Abstract
PURPOSE
Recent findings suggest a role for heart failure in the etiology of osteoporotic fractures, yet the temporal sequence of occurrence of the two conditions needs clarification.
METHODS
Using the Rochester Epidemiology Project, the authors conducted a 2-phase study: A case-control study compared osteoporotic fracture history among Olmsted County, Minnesota, residents newly diagnosed with heart failure in 1979-2002 to age- and sex-matched community controls without heart failure (961 pairs; mean age 76 years; 54% women). Both groups were then followed to July 2009 to evaluate their subsequent fracture risk in a cohort study.
RESULTS
Prior fractures were more frequent in heart failure cases than controls (23.1% versus 18.8%, P = 0.02). The adjusted odds ratio (OR) for heart failure associated with prior fracture was 1.39 (95% confidence interval (CI): 1.07, 1.81), mainly driven by hip fractures (OR: 1.82; 95% CI: 1.25, 2.66) with little or no association with other fractures. Over a mean follow-up of 7.5 years, 444 individuals developed subsequent osteoporotic fractures. The adjusted fracture risk was marginally elevated in heart failure patients compared with controls (hazard ratio (HR): 1.32; 95% CI: 0.98, 1.79), again largely attributable to hip fractures (HR: 1.58; 95% CI: 1.03, 2.41).
CONCLUSIONS
In this community, the association with fracture risk was about as strong before as after the diagnosis of heart failure and was nearly entirely attributable to hip fractures. Additional work is needed to identify common underlying mechanisms for heart failure and hip fracture, which may define prevention opportunities.
Keywords: Heart failure, Fractures, Hip fracture, Osteoporosis, Epidemiology, Prevention
INTRODUCTION
There is increasing awareness of an association between osteoporosis and cardiovascular disease. Indeed, low bone mineral density (BMD) has been linked to increased cardiovascular disease morbidity and mortality.1-9 Further, aortic and coronary calcifications, endothelial dysfunction, and inflammation, all implicated in the initiation and progression of the atherosclerotic lesion,10,11 are associated with low BMD and fractures.12-16 This suggests common pathophysiological mechanisms underlying both diseases,17,18 but a mechanistic basis for the relationship is unclear. Moreover, both entities may share common etiologic factors such as diabetes, dyslipidemia, smoking, hypertension, and estrogen deficiency,18-21 which adds to the complexity of this relationship as these could act as confounding factors. Finally, the burden of cardiovascular disease in the community has shifted in recent decades toward women and the elderly, where osteoporosis is quite prevalent,22 so any association could simply reflect disease coexistence rather than causality.23 Recently, two large epidemiological studies revealed a substantial increase in osteoporotic fractures, particularly in the hip, associated with heart failure,24,25 suggesting heart failure as a possible cause of fracture. Still, it is uncertain whether osteoporosis precedes cardiovascular disease 1,3-5 or follows it.24-27 Since the relationship of fractures with cardiovascular disease seems strongest for heart failure,24,25,27 this population-based study was undertaken to test the null hypothesis of no association between heart failure and fractures generally, as well as specific fracture sites, and to determine if any association was as strong prior to the recognition of heart failure as afterward, which would argue against the notion that heart failure per se causes fractures.28
METHODS
Study setting
Olmsted County, Minnesota, is isolated from other urban centers, and medical care is delivered to local residents by few providers. Consequently, it has been possible through the Rochester Epidemiology Project to link the inpatient and outpatient medical records from all sources of care used by the population, thus providing a unique infrastructure to analyze disease determinants and outcomes.29 Access to this comprehensive contemporary documentation was used to assess the relationship between heart failure and fractures.
Study design
Following approval by the appropriate Institutional Review Boards, this study was conducted in 2 complementary stages: First, we performed an incident case-control study with newly diagnosed heart failure patients serving as cases and age- and sex-matched subjects, selected from the general population, constituting the comparison group. Prior fractures (verified by dates, classified by anatomic site, and dichotomized by current convention into major osteoporotic fractures versus all others [nonosteoporotic])30 were the primary exposure of interest, whereas known risk factors for heart failure were treated as potential confounders. Subsequently, the heart failure patients and their matched controls were followed forward in time to compare their long-term fracture risk using a cohort design.
Selection of cases and controls
Case subjects were Olmsted County residents with an incident diagnosis of heart failure in 1979-2002. Framingham criteria31 (Table 1) were used to define heart failure using methods previously described.32 The index date was defined as the first evidence of heart failure in the medical record.
Table 1.
Framingham Heart Study Criteria for Heart Failurea
| Major criteria: |
| • Paroxysmal nocturnal dyspnea |
| • Neck vein distention |
| • Rales |
| • Radiographic cardiomegaly (increasing heart size on chest radiography) |
| • Acute pulmonary edema |
| • S3 gallop |
| • Increased central venous pressure (>16 cm H2O at right atrium) |
| • Hepatojugular reflux |
| • Weight loss >4.5 kg in 5 days in response to treatment |
| Minor criteria: |
| • Bilateral ankle edema |
| • Nocturnal cough |
| • Dyspnea on ordinary exertion |
| • Hepatomegaly |
| • Pleural effusion |
| • Decrease in vital capacity by one third from maximum recorded |
| • Tachycardia (heart rate>120 beats/min.) |
Diagnosis of heart failure requires the simultaneous presence of at least 2 major criteria or 1 major criterion in conjunction with 2 minor criteria
Control subjects were also selected from the Olmsted County population. In any 3-year period, over 90% of residents are seen at Mayo Clinic, and the majority are attended annually by some local health care provider.29 Thus, the Rochester Epidemiology Project medical records linkage system provides a virtually complete enumeration of the population from which to sample controls. Control subjects were individually matched (1:1) to cases on age (±3 years) and sex. The index date for the control corresponds to the incidence date of the matched heart failure case. Since information on exposures prior to the index date was obtained from these community medical records, this ensures similar opportunities for ascertainment of risk factors in the two groups and avoids biases inherent in many case-control studies (e.g., differential recall, non-response bias, and survivor bias). Potential controls with heart failure prior to the index date were excluded.
Follow-up
All participants were then followed through their community medical records.29 Follow-up began at the index date (January 1979 to December 2002) and lasted until death or the most recent clinical contact, whichever came first (last follow up, July 2009). Death was ascertained using multiple sources, as described previously.32
Fracture data
Each subject's complete inpatient and outpatient medical record at each local provider of medical care was searched electronically for the occurrence of any fracture through the comprehensive diagnostic and surgical indices that are part of the Rochester Epidemiology Project.29 Of note, the diagnostic index records all diagnoses made over time, not only chief complaints. Thus, fractures found incidentally on work-ups for other problems are included in this system, as are symptomatic fractures presenting for treatment. Fractures were classified by anatomic site, but information on the degree of trauma involved in each fracture event was not available. Thus, “osteoporotic” fractures were taken to be those of the proximal femur, lumbar/thoracic vertebrae, distal forearm or humerus, the major osteoporotic fracture sites now linked to osteoporosis.30 Traditionally, these are further restricted to fractures resulting from moderate trauma, but nothing about osteoporosis protects bones from severe trauma, and this convention is now questioned.33,34 All fractures were included in this analysis; and the date of first fracture event both before and after the index time, overall and by anatomic site, was used as the diagnosis date.
Risk factors
When the cases and controls were assembled, data on risk factors prior to the index date were abstracted from the entire community medical record for both groups. Myocardial infarction was ascertained using standardized criteria. Diabetes mellitus was defined according to National Diabetes Data Group criteria.35 Clinical definitions were used to assess hypertension and dyslipidemia. Body mass index (BMI, kg/m2) was calculated using the weight and earliest adult height. Smoking was dichotomized as current versus no current smoking. Comorbidity was assessed by the Charlson index,36 which consists of 19 comorbid conditions weighted according to the degree to which they predict mortality (1,2,3 or 6 points for each), and analyzed categorically (no comorbidity for 0 points, moderate comorbidity for 1 to 2 points, and severe comorbidity for 3 points or more). Revascularization procedures included percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass grafting (CABG).
Statistical analysis
For the case-control study, a matched analysis was employed using conditional logistic regression with age and gender as stratification variables.37 A model was developed to estimate the odds ratio (OR) for heart failure associated with prior fracture. As noted above, fractures were divided into “osteoporotic” or “nonosteoporotic”, and the former group was subsequently refined by anatomic site (i.e., hip, forearm, spine, and humerus). For each of these two categories, “no fracture” was defined as the reference condition. Multivariable adjustment was performed for known cardiovascular risk factors and comorbidities. Stratified analysis was used to assess ORs in subgroups, and the heterogeneity of these estimates was then tested.
For the follow-up study, the risk of subsequent fractures in heart failure patients was compared directly with that in their matched controls utilizing a stratified proportional hazards model with the case/control pairs forming the strata. Hazard ratios (HRs) compared the rate of occurrence of fractures in heart failure versus non-heart failure subjects. Multivariable adjustment was made for suspected risk factors for fractures18 and predictors of fracture risk in bivariate screening (with P < 0.20). Stratified analysis by gender was also performed and the heterogeneity of the HRs was tested. For both unadjusted and adjusted models, the assumption of proportional hazards was examined and satisfied for the variables considered.
Finally, the cumulative incidence of a new fracture was projected for up to 10 years following the recognition of heart failure. In the customary Kaplan-Meier approach, patients who die are censored; when the death rate is high, however, this may overestimate the cumulative fracture incidence. Therefore, death was treated as a competing event in this analysis.38 Analyses were performed using SAS 8.2 (SAS Institute Inc., Cary, NC).
RESULTS
The study included 1,922 subjects, 961 incident heart failure cases (mean age: 75.5 [SD 12.7] years; 54% women) and 961 age- and sex-matched controls. Subject characteristics by heart failure status are presented in Table 2. On average, heart failure patients had a higher frequency of prior myocardial infarction, revascularization procedures, hypertension, diabetes, and smoking. In addition, they had higher mean BMI and more comorbidities than controls. A history of any osteoporotic fracture was more prevalent among heart failure cases than among controls, whereas the distribution of nonosteoporotic fractures was similar. Regarding specific anatomic fracture sites, a difference in prevalence between cases and controls was shown for hip fractures but not for other types of fracture (Table 2).
Table 2.
Pertinent Clinical Characteristics Among Olmsted County, Minnesota, Residents With Heart Failure First Diagnosed in 1979-2002, Compared to Age- and Sex-Matched Community Controls
| Heart Failure Status |
|||
|---|---|---|---|
| Characteristic | Cases | Controls | P Value |
| n | 961 | 961 | |
| Age, mean (SD), yrs | 75.5 (12.7) | 75.4 (12.6) | 0.98 |
| Female, n (%) | 517 (54) | 517 (54) | 1.00 |
| Risk Factor History | |||
| Myocardial infarction, n (%) | 208 (22) | 80 (8) | <0.001 |
| Coronary heart disease, n (%) | 472 (49) | 272 (28) | <0.001 |
| Hypertension, n (%) | 643 (67) | 563 (59) | <0.001 |
| Diabetes, n (%) | 181 (19) | 78 (8) | <0.001 |
| Hyperlipidemia, n (%) | 279 (29) | 302 (31) | 0.25 |
| Current smoking, n (%) | 155 (16) | 84 (9) | <0.001 |
| Body mass index, mean (SD) | 26.9 (6.4) | 25.4 (6.5) | <0.001 |
| CABG, n (%) | 82 (9) | 27 (3) | <0.001 |
| PCI, n (%) | 58 (6) | 34 (4) | 0.01 |
| Comorbidity index, n (%) | |||
| 0 points | 206 (21) | 333 (35) | <0.001 |
| 1-2 points | 431 (45) | 395 (41) | |
| ≥ 3 points | 324 (34) | 233 (24) | |
| Fracture (Fx) History | |||
| Any osteoporotic Fx, n (%) | 222 (23) | 181 (19) | 0.02 |
| Hip Fx, n (%) | 99 (10) | 65 (7) | 0.006 |
| Forearm Fx, n (%) | 89 (9) | 82 (9) | 0.57 |
| Spine Fx, n (%) | 71 (7) | 55 (6) | 0.14 |
| Humerus Fx, n (%) | 29 (3) | 30 (3) | 0.89 |
| Any nonosteoporotic Fx, n (%) | 373 (39) | 360 (37) | 0.54 |
CABG=coronary artery bypass grafting; Fx=fracture; PCI=percutaneous coronary interventions; SD=standard deviation
Prior fractures and heart failure
Based on review of original medical record documentation spanning an average of 40 years (median, 43 years) prior to index in the heart failure cases and 40 years (median, 42 years) in controls, we found a moderate association between prior osteoporotic fractures and incident heart failure (Table 3). After adjusting for cardiovascular risk factors, comorbidities, and nonosteoporotic fracture, the OR for heart failure associated with any osteoporotic fracture was 1.39 (95% confidence interval (CI): 1.07, 1.81). However, examination of specific anatomic sites revealed that the overall relationship was almost entirely attributable to a strong association between hip fracture and heart failure (adjusted OR: 1.82; 95% CI: 1.25, 2.66), with no apparent associations for other sites (adjusted OR: 1.14; 95% CI: 0.83, 1.55, for any non-hip osteoporotic fracture). The adjusted association between hip fracture and heart failure applied both to women (OR: 1.63; 95% CI: 1.05, 2.53) and men (OR: 2.47; 95% CI: 1.14, 5.33) (P for heterogeneity in ORs = 0.36) and did not change by study period (for 1979-1990, OR: 1.84; 95% CI: 1.04, 3.27 and for 1991-2002, OR: 1.80; 95% CI: 1.07, 3.03) (P for heterogeneity in ORs = 0.96). We further examined this association by time from hip fracture to heart failure. The adjusted ORs were 1.66 (95% CI: 0.97, 2.84) for fractures occurring more than 5 years before recognition of heart failure and 1.96 (95% CI: 1.21, 3.16) for fractures occurring within 5 years of heart failure (P for heterogeneity in ORs = 0.65). In sensitivity analyses, we adjusted further for cardiovascular disease medication use prior to or at the time of the index date (i.e., diuretics, beta-blockers, angiotensin converting enzyme inhibitors, and statins), which did not materially affect any of the results.
Table 3.
Odds Ratios (ORs) for Heart Failure Associated With Prior Fracture (Fx), Overall and by Fracture Type, Among Olmsted County, Minnesota, Residents With Heart Failure First Diagnosed in 1979-2002, Compared to Age- and Sex-Matched Community Controls
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| Exposure | OR | 95% CI | OR | 95% CI |
| Any osteoporotic Fx | 1.36 | 1.07, 1.72 | 1.39 | 1.07, 1.81 |
| Specific Fracture Types b | ||||
| Hip Fx | 1.66 | 1.18, 2.35 | 1.82 | 1.25, 2.66 |
| Forearm Fx | 1.14 | 0.82, 1.57 | 1.10 | 0.77, 1.56 |
| Spine Fx | 1.28 | 0.88, 1.85 | 1.15 | 0.76, 1.74 |
| Humerus Fx | 1.07 | 0.63, 1.81 | 0.77 | 0.43, 1.38 |
| Any nonosteoporotic Fx | 1.06 | 0.88, 1.28 | 0.97 | 0.78, 1.20 |
The ORs are derived from conditional logistic regressions with age and gender as stratification variables.
CI=confidence interval; Fx=fracture
Adjusted for prior myocardial infarction, hypertension, hyperlipidemia, diabetes, smoking, BMI, Charlson comorbidity index, coronary heart disease, CABG, PCI and nonosteoporotic fracture.
All fracture types were included in one multivariable model.
Heart failure and subsequent fracture risk
Follow-up data were available for 1908 (99%) subjects; the overall mean (SD) follow-up was 7.5 (6.1) years. The risk of death was substantially greater in heart failure patients (adjusted HR: 2.40; 95% CI: 2.03, 2.83) than in controls. Nonetheless, 444 individuals (195 cases and 249 controls) developed osteoporotic fractures. The incidence rates of osteoporotic fracture per 1,000 person years were 40.1 for heart failure cases versus 32.6 for controls (P = 0.04). Although not statistically significant at the 5% level, the overall adjusted HR for osteoporotic fracture (1.32; 95% CI: 0.98, 1.79; P = 0.07) was elevated in cases compared with controls (Table 4). Evaluation of specific fracture types as separate outcomes again revealed an increased risk of hip fracture (adjusted HR: 1.58; 95% CI: 1.03, 2.41) among heart failure patients. In addition, the risk of humerus fractures was higher in heart failure patients compared with controls (unadjusted HR: 3.20; 95% CI: 1.17, 8.74), however it was based on 44 cases only. No additional associations were detected for any other fracture types (Table 4). The association between heart failure and hip fracture risk did not differ materially between women (adjusted HR: 1.45; 95% CI: 0.89, 2.36) and men (adjusted HR: 1.73; 95% CI: 0.43, 6.92) (P for heterogeneity in HRs = 0.81) and was largely constant over the follow-up period (r = −0.005 for correlation of the hazard function with time).
Table 4.
Hazard Ratios (HRs) for Subsequent Fracture (Fx) Associated With Heart Failure Status, Overall and By Fracture Type, Among Olmsted County, Minnesota, Residents With Heart Failure First Diagnosed in 1979-2002, Compared to Age- and Sex-Matched Community Controls
| Outcome | No. of Events |
Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
| Any osteoporotic Fx | 444 | 1.33 | 1.03, 1.72 | 1.32 | 0.98, 1.79 |
| Specific Fracture Types | |||||
| Hip Fx | 248 | 1.47 | 1.05, 2.06 | 1.58 | 1.03, 2.41 |
| Forearm Fx | 85 | 1.05 | 0.58, 1.88 | 0.68 | 0.28, 1.67 |
| Spine Fx | 159 | 1.22 | 0.81, 1.84 | 0.99 | 0.59, 1.67 |
| Humerus Fx | 44 | 3.20 | 1.17, 8.74 | -b | |
| Any nonosteoporotic Fx | 467 | 1.09 | 0.86, 1.38 | 1.22 | 0.93, 1.62 |
The HRs are derived from stratified Cox proportional hazards models with the matched pairs forming the strata.
CI=confidence interval; Fx=fracture
Adjusted for prior osteoporotic fracture, prior nonosteoporotic fracture, smoking, diabetes, hypertension, hyperlipidemia, BMI, and Charlson comorbidity index.
Coefficient did not converge.
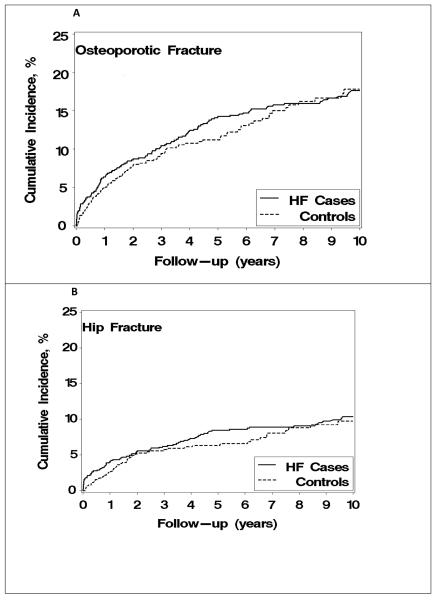
As a complementary analytical approach, we further accounted for death as a competing risk and observed a higher fracture incidence in heart failure patients compared with controls. Again, this difference was largely driven by hip fractures (Figure 1).
Figure 1.
Cumulative incidence of any osteoporotic fracture (Panel A) or hip fracture specifically (Panel B) among Olmsted County, Minnesota, residents with heart failure (HF) first diagnosed in 1979-2002, compared to age- and sex-matched community controls, with death considered a competing event.
DISCUSSION
In this community study, we have shown that newly diagnosed heart failure patients have a higher adjusted prevalence of prior osteoporotic fractures. These patients also experienced a higher adjusted risk of fractures after the heart failure diagnosis, although this association was of borderline statistical significance. Importantly, however, this excess burden of fractures was largely restricted to hip fractures, which both preceded and followed heart failure at significantly higher than expected rates.
Our findings are consistent with a previous study of Swedish women showing that among 1,327 incident hip fracture cases and 3,170 randomly selected population-based controls, the association between cardiovascular disease and hip fracture was more strongly related to heart failure and stroke than to ischemic heart disease.27 Likewise, a recent cohort study of 31,936 Swedish twins demonstrated a substantial increase in hip fracture risk after a diagnosis of heart failure and stroke and underscored the genetic component of the association 25 Furthermore, in a study of 16,294 elderly Canadian patients with cardiovascular disease followed for 1 year, a new diagnosis of heart failure was associated with a 6.3-fold higher risk of developing hip fracture, compared with other cardiovascular disease diagnoses.24 The authors concluded that “heart failure is a risk factor for orthopedic fracture.”24 However, this study relied on administrative data and is thus exposed to misclassification as acknowledged by the authors.24 Our study augments previous reports by examining, in a population-based cohort with rigorous ascertainment of exposure and outcomes, whether there is a higher prevalence of osteoporotic fractures both before and after the diagnosis of heart failure, suggesting a common underlying pathway for both disorders.
The actual basis for the association of heart failure with fracture is unclear. Although traditionally viewed as unrelated disorders of aging, several lines of evidence join both illnesses: 1) reduced BMD and increased bone turnover are associated with increased cardiovascular risk;1-6,7 ,8,9,39 2) cardiovascular risk factors such as vascular calcification, inflammation, and endothelial dysfunction10,11 are associated with low BMD and fractures;12-16 and 3) cardiovascular disease has been associated with an increased risk of falls40,41 and hip fractures,24-27 while others reported increased cardiovascular events in osteoporotic patients.1,3-5 It has also been suggested that the association could reflect shared risk factors, particularly estrogen deficiency, which has been linked to both coronary disease42 and osteoporosis,43 although more complex pathophysiological mechanisms may operate.17,18 In addition, traditional cardiovascular risk factors such as dyslipidemia, hypertension, smoking, and diabetes have been reported as associated to osteoporosis.18
None of these mechanistic possibilities could be directly evaluated in the present study. However, if the relationship was mediated through osteoporosis, one would have expected a stronger association with other fractures, especially vertebral fractures, than was seen here. Vertebral fractures, long considered the quintessential osteoporotic fracture,22 were not increased among heart failure patients despite the relatively complete ascertainment of such fractures in this data system.44 The other major factor in fracture etiology is trauma, especially falls. Distal forearm fractures are almost always due to falls, but such falls typically occur when relatively healthy people fall forward onto an outstretched arm.22,45 Forearm fractures were not increased among heart failure patients either. Conversely, falls leading to hip fractures, which were associated with heart failure, occur more often in frail individuals who fall over backwards or to the side and land on the hip.22,46 Given the excess burden of frailty among patients with heart failure,47,48 an important clinical implication of the present data is the need to focus on preventing falls in the heart failure population. Indeed, it was recently pointed out that falling rather than osteoporosis is the strongest single risk factor for fractures in the elderly, a risk that may be reduced by up to 50% by appropriate intervention.49
Some limitations of our study should be acknowledged to aid in data interpretation. As in any observational study, the observed associations could reflect residual confounding due to unmeasured variables or under-ascertainment of measured factors by medical record review. Data on the use of pharmacological treatments possibly related to fracture risk, such as bisphosphonates, corticosteroids, and specific diuretic agents, were not available, and measurements of BMD or biochemical markers of bone turnover were not routinely performed so the role of bone loss in fracture risk could not be examined. Moreover, we could not directly evaluate the many potential pathophysiologic mechanisms that might account for an association between heart failure and hip fracture. Lastly, since the study population was mainly white, these data need replication in other racial and ethnic groups.
The present investigation also has several strengths. We capitalized on the comprehensive data resources of the Rochester Epidemiology Project to examine osteoporotic fractures occurring before and after heart failure. We report on a large, population-based inception cohort registered at the time their heart failure was first confirmed by standardized criteria.32 The controls were randomly selected from an enumeration of the Olmsted County population, and therefore should have been representative of community residents generally.29 Furthermore, the clinical characteristics were recorded prior to any knowledge of resulting fracture outcomes, which were documented in the detailed inpatient and outpatient medical records that spanned each subject's entire period of residency in the community. Finally, fracture ascertainment should be nearly complete since the vast majority come to medical attention either directly or indirectly.44
In conclusion, we found that prior fracture is associated with heart failure at least as strongly as heart failure is associated with subsequent fracture. In both instances, the increased risk is driven by hip fractures rather than other types of fractures. Hip fracture commonly results from falls in frail individuals, suggesting that fracture prevention in elderly patients with heart failure should aim more directly at reducing falls.
ACKNOWLEDGMENTS
The authors are indebted to Ruoxiang (Rochelle) Jiang for computer programming and data analysis, Mary G. Roberts and Kristie K. Shorter for administrative assistance, and Dr. Walter K. Kremers for statistical consultation.
Funding Sources: This work was supported by grants from the National Institutes of Health (R01 AR30582 and P01 AG04875 to L.J.M.; R01 HL59205 and R01 HL72435 to V.L.R.). Dr. Roger is an Established Investigator of the American Heart Association. The funding sources played no role in the design, conduct, or reporting of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict Of Interest: None.
REFERENCES
- 1.Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR, for the Study of Osteoporotic Fractures Research Group. Association between low bone density and stroke in elderly women The Study of Osteoporotic Fractures. Stroke. 1993;24:940–946. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 2.Browner WS, Seeley DG, Vogt TM, Cummings SR, for the Study of Osteoporotic Fractures Research Group Non-trauma mortality in elderly women with low bone mineral density. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 3.Farhat GN, Newman AB, Sutton-Tyrrell K, et al. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18:999–1008. doi: 10.1007/s00198-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 4.Samelson EJ, Kiel DP, Broe KE, et al. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 5.Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12:259–265. doi: 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- 7.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 8.Johansson C, Black D, Johnell O, Odén A, Mellström D. Bone mineral density is a predictor of survival. Calcif Tissue Int. 1998;63:190–196. doi: 10.1007/s002239900513. [DOI] [PubMed] [Google Scholar]
- 9.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study. Ann Epidemiol. 2003;13:692–697. doi: 10.1016/s1047-2797(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 10.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, Kauppila LI, O'Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 12.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 13.Sanada M, Taguchi A, Higashi Y, et al. Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis. 2004;176:387–392. doi: 10.1016/j.atherosclerosis.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Koh JM, Khang YH, Jung CH, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;16:1263–1271. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 15.Chow JT, Khosla S, Melton LJ, 3rd, Atkinson EJ, Camp JJ, Kearns AE. Abdominal aortic calcification, BMD, and bone microstructure: a population-based study. J Bone Miner Res. 2008;23:1601–1612. doi: 10.1359/JBMR.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 19.Anagnostis P, Karagiannis A, Kakafika AI, Tziomalos K, Athyros VG, Mikhailidis DP. Atherosclerosis and osteoporosis: age-dependent degenerative processes or related entities? Osteoporos Int. 2009;20:197–207. doi: 10.1007/s00198-008-0648-5. [DOI] [PubMed] [Google Scholar]
- 20.Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A. Role of vascular factors in osteoporosis. J Gerontol A Biol Sci Med Sci. 2003;58:362–366. doi: 10.1093/gerona/58.4.m362. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard P, Rejnmark L, Mosekilde L. Hypertension is a risk factor for fractures. Calcif Tissue Int. 2009;84:103–111. doi: 10.1007/s00223-008-9198-2. [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 23.Sinnott B, Syed I, Sevrukov A, Barengolts E. Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int. 2006;78:195–202. doi: 10.1007/s00223-005-0244-z. [DOI] [PubMed] [Google Scholar]
- 24.van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA. Heart failure is a risk factor for orthopedic fracture: a population-based analysis of 16,294 patients. Circulation. 2008;118:1946–1952. doi: 10.1161/CIRCULATIONAHA.108.784009. [DOI] [PubMed] [Google Scholar]
- 25.Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 26.Collins TC, Ewing SK, Diem SJ, et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaelsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–1362. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 28.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 30.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 31.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 32.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 33.Sanders KM, Pasco JA, Ugoni AM, et al. The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: the Geelong Osteoporosis Study. J Bone Miner Res. 1998;13:1337–1342. doi: 10.1359/jbmr.1998.13.8.1337. [DOI] [PubMed] [Google Scholar]
- 34.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 35.Anonymous Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons, Inc.; New York: 1989. [Google Scholar]
- 38.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Szulc P, Samelson EJ, Kiel DP, Delmas PD. Increased bone resorption is associated with increased risk of cardiovascular events in men: the MINOS study. J Bone Miner Res. 2009;24:2023–2031. doi: 10.1359/JBMR.090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerse N, Parag V, Feigin VL, et al. Falls after stroke: results from the Auckland Regional Community Stroke (ARCOS) Study, 2002 to 2003. Stroke. 2008;39:1890–1893. doi: 10.1161/STROKEAHA.107.509885. [DOI] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Weiss TW, McHorney CA, Miller PD, Siris ES. Predictors of falls among postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA) Osteoporos Int. 2009;20:715–722. doi: 10.1007/s00198-008-0748-2. [DOI] [PubMed] [Google Scholar]
- 42.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 43.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 44.Melton LJ, 3rd, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 45.Kelsey JL, Prill MM, Keegan TH, et al. Reducing the risk for distal forearm fracture: preserve bone mass, slow down, and don't fall! Osteoporos Int. 2005;16:681–690. doi: 10.1007/s00198-004-1745-8. [DOI] [PubMed] [Google Scholar]
- 46.Parkkari J, Kannus P, Palvanen M, et al. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int. 1999;65:183–187. doi: 10.1007/s002239900679. [DOI] [PubMed] [Google Scholar]
- 47.Singh M, Alexander K, Roger VL, et al. Frailty and its potential relevance to cardiovascular care. Mayo Clin Proc. 2008;83:1146–1153. doi: 10.4065/83.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 49.Järvinen TLN, Sievänen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336:124–126. doi: 10.1136/bmj.39428.470752.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]