scholar]
- 8.Cone RA. Barrier properties of mucus. Adv Drug Del Rev. 2009;61(2):75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Autumn K, Sitti M, Liange YA, Peattie AM, Hansen WR, Sponberg S, et al. Evidence for van der waals adhesion in gecko setae. Proc Natl Acad Sci U S A. 2002;99:12252–12256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Autumn K, Liange YA, Hsieh ST, Zesch W, Chan WP, Kenny TW, et al. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–685. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- 11.Spolenak R, Gorb S, Arzt E. Adhesion design maps for bio-inspired attachment systems. Acta Biomater. 2005;1:5–13. doi: 10.1016/j.actbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Prud'homme RK. Thermodynamic limits on drug loading in nanoparticle cores. J Pharm Sci. 2008;97(11):4904–4914. doi: 10.1002/jps.21342. [DOI] [PubMed] [Google Scholar]
- 13.Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J Controlled Release. 2005;102(2):313–332. doi: 10.1016/j.jconrel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Fischer KE, Aleman BJ, Tao SL, Daniels RH, Li EM, Bunger MD, et al. Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano Lett. 2009;9(2):716–720. doi: 10.1021/nl803219f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, et al. Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 16.Papra A, Gadegaard N, Larsen NB. Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir. 2001;17(5):1457–1460. [Google Scholar]
- 17.Cui Y, Lauhon LJ, Gudiksen MS, Wang J, Lieber CM. Diameter-controlled synthesis of single-crystal silicon nanowires. Appl Phys Lett. 2001;78(15):2214–2216. [Google Scholar]
- 18.Ainslie KM, Tao SL, Popat KC, Daniels H, Hardev V, Grimes CA, et al. In vitro inflammatory response of nanostructured titania, silicon oxide, and polycaprolactone. J Biomed Mater Res Part A. 2008;91A(3):647–655. doi: 10.1002/jbm.a.32262. [DOI] [PubMed] [Google Scholar]
- 19.Werner U, Kissel T. In-vitro cell culture models of the nasal epithelium: A comparative histochemical investigation of their suitability for drug transport studies. Pharm Res. 1996;13(7):978–988. doi: 10.1023/a:1016038119909. [DOI] [PubMed] [Google Scholar]
- 20.Gospodarowicz D, Moran J, Braun D, Birdwell C. Clonal growth of bovine vascular endothelial cells: Fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. Frictional adhesion: A new angle on gecko attachment. J Exp Biol. 2006;209:3569–3579. doi: 10.1242/jeb.02486. [DOI] [PubMed] [Google Scholar]
- 22.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champion J, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S-D, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 25.Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2009;6(1):12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 26.Weinbaum S, Guo P, You L. A new view of mechanotransduction and strain amplification in cells with microvilli and cell processes. Biorheology. 2001;38:119–142. [PubMed] [Google Scholar]
- 27.Gorelik J, Shevchuk AI, Frolenkov GI, Diakonov IA, Lab MJ, Kros CJ, et al. Dynamic assembly of surface structures in living cells. Proc Natl Acad Sci U S A. 2003;100(10):5819–5822. doi: 10.1073/pnas.1030502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampath P, Pollard TD. Effects of cytochalasin, phalloidin and ph on the elongation of actin filaments. Biochemistry. 2002;30(7):1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y-Y, Sibley E, Tang S-C. Transient cytochalasin-d treatment induces apically administered raav2 across tight junctions for transduction of enterocytes. J Gen Virol. 2008;89(12):3004–3008. doi: 10.1099/vir.0.2008/001446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.