Abstract
Glial cells were long considered end products of neural differentiation, specialized supportive cells with an origin very different from that of neurons. New studies have shown that some glial cells—radial glia (RG) in development and specific subpopulations of astrocytes in adult mammals—function as primary progenitors or neural stem cells (NSCs). This is a fundamental departure from classical views separating neuronal and glial lineages early in development. Direct visualization of the behavior of NSCs and lineage-tracing studies reveal how neuronal lineages emerge. In development and in the adult brain, many neurons and glial cells are not the direct progeny of NSCs, but instead originate from transit amplifying, or intermediate, progenitor cells (IPCs). Within NSCs and IPCs, genetic programs unfold for generating the extraordinary diversity of cell types in the central nervous system. The timing in development and location of NSCs, a property tightly linked to their neuroepithelial origin, appear to be the key determinants of the types of neurons generated. Identification of NSCs and IPCs is critical to understand brain development and adult neurogenesis and to develop new strategies for brain repair.
Keywords: neural stem cells, radial glia, astrocytes, cortical development, adult neurogenesis, subventricular zone
INTRODUCTION
The identification of progenitor cells and the lineages they produce is key to our understanding of how the enormous diversity of cell types is produced in the brain, and this information will guide future attempts to harness stem cells for brain repair. Nerve cells (neurons) and glial cells (the supporting elements) in the central nervous system (CNS) have classically been thought to derive from distinct precursor pools that diverge early during embryonic development. The separation of glial and neuronal lineages has a long history. Rudolf Virchow, who first suggested the presence of supporting cells in the CNS in 1846 and called these cells glia, assumed that like other support cells in the body they too had a mesenchymal origin. Four decades later, Wilhelm His demonstrated a CNS origin for glial cells, leading to the rejection of the mesenchymal hypothesis (for historical perspective see Jacobson 1991). His nevertheless concluded that neurons and glial cells were produced from two separate progenitor pools. This concept became highly influential throughout most of the past century. Neurons were proposed to derive from specialized progenitors (neuroblasts). Spongioblasts [corresponding to what we now call radial glia (RG)] were thought to be precursors to astroglial cells, a proposition that was extensively supported by descriptions of the transformation of RG into astrocytes during late embryonic development (see below). The separate origins of neurons and glia became widely accepted. Surprisingly, however, recent studies have shown that cells considered part of this glial lineage—RG and a subpopulation of astrocytes—are in fact the neural stem cells that give rise to differentiated neurons and glial cells during development and in the postnatal brain.
We use the term neural stem cell (NSC) to refer to the primary progenitor cells at different developmental stages that initiate lineages leading to the formation of differentiated neurons or glial cells. These end products, however, are not always generated directly from the division of NSCs and can be generated through one or multiple stages of amplification by precursors with more restricted potential. We refer to transit amplifying cells that are derived from NSCs as intermediate progenitor cells (IPCs). IPCs can generate neurons (nIPCs) or generate glial cells, including oligodendrocytes (oIPCs) or astrocytes (aIPCs) (Figure 1). The term glial cell becomes somewhat confusing because it refers to both a progenitor population as well as a differentiated population of parenchymal astrocytes, oligodendrocytes, and ependymal cells (as discussed below, this problem also applies to the term astrocyte). However, at least some of the functions attributed to terminally differentiated astrocytes (supporting neuronal function and regulating metabolic activity) are likely represented in the adult and earlier progenitor cells including RG. Short of proposing an entirely new nomenclature, which could add confusion, we use the term glia more generally to refer both to glial cells that have specialized traditional glial functions and to those that, in addition, retain progenitor capacity.
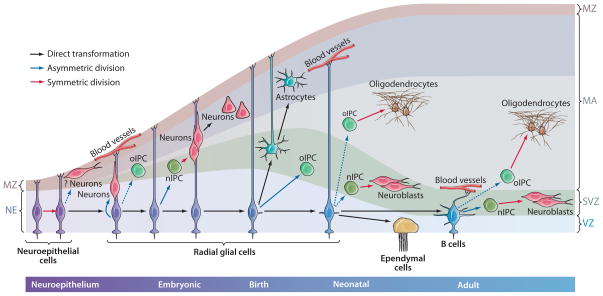
Figure 1.
Glial nature of neural stem cells (NSCs) in development and in the adult. Neuroepithelial cells in early development divide symmetrically to generate more neuroepithelial cells. Some neuroepithelial cells likely generate early neurons. As the developing brain epithelium thickens, neuroepithelial cells elongate and convert into radial glial (RG) cells. RG divide asymmetrically to generate neurons directly or indirectly through intermediate progenitor cells (nIPCs). Oligodendrocytes are also derived from RG through intermediate progenitor cells that generate oligodendrocytes (oIPCs). As the progeny from RG and IPCs move into the mantel for differentiation, the brain thickness, further elongating RG cells. Radial glia have apical-basal polarity: apically (down), RG contact the ventricle, where they project a single primary cilium; basally (up), RG contact the meninges, basal lamina, and blood vessels. At the end of embryonic development, most RG begin to detach from the apical side and convert into astrocytes while oIPC production continues. Production of astrocytes may also include some IPCs (see Figure 2) not illustrated here. A subpopulation of RG retain apical contact and continue functioning as NSCs in the neonate. These neonatal RG continue to generate neurons and oligodendrocytes through nIPCs and oIPCS; some convert into ependymal cells, whereas others convert into adult SVZ astrocytes (type B cells) that continue to function as NSCs in the adult. B cells maintain an epithelial organization with apical contact at the ventricle and basal endings in blood vessels. B cells continue to generate neurons and oligodendrocytes through (n and o) IPCs. This illustration depicts some of what is known for the developing and adult rodent brain. Timing and number of divisions likely vary from one species to another, but the general principles of NSC identity and lineages are likely to be preserved. Solid arrows are supported by experimental evidence; dashed arrows are hypothetical. Colors depict symmetric, asymmetric, or direct transformation. IPC, intermediate progenitor cell; MA, mantle; MZ, marginal zone; NE, neuroepithelium; nIPC, neurogenic progenitor cell; oIPC, oligodendrocytic progenitor cell; RG, radial glia; SVZ, subventricular zone; VZ, ventricular zone.
Many recent publications have described molecular pathways that regulate ventricular zone (VZ) and subventricular zone (SVZ) progenitors as well as progress characterizing NSCs in vitro. Here we mention some of this work, but the focus of the present review is on identifying the lineages leading from NSCs to differentiated cell types at different stages in development and in the adult brain. The process is more complex and elegant than previously thought and involves a series of distinct progenitor cell types that are lineally related and coexist not only in the embryo throughout the period of neurogenesis but also in the adult in restricted germinal regions where neurons continue to be produced. RG and a subpopulation of adult astrocytes are the founder cells for most, if not all, neurogenic lineages in the CNS. These NSCs undergo distinct modes of cell division, giving rise to a remarkable diversity of glial and neuronal cell types in the brain and CNS.
TRANSFORMATION OF NEUROEPITHELIAL CELLS AND THE ORIGIN OF RADIAL GLIA
Although we describe neurogenesis and gliogenesis specifically in the cerebral cortex, much of our current understanding of cortical development is applicable to other CNS regions as well. Neurons and macroglia ultimately derive from a pseudostratified neuroepithelium of ectodermal origin that lines the cerebral ventricles early in embryonic development. At approximately the time when cortical neurogenesis begins, around E9–10 in the mouse, neuroepithelial cells begin to acquire features associated with glial cells (Figure 1): the RG cells. Whereas these cells remain in contact with both pial and ventricular surfaces, their cell bodies are retained within the ventricular zone (VZ), a defined region next to the ventricles (Boulder Comm. 1970). Progressive thickening of the cortex throughout neurogenesis is accompanied by lengthening of the pial-directed radial processes of RG. These morphological changes are accompanied by the acquisition of 24-nm microtubules and 9-nm intermediate filaments within the radial fiber, as well as glycogen storage granules in the subpial end feet (Choi & Lapham 1978, Bruckner & Biesold 1981, Gadisseux & Evrard 1985). The RG cells also begin to express astroglial markers such as the astrocyte-specific glutamate transporter (GLAST), brain lipid-binding protein (BLBP), and Tenascin C (TN-C) (for review, see Campbell & Gotz 2002). They also express a variety of intermediate filament proteins including nestin, vimentin, the RC1 and RC2 epitopes (Mori et al. 2005), and in some species the astroglial intermediate filament, glial fibrillary acidic protein (GFAP) (Choi & Lapham 1978, Levitt & Rakic 1980, Benjelloun-Touimi et al. 1985, Eng 1985, Choi 1988). At about the same time, the tight junctional complexes that couple neuroepithelial cells convert to adherens junctions (Aaku-Saraste et al. 1997, Stoykova et al. 1997), and the cells begin to make astrocyte-like specialized contact with endothelial cells of the developing cerebral vasculature (Takahashi et al. 1990, Misson et al. 1991). These changes mark the transition of epithelial cells into RG, but the transition is not abrupt (Gotz & Huttner 2005). Like neuroepithelial cells, RG cells maintain apical-basal polarity, line the lateral ventricles, and undergo interkinetic nuclear migration, thereby maintaining a pseudostratified epithelium within the VZ. A recent study indicates that basal contacts of RG with the meninges and retinoic acid signaling at this location are essential for the transition from neuroepithelial symmetric proliferation to asymmetric neurogenic proliferation (J. Siegenthaler, A. Ashique, K. Zarbalis, K. Patterson, J.H. Hecht et al., unpublished observation).
The apical end feet of RG are anchored to each other through adherens junctions. These junctions are critical to maintain VZ integrity and RG behavior. The mouse homologs of the Drosophila endocytic protein genes, Numb and Numbl, are essential to maintain the polarized structure of RG cells and specifically of adherens junctional complex components such as cadherins (Rasin et al. 2007). Inactivation of Numb and Numbl in RG cells decreases basolateral insertion of cadherins, disrupting adherens junctions and polarity. This disruption results in RG becoming disorganized and dispersing basally, leading to disorganized cortical lamination. In contrast, overexpression of Numb appears to strengthen RG polarization. RG cell adhesions and polarity are therefore critical for cortical architecture, and Numb and Numbl play a role in the organization of adherens junctional complexes in RG apical end feet. Inactivation of Numb and Numbl during postnatal development similarly disrupts epithelial organization in ependymal cells (Kuo et al. 2006), likely through disorganization of apical junctional complexes as observed in RG during embryonic development. The consequences of epithelial disorganization on neurogenesis, however, can be surprisingly minor. Deletion of the atypical protein kinase C, known to contribute to adherens junction formation, leads to loss of junctional integrity and disordered VZ architecture. Despite the loss of apical attachment, cortical neurons are generated normally (Imai et al. 2006). Also, randomizing spindle orientation, either by knocking out the G protein regulator, LGN, or overexpressing mouse inscuteable, leads to loss of apical anchoring of RG cells and movement away from the ventricle (Konno et al. 2008). Despite the disorganization and shift of mitotic cells out of the epithelial layer, however, neuron production appears normal. Not all manipulations that disrupt junctions, however, leave cell fate unchanged. When the small Rho-GTPase, cdc42, which acts to regulate adherens junctions, is deleted in RG, adherens junctions are lost; but in this case, RG appear to change fate and transform into IPCs (see below) (Cappello et al. 2006). These disparate results may be explained by effects of cdc42 deletion on processes other than junctional integrity alone.
RG cells appear to serve as neural precursors throughout the CNS (Figures 1 and 2). When the promoter for BLBP, a protein expressed by RG cells throughout the developing brain and spinal cord (Feng et al. 1994, Kurtz et al. 1994, Hartfuss et al. 2001), was used to drive Cre-recombinase gene expression in a Cre/loxP fate mapping experiment, large numbers of neurons in virtually all brain regions were labeled (Anthony et al. 2004). Excitatory projection neurons and interneurons as well as glial cells were all labeled, indicating an RG cell origin. A similar fate mapping experiment, based on expression of the human GFAP promoter, which is expressed by RG at a slightly later stage, failed to label neurons derived from the ventral telencephalon (Malatesta et al. 2003), possibly because the hGFAP promoter may not be active ventrally in the mouse until later stages of development (Anthony et al. 2004).
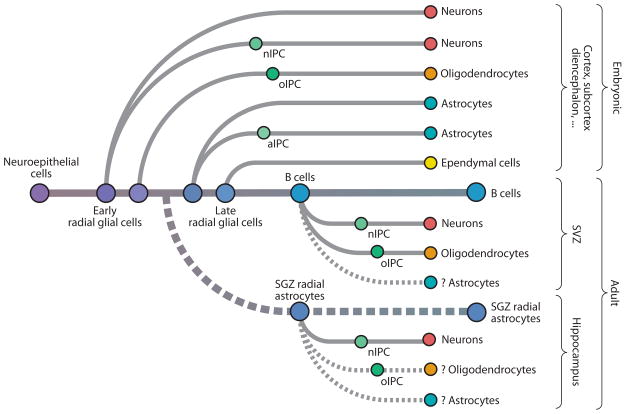
Figure 2.
Lineage tree of NSCs. Purple to blue dots represent NSCs at different stages in development from neuroepithelial cells through early and late RG to adult NSCs (SVZ B cells and SGZ radial astrocytes). The derivation of embryonic progeny is depicted in the upper half, whereas the lower half shows lineages derived in the postnatal and adult brain. Solid lines indicate lineage conversions for which experimental evidence is available; dashed arrows are hypothetical. Intermediate progenitor cells (IPCs) for neurons (nIPCs), for oligodendrocytes (oIPCs), and for astrocytes (aIPCs) are indicated along each lineage leading to differentiated progeny. Note that in some instances this transit-amplifying step is bypassed. NSC, neural stem cell; RG, radial glia; SGZ: subgranular zone; SVZ, subventricular zone.
INTERKINETIC NUCLEAR MIGRATION
One aspect of neuroepithelial cell activity retained by RG cells is the complex mitotic behavior known as interkinetic nuclear migration (INM). INM was first inferred on the basis of an analysis of nuclear position at different phases of the cell cycle (Sauer 1935) and later confirmed by thymidine analog labeling (Sauer & Walker 1959, Sidman et al. 1959, Fujita 1962, Hayes & Nowakowski 2000). Nuclei undergoing S phase of the cell cycle form a layer several cell-body diameters from the ventricle, at the apical side of the VZ, while nuclei in M phase line up along the surface of the ventricle, and nuclei in G1 and G2 phases are transitioning between the S and M phases in the mid region. INM may be a cell-autonomous feature of neuroepithelial or RG-like cells because NSCs derived from embryonic stem cell lines both express RG markers and undergo nucleokinesis as they progress through the cell cycle (Conti et al. 2005, Glaser & Brustle 2005, Elkabetz et al. 2008). Although the mechanism of INM has been explored (reviewed in Miyata 2008), little is known about its functional significance. Cells can still enter mitosis when nucleokinesis is arrested by manipulations that disrupt microtubules or depolymerize actin (Karfunkel 1972, Murciano et al. 2002). However, manipulations that block INM also interfere with cell cycle progression. For example, loss of the microtubule-associated protein LIS1 arrests nucleokinesis and reduces cell proliferation (Tsai et al. 2005), whereas arresting cells in G2/M phase with 5-azacytidine, or in S phase with cyclophosphamide, also stops INM (Ueno et al. 2006). These observations suggest that nuclear migration is linked to cell cycle progression.
Del Bene et al. (2008) recently proposed that INM regulates neurogenesis by modulating the exposure of progenitor cell nuclei to neurogenic or proliferative signals, specifically to Notch. Notch activation maintains cells in a proliferative state (Dorsky et al. 1997, Gaiano & Fishell 2002, Silva et al. 2003, Zhang et al. 2008) and is critical to maintain RG cell identity and self-renewal (Gaiano et al. 2000). Notch also activates target genes such as hes1 and hes5 (Gaiano et al. 2000, Iso et al. 2003) to antagonize proneural genes and block cell differentiation. Del Bene et al. (2008) also demonstrated an apical-basal Notch gradient within the neuroepithelium of the zebrafish retina with high levels at the apical side. Disrupting the dynein/dynactin motor protein complex, which helps couple the nuclear envelope to the centrosome (Iso et al. 2003, Tsai & Gleeson 2005), resulted in more rapid migration of nuclei to the basal side and slower migration to the apical side. Cells subsequently exited the cell cycle prematurely, presumably because of decreased exposure to Notch signaling (Del Bene et al. 2008). The apical distribution of Notch and the involvement of dynein complex in apically directed nuclear migration may also help explain the arrested INM and premature cell cycle exit observed in animal models of the smooth brain disease, classical lissencephaly. The causative gene of classical lissencephaly, Lis1, interacts with the cytoplasmic dynein pathway (Tsai et al. 2005). Neuroepithelial and RG cells undergo INM, but IPCs do not. Thus, INM, a feature of CNS stem cells, is not shared by progenitors with more limited potential.
ASYMMETRIC RADIAL GLIA CELL DIVISIONS IN THE VENTRICULAR ZONE
During the period of cortical neurogenesis, RG cells maintain their marked apico-basal polarity and undergo asymmetric cell division to self-renew and also to produce a daughter that is either a neuron or an IPC (Haubensak et al. 2004; Noctor et al. 2004, 2007) (Figure 3). Fluorescent marker studies have demonstrated that the RG pial process does not retract during mitosis but instead remains in place throughout the cell cycle (Miyata et al. 2001, Noctor et al. 2001, Tamamaki et al. 2001, Gotz et al. 2002, Weissman et al. 2003). During M phase, cytoplasm within the radial fiber can be seen streaming toward the cell body, and varicosities appear at intervals along the attenuated fiber (Miyata et al. 2001, Noctor et al. 2001, Tamamaki et al. 2001, Weissman et al. 2003). Following mitosis, cytoplasm appears to flow in the opposite direction, restoring the fiber to a more uniform caliber. Time-lapse imaging of retrovirally labeled RG has also demonstrated that daughter neurons often migrate along parental RG fibers and that neuronal migration continues even while the guiding RG cell is dividing (Noctor et al. 2001, 2008).
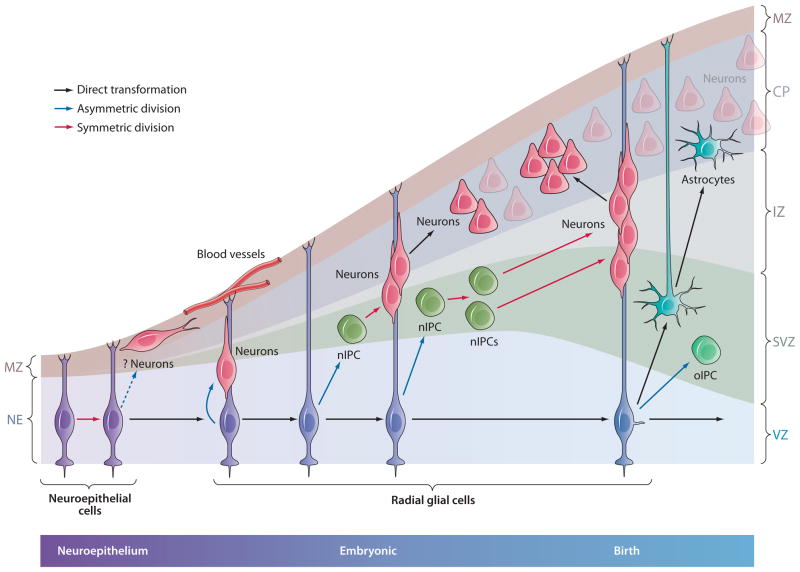
Figure 3.
Three modes of neurogenesis during cortical development. RG in cortex generate neurons (a) directly through asymmetric division; (b) indirectly by generation of nIPCs and one round of amplification; or (c) indirectly again through nIPCs, but with two rounds of division and further amplification. This additional amplification stage may be fundamental to increase cortical size during evolution (see text). Subpopulations of nIPCs are likely to divide more than once in subcortical brain regions, but this has not yet been documented. For additional details, see Figure 1. CP, cortical plate; IZ, intermediate zone; MZ, marginal zone; nIPC, neurogenic intermediate progenitor cell; RG, radial glia; SVZ, subventricular zone; VZ, ventricular zone.
In the central nervous system of Drosophila, cleavage plane orientation during division is a critical determinant of daughter cell fate (Doe & Skeath 1996). The role of cleavage plane orientation as a determinant of symmetry of division or cell fate in neuroepithelial or RG cell division, however, has been controversial; reports either support or challenge the application of this model (Sauer 1935, Smart 1973, Landrieu & Goffinet 1979, Chenn & McConnell 1995, Zhong et al. 1996, Huttner & Brand 1997, Haydar et al. 2003, Noctor et al. 2008). In the developing mammalian neuroepithelium, cleavage plane orientation remains predominantly vertical (planar division) during the period of symmetrical division prior to neurogenesis, and throughout the period of predominantly asymmetrical division during neurogenesis (Smart 1973, Zamenhof 1985, Miyata 2007, Noctor et al. 2008). Thus, cleavage plane angle alone is unlikely to be a determinant of symmetry of division or cell fate. This conclusion is bolstered by recent studies randomizing spindle rotation and cleavage orientation by deleting the G protein regulator, LGN, in brain or spinal cord neuroepithelium, a manipulation that disorganized the epithelium but did not significantly alter cell fate (Morin et al. 2007, Konno et al. 2008).
Some studies, based on dye labeling and time-lapse imaging, report that neurons rather than RG cells can inherit the radial process during division and that the daughter neuron migrates to the cortical plate through somal translocation (Miyata et al. 2001, Nadarajah et al. 2001, Tamamaki et al. 2001, Nadarajah 2003). Under this scheme, the RG daughter cell would presumably regrow a pial process or could become a short neural precursor (Gal et al. 2006). However, time-lapse imaging of retroviral labeled RG cells followed by fate determination using marker expression or electrophysiology shows that in most cases the RG cell inherits the radial process (Noctor et al. 2001, 2007, 2008; Weissman et al. 2003). Some RG cells have been observed to translocate toward the cortical plate following division, but immunostaining or electrophysiological analysis suggests that these RG are not translocating neurons but rather are RG cells that are beginning their transformation into astrocytes (Noctor et al. 2004, 2008) (Figures 1 and 3). Some investigators have also proposed that neurons may inherit the radial process at early stages in cortical development, such as during preplate formation, and migrate by translocation (Brittis et al. 1995, Nadarajah & Parnavelas 2002), but this idea has not been confirmed.
INTERMEDIATE PROGENITOR CELLS
Although the VZ was long thought to be the predominant site of neurogenesis, mitotic figures were nonetheless observed in the SVZ more than 100 years ago (Magini 1888, Retzius 1894). Throughout most of the twentieth century, the SVZ was presumed to be a site of gliogenesis (Altman & Bayer 1990a, Takahashi et al. 1995). Smart (1973) suggested that mitotic figures observed in the SVZ might represent cortical neurogenesis in an abventricular location, and Valverde et al. (1995) suggested that mitotic figures observed in the cortical preplate at stages prior to the appearance of the SVZ were also possible sites of neurogenesis. Moreover, a series of cell marker genes including Svet1 (Tarabykin et al. 2001), Cux1, and Cux2 (Nieto et al. 2004, Zimmer et al. 2004), are expressed first by embryonic progenitor cells in the SVZ and later by cortical neurons in specific layers. Recent work imaging the division of precursor cells within the SVZ has directly demonstrated that SVZ cells are derived from RG and subsequently divide to generate neurons (Miyata et al. 2004, Noctor et al. 2004) (Figures 1 and 3). It now appears that, throughout most of neurogenesis, RG either generate daughter neurons directly or produce a second, more restricted nIPC, also referred to as a basal progenitor cell, which populates the embryonic SVZ (Haubensak et al. 2004; Miyata et al. 2004; Noctor et al. 2004, 2007). The embryonic SVZ is therefore increasingly appreciated as a major site of neurogenesis (Tarabykin et al. 2001; Smart et al. 2002; Nieto et al. 2004; Noctor et al. 2004, 2007; Zimmer et al. 2004; Zecevic et al. 2005; Martínez-Cerdeño et al. 2006; Pontious et al. 2008). nIPCs, which frequently have multipolar processes, do not appear to contact the ventricle or pial surface, but they do display a predilection to divide with a cleavage plane parallel to the ventricular surface (Haubensak et al. 2004; Noctor et al. 2004, 2008). Whereas neuroepithelial and RG cells undergo INM, nIPCs do not (Haubensak et al. 2004, Miyata et al. 2004, Noctor et al. 2004).
Recent work suggests that Notch signaling among SVZ IPCs and between IPCs and RG may be involved in the regulation of progenitor proliferation. IPCs, as well as migrating neurons, were shown to activate Notch in RG through expression of mind bomb-1 (mib1) (Yoon et al. 2008), an essential component for both Deltalike- and Jagged-mediated Notch ligand signaling (Koo et al. 2007). Thus IPCs and newborn neurons may maintain RG proliferation through Notch activation mediated by contact with RG radial fibers. Other studies suggest that although Notch activation promotes proliferation of IPCs as well as RG, the two cell types differ with respect to Notch signal transduction. RG signal through the canonical Notch effector C-promoter binding factor 1 (CBF1), whereas IPCs generally do not (Mizutani et al. 2007). Very similar differences in downstream Notch signal transduction pathways characterize the difference between stem and progenitor cells in the hematopoietic system (Duncan et al. 2005).
nIPCs are also present within the VZ, particularly at early time periods, prior to the formation of a distinct SVZ (Noctor et al. 2007). This mixture of RG cells and nIPCs within the VZ may account for previous observations of both RG cells and non-RG cells undergoing mitosis in the embryonic VZ (Levitt et al. 1981, Misson et al. 1988, Gal et al. 2006). Unlike RG cells, which predominantly divide asymmetrically during the period of peak neurogenesis, nIPCs undergo symmetrical divisions to produce two neurons or may divide symmetrically to produce two additional IPCs (Haubensak et al. 2004; Miyata et al. 2004; Noctor et al. 2004, 2007; Wu et al. 2005). In rodent cortex, the symmetric nIPC-producing divisions appear to represent ~10% of nIPC divisions (Haubensak et al. 2004, Noctor et al. 2004). The number of times IPCs divide may vary in different brain regions and in different species. For example, the primate brain contains a large abventricular proliferative zone referred to as the outer SVZ (Smart et al. 2002). The outer SVZ in the fetal primate brain contains large numbers of mitotic cells, and we suggest that some of these may correspond to IPCs that could contribute to the enormous cortical expansion observed in primate cortex (Kriegstein et al. 2006) (Figure 3). IPCs are therefore functionally analogous to transit amplifying cells present in stem cell lineages in other tissues. The concept of transit amplifying cells within the brain is relatively new and has also been applied to adult NSC lineages (see below) (Figure 2).
DIVERSITY OF NEURAL PROGENITOR CELLS
Collectively, RG give rise to other glial cells as well as an astonishing assortment of neuronal types. This diversity in molecular and morphological characteristics of neurons underlies neural circuit formation. For a long time, researchers thought that neuronal phenotype was determined largely by the microenvironment in which cells settle and mature. However, recent advances indicate that neuronal characteristics, including their unique morphology, are determined largely by intrinsic cellular mechanisms that are established as new neurons are produced.
Different types of neurons are derived from RG in different subregions of the VZ (see Figure 4). This process has been extensively studied in the developing spinal cord where gradients of morphogens (BMPs) and sonic hedgehog (SHH) establish discrete dorsoventral territories of transcription factor expression, each associated with the production of different types of neurons (Hochstim et al. 2008). Similarly, investigators have observed segregation of progenitor zones, each associated with distinct transcription factor expression, in the developing forebrain (Campbell 2003, Puelles & Rubenstein 2003, Guillemot 2005, Flames et al. 2007, Long et al. 2009). Neuroepithelial cells and RG must interpret these gradients of morphogen and, in response, unfold unique programs of transcription factor expression to produce the large diversity of neuronal types within the forebrain. Therefore, RG must be highly heterogeneous in terms of their progenitor function, depending on the set of transcription factors they express. Pax6, Emx1, Gsh1, Gsh2, Er81, Sp8, Nkx2.1, Dlx1, Dlx2, and Olig2 have been implicated in the generation of different subsets of forebrain neurons. These likely represent just a small subset of a much larger group of transcription factors (Long et al. 2009) that, together with proneural genes such as Ngn2 and Mash1 (Guillemot 2007), presumably determine the specific cell subtype produced by RG and IPCs. Thus although the embryonic VZ may appear to be a relatively uniform and continuous proliferative zone surrounding the ventricle, in fact it is composed, from the earliest stage of neurogenesis, of RG cells that are heterogeneous with respect to the cell types they will produce. Interestingly, similar heterogeneity has been recently observed in the organization of adult NSCs (see below).
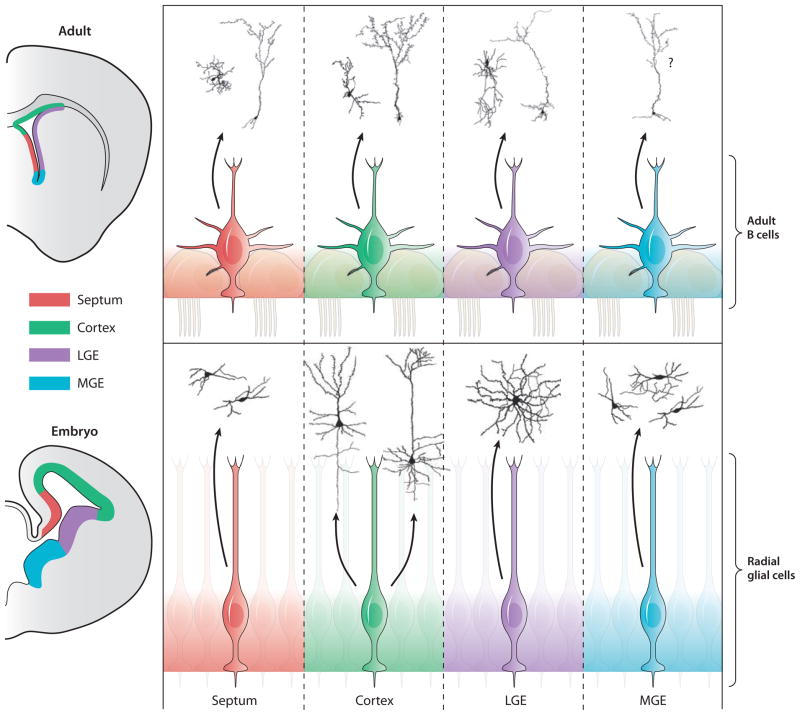
Figure 4.
Regional specification of NSCs in the embryo and the adult brain. The left two panels show cross sections of the mouse forebrain from embryonic and adult brain showing four subdivisions of the VZ in different colors. On the right, RG in embryonic development (lower four panels) and type B cells in the adult brain (upper four panels) from the major forebrain subregions (septum, cortex, LGE, and MGE) are illustrated using the same colors as on the left. NSCs in the different subregions generate different types of neurons. Combinations of transcription factor expression (see text for additional details) define the different subdomains and the types of neurons produced. Representative camera lucida drawings of neuronal types produced within each subregion are illustrated. For example, neurons derived from cortical (green) RG in development give rise to large pyramidal neurons. In the adult, cortical progenitors give rise to olfactory bulb interneurons. NSCs in each subregion may give rise to an even wider diversity of cell types. Although in some cases (e.g., embryonic cortex) neuron progeny migrate radially, more frequently, immature neurons migrate tangentially for some distance before differentiating. Therefore, although mature neurons are shown deriving from distinct proliferative regions, we do not denote ultimate location, but rather that different neuronal phenotypes are derived from distinct pools of NSCs. LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; NSCs, neural stem cells; RG, radial glia; VZ, ventricular zone.
In addition to location, timing in development must determine the types of neurons or glial cells derived from RG. The NSCs and IPCs in different locations go through a stereotypic sequence by which specific types of neurons are produced at different developmental stages (Desai & McConnell 2000), a phenomenon that appears to be cell-autonomous (Shen et al. 2006). Deep-layer cortical projection neurons are generated early, for example, and thalamic recipient neurons are generated at later times (see below). Investigators have recently reported a similar regional specification for oligodendrocyte (Kessaris et al. 2006) and astrocyte (Hochstim et al. 2008) production. Dynamic programs of transcription factor expression are believed to control NSC behavior in time and space. The identification of the NSCs in the embryo and the adult indicates the precise cell types in which these transcriptional programs must be at play.
Do neurons, astroctyes, and oligodendrocytes arise from distinct fate-restricted progenitors, or do multipotential progenitors contribute cells to neuronal as well as glial lineages? Can individual progenitor cells generate a diversity of neuron cell types, or are there progenitor cells restricted to generate neurons of a single class? The evidence suggests that all these progenitor types may coexist in the embryonic cortex. The coexistence of neuronal and glial progenitor cells in the embryonic primate cortex was suggested by the finding of two classes of cycling cells in the VZ and SVZ: GFAP positive and negative (Levitt et al. 1981, 1983). A series of studies based on clonal analysis demonstrated that fate-restricted progenitors producing only neurons or only glia coexist at early stages of cortical development in the rodent (Luskin et al. 1988, 1993; Price & Thurlow 1988; Grove et al. 1993; Krushel et al. 1993; Davis & Temple 1994; Williams & Price 1995; Mione et al. 1997; McCarthy et al. 2001; Anthony et al. 2004; Wu et al. 2006; Battiste et al. 2007). In contrast, other studies using retroviral labeling and clonal analysis have also shown that both neurons and oligodendrocytes or neurons and astrocytes can derive from a single precursor cell (Walsh & Cepko 1988, 1992; Parnavelas et al. 1991; Williams et al. 1991; Reid et al. 1995; He et al. 2001; Yung et al. 2002). Additionally, individual neurogenic progenitor cells can contribute neurons to multiple layers (Luskin et al. 1988), and even in isolated cell cultures, NSCs can generate neurons in a precise temporal and laminar order, echoing the in vivo sequence (Shen et al. 2006). Taken together these studies suggest that although some single multipotential progenitor cells exist that are intrinsically capable of generating a diversity of neuronal and glial cell types, fate-restricted progenitor cells are also present, suggesting heterogeneity of RG proliferative behavior. Although it is unlikely that all RG cells undergo the same sequence of neurogenic and progenitor cell divisions, some of the results based on retroviral labeled clones or clones expanded in vitro could be explained by IPC-derived clones. Following retroviral infection, either the self-renewed RG cell or the daughter neuron or IPC could inherit the retroviral label. Even though neuron-only clones predominate in most studies, many clones consist of only two neurons, and such clones could correspond to the terminal symmetric division of a labeled nIPC cell. In this way many of the clones restricted to a single cell type may have resulted from labeling an IPC or transit amplifying type cell, whereas RG or neuroepithelial cell labeling might be expected to produce clones of mixed cell types. Similarly, clones of astroglial cells could represent retroviral labeling of the terminal glial branches of a multipotent lineage tree (Figure 2).
As indicated above, evidence supports cell-autonomous construction of specific developmental lineages. Single cells isolated from E10 embryonic cortex and grown in culture are multipotent and sequentially generate neuronal and then glial restricted progenitors, resembling the in vivo developmental sequence (Qian et al. 2000). Similarly, embryonic stem cells can be turned into NSCs that are capable of producing neurons and then astroglial cells (Gaspard et al. 2008). Stem cells in culture can even sequentially produce subtypes of neurons over time whose identities match the layer-specific temporal pattern observed in vivo (Gaspard et al. 2008). These data indicate that at least some individual multipotent progenitor cells can follow a clockwise developmental sequence and generate a changing set of distinct neural cell types with maturation. However, these results do not rule out the possibility that environmental signals could contribute. For example, cells can interact with each other in cell culture, and cell progeny could provide fate-modulating signals that could influence clone composition.
TRANSFORMATION OF RADIAL GLIA AND PROLIFERATION OF ASTROCYTES
RG cell bodies reside in the VZ throughout the period of cortical development, participating in neurogenesis and guiding neuronal migration. At the end of this developmental period, most RG cells lose their ventricular attachment and migrate toward the cortical plate by a process of somal translocation. In mammals, most RG cells transform into astrocytes (Figures 1 and 3). The transformation of RG to multipolar astrocytes was initially inferred from observations of developmental changes in RG features in species as diverse as rodents, marsupials, and primates (Morest 1970, Choi & Lapham 1978, Schmechel & Rakic 1979, Misson et al. 1991) [see also (Ramón y Cajal 1995) and references therein for earlier work on the transformation of RG (epithelial cells, spongioblasts) into astrocytes]. The morphology of RG changes from bipolar spanning the cortex to unipolar that no longer contacts the ventricle to multipolar with a regressing radial process, thus progressively taking on astrocytic morphology. Moreover, the disappearance of RG is coincident with the appearance of increasing numbers of astrocytes. The inferred lineage relationship of RG and astrocytes is supported by mutual expression of the same markers such as RC1 (Misson et al. 1991) and has been demonstrated more directly by labeling RG cells in newborn ferrets with a fluorescent dye (DiI) and noting the appearance of DiI-labeled astrocytes coincident with the disappearance of labeled RG (Voigt 1989). More recently, Noctor et al. (2008) directly visualized the translocation of RG and transformation to astrocytes by using retroviral labeling and time-lapse imaging (Noctor et al. 2008). Although this process of transformation from RG to astrocytes has been best studied in cortex, a very similar transformation occurs in the subcortical telencephalon and other regions of the neuraxis (Levitt & Rakic 1980, Yang et al. 1993, Barry & McDermott 2005). As discussed below, RG transformation into astrocytes continues postnatally in some regions of the rodent brain, whereas in some vertebrates, this transformation does not take place and RG persist into adult life (see below).
Whether astrocytes, like neurons, are spatially restricted to specific locations on the basis of their sites of origin or instead migrate widely is not well known. A recent study suggests that tangential dispersal of astrocytes in the developing spinal cord may be limited (Hochstim et al. 2008). Depending on their position, superficially located spinal cord astrocytes express Reelin, Slit1, or both. These cells are derived from restricted domains of the ventral spinal cord that can be distinguished by expressing Pax6 or Nkx6.1. This positional identity in the origin of distinct groups of astrocytes suggests that, during their transformation from RG, astrocytes are retained within restricted radial domains, perhaps closely related to the position of the RG cell processes. A similar restriction in tangential dispersal is observed post-natally in mice during the transformation of striatal RG cells into parenchymal astrocytes (F. Merkle and A. Alvarez-Buylla, unpublished observation). This observation suggests that astrocytic conversion and dispersal may be strictly controlled. Astrocytes may retain fundamental properties and positional information linked to the original array of RG in the developing brain (Rakic 1988).
Some astrocytes may divide locally before terminal differentiation and represent a population of aIPCs (Figure 2). This process of astrocytic amplification may occur in the postnatal murine cortex (Mares & Bruckner 1978, Hajós et al. 1981, Ichikawa et al. 1983). Interestingly, astrocytes take cortical positions that mirror the inside-out laminar birth date pattern of cortical neurons with later-born glia taking up superficial cortical positions (Ichikawa et al. 1983). Whether astrocyte progenitor cells represent transformed RG or whether they derive from a distinct neuroepithelial progenitor cell is not clear. Clonal analysis supports both possibilities. Single embryonic progenitor cells in culture produce neurons first, then glial cells (Qian et al. 2000). However, retroviral labeling of progenitors in vivo at stages of midcorticogenesis often yields astrocyte-only clones (Grove et al. 1993, Luskin et al. 1993, McCarthy et al. 2001), suggesting that at least some glial-restricted progenitor cells are present at embryonic ages. Retroviral analysis of progenitor cells labeled at neonatal ages has also produced evidence for oligodendrocyte-only clones (Luskin et al. 1993, Luskin & McDermott 1994) as well as for mixed clones containing both oligodendrocytes and astrocytes (Levison & Goldman 1993), suggesting the possible coexistence of both restricted and bipotential glial progenitors neonatally. However, as discussed above, we do not know if these glial colonies are derived from primary progenitors or from IPCs. Direct visualization of retrovirally labeled RG has demonstrated that the same RG cell that produces neurons can then transform into an astroglial cell (Noctor et al. 2008), confirming that the neurogenic to gliogenic transition occurs in at least some individual progenitor cells in situ.
NEUROGENESIS AND GLIOGENESIS CONTINUE POSTNATALLY
NSCs are present in the developing brain and persist in restricted regions of postnatal and adult brains. These cells continue to produce not only glial cells but also neurons (Gage 2002, Alvarez-Buylla & Lim 2004, Nottebohm 2004, Ming & Song 2005). In the early neonatal (Luskin 1993) and adult mammalian brains (Lois & Alvarez-Buylla 1994), new neurons are generated primarily in the SVZ in the walls of the lateral ventricles. These young neurons migrate to the olfactory bulb where they continually replace local interneurons (Imayoshi et al. 2008). Neurogenesis also continues in the adult hippocampus, specifically in the subgranular layer (SGL) of the dentate gyrus (Gould & Cameron 1996, Kempermann et al. 1997). In adult birds, neurogenesis is more widespread; new neurons are born in a VZ in the walls of the lateral ventricle, migrate widely, and incorporate throughout most of the telencephalon (Nottebohm 1985). Adult neurogenesis also continues in poikilotherms where, in some species, it is associated with continuous growth of the CNS (García-Verdugo et al. 2002). NSCs are retained within specialized germinal centers in adult brains of all these vertebrates. In good agreement with the developmental data reviewed above, the primary progenitors for the continual generation of neurons in postnatal animals correspond to cells with glial characteristics: RG or astrocytes.
RADIAL GLIA PERSIST IN SOME VERTEBRATES AND FUNCTION AS PRIMARY PROGENITORS OF NEW NEURONS
Similar to development, RG that persist post-natally have their cell bodies in the region surrounding the ventricular walls and project a long thin process deep into the forebrain. In adult birds, this radial process guides the initial migration of young neurons from their birth place in the VZ to distant locations within the telencephalon. RG cells in adult songbirds divide, undergo interkinetic nuclear migration, and maintain a characteristic primary cilium on their apical ventricular surface (Alvarez-Buylla et al. 1998). This proliferation occurs in neurogenic hot spots and correlates with the production of new neurons (Alvarez-Buylla et al. 1990). Retroviral lineage-tracing studies show that RG and new neurons descend from the same lineage (Goldman et al. 1996). The above study suggested that young neurons in adult songbirds are derived from RG. Interestingly, RG are the predominant glial cell type in some regions in the CNS of fish and reptiles, and some of these cells continue to divide in adulthood (Stevenson & Yoon 1981, Ramón y Cajal 1995, Kalman 1998). More recent observations suggest that these RG cells in poikilotherms could also function as stem cells (García-Verdugo et al. 2002, Weissman et al. 2003, Zupanc 2006). Therefore, in many vertebrate species, some RG cells do not convert into astrocytes at the end of development but persist postnatally. These cells not only have morphological characteristics similar to RG in the developing mammalian brain, but also serve a similar function: They are the primary progenitors in the generation of neurons and glia, and their long fibers guide the migration of young neurons.
NEURAL STEM CELLS IN THE ADULT SUBVENTRICULAR ZONE
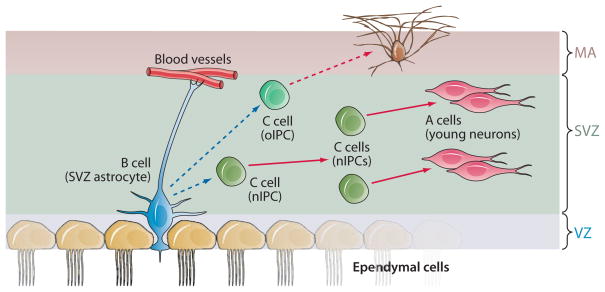
As indicated above, in adult mammals, neurogenesis is most prominent in the SVZ in the walls of the lateral ventricles (Figure 5), a region highly related to the embryonic SVZ, which as described above is the site of significant IPC proliferation earlier in development. The SVZ contains relatively quiescent NSCs, known as B cells, which give rise to actively proliferating C cells that function as the IPCs or transit amplifying progenitors in the adult brain SVZ (Doetsch et al. 1999b). Type C cells give rise to immature neuroblasts (A cells), which migrate in chains to the olfactory bulb (Lois & Alvarez-Buylla 1994, Belluzzi et al. 2003), where they differentiate into interneurons (Carleton et al. 2003). Despite their NSC properties, type B cells display ultrastructural characteristics and markers of astroglial cells, including the expression of GFAP, GLAST, and other astroglial markers (Doetsch et al. 1997, Colak et al. 2008, Platel et al. 2008). Thus type B cells are also frequently referred to as SVZ astrocytes.
Figure 5.
Schematic of progenitor types and lineages in the adult brain SVZ. NSCs in the wall of the lateral ventricles of adult rodents correspond to type B cells (SVZ astrocytes). These cells retain epithelial properties, including the extension of a thin apical process that ends on the ventricle and a basal process ending on blood vessels. B cells give rise to C cells, which correspond to nIPCs. B cells also generate oligodendrocytes through oIPCs. Dashed arrows illustrate hypothetical modes of division: blue for asymmetric and red for symmetric divisions. Investigators do not currently know how many times C cells divide. See Figure 1 for other information. nIPCs, neurogenic intermediate progenitor cells; NSCs, neural stem cells; oIPCs, oligodendrocytic intermediate progenitor cells; SVZ, subventricular zone.
The astrocytic nature of adult SVZ NSCs has now been demonstrated in several studies. Initial evidence indicated that following a six-day treatment with cytosine-β-d-arabinofuranoside (Ara-C), which kills actively dividing type C and A cells, SVZ B cells are activated and regenerate C cells, which in turn give rise to new neuroblasts (Doetsch et al. 1999a). A replication-competent avian leukosis (RCAS) retrovirus carrying a reporter gene has been used specifically to label mammalian SVZ astroglial cells (and their progeny) in transgenic mice (G-tva) expressing the receptor for this avian retrovirus under the control of the GFAP promoter (Holland & Varmus 1998). RCAS-labeled SVZ astrocytes generate olfactory bulb neurons (Doetsch et al. 1999b) (Figure 5). Further evidence that B cells correspond to the adult SVZ NSCs comes from studies using transgenic mice expressing herpes-simplex virus thymidine kinase (TK) from the GFAP promoter. Ganciclovir (GCV) treatment in these mice leads to the selective death of cells expressing TK and to a severe reduction in the number of new neurons produced in the adult brain and a depletion of neurosphere-forming cells from the SVZ (Imura et al. 2003, Morshead et al. 2003). Together these data indicate that NSCs in the SVZ are contained within a population of cells classically considered as differentiated astrocytes.
Recent studies indicate that type B cells retain some important properties of RG. Type B cell bodies are generally located just under the ependymal cell layer but have short processes that extend through the ependymal layer with small apical endings on the ventricle (Mirzadeh et al. 2008, Shen et al. 2008). These apical endings form junctional complexes among themselves that are virtually identical to those that join RG earlier in development. These junctional complexes are very different from those that link apical ependymal membranes or B cells to ependymal cells (Mirzadeh et al. 2008). The ventricular contacts of SVZ B cells can be observed in whole mount preparations of the ventricular surface as small apical surfaces containing a single primary cilium. This feature is very different from ependymal cells, which as mentioned above have very large apical surfaces and contain at least 50 long motile cilia. A novel type of ependymal cells (E2 cells) appears to contain only two long cilia (Mirzadeh et al. 2008). The function of E2 cells remains unknown. Interestingly, the apical processes of SVZ B cells cluster together in the center of structures resembling pinwheels. The periphery of each pinwheel is formed by the large apical surfaces of ependymal cells.
The SVZ also contains blood vessels that extend a substantial extracellular matrix next to type B and C cells (Mercier et al. 2002). However, SVZ type B and C cells are also closely associated with blood vessels (Shen et al. 2008, Tavazoie et al. 2008). Type B cells that contact the ventricle through their small apical endings have relatively long basal processes, frequently oriented tangentially with specialized end feet on blood vessels (Mirzadeh et al. 2008). Proliferating SVZ cells are frequently associated with blood vessels (Tavazoie et al. 2008) or the extracellular matrix around them (Kerever et al. 2007), which suggests that factors derived from the vasculature may regulate both primary progenitors and IPCs in the SVZ. Adult SVZ B cells thus retain apical-basal polarity and are part of the ventricular epithelium, as were RG earlier in development.
As has been suggested for birds, the walls of the lateral ventricles where neurogenesis continues in the adult contain not only differentiated ependymal cells but also remnants of a VZ (Figure 5). In the case of the rodent lateral ventricles, the VZ-like structure is present in the center of pinwheels, where adult B cells, like RG, have characteristic end feet. Thus, adult NSCs in the SVZ appear to maintain many epithelial characteristics that allow them to bridge between blood vessels underlying the SVZ and the ventricular surface. Adult stem cells likely integrate information arising at both apical and basal compartments to regulate their proliferation and differentiation, a process mirroring that of RG during development (Figure 1).
Unlike ependymal cells that line most of the ventricular surface and extend a large number of long motile cilia, B cells have short, single primary cilia extending into the ventricle (Doetsch et al. 1999c, Mirzadeh et al. 2008). The function of this organelle in NSCs remains unknown, but as discussed below, recent work indicates that primary cilia are important sites for signal reception, particularly sonic hedgehog (Shh) (Huangfu & Anderson 2005, Singla & Reiter 2006). Shh is a critical morphogen and growth factor during development. Shh binding to receptor Patch results in disinhibition of Smoothened (Smo), an effector of Hh signaling. Conditional ablation of Smo using NestinCre or an inducible NestinCreERT2 results in postnatal depletion of SVZ B and C cells (Machold et al. 2003), which suggests that Shh signaling is important for the postnatal maintenance of SVZ B cells and possibly for the regulation of proliferation in type C cells. As described below in more detail, conditional deletion of primary cilia in GFAP-expressing progenitors of the dentate gyrus also results in the postnatal depletion of NSCs. These animals also display decreased proliferation in the SVZ (Y.-G. Han & A. Alvarez-Buylla, unpublished observation), which is consistent with aforementioned observations that Shh plays a critical role in SVZ stem cell maintenance.
No single molecular marker is available to identify adult SVZ primary progenitors. Although SVZ B cells, like embryonic RG, express Nestin and Sox2, they also express GFAP and GLAST, which are more frequently associated with astrocytes. Other cells both within and outside the SVZ also contain some of these alleged NSC markers. Initial experiments suggested that neurospheres, which self-renew in vitro and can generate oligodendrocytes, astrocytes, and neurons (Weiss et al. 1996), are derived from the SVZ primary progenitors (Morshead et al. 1994), presumably corresponding to the NSCs in vivo. However, neurosphere-forming ability is not restricted to primary progenitors; both SVZ type B and C cells can generate neurospheres (Doetsch et al. 2002). Similarly, progenitors for oligodendrocytes can, upon stimulation with growth factors, make multipotent neurospheres (Kondo & Raff 2000). Lewis X (LeX) is an extracellular carbohydrate expressed by embryonic pluripotent stem cells that is also enriched in a subpopulation of GFAP-expressing SVZ cells (Capela & Temple 2002). LeX has been used to enrich the population of SVZ cells that can form neurospheres in vitro and may be a possible SVZ stem cell marker. Marker combination has also been employed to isolate SVZ cells with the potential to form neurospheres. For example, a subpopulation of adult VZ cells that is positive for CD133 but negative for CD24 generated neurospheres in vitro (Coskun et al. 2008). A previous study suggested that multiciliated ependymal cells could function as NSCs (Johansson et al. 1999). However, other studies indicated that ependymal cells do not generate neurospheres with NSC properties in vitro (Chiasson et al. 1999, Capela & Temple 2002) and that ependymal cells become postmitotic during development (Spassky et al. 2005). Ependymal cells can be distinguished by their large apical surface, long cilia with 9+2 microtubule structure, and S100β staining. In whole mount preparations of the ventricular wall, all cells with a large apical surface stained positive for CD24, and these cells costained with S100β (Mirzadeh et al. 2008). None of these cells stained positively for proliferative markers or incorporated BrdU. Although ependymal cells are also CD133+, the only cellular profiles in the ventricular wall that are CD133+/CD24− are cells in the center of pinwheels that correspond to the small apical end feet of type B cells. Therefore, CD133+/CD24− cells in the ventricular walls are likely to be type B cells. The overall evidence indicates that ependymal cells are post-mitotic differentiated cells that originate from RG during perinatal development (Spassky et al. 2005). With age, these cells die. Recent observations indicate that in aging animals some SVZ astrocytes can develop multiple cilia similar to those observed in ependymal cells (Luo et al. 2008), which further suggests that cells with multiple cilia do not divide.
Better markers may be emerging to define the subpopulation of cells in the adult VZ that function as stem cells. One promising candidate is the nuclear orphan receptor tailless, which appears to be expressed in SVZ type B cells and to be essential for neurogenesis (Liu et al. 2008). Markers are also required to identify NSCs better in the adult human brain. It remains controversial whether neurogenesis and migration of neuroblasts to the olfactory bulb continue in adult humans (Curtis et al. 2007, Sanai et al. 2007). A prominent band of SVZ astrocytes exists in the adult human brain, in an abventricular location in the walls of the anterior lateral ventricles (Sanai et al. 2004, Quinoñes-Hinojosa et al. 2006, Oldham et al. 2008). Some of these cells can function in vitro as stem cells. Transcriptional analysis suggests that periventricular astrocytes in humans are distinct from parenchymal astrocytes (Oldham et al. 2008), which may lead to a better definition of the astrocytes in the human SVZ that, like B cells in rodents, continue to function as stem cells in the adult. Although both parenchymal and SVZ astrocytes appear to be directly derived from RG, only SVZ B cells retain expression of critical determinants for their function as NSCs.
NEURAL STEM CELLS IN THE ADULT HIPPOCAMPUS POSSESS ASTROGLIAL CHARACTERISTICS
Another major region that produces new neurons in the adult mammalian brain (including humans) is the dentate gyrus of the hippocampus (Altman & Das 1965, Kaplan & Hinds 1977, Eriksson et al. 1998, Gould et al. 1999, Gage 2000, Kempermann et al. 2004). New neurons in the adult dentate gyrus have been implicated in learning and memory (Shors et al. 2002, Zhao et al. 2008), and aberrant neurogenesis in this brain region has been linked to depression (Dranovsky & Hen 2006), neuroinflammation (Monje et al. 2003), and epilepsy (Parent et al. 2006). There is great interest in understanding where these new neurons are coming from and how their production is regulated. The new hippocampal neurons are born in the subgranular zone (SGZ), which is located at the interface of the granule cell layer and the hilus (Figure 5). The SGZ contains two types of dividing cells: astrocytes and darkly stained small cells with small basophilic nuclei (Altman & Das 1965, Kaplan & Hinds 1977, Cameron et al. 1993, Palmer et al. 2000). Consistent with observations in development and in the adult SVZ, radial astrocytes in the SGZ function as the primary precursors of the new neurons in the dentate gyrus (Seri et al. 2001, Fukuda et al. 2003, Garcia et al. 2004, Steiner et al. 2004). Radial astrocytes in the SGZ, also referred to as type I progenitors (Filippov et al. 2003, Fukuda et al. 2003), have a prominent process that crosses the granule cell layer as well as smaller horizontally oriented processes along the SGZ (Kosaka & Hama 1986, Seri et al. 2004). Unlike other astrocytes in the SGZ that express only GFAP, these cells express both GFAP and Nestin (Seri et al. 2004, Steiner et al. 2006). Similar to what is observed in the SVZ, the SGZ is located next to an extensive vascular niche (Palmer et al. 2000), suggesting that factors derived from blood vessels influence the behavior of NSCs in the SGZ. Radial astrocytes do not give rise to neurons directly but generate nIPCs, which correspond to the small basophilic cells that are darkly stained by hematoxylin, referred to as type D cells (Seri et al. 2004) or type II progenitors (Filippov et al. 2003, Fukuda et al. 2003). Immature D cells (D1 cells) appear to divide and function as nIPCs (Figure 5). More mature darkly stained D cells (D2–D4 cells) have a prominent process and have properties of neurons at different stages of maturation, characterized by the expression of doublecortin, PSA-NCAM, Tuc4, NeuroD, Prox1, and NeuN (Seki & Arai 1993, Fukuda et al. 2003, Seri et al. 2004). These cells also progressively acquire electrophysiological characteristics of new neurons (Song et al. 2002, Filippov et al. 2003).
A recent study suggests that SGZ Sox2+ cells lacking radial processes function as the primary progenitors of new neurons and glial cells in the adult hippocampus (Suh et al. 2007). Both radial astrocytes and nonradial cells, presumably D cells, express Sox2. This study found that after BrdU injection, only nonradial cells incorporate this proliferative marker. Many BrdU-labeled, Sox2-expressing radial astrocytes appear in mice that exercise in a running wheel, a condition that dramatically increases neurogenesis in the SGZ (Van Praag et al. 1999). However, Suh et al. (2007) suggested that in controls without the running wheel, nonradial cells function as stem cells in the SGZ. They used Cre-mediated recombination in a retroviral lineage-tracing strategy to show that neurons are derived from cells expressing Cre under the Sox2 promoter. However, cells that activate the retroviral Sox2 promoter must have divided at least once before the retroviral DNA is integrated and Cre can be expressed. The reporter gene used following Cre-mediated recombination could initiate its expression only in self-renewing primary progenitors or in type D cells, the nIPCs in the SGZ. Because Suh et al. observed very few, if any, self-renewing cells labeled by this method, their retroviral lineage-tracing experiment must have begun with nIPCs rather than with primary progenitors. This result would be consistent with the division of radial astrocytes, generating D cells in which the Sox2 promoter remains active and leads to the generation of new neurons. Yet it remains intriguing why this study failed to observe proliferating Sox2+ radial astrocytes in nonrunning animals. Retroviral lineage-tracing experiments using the RCAS-TVA system (Holland & Varmus 1998) specifically to target GFAP- or Nestin-expressing cells in the SGZ indicate that radial astrocytes not only divide but also generate the neurons in the adult dentate gyrus (Seri et al. 2004). This and other studies (Dayer et al. 2003, Kempermann et al. 2003) support the interpretation that radial astrocytes function as primary progenitors. It will be interesting to determine the precise time of Sox2 activation in these cells and IPCs during the generation of new neurons in the adult dentate gyrus.
In addition to their role as neuronal precursors, radial astrocytes may also retain the classical astrocytic functions of supporting neuronal and synaptic activity in the granule and molecular layers of the dentate gyrus. The electrophysiological properties of radial astrocytes are similar to those of other astrocytes in the brain (Fukuda et al. 2003). Much like RG in the developing cortex, SGZ radial astrocytes are arranged in a regular array along the blades of the dentate gyrus (Figure 5). Their progeny, the D cells (type II progenitors), are closely associated with the radial astrocytes creating regular clusters of young neurons along the SGZ of the postnatal dentate gyrus. The prominent radially oriented process of these astrocytes could play a fundamental role in the collection of signals that regulate their own proliferation as well as the proliferation and differentiation of D cells. A radial astrocyte could receive information along its main shaft, which is near the cell bodies of many granule neurons, as well as from endings of the radial process in the molecular layer where the dentate gyrus receives internal and external input. Neurogenesis in the dentate gyrus is regulated by multiple physiological and environmental signals including adrenal steroids (Gould et al. 1992, Cameron et al. 1998), glutamate receptor activation (Gould et al. 1994), seizures (Parent et al. 1997), enriched environmental conditions (Kempermann et al. 1997), exercise (Kempermann et al. 1998), inflammation (Monje et al. 2003), and antidepressants (Santarelli et al. 2003). These different conditions may affect the behavior of radial astrocytes directly and/or indirectly through the level of neuronal activity within the dentate gyrus, changes that could be detected by the radial astrocytes through their processes. This, in turn, may induce changes in the proliferation rate of these cells and their progeny. The identification of primary precursors and of the intermediate cellular stages in the production of new hippocampal neurons in the dentate gyrus is an essential step to further understand the function and regulated production of these new neurons.
A LINK BETWEEN EMBRYONIC AND ADULT NEURAL STEM CELLS
The above studies indicate that the primary progenitors in the adult SVZ and SGZ correspond to cells classically considered astrocytes. Under normal conditions, only SGZ and SVZ astrocytes have been shown to function as neuronal progenitors. Cultured astrocytes have been induced to function as neuronal progenitors by introducing transcription factors (Heins et al. 2002, Berninger et al. 2007). However, it remains largely unknown which molecular characteristics distinguish astroglial cells with progenitor properties from those with classical astroglial support functions that do not function as NSCs. NSCs are likely contained within the neuroepithelial-RG-astrocyte lineage (Alvarez-Buylla et al. 2001). Selective labeling of RG has demonstrated a direct link between these cells and adult SVZ NSCs (Figure 1). These cells can be selectively labeled by small distal injections of adenovirus-carrying Cre recombinase along the RG process. The injected adenovirus is transported retrogradely into the RG cell body, resulting in the recombination of reporter genes in a subset of these progenitor cells. This labeling method revealed that adult SVZ B cells functioning as adult NSCs are derived from RG (Merkle et al. 2004). It also showed that neonatal RG give rise to oligodendrocytes, earlier generated olfactory bulb interneurons, parenchymal astrocytes, and ependymal cells. It is not known whether single RG are multi-potent or whether different subpopulations of RG cells give rise to these different cell types.
As discussed above, adult SVZ cells retain key characteristics of RG. One distinctive property of RG is their long basal process that during embryonic development ends on the surface of the brain. B cells in the adult SVZ also have a prominent basal process that does not end on the brain surface but projects radially or tangentially (depending on location) and terminates in specialized endings on blood vessels (Mirzadeh et al. 2008, Shen et al. 2008). During development, RG also establish branch contacts with the vasculature, and these contacts may be analogous to those observed in adult progenitor cells. As discussed above, a VZ compartment has been revealed for adult SVZ B cells. An apical process that intercalates between ependymal cells connects B cells in the SVZ with the surface of the ventricle. These apical processes, just like those of RG, have specialized apical junctions and a primary cilium. The findings above indicate that adult SVZ NSCs share basic properties with embryonic RG, suggesting that adult SVZ NSCs are modified RG that retain progenitor function throughout life (Figure 1).
Although direct experimental evidence for a link beween radial glia in the embryo and adult SGZ radial astrocytes in the dentate gyrus is not available, classical anatomical studies suggest that RG in the dentate neurepithelium (Altman & Bayer 1990b) convert into the different types of dentate gyrus astrocytes, including radial astrocytes (Eckenhoff & Rakic 1989). It is therefore possible that SGZ radial astrocytes are derived from these radial glia (Figure 5). The primary cilium, an organelle strategically localized at the apical surface in RG and in SVZ B cells, is also present in radial astrocytes and their embryonic progenitors (Breunig et al. 2008, Han et al. 2008). As indicated above, the primary cilium has been linked to Shh signaling (Singla & Reiter 2006, Eggenschwiler & Anderson 2007). In the dentate gyrus, the primary cilium is essential for embryonic SGZ progenitors to proliferate and generate postnatal radial astrocytes that continue to produce neurons in the dentate gyrus throughout life (Breunig et al. 2008, Han et al. 2008). At a critical developmental stage when embryonic progenitors divide to generate postnatal radial astrocytes, Shh signaling is essential for the formation and/or maintenance of this population of postnatal NSCs (Han et al. 2008). Other hippocampal astrocytes are not affected by the lack of primary cilia or Shh signaling, which suggests that NSCs, compared with other astrocytic cells in the adult hippocampus, are unique in their requirements for Shh and primary cilia.
NEURAL STEM CELLS IN THE ADULT BRAIN DIFFER BY LOCATION
During development, NSCs correspond to RG, whereas in the adult brain, a subpopulation of astrocytes (B cells) in the SVZ and radial astrocytes in the SGZ function as NSCs. As discussed above, neuroepithelial cells and RG in different locations of the developing nervous system become regionally specialized for producing specific subtypes of neurons. This parcelation of NSCs has been observed at multiple levels of the neuraxis from the spinal cord (Ericson et al. 1997) to the forebrain (Campbell 2003, Guillemot 2005) (Figure 4).
Recent work in the SVZ-olfactory bulb system shows that postnatal RG and adult SVZ B cells are also heterogeneous and regionally specified; primary progenitors in different locations of the SVZ generate different types of neurons for the olfactory bulb (Figure 4). Expression and functional analysis provided initial evidence that neuroblasts migrating in the rostral migratory stream (RMS) are heterogeneous before they enter the olfactory bulb (Hack et al. 2005, Kohwi et al. 2005). Pax6 labels a subpopulation of neuroblasts and is essential for the generation of dopaminergic periglomerular neurons and a subpopulation of superficial granule cells. The transcription factor SP8, which is highly expressed by the developing dorsal LGE, cortex, and septum, has also been associated with the formation of another subpopulation of olfactory bulb interneurons, the calretinin+ (CalR+) cells (Waclaw et al. 2006). These studies suggest that differential transcription factor expression somewhere in the SVZ or RMS results in the generation of different types of interneurons. However, this restriction in potential could occur in primary (type B cells) or secondary progenitors (type C or A cells).
Distinct territories of transcription factor expression are present in the developing telencephalon (Campbell 2003, Long et al. 2009), and this regional code may be preserved to some degree in the postnatal brain (Stuhmer et al. 2002, Stenman et al. 2003, Parras et al. 2004, Waclaw et al. 2006, Kohwi et al. 2007, Young et al. 2007) (Figure 4). Lineage-tracing experiments, using Cre recombinase under the control of transcription-factor promoters that are expressed in different locations of the developing and postnatal SVZ, suggest that different types of interneurons in the olfactory bulb are derived from progenitors in different locations in this germinal niche (Young et al. 2007). For example, dopaminergic and calbindin+ perioglomerular cells (PGCs) in the olfactory bulb are derived from territories in the SVZ that express Gsh2. However, these regions generate very few olfactory bulb CalR+ neurons. A fraction of the CalR+ cells are derived from progenitors that expressed Emx1 (Kohwi et al. 2007, Young et al. 2007), which is classically considered a pallial marker, but a small subpopulation of subpallial progenitors also expresses this transcription factor (Willaime-Morawek et al. 2006).
Direct evidence for the origin of different types of olfactory bulb interneurons from particular sublocations of the postnatal SVZ comes from regional labeling of neonatal RG and adult SVZ B cells (Merkle et al. 2004). This work shows that NSCs in different locations in the postnatal SVZ generate different types of olfactory bulb interneurons (Figure 4). This labeling method confirms that pallial RG on the dorsal SVZ give rise to olfactory bulb interneurons, including a subpopulation of the CalR+ cells (Merkle et al. 2007, Ventura & Goldman 2007). In contrast, the majority of calbindin-expressing PGCs are derived from ventrolateral SVZ. Different types of granule cells (GCs) are also derived from unique domains of the SVZ. Dorsal targeting results in the generation of primarily superficial GCs, whereas ventral targeting gives rise to deep GCs. The majority of calretinin+ PGCs and GCs are derived from the medial wall of the lateral ventricle facing the septum. Progenitor cells in different locations of the SVZ also specify the branching pattern of the dendritic trees of SVZ-derived interneurons (Kelsch et al. 2007). The above studies indicate that the primary progenitors for the postnatal generation of new neurons in the SVZ are regionally specified and that the patterning of NSCs occurs, to a large extent, before birth. Thus, RG and adult SVZ B cells on the walls of the lateral ventricle must inherit a regional pattern of gene expression from VZ cells in the embryo. Culture and transplantation experiments suggest that this specification is cell-autonomous and not easily modified by homotopic and heterotopic grafting (Kelsch et al. 2007, Merkle et al. 2007, Young et al. 2007). Taken together, these studies suggest that RG and SVZ B cells are regionally heterogeneous and specified for the production of different types of neurons.
The above regional labeling experiments show that restricted groups of RG in neonatal mice give rise to specific subtypes of neurons and that the relative position of NSCs, as RG transform into adult SVZ B cells (Figure 1), does not change significantly. NSCs do not move tangentially during postnatal development. It appears that once NSCs acquire positional specification, they remain in that particular location, being displaced only by differential growth of the brain (Figure 4). We do not know whether similar regional specification takes place during the transformation of the embryonic dentate gyrus neuroepithelial cells to adult SGZ radial astrocytes (as discussed above) (Figure 6). Regional specification allows the preservation of relative positional information as NSCs transform from neuroepithelial cells to RG and to adult progenitor astrocytes. It will be interesting to determine how the dynamic molecular mechanisms for primary progenitor specification in the embryo are maintained in populations of progenitor cells that preserve region-specific production of different types of neurons throughout life. It is clearly within RG and adult NSCs that these critical transcriptional programs are retained (Figure 4). Again, the identification of NSCs during development and in the adult should aid in understanding how this specification is attained.
Figure 6.
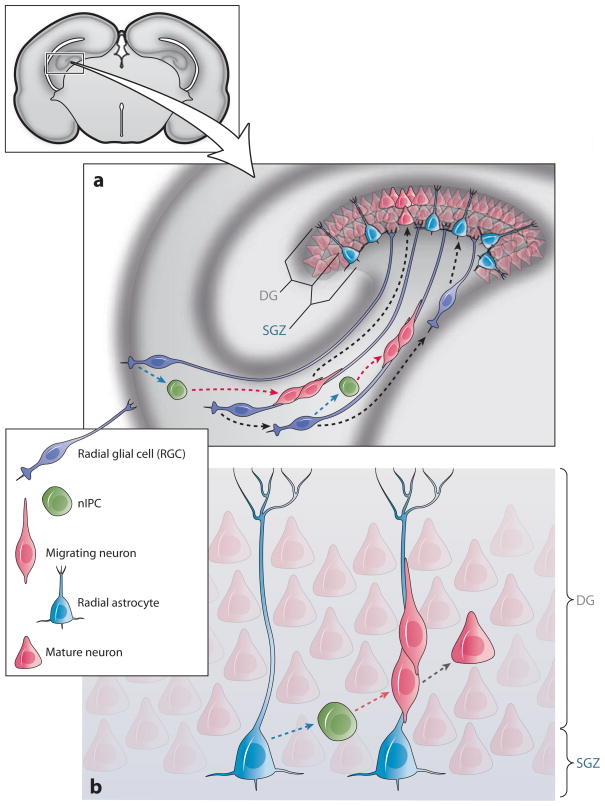
Schematic of progenitor types and lineages in the developing and adult brain dentate gyrus (DG) in the hippocampus. The subgranular zone (SGZ) is unique among forebrain germinal regions because it is detached from the walls of the ventricles. (a) Depicts a possible link between RG in the VZ facing the lateral ventricle (dentate neuroepithelium) and the developing SGZ. These RG migrate away from this region while they continue to generate nIPCs and early-born neurons. RG generate radial astrocytes, which become localized to the SGZ. These astrocytic cells have a prominent process (a, b) that traverses the dentate granule cell layer and branches in the deep molecular layer. (b) Radial astrocytes (also known as type I progenitors, see text) generate nIPCs (D cells or type II progenitors), which in turn generate young neurons. Young neurons remain tightly associated to radial processes of radial astrocytes before differentiating into granule cells. Dashed arrows indicate hypothetical symmetric (red) and asymmetric (blue) divisions. Black dashed arrows indicate hypothetical transformation. The inset in the upper left corner shows a cross section of the rodent forebrain to illustrate the location of the developing dentate gyrus in the hippocampus. nIPCs, neurogenic intermediate progenitor cells; RG, radial glia; SGZ, subgranular zone; VZ, ventricular zone.
GENERATION OF OLIGODENDROCYTES
RG in development and SVZ B cells in the adult rodent brain generate oligodendrocytes in addition to neurons (Figures 1–3 and 5). Cre/loxP fate-mapping experiments using RG promoters to drive expression demonstrate that RG give rise to neurons and oligodendrocytes throughout the CNS (Fogarty et al. 2005, Casper & McCarthy 2006). Oligodendrocytes originate in multiple locations during development. The earliest cortical oligodendrocytes arise from Nkx2.1-expressing precursor cells located in the ventral telencephalon and arrive in the cortex beginning at ~E16 in the mouse. They originate from the VZ (presumably from RG) of the medial ganglionic eminence (MGE) (Pringle & Richardson 1993, Spassky et al. 1998, Tekki-Kessaris et al. 2001). A subpopulation of these oligodendrocytes that migrate into dorsal cortex and hippocampus arises from progenitor cells that also produce GABAergic neurons (He et al. 2001, Nery et al. 2001). In the ventral telencephalon, the Dlx homeobox transcription factors Dlx1 and Dlx2, which are required for GABAergic neuron production, negatively regulate Olig2-dependent oligodendrocyte formation, thereby modulating the neuron-to-oligodendrocyte switch in multipotent precursor cells (Petryniak et al. 2007). Secondary waves of oligodendrocytes are generated more dorsally. Gsh2-expressing precursor cells in the lateral and/or caudal ganglionic eminence(s) (LGE/CGE) also generate oligodendrocytes that migrate to the dorsal cortex (Kessaris et al. 2006). After E18, yet another wave of cortical oligodendrocytes arise, this time from Emx1-expressing precursor cells presumably from dorsal cortex itself (Gorski et al. 2002, Kessaris et al. 2006). Interestingly, most of the early-generated oligodendrocytes disappear after birth so that the majority oligodendrocytes present in adult cortex appear to have been derived from the Emx1-expressing progenitors (Kessaris et al. 2006).
Oligodendrocyte progenitor cells (generally referred to as OPCs) are NG2-expressing proliferating cells distributed throughout the brain (Noble 2000, Aguirre et al. 2007, Barres 2008, Rivers et al. 2008). OPCs likely correspond to IPCs that are committed to the oligodendroglial lineage (i.e., oIPCs). However, unlike IPCs that actively proliferate in the VZ or SVZ, NG2+ OPCs are found in white and gray matter, where they may be quiescent and proliferate symmetrically in response to local signals. The origin of these cells remains unclear, but during development they are likely derived from RG. Oligodendrocytes continue to be born postnatally, and there is evidence that at least a subpopulation of these cells, including new NG2+ progenitors, is derived from the SVZ (Figure 5) (Levison & Goldman 1993, Nait-Oumesmar et al. 1999, Menn et al. 2006, Aguirre et al. 2007). Labeling of neonatal RG on the lateral ventricle walls results in labeled oligodendrocytes that migrate into the corpus callosum and other white matter tracts (Merkle et al. 2004). Menn et al. (2006) used retroviral labeling via the RCAS-tva system (see above) to show that a subpopulation of myelinating and nonmyelinating oligodendrocytes in the adult brain originate from SVZ B cells (Menn et al. 2006). Adult SVZ B cells also give rise to parenchymal NG2+ OPCs. We still do not know whether single RG during development or adult SVZ B cells can give rise to both neurons and oligodendrocytes, but studies in vitro suggest that such bipotential cells probably do exist (He et al. 2001, Menn et al. 2006). In contrast to the adult SVZ, very few, if any, oligodendrocytes appear to be generated in the SGZ of the adult hippocampus under normal conditions. Overexpression of Mash1 (also known as Ascl1) in adult hippocampal cells redirects differentiation of these postnatal progenitors from neuronal production to oligodendrocyte production (Jessberger et al. 2008).
The above suggests that RG and adult NSCs serve as primary progenitors for the generation of oligodendrocytes. However, evidence also supports a small (1%–3% compared with VZ/SVZ progenitors) source of oligodendrocytes (possibly of some neurons) outside the VZ or SVZ (Costa et al. 2007). Precursors that can be isolated from the cortical marginal zone largely express Olig2 and most generate clones consisting of astrocytes and oligodendrocytes in vitro, although some can generate neurons in culture. Fate-mapping studies suggest that these MZ oligodendrocyte progenitors are also ultimately derived from the VZ, presumably from RG that express ventral (Nkx2.1- and Gsh2-expressing) or dorsal (Emx1-expressing) markers. Like neuronal progenitor cells, oligodendrocyte precursor cells are likely to be heterogeneous and to be derived from regionally specified progenitors with unique transcription-factor expression patterns in the VZ and SVZ. For example, Mash1 cooperates with Olig2 to generate a specific subpopulation of oligodendrocytes produced early in development (Parras et al. 2007). A subpopulation of cortical oligodendrocytes is derived from progenitors expressing Dlx genes in the ventral forebrain (He et al. 2001). In the SVZ, Olig2 appears to repress neuronal fate and to promote not only oligodendrogenesis (Hack et al. 2005) but also differentiation into astrocytes (Marshall et al. 2005). As discussed for neurons (see Figure 4), temporal and regional transcription-factor programs appear to specify different populations of oligodendrocytes. The primary progenitors for these ensheathing and myelinating glial cells are also cells classically considered part of the astroglial lineage.
CONCLUSION
RG and astrocytes are closely related, and for most of the past century, both cell types were considered support elements: during development, as scaffolding for brain assembly, and in the adult, as cells supporting neuronal and synaptic function. As reviewed above, RG and a subpopulation of astrocytes are now considered NSCs. They produce, depending on their location and developmental stage, specific types of neurons and glial cells. This new view is a departure from the classical concept that separated glial and neuronal cell origins. RG were, as their name implies, strictly associated with the production of glial cells including astrocytes, whereas neurons were thought to originate from a separate pool of neuron-restricted progenitors. It is interesting that this view prevailed for so long, given the undeniable evidence of an epithelial origin of the brain.
The CNS originates from an epithelium, a palisade of cells that very early in development acquires positional information essential to produce the diversity of neuronal and glial types that form the brain. The neural “stem cell” must therefore be a type of neuroepithelial cell. Cajal saw RG as epithelial cells and adult astrocytes as “nothing more than displaced and transformed neuroepithelial cells” (Ramón y Cajal 1995). Given this perspective, it is not surprising that subpopulations of these cells that have classically been termed glia are now identified as NSCs. Thus neural progenitors at different developmental ages are contained within the neuroepithelial-RG-astrocyte lineage (Figure 1). It is these cells that retain NSC properties at different developmental stages and in the postnatal brain. From this trunk emerge differentiated neurons, ependymal cells, and astrocytes, as well as nIPCs that generate neurons and oIPCs that generate oligodendrocytes (Figure 2). During the proliferation of these IPCs, initial programs encoded within primary progenitors unfold, resulting in the formation of young neurons or glial cells with unique characteristics. The identification of IPCs is therefore fundamental to our understanding of how progenitor proliferation is coupled to the acquisition of specific properties.
Clues for novel approaches to brain repair are also likely to emerge from the identification of NSCs during development and in the adult. Several studies have suggested that the adult SVZ may be an important reservoir of precursor cells for brain repair (Lindvall & Kokaia 2006, Martino & Pluchino 2006). Although NSCs and some level of SVZ proliferation have been described in the human brain (Eriksson et al. 1998, Curtis et al. 2003, Sanai et al. 2005), the degree to which these cells generate new neurons and the extent of neuronal migration in the adult human brain remain controversial (Curtis et al. 2007, Sanai et al. 2007). New data suggesting that adult rodent type B cells, like RG in development, are heterogeneous and spatially restricted contrasts with the generalized view that developing and adult NSCs may be able to produce a wide array of neuronal subtypes as may be required for specific therapeutic applications. Instead, an extraordinary level of prespecification appears to exist in terms of the types of neurons derived from different locations within progenitor zones (see Figure 4). If the human brain resembles the rodent in terms of spatial specification, progenitor domains are likely spatially separated, and different subtypes of neurons may arise from distinct SVZ regions. Neuronal subtypes that may be relevant for brain repair may need to be collected from specific locations of the adult germinal niche. Similarly, RG and nIPCs derived from a specific age and location in the embryonic brain may be committed to the generation of specific neuronal types. Deciphering how combinations of transcription factors expressed in different locations of the embryonic and adult SVZs result in the generation of distinct subsets of neurons may help derive specific subtypes of neurons for brain repair. Time and space appear to be the chief regulators of primary progenitors in the brain. Identification of RG and subpopulations of adult astrocytes that function as the NSCs in the developing and mature nervous system is an essential step to understand how these temporal and spatial programs emerge. In addition, identification of specific astroglial cells as NSCs serves to blur the traditional distinction between glial cells and neurons and provides a more integrated view of the origins of cellular diversity in the nervous system.
Acknowledgments
This work was supported by grants from the NIH to A.K. and A.A.-B., and by the John G. Bowes Research Fund. We thank John Rubenstein, Sala Major, Thuhien Nguyen, and David Hansen for comments on the manuscript. We also thank Kenneth Xavier Probst of Xavier Studio for assisting with the design and preparation of Figures.
Glossary
- RG
radial glia
- IPC
intermediate progenitor cell
- nIPC
intermediate progenitor cell that generates neurons
- oIPC
intermediate progenitor cell that generates oligodendrocytes
- aIPC
intermediate progenitor cell that generates astrocytes
- VZ
ventricular zone
- SVZ
subventricular zone
- NSC
neural stem cell
- GLAST
astrocyte-specific glutamate transporter
- GFAP
glial fibrillary acidic protein
- INM
interkinetic nuclear migration
- SGZ
subgranular zone
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Arnold Kriegstein, Email: kriegsteina@stemcell.ucsf.edu.
Arturo Alvarez-Buylla, Email: abuylla@stemcell.ucsf.edu.
LITERATURE CITED
- Aaku-Saraste E, Oback B, Hellwig A, Huttner WB. Neuroepithelial cells downregulate their plasma membrane polarity prior to neural tube closure and neurogenesis. Mech Dev. 1997;69:71–81. doi: 10.1016/s0925-4773(97)00156-1. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Horizontal compartmentation in the germinal matrices and intermediate zone of the embryonic rat cerebral cortex. Exp Neurol. 1990a;107:36–47. doi: 10.1016/0014-4886(90)90061-v. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990b;301:325–42. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM, Mateo A, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–37. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–86. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–9. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–90. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–97. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, et al. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–93. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–18. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjelloun-Touimi S, Jacque CM, Derer P, De Vitry F, Maunoury R, Dupouey P. Evidence that mouse astrocytes may be derived from the radial glia. An immunohistochemical study of the cerebellum in the normal and reeler mouse. J Neuroimmunol. 1985;9:87–97. doi: 10.1016/s0165-5728(85)80009-6. [DOI] [PubMed] [Google Scholar]
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–64. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulder Comm. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–61. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008;105:13127–32. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittis PA, Meiri K, Dent E, Silver J. The earliest patterns of neuronal differentiation and migration in the mammalian central nervous system. Exp Neurol. 1995;134:1–12. doi: 10.1006/exnr.1995.1031. [DOI] [PubMed] [Google Scholar]
- Bruckner G, Biesold D. Histochemistry of glycogen deposition in perinatal rat brain: importance of radial glial cells. J Neurocytol. 1981;10:749–57. doi: 10.1007/BF01262651. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–54. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Wooley CS, McEwen BS, Gould E. Differentiation of newly born neuron and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–56. doi: 10.1016/s0959-4388(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–38. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–75. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–18. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–84. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–41. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–71. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH. Prenatal gliogenesis in the developing cerebrum of the mouse. Glia. 1988;1:308–16. doi: 10.1002/glia.440010503. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW. Radial glia in the human fetal cerebrum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res. 1978;148:295–311. doi: 10.1016/0006-8993(78)90721-7. [DOI] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–46. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026–31. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Kessaris N, Richardson WD, Gotz M, Hedin-Pereira C. The marginal zone/layer I as a novel niche for neurogenesis and gliogenesis in developing cerebral cortex. J Neurosci. 2007;27:11376–88. doi: 10.1523/JNEUROSCI.2418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–49. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci USA. 2003;100:9023–27. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372:263–66. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–65. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–72. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Skeath JB. Neurogenesis in the insect central nervous system. Curr Opin Neurobiol. 1996;6:18–24. doi: 10.1016/s0959-4388(96)80004-3. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999b;97:1–20. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999c;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, García-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–34. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–43. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–22. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. Radial organization of the hippocampal dentate gyrus: a Golgi, ultrastructural, and immunocytochemical analysis in the developing rhesus monkey. J Comp Neurol. 1989;223:1–21. doi: 10.1002/cne.902230102. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–73. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–14. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harbor Symp Quant Biol. 1997;62:451–66. [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–17. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–82. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–95. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–59. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Fujita S. Kinetics of cellular proliferation. Exp Cell Res. 1962;28:52–60. doi: 10.1016/0014-4827(62)90311-7. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–66. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadisseux JF, Evrard P. Glial-neuronal relationship in the developing central nervous system. A histochemical-electron microscope study of radial glial cell particulate glycogen in normal and reeler mice and the human fetus. Dev Neurosci. 1985;7:12–32. doi: 10.1159/000112273. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–38. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–13. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–90. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–56. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- García-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–75. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–57. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Glaser T, Brustle O. Retinoic acid induction of ES-cell-derived neurons: the radial glia connection. Trends Neurosci. 2005;28:397–400. doi: 10.1016/j.tins.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996;30:505–20. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–14. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Hartfuss E, Malatesta P. Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res Bull. 2002;57:777–88. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Regulation of neuronal birth, migration and death in the rat dentate gyrus. Dev Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Wooley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–50. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, McEwen BS. Blockade of NMDA receptors increases cell death and birth in the developing rat dentate gyrus. J Comp Neurol. 1994;340:551–65. doi: 10.1002/cne.903400408. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999;96:5263–67. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Williams BP, Li DQ, Hajihosseini M, Friedrich A, Price J. Multiple restricted lineages in the embryonic rat cerebral cortex. Development. 1993;117:553–61. doi: 10.1242/dev.117.2.553. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr Opin Cell Biol. 2005;17:639–47. doi: 10.1016/j.ceb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–72. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hajós F, Woodhams PL, Bascó E, Csillag A, Balázs R. Proliferation of astroglia in the embryonic mouse forebrain as revealed by simultaneous immunocytochemistry and autoradiography. Acta Morphol Acad Sci Hung. 1981;29:361–64. [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, García-Verdugo JM, Aguilar A, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar TF, Ang E, Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci USA. 2003;100:2890–95. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Exploiting the dynamics of S-phase tracers in developing brain: interkinetic nuclear migration for cells entering versus leaving the S-phase. Dev Neurosci. 2000;22:44–55. doi: 10.1159/000017426. [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal fore-brain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–62. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–15. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133:510–22. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA. 1998;95:1218–23. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–30. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Brand M. Asymmetric division and polarity of neuroepithelial cells. Curr Opin Neurobiol. 1997;7:29–39. doi: 10.1016/s0959-4388(97)80117-1. [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Shiga T, Hirata Y. Spatial and temporal pattern of postnatal proliferation of glial cells in the parietal cortex of the rat. Brain Res. 1983;285:181–87. doi: 10.1016/0165-3806(83)90050-0. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, et al. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–44. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–32. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–55. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Developmental Neurobiology. New York: Plenum; 1991. [Google Scholar]
- Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–93. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kalman M. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunohistochemical staining against glial fibrillary acidic protein (GFAP) Anat Embryol. 1998;198:409–33. doi: 10.1007/s004290050193. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–94. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Karfunkel P. The activity of microtubules and microfilaments in neurulation in the chick. J Exp Zool. 1972;181:289–301. doi: 10.1002/jez.1401810302. [DOI] [PubMed] [Google Scholar]
- Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–99. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–52. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–95. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dendate gyrus. J Neurosci. 1998;18:3206–12. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–57. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–79. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–91. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–57. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Koo BK, Yoon MJ, Yoon KJ, Im SK, Kim YY, et al. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS ONE. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Hama K. Three-dimensional structure of astrocytes in the rat dentate gyrus. J Comp Neurol. 1986;249:242–60. doi: 10.1002/cne.902490209. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–90. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Krushel LA, Johnston JG, Fishell G, Tibshirani R, van der Kooy D. Spatially localized neuronal cell lineages in the developing mammalian forebrain. Neuroscience. 1993;53:1035–47. doi: 10.1016/0306-4522(93)90487-z. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–64. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnütgen F, Brüning G, Spener F, Müller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–49. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Landrieu P, Goffinet A. Mitotic spindle fiber orientation in relation to cell migration in the neocortex of normal and reeler mouse. Neurosci Lett. 1979;13:69–72. doi: 10.1016/0304-3940(79)90077-6. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–12. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Early divergence and changing proportions of neuronal and glial precursor cells in the primate cerebral ventricular zone. Dev Biol. 1983;96:472–84. doi: 10.1016/0012-1606(83)90184-7. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–40. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–96. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Liu H, Belz T, Bock D, Takacs A, Wu H, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–78. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–48. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol. 2009;512:556–72. doi: 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Shook BA, Daniels SB, Conover JC. Subventricular zone-mediated ependyma repair in the adult mammalian brain. J Neurosci. 2008;28:3804–13. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB, McDermott K. Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia. 1994;11:211–26. doi: 10.1002/glia.440110302. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–50. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–47. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Magini J. Nouvelles recherches histologiques sur le cerveau du foetus. Arch Ital Biol. 1888;10:384–87. [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–64. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Mares V, Bruckner G. Postnatal formation of non-neuronal cells in the rat occipital cerebrum: an autoradiographic study of the time and space pattern of cell division. J Comp Neurol. 1978;177:519–28. doi: 10.1002/cne.901770310. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25:7289–98. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–61. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Turnbull DH, Walsh CA, Fishell G. Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J Neurosci. 2001;21:6772–81. doi: 10.1523/JNEUROSCI.21-17-06772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, García-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–88. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–84. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G-L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mione MC, Cavanagh JFR, Harris B, Parnavelas JG. Cell fate specification and symmetrical/asymmetrical divisions in the developing cerebral cortex. J Neurosci. 1997;17:2018–29. doi: 10.1523/JNEUROSCI.17-06-02018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, García-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Mitotic cycling of radial glial cells of the fetal murine cerebral wall: a combined autoradiographic and immunohistochemical study. Brain Res. 1988;466:183–90. doi: 10.1016/0165-3806(88)90043-0. [DOI] [PubMed] [Google Scholar]
- Misson JP, Takahashi T, Caviness VS., Jr Ontogeny of radial and other astroglial cells in murine cerebral cortex. Glia. 1991;4:138–48. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- Miyata T. Asymmetric cell division during brain morphogenesis. Prog Mol Subcell Biol. 2007;45:121–42. doi: 10.1007/978-3-540-69161-7_6. [DOI] [PubMed] [Google Scholar]
- Miyata T. Development of three-dimensional architecture of the neuroepithelium: role of pseudostratification and cellular ‘community’. Dev Growth Differ. 2008;50(Suppl 1):S105–12. doi: 10.1111/j.1440-169X.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–41. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–45. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–55. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–65. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morest DK. A study of neurogenesis in the forebrain of opossum pouch young. Z Anat Entwickl. 1970;130:265–305. doi: 10.1007/BF00520999. [DOI] [PubMed] [Google Scholar]
- Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- Morin X, Jaouen F, Durbec P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci. 2007;10:1440–48. doi: 10.1038/nn1984. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Murciano A, Zamora J, López-Sánchez J, Frade JM. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol Cell Neurosci. 2002;21:285–300. doi: 10.1006/mcne.2002.1174. [DOI] [PubMed] [Google Scholar]
- Nadarajah B. Radial glia and somal translocation of radial neurons in the developing cerebral cortex. Glia. 2003;43:33–36. doi: 10.1002/glia.10245. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–32. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–66. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–40. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol. 2004;479:168–80. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Noble M. Precursor cell transitions in oligodendrocyte development. J Cell Biol. 2000;148:839–42. doi: 10.1083/jcb.148.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdño V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol. 2007;64:639–42. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdño V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adulthood. In: Nottebohm F, editor. Hope for a New Neurology. New York: NY Acad. Sci; 1985. pp. 143–61. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann NY Acad Sci. 2004;1016:628. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Oldham MC, Konopka G, Iwamoto K, Langfelder P, Kato T, et al. Functional organization of the transcriptome in human brain. Nat Neurosci. 2008;11:1271–82. doi: 10.1038/nn.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cereb Cortex. 1991;1:463–68. doi: 10.1093/cercor/1.6.463. [DOI] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27:4233–42. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–33. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2008;57:66–78. doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Price J, Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988;104:473–82. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Richardson WD. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993;117:525–33. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–76. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–76. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System of Man and Vertebrates. New York: Oxford Univ. Press; 1995. [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–27. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- Reid CB, Liang I, Walsh C. Systematic widespread clonal organization in cerebral cortex. Neuron. 1995;15:299–310. doi: 10.1016/0896-6273(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Retzius G. Die Neuroglia des Gehirns beim Menschen und bei Säugetieren. Biol Unters. 1894;6:1–24. [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- Sanai N, Berger MS, García-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;318:393. doi: 10.1126/science.318.5849.393a. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–44. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sauer FC. Mitosis in the neural tube. J Comp Neurol. 1935;62:377–405. [Google Scholar]
- Sauer ME, Walker BE. Radioautographic study of interkinetic nuclear migration in the neural tube. Proc Soc Exp Biol Med. 1959;101:557–60. doi: 10.3181/00379727-101-25014. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol. 1979;156:115–52. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–58. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–84. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Miale IL, Feder N. Cell proliferation and migration in the primitive ependymal zone: an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959;1:322–33. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- Silva AO, Ercole CE, McLoon SC. Regulation of ganglion cell production by Notch signaling during retinal development. J Neurobiol. 2003;54:511–24. doi: 10.1002/neu.10156. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Smart IH. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat. 1973;116:67–91. [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–45. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, et al. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18:8331–43. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–14. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–74. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JA, Yoon MG. Mitosis of radial glial cells in the optic tectum of adult goldfish. J Neurosci. 1981;1:862–75. doi: 10.1523/JNEUROSCI.01-08-00862.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Gotz M, Gruss P, Price J. Pax6-dependent regulation of adhesive patterning, R-cadherin expression and boundary formation in developing forebrain. Development. 1997;124:3765–77. doi: 10.1242/dev.124.19.3765. [DOI] [PubMed] [Google Scholar]
- Stuhmer T, Puelles L, Ekker M, Rubenstein JL. Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb Cortex. 2002;12:75–85. doi: 10.1093/cercor/12.1.75. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2(+) neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–28. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Misson JP, Caviness VS., Jr Glial process elongation and branching in the developing murine neocortex: a qualitative and quantitative immunohistochemical analysis. J Comp Neurol. 1990;302:15–28. doi: 10.1002/cne.903020103. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness V., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995;15:6058–68. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–93. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, et al. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–54. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–45. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46:383–88. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Ueno M, Katayama K, Yamauchi H, Nakayama H, Doi K. Cell cycle progression is required for nuclear migration of neural progenitor cells. Brain Res. 2006;1088:57–67. doi: 10.1016/j.brainres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Valverde F, De Carlos JA, López-Mascaraque L. Time of origin and early fate of preplate cells in the cerebral cortex of the rat. Cereb Cortex. 1995;5:483–93. doi: 10.1093/cercor/5.6.483. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdélyi F, Szabó G, et al. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–16. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–45. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992;255:434–40. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–93. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–59. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Willaime-Morawek S, Seaberg RM, Batista C, Labbé E, Attisano L, et al. Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol. 2006;175:159–68. doi: 10.1083/jcb.200604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995;14:1181–88. doi: 10.1016/0896-6273(95)90265-1. [DOI] [PubMed] [Google Scholar]
- Williams BP, Read J, Price J. The generation of neurons and oligodendrocytes from a common precursor cell. Neuron. 1991;7:685–93. doi: 10.1016/0896-6273(91)90381-9. [DOI] [PubMed] [Google Scholar]
- Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–90. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- Wu SX, Goebbels S, Nakamura K, Nakamura K, Kometani K, et al. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–77. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Lieska N, Shao D, Kriho V, Pappas GD. Immunotyping of radial glia and their glial derivatives during development of the rat spinal cord. J Neurocytol. 1993;22:558–71. doi: 10.1007/BF01189043. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Koo BK, Im SK, Jeong HW, Ghim J, et al. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–31. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–96. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF. Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci USA. 2002;99:16273–78. doi: 10.1073/pnas.232586699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof S. Quantitative studies of mitoses in cerebral hemispheres of fetal rats. Brain Res. 1985;352:306–9. doi: 10.1016/0165-3806(85)90120-8. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–22. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XP, Zheng G, Zou L, Liu HL, Hou LH, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008;307:101–8. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhong WM, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–20. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A. 2006;192:649–70. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]