Abstract
We previously found that chronic alcohol consumption (20% w/v in drinking water) that models the level consumed by human alcoholics, when administered to female C57BL/6 mice inhibits B16BL6 melanoma metastasis to the lung; however, the mechanism is not known. Chronic alcohol consumption increases IFN-γ producing NK, NKT, CD4+, and CD8+ T cells. To examine the impact of IFN-γ on metastasis, we inoculated B16BL6 melanoma cells i.v. into control and chronic alcohol drinking IFN-γ knockout (KO) mice. Knockout of the ifn-γ gene abrogated the anti-metastatic effects associated with alcohol consumption. We examined metastasis in common gamma-chain (γC) KO mice, which are deficient in NK, NKT and CD8+ T cells, and in Vα14Jα281−/− KO mice, which are deficient in invariant NKT (iNKT) cells, in order to assess the importance of specific IFN-γ producing cell types to this effect. We found that the antimetastatic effect of alcohol was still present in γC KO mice and also in γC KO mice depleted of Gr-1+ cells. Knockout of iNKT cells reduced the degree but not the antimetastatic effect associated with alcohol. These results indicate that the anti-metastatic effect induced by chronic alcohol consumption is IFN-γ dependent and that multiple IFN-γ producing cell types contribute to this effect.
Keywords: Alcohol, IFN-γ, Knockout, Mice, Melanoma, Metastasis
Introduction
There is strong epidemiological linking alcohol consumption with an increased risk of developing cancer, including but not limited to breast cancer, esophageal cancer, liver cancer, and colorectal cancer [1–5]. It is now also becoming clear that alcohol consumption also increases the risk of developing melanoma skin cancer [6–10]. Since the incidence of melanoma continues to increase every year, it is important so study the impact of alcohol consumption on this disease. While information linking alcohol consumption to cancer risk continues to accumulate, it is not at all clear what effect chronic alcohol consumption has on cancer progression. Thus, it is not known to any great extent whether the cancers associated with alcohol consumption are more or less prone to metastasize.
Alcohol consumption is a well-known immunosuppressant, and our previous research indicated that chronic high alcohol consumption suppresses natural killer (NK) cell cytolytic activity [11–16]. NK cells, which are the first line of immune defense against tumors, play an initial role in adaptive antitumor immune responses, and were found several years ago to have an important role in the control of melanoma metastasis [17, 18]. Thus, we previously examined the hypothesis that alcohol consumption would enhance metastasis of melanoma. Using the well characterized B16BL6 melanoma model, which is highly invasive and metastatic, we found to our surprise that 20% w/v alcohol consumption inhibited both spontaneous metastasis to the lung after intradermal inoculation into the pinna of the ear as well as experimental metastasis after i.v. inoculation via the lateral tail vein [19, 20]. The effect of alcohol on B16BL6 melanoma metastasis is dose dependent [20] and independent of perforin and granzymes related NK cell cytolytic activity, as demonstrated in beige mice [21].
The mechanism underlying the inhibition of melanoma metastasis associated with alcohol consumption is not known. While multiple factors are likely involved, it is known that IFN-γ is a key player in controlling melanoma metastasis [22, 23]. This cytokine, which can be produced by a number of cells including but not limited to NK cells, NKT cells, T cells, and macrophages, is also known to have a significant impact on metastasis in other tumors [24–29]. We previously reported that chronic alcohol consumption increases IFN-γ producing CD3−NK1.1+, CD4+ and CD8+ T cells [30, 31]. Herein we also report that chronic alcohol consumption also increases IFN-γ producing CD3+NK1.1+ (NKT) cells. This information provided a strong rationale to examine the hypothesis that enhancement of IFN-γ by chronic alcohol consumption is responsible for the decrease in B16BL6 melanoma metastasis to the lung. Herein, we report that knockout of the ifn-γ gene in mice abrogates the antimetastatic effect induced by chronic alcohol consumption, suggesting that the effect is IFN-γ dependent.
Materials and methods
Animals and alcohol administration
Female C57BL6 mice at 6–7 weeks of age were purchased from NIH Charles River Laboratories (Wilmington, MA) and acclimated in the Wegner Hall Vivarium at Washington State University for 1 week before using them in experiments. These mice were used to determine the effect of chronic alcohol administration on the percentage of IFN-γ producing NKT cells in the spleen. IFN-γ knockout (KO) mice (B6.129S7-Ifngtm1Ts/J) and breeding stock for γC KO mice (B6.129S4-Il2rgtm1Wjl/J) were purchased from the Jackson Laboratory (Bar Harbor, ME). Vα14Jα281−/− NKT KO mice were originally obtained through Dr. M. Taniguchi at Chiba University (Chiba, Japan). The γC KO and the iNKT KO mice were bred and housed in the Wegner Hall Vivarium. Female KO mice from the three different strains all on a C57BL/6 background were utilized in experiments at 7–8 weeks of age. All mice were single housed in air filter topped polystyrene cages. Experimental mice were randomly divided into two groups. One group was provided with sterilized double distilled, deionized water as a control and the other another group was provided with 20% w/v alcohol (Everclear, St. Louis, MO) as the sole drinking fluid. Both groups had free access to Purina 5001 Rodent Laboratory Chow. We previously validated this chronic model of human alcoholism [32]. Mice given 20% w/v alcohol in the drinking water consume >30% of their calories from alcohol, which is similar to the intake of human alcoholics. Mice consumed alcohol for 3 months prior to using them in experiments. Mice were then injected with melanoma, and they continued to receive alcohol or water until the experiments were terminated. The animal protocols used in this research were approved by the Institutional Animal Care and Use Committee at Washington State University.
Tumor cell culture, tumor inoculation and lung colony counting
B16BL6 melanoma cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, and 1% penicillin and streptomycin. Tumor cells at 50–70% confluence were detached with 2 mM ethylene glycol tetracetic acid and resuspended in phosphate buffered magnesium- and calcium-free normal saline (CMF). Mice were inoculated with 5 × 104 melanoma cells in 200 μl of CMF i.v. via the lateral tail vein and then assessed for lung metastasis 3 weeks later. The lungs were fixed at necropsy with Fekete’s solution and tumor colonies on the 5 lobes of the lung were counted using a stereo microscope.
Isolation and phenotyping of splenocytes and peripheral blood leukocytes (PBL)
Splenocytes from C57BL/6 mice and PBL from γC KO mice were isolated and stained with appropriately labeled antibodies, and the cellular phenotype was determined by flow cytometry according to previously published procedures [33]. Cells were labeled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP)-Cy5.5, or PE-Cy5.5 labeled anti-mouse antibodies. Anti-CD11b (M1/70), anti-CD4 (L3T4), and anti-CD8 (53-6.7) were purchased from BD Biosciences Pharmingen (San Diego, CA). Anti-NK1.1 (PK136), anti-Ly6G/Ly-6C (Gr-1) (RB6-8C5), and anti-CD3 (145-2C11) were obtained from BioLegend (San Diego, CA).
Gr-1+ cell depletion
Gr-1+ cells were depleted by injection of 200 μg of purified rat anti-mouse Gr-1 monoclonal antibody (Clone RB6-8C5, National Cell Culture Center, Biovest International, Inc., Minneapolis, MN) in 200 μl of PBS via the lateral tail vein 24 h before and again 30 min before melanoma inoculation. Control water and alcohol drinking mice were injected with purified rat anti-IgG2b (BioLegend, San Diego, CA).
Intracellular staining of IFN-γ
Splenocytes from γC KO mice were isolated according to standard procedures and after activation and staining were analyzed for IFN-γ by flow cytometry as previously described [33]. Splenocytes were activated with 80 ng/ml alpha-galactosylceramide (α-GalCer) in RPMI1640 medium containing 10% fetal bovine serum and GolgiStop for 5–6 h, fixed, and permeabilized by Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences, San Jose, CA) before staining for IFN-γ. Cells were then stained with anti-IFN-γ (XMG1.2) antibody (BD Biosciences Pharmingen, San Diego, CA) and analyzed by flow cytometry [33].
Statistical analysis
Data were analyzed with the Microsoft Excel statistical program or GraphPad Prism 5 (GraphPad Software Inc.). The results are expressed as the mean ± SD. Differences between groups were determined using two-tailed unpaired Student’s t test. Values are considered different at P < 0.05.
Results
The antimetastatic effects induced by chronic alcohol consumption are abrogated in IFN-γ KO mice
IFN-γ plays a crucial role in controlling tumor metastasis [24–27]. Chronic alcohol consumption significantly increases the percentage of IFN-γ producing splenic CD3−NK1.1+, CD4+ T, and CD8+ T cells [30, 31]. Herein, we determined that alcohol consumption also increased the percentage of IFN-γ producing cells in the CD3+NK1.1+ (NKT) splenic cell population (46.4 ± 2.3% (SD) in water drinking mice compared to 55.1 ± 7.0% (SD) in alcohol drinking mice (P = 0.015, n = 7)). Since the mean fluorescence intensity of these cells was not different, this indicates that it is the proportion of CD3+NK1.1+ cells that is increased by alcohol rather than the amount of IFN-γ that is produced by these cells. Together these data provided a strong rationale to explore the hypothesis that the antimetastatic activity associated with alcohol consumption in the B16BL6 melanoma tumor model resulted from elevated IFN-γ producing cells. The results in Fig. 1 indicate that knocking out the ifn-γ gene abrogates the antimetastatic effects associated with alcohol consumption.
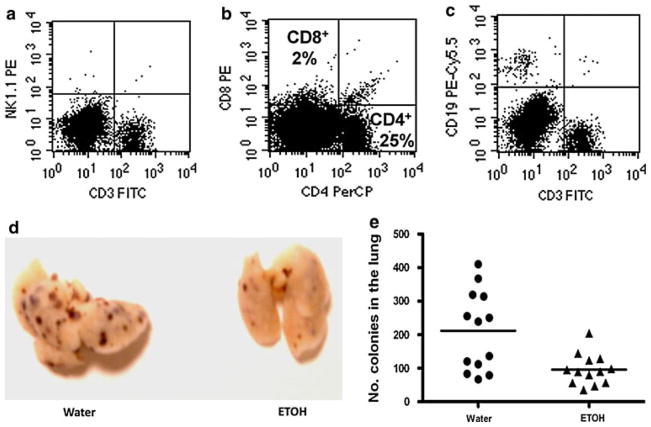
Fig. 1.
The effect of chronic alcohol consumption on experimental lung metastasis of B16BL6 melanoma is abrogated in IFN-γ KO mice. Female IFN-γ KO mice received alcohol for 3 months and then were inoculated via tail vein with 5 × 104 B16BL6 melanoma cells. Mice were euthanized 3 weeks after tumor inoculation. The figure shows the distribution of lung colony numbers in which no differences were observed between water and ETOH (alcohol) drinking groups (P = 0.49). The bar indicates the mean number of lung tumor colonies. Data are representative of two experiments with similar results
Depletion of NK, NKT, CD8+ T cells, and B cells does not abrogate the antimetastatic effects induced by chronic alcohol consumption
We next sought to determine the cell type that played the key role in producing IFN-γ associated with inhibition of B16BL6 melanoma metastasis. NK cells, NKT cells and CD8+ T cells are major producers of IFN-γ; therefore, we used a murine model for X-linked severe combined immunodeficiency disease in humans that results in marked deficiency or lack of NK and T cells. Previous studies in male mice lacking γc expression (γC−/Y) indicated an absence of NK and CD8+ T cells in the spleen and greatly diminished percentages of B cells [34]. Herein, we showed similar results in the PBL from female γC KO (γC−/−) mice (Fig. 2a–c). The results in Fig. 2d and e show that the antimetastatic effect of alcohol consumption is not impaired (Fig. 2d, e), and they suggest that NK, NKT, and CD8+ T cells are not critical to the antimetastatic effect.
Fig. 2.
Chronic alcohol consumption decreases lung metastasis of B16BL6 melanoma in γC KO mice. Female γC KO mice received alcohol for 3 months and then were inoculated via the tail vein with 5 × 104 B16BL6 melanoma cells. Mice were euthanized 3 weeks after tumor inoculation. a Dot plot indicating the deficit of NK (upper left quadrant) and NKT (upper right quadrant) in peripheral blood. b Dot plot indicating the presence of CD8+ T cells (upper left quadrant) and CD4+ T cells (lower right quadrant) in peripheral blood. c Dot plot indicating the presence of CD19+ B cells (upper left quadrant) in the peripheral blood. d Representative example of B16BL6 melanoma colonies on the lungs of mice given water or ETOH (alcohol). e Distribution of the number of tumor colonies on the lung of water and alcohol drinking mice. The bar indicates the mean number of lung tumor colonies. Water drinking group different from ETOH (alcohol) drinking group, P = 0.003
Depletion of Gr-1+ cells does not abrogate the antimetastatic effects of alcohol consumption in γC KO mice
Flow cytometric analysis revealed that CD11b+ cells are a dominant population in the PBL of γC KO mice (Fig. 3a). These cells are a heterogeneous cell population comprised of neutrophils, monocytes, and marcophages, and we found that 40% of the PBL were CD11b+Gr-1+ cells. These CD11b+Gr-1+ cells produce IFN-γ upon activation [26]. The percentage of these cells increases significantly in the blood of γC KO mice 2 days after i.v. B16BL6 inoculation (Fig. 3b). Thus, the CD11b+Gr-1+ cells could play a role in the inhibition of B16BL6 lung colonization in the alcohol drinking mice. To examine this hypothesis, anti-Gr-1 monoclonal antibody was used to deplete the Gr-1+ cells. Figure 3c shows that the majority of the Gr-1+ cells were depleted after two injections of anti-Gr-1 antibody. However, the depletion of Gr-1+ cells did not abrogate the antimetastatic effects induced by chronic alcohol consumption in the γC KO mice (Fig. 3d). Liver tumor colonies were observed in 10 out of 10 water drinking mice and in 7 out of 10 alcohol consuming mice. The total number of liver tumor colonies observed in the water drinking mice was 42 compared to 23 in the alcohol consuming mice; however, the difference between the two groups was not significant (P = 0.2 by Mann–Whitney test).
Fig. 3.
The antimetastatic effect of chronic alcohol consumption is sustained in CD11b+Gr-1+ depleted γC KO mice. a Dot plot showing a high proportion of CD11b+Gr-1+ cells (gray dots) in the PBL of water drinking γC KO mice. b Dynamic changes in the percentage of CD11b+Gr-1+ cells in the PBL of γC KO mice before (day 0) and after (day 1 and day 2) inoculation of 5 × 104 B16BL6 melanoma via the lateral tail vein. * Different from day 0, P < 0.05. c Dot plot showing depletion of CD11b+Gr-1+ cells in the PBL of water drinking γC KO mice. d Numbers of B16BL6 melanoma colonies on the lung of water and ETOH (alcohol) drinking γC KO mice after depletion of Gr-1+ cells. Water drinking group different from ETOH (alcohol) drinking group, P = 0.03
Depletion of iNKT cells did not abrogate the antimetastatic effects induced by chronic alcohol consumption
Although γC KO mice are essentially devoid of NK1.1+CD3+ NKT cells in the blood (Fig. 2a), when splenocytes from γC KO mice were stimulated with αGalCer in vitro, the percentage of IFN-γ producing cells were significantly higher in the alcohol drinking than in the water drinking mice (Fig. 4a, b). Additional phenotypic analysis indicated that all of the IFN-γ producing cells are Gr-1−CD 11b−CD3+. Gating on only the IFN-γ producing cells, we found that all cells are CD3+. Eleven percent of these cells are CD3+NK1.1+. No CD3−NK1.1+ cells were found in the IFN-γ producing cell population. The basis for the increase is not clear; however, since αGalCer is the T-cell receptor ligand of iNKT cells, this led us to further examine if iNKT cells play a role in controlling B16BL6 lung metastasis in alcohol drinking mice. The results in Fig. 4c indicate that iNKT KO mice retain their sensitivity to the antimetastatic effect of alcohol consumption, although the magnitude of the difference between the lung colony numbers in the alcohol and water drinking mice is not as great as in the γC KO mice (Fig. 2e).
Fig. 4.
Chronic alcohol consumption increases αGalCer induced IFN-γ producing cells in the splenocytes from γC KO mice and inhibits B16BL6 melanoma lung metastasis in iNKT KO mice. a Histogram of the IFN-γ producing splenocytes from water and ETOH (alcohol) drinking γC KO mice. b Percentage of IFN-γ producing cells in the splenocytes of water drinking and ETOH (alcohol) drinking γC KO mice after stimulation for 5–6 h with αGalCer in vitro. Water drinking group different from ETOH (alcohol) drinking group, P = 0.04. c Number of colonies in the lung of water drinking and alcohol drinking iNKT mice. Water drinking group different from ETOH (alcohol) drinking group, P = 0.007
Discussion
Little information exists regarding the effects of chronic alcohol consumption on tumor progression and metastasis. Using a mouse model of chronic alcohol consumption that mimics intake in human alcoholics, we found that B16BL6 melanoma metastasis to the lung is greatly decreased between 42 and 94% [19–21]. Our previous findings [19, 21] and the results of others [24, 25] indicate that NK cell cytolytic activity is not the major mechanism underlying control of melanoma metastasis. Until now the mechanisms associated with this effect have not been elucidated. The results of this investigation showed that IFN-γ is essential for the antimetastatic activity associated with alcohol consumption. These results are consistent with other well-documented reports that IFN-γ is the crucial factor that controls the metastasis of mesothelioma, breast cancer, as well as melanoma [24–29]. A variety of IFN-γ producing cells were found to have a primary role in controlling metastasis in these studies. IFN-γ produced by tumor specific T cells was found to play a key role in control of mesothelioma metastasis [28]. Phagocytic cells were found to be the main source of IFN-γ production that controls 4T1 breast cancer metastasis [26]. In this model, even small amounts of endogenous of IFN-γ produced by hematopoietic cells within the tumor microenvironment had an impact on metastasis [29].
In the present study, we were unable to identify one specific cell type that was crucial to the production of IFN-γ and to the antimetastatic effect of alcohol consumption. We found that knockout of NK, NKT, and CD8+ T cells that are major producers of IFN-γ and also depletion of Gr-1+ cells that also can produce IFN-γ did not abrogate the antimetastatic effects of alcohol as expected. Even though Cao et al. [34] found that IFN-γ production was dramatically reduced in γC KO mice, a very small amount was still produced after stimulation of splenocytes with anti-CD3 and anti-CD28. Thus, it is possible that alcohol consumption could facilitate production of IFN-γ in residual cell populations of the γC KO mice and that the additional amount of IFN-γ produced was sufficient to maintain the antimetastatic effect in the alcohol drinking mice. This is not an unreasonable possibility given the similar finding of duPre et al. [29] in the 4T1 model of breast cancer. We did not exclude a role for monocytes and macrophages in the γC KO mice. It is also well known that alcohol consumption increases the permeability of the gut to bacteria, which can lead to an increase in the concentration of the bacterial endotoxin, lipopolysaccharide, in the blood. This in turn can activate monocytes and macrophages to produce IFN-γ [35, 36]. Lipopolysaccharide also is known to induce tumor regression as well as inhibit tumor growth in other experimental tumor systems, an effect that is blocked by neutralizing IFN-γ [22].
We also found that treating splenocytes from γC KO mice with αGalCer enhanced IFN-γ production in alcohol drinking mice compared to water drinking mice (Fig. 4b). While we do not understand the underlying basis for this finding, it is possible that alcohol facilitates production of IFN-γ in a unique population of iNKT cells that are not dependant on the γC receptor for activity. This finding provided additional rationale for examining the contribution of the specific role of iNKT cells to the antimetastatic effect of alcohol. However, we found again that the anti-metastatic effect of alcohol consumption was not abrogated in the iNKT KO mice.
In summary, we showed that chronic alcohol consumption facilitates the generation of IFN-γ producing cells and that IFN-γ is essential to inhibition of murine B16BL6 melanoma lung metastasis. Our findings support those of duPre et al. [29] suggesting that only small amounts of IFN-γ are necessary to inhibit metastasis. In addition, we found that no one IFN-γ producing cell type was the source of the antimetastatic effect in melanoma associated with chronic alcohol consumption. The underlying mechanism associated with IFN-γ is still unclear and is the subject of ongoing research.
Acknowledgments
The authors thank Dr. Danny Welch (University of Alabama, Birmingham, AL, USA) for reading the manuscript and for his constructive comments. This work was supported by the National Institute on Alcohol Abuse and Alcoholism grants R01A A07293 and K05AA017149.
Abbreviations
- α-GalCer
Alpha-galactosylceramide
- CMF
Phosphate buffered magnesium- and calcium-free normal saline
- γC KO
Common gamma-chain knockout
- FITC
Fluorescein isothiocyanate
- iNKT
Invariant natural killer T cells
- KO
Knockout
- NK
Natural killer
- PBL
Peripheral blood leukocytes
- PE
Phycoerythrin
- PerCP
Peridinin chlorophyll protein
References
- 1.Nelson S, Kolls JK. Alcohol, host defense and society. Nat Rev Immunol. 2002;2(3):205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 2.Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39(3):155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 3.Allen NE, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 4.Bagnardi V, et al. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85(11):1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi SW, et al. Alcohol consumption and digestive cancer mortality in Koreans: the Kangwha Cohort Study. J Epidemiol. 2010;20(3):204–211. doi: 10.2188/jea.JE20090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millen AE, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13(6):1042–1051. [PubMed] [Google Scholar]
- 7.Le Marchand L, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164(3):232–245. doi: 10.1093/aje/kwj115. [DOI] [PubMed] [Google Scholar]
- 8.Mukamal KJ. Alcohol consumption and self-reported sunburn: a cross-sectional, population-based survey. J Am Acad Dermatol. 2006;55(4):584–589. doi: 10.1016/j.jaad.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Benedetti A, Parent ME, Siemiatycki J. Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal. Cancer Detect Prev. 2009;32(5–6):352–362. doi: 10.1016/j.canep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Lie JA, Andersen A, Kjaerheim K. Cancer risk among 43000 Norwegian nurses. Scand J Work Environ Health. 2007;33(1):66–73. [PubMed] [Google Scholar]
- 11.Blank SE, Duncan DA, Meadows GG. Suppression of natural killer cell activity by ethanol consumption and food restriction. Alcohol Clin Exp Res. 1991;15(1):16–22. doi: 10.1111/j.1530-0277.1991.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 12.Blank SE, et al. Modulation of NK cell activity by moderate intensity endurance training and chronic ethanol consumption. J Appl Physiol. 1992;72:8–14. doi: 10.1152/jappl.1992.72.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Blank SE, et al. Ethanol-induced changes in peripheral blood and splenic NK cells. Alcohol Clin Exp Res. 1993;17(3):561–565. doi: 10.1111/j.1530-0277.1993.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 14.Meadows GG, Blank SE, Duncan DD. Influence of ethanol consumption on natural killer cell activity in mice. Alcohol Clin Exp Res. 1989;13:476–479. doi: 10.1111/j.1530-0277.1989.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 15.Meadows GG, et al. Alcohol, immunity, and cancer. CRC Press; Boca Raton: 1993. Modulation of natural killer cell activity by alcohol; pp. 55–85. [Google Scholar]
- 16.Gallucci RM, Pfister LJ, Meadows GG. Effects of ethanol consumption on enriched natural killer cells from C57BL/6 mice. Alcohol Clin Exp Res. 1994;18(3):625–631. doi: 10.1111/j.1530-0277.1994.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi K, Kärre K, Klein G. Lung colonization and metastasis by disseminated B16 melanoma cells: H-2 associated control at the level of the host and the tumor cell. Int J Cancer. 1985;36:503–510. doi: 10.1002/ijc.2910360415. [DOI] [PubMed] [Google Scholar]
- 18.Hanna N. Expression of metastatic potential of tumor cells in young nude mice is correlated with low levels of natural killer cell-mediated cytotoxicity. Int J Cancer. 1980;26:675–680. doi: 10.1002/ijc.2910260521. [DOI] [PubMed] [Google Scholar]
- 19.Meadows GG, et al. Alcohol consumption suppresses metastasis of B16-BL6 melanoma in mice. Clin Exp Metastasis. 1993;11:191–199. doi: 10.1007/BF00114977. [DOI] [PubMed] [Google Scholar]
- 20.Blank SE, Meadows GG. Ethanol modulates metastatic potential of B16BL6 melanoma and host responses. Alcohol Clin Exp Res. 1996;20:624–628. doi: 10.1111/j.1530-0277.1996.tb01664.x. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer JH, et al. The modulation of B16BL6 melanoma metastasis is not directly mediated by cytolytic activity of natural killer cells in alcohol-consuming mice. Alcohol Clin Exp Res. 2000;24(6):837–844. [PubMed] [Google Scholar]
- 22.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 23.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15(2):148–154. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 24.Smyth MJ, et al. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the anti-metastatic effect of α-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa Y, et al. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31(6):1720–1727. [PubMed] [Google Scholar]
- 26.Pulaski BA, Smyth MJ, Ostrand-Rosenberg S. Interferon-gamma-dependent phagocytic cells are a critical component of innate immunity against metastatic mammary carcinoma. Cancer Res. 2002;62(15):4406–4412. [PubMed] [Google Scholar]
- 27.Ostrand-Rosenberg S, et al. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169(10):5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 28.Rudge G, et al. Infiltration of a mesothelioma by IFN-gamma-producing cells and tumor rejection after depletion of regulatory T cells. J Immunol. 2007;178(7):4089–4096. doi: 10.4049/jimmunol.178.7.4089. [DOI] [PubMed] [Google Scholar]
- 29.duPre SA, Redelman D, Hunter KW., Jr Microenvironment of the murine mammary carcinoma 4T1: endogenous IFN-gamma affects tumor phenotype, growth, and metastasis. Exp Mol Pathol. 2008;85(3):174–188. doi: 10.1016/j.yexmp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78(5):1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Meadows GG. Chronic alcohol consumption perturbs the balance between thymus-derived and bone marrow-derived natural killer cells in the spleen. J Leukoc Biol. 2008;83(1):41–47. doi: 10.1189/jlb.0707472. [DOI] [PubMed] [Google Scholar]
- 32.D’Souza El-Guindy NB, et al. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol Clin Exp Res. 2010;34(9):1489–1511. doi: 10.1111/j.1530-0277.2010.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Meadows GG. Chronic alcohol consumption enhances myeloid-derived suppressor cells in B16BL6 melanoma-bearing mice. Cancer Immunol Immunother. 2010;59(8):1151–1159. doi: 10.1007/s00262-010-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao X, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2(3):223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 35.Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut–liver–brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16(11):1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16(11):1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]