Abstract
Leishmania parasites, which afflict 12 million people in 88 countries, exist as promastigotes transmitted by insect vectors and as amastigotes residing in mammalian macrophages. Promastigote cells arranged in rosettes have also been described but universally disregarded as a distinct stage in the life cycle. We present evidence that only rosettes of Leishmania major promastigotes express cell surface polysialic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA). Expression of rosette-specific PSA antigens was mosaic, with individual promastigotes expressing PSA, NeuPSA or both. A 50kDa protein was detected by Western blot analysis of a detergent-insoluble cell fraction with both PSA and NeuPSA-reactive antibodies. Frequencies of rosette formation as well as cell surface PSA/NeuPSA expression were temperature dependent. Rosettes also engaged in an unusual swarming behaviour, congregating into extended clusters. Distinct structures resembling cellular fusion bodies were formed in and released from rosettes. The results indicate that rosettes are an unrecognized stage in the life cycle of Leishmania. We hypothesize that rosettes initiate mating in Leishmania during which PSA/NeuPSA expression plays an important role. Recognizing rosettes as a distinct form of the Leishmania life cycle opens new possibilities for treatment or prevention of disease and, possibly, in vitro genetic recombination without passage of cells through insect vectors.
Keywords: carbohydrate, polysialic acid, aggregation, kinetoplastid
Leishmaniasis is a disease caused by diverse species of protozoan parasites afflicting 12 million individuals in addition to livestock and domesticated animals in 88 countries. The leishmanial parasite is digenetic, alternating between the promastigote form free-living in the gut of insect vectors and an intracellular amastigote form that resides in the phagolysosome of mammalian macrophages. Several promastigote morphologies have been reported, including procyclic, nectomonad, leptomonad, metacyclic, and haptomonad forms (Kamhawi 2006), as well as groups of promastigotes growing in clusters referred to as rosettes (Greenblatt et al. 1985; Noguchi and Tilden 1926; Trager 1953). To date, no specialized morphological forms of Leishmania that could be attributed to a sexual stage have been identified despite mounting evidence for a functional sexual cycle (Akopyants et al. 2009; Bastien et al. 1992; Chargui et al. 2009; Kelly et al. 1991; Yahiaoui et al. 1996). Consequently genetic manipulation of this organism in the laboratory remains limited. A more complete understanding of the parasite life cycle would greatly improve the ability to study the natural genetics of these organisms, and aid in the preparation of laboratory constructs with genetic traits useful for the development of vaccines and chemotherapeutic approaches to treatments for leishmanial infections.
Of the various morphological forms of Leishmania that have thus far been reported, rosettes have largely been ignored, despite their repeated observation in the midgut of insect vectors (Feliciangeli et al. 1988; Gontijo et al. 1995; Lainson and Shaw 1973; Leishman 1911). Rosettes have been considered characteristic of a “feeding frenzy” (Pommerville and Alcamo 2004), the result of delayed multiplication (Mehlhorn and Armstrong 2001), or an artifact of flagellar entanglement during in vitro cultivation (Mehlhorn and Armstrong 2001; Schuster and Sullivan 2002). However, rosettes share a striking resemblance to early mating stages of the green alga Chlamydomonas, in which cell-cell contact between opposite mating types is achieved through a process known as agglutination. Initial contact between mating individuals begins through the entanglement of their flagella, forming large clumps of rapidly twitching cells (Wilson 2008). This process is mediated by specific high molecular weight adhesion glycoproteins known as agglutinins located along the length of the flagella (Adair et al. 1982).
Recently, α 2,3- and α 2,6-linked sialic acids have been reported in the promastigote of Leishmania donovani in the insect vector (Chatterjee et al. 2003; Mukhopadhyay and Mandal 2006). However, the expression of poly α 2,8 N-acetyl neuraminic acid (PSA) or its partially de-N-acetylated derivative NeuPSA has not been reported for any leishmanial species. Our interest in investigating the presence of PSA and NeuPSA in human pathogens that express sialic acid antigens (Moe et al. 2009) has led us to the discovery that in Leishmania only promastigote cells organized in rosettes express PSA/NeuPSA. Therefore, rosettes may represent a distinct stage in the life cycle of these organisms. Additional studies to determine the culture conditions that promote rosette formation resulted in the observation of a previously undescribed swarming behavior by rosettes, and the generation of unusual structures corresponding to promastigote fusion bodies arising from rosettes. We discuss the implications of these results with regard to a sexual stage in Leishmania.
MATERIALS AND METHODS
Monoclonal antibodies (mAbs)
The murine mAbs SEAM 2 (IgG3) and SEAM 12 (IgG2a) were produced using N-propionyl Neisseria meningitidis group B capsular polysaccharide (N-Pr PSA)-tetanus toxoid conjugate vaccine (Granoff et al. 1998). Poly α 2,8 N-acetyl neuraminic acid (PSA) is chemically identical to long chain polysialic acid (PSA) expressed by humans during fetal development (Finne et al. 1983b). The mAb 2-1-B (IgM) was produced by immunizing mice with live N. meningitidis group B bacteria (Mandrell and Zollinger 1982). Both SEAM 12 (Granoff et al. 1998) and 2-1-B (Mandrell and Zollinger 1982) are specific for PSA. SEAM 2 is specific for poly α 2,8 N-acyl neuraminic acid that contains 40--60% de-N-acetyl neuraminic acid residues (Moe et al. 2009). All three mAbs are only reactive with PSA derivatives having a degree of polymerization (Dp) greater than ~10. Anti-leishmanial major surface protease (anti-gp63) was obtained from Cedarlane Laboratories, Ontario, CANADA. Irrelevant murine IgG2a and IgG3 mAbs used as negative controls were obtained from Southern Biotech, Birmingham, AL. Control IgM was obtained from Abcam, Cambridge, MA. Goat anti-mouse class or subclass-specific secondary antibodies conjugated to FITC, AlexaFluor 488, or AlexaFluor 546 were obtained from Invitrogen, Carlsbad, CA.
Specific monoclonal (mAb) antibody inhibitors
Colominic acid (i.e. PSA) is a specific inhibitor of mAb 2-1-B and mAb SEAM 12 but not mAb SEAM 2 (Granoff et al. 1998). N-Pr PSA is a specific inhibitor of mAb SEAM 2 and 12 but not mAb 2-1-B (Granoff et al. 1998) and N-TcAc PSA inhibits binding of mAb SEAM 2 but not mAb SEAM 12 or mAb 2-1-B (Moe et al. 2009).
Parasites
Promastigotes of L. major strain LV39 (also known as MRHO/SU/59/P) were obtained from Richard Titus, Colorado State University, and have been previously described (Neal 1964). Cultures were maintained in Dulbecco’s Modified Eagle Medium without bicarbonate (pH 7.0--7.4) and supplemented with 10% (v/v) heat inactivated fetal calf serum, 20 mM HEPES buffer, 0.01 mg/ml biotin, 2 mM Glutamax, 100 U/ml of Penicillin and 100 µg/ml Streptomycin, and 50 µM each of adenine, guanine, hypoxanthine, xanthine, and uracil. Promastigote cells can be cultured in this medium either in the absence or presence of 5% (v/v) exogenous CO2, which lowers the pH of the medium by approximately 0.5 pH units. All data presented in this study were generated for cells grown in ambient atmosphere without exogenous CO2. However, similar observations have also been made for cells grown in the presence of 5% (v/v) CO2.
Rosette Preparations
Log phase promastigote cultures were enriched for rosettes by multiple centrifugations at ~50 × g in 30 mM sucrose in PBS.
Immunofluorescence and Flow Cytometry
For examination of fusion bodies, cells were methanol/acetone fixed to glass slides as previously described (Das et al. 2001). For all other experiments, cells were fixed by a brief treatment with 1% (v/v) formalin. Cell preparations were blocked with 3% (v/v) goat serum, 30 mM sucrose in PBS for 1 h to overnight. All cell blocking, mAb binding, and washing steps were performed in the cold either at 4 °C or on ice. For some experiments cells were permeabilized by an additional 10-min incubation with 0.05% (v/v) Triton X-100, 30 mM sucrose in PBS. For anti-PSA derivative mAb inhibition of binding experiments, PSA derivatives specifically reactive with each mAb were pre-incubated with the primary mAb for 20 min prior to combining with cell preparation (final concentration 0.1 mg/ml each derivative). The SEAM 2 inhibitor, N-trichloroacetyl PSA (N-TcAc PSA), and SEAM 12 inhibitor (N-Pr PSA) were prepared as described previously (Moe et al. 2009). PSA and E. coli K1 capsular polysaccharide known as colominic acid were obtained from Sigma-Aldrich, Saint Louis, MO.
Confocal Microscopy
Following immunofluorescent labeling, cells were adhered to multi-well LabTek™ microscope slides, and a hardening mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) was applied. Confocal images were obtained using a Zeiss Meta510 confocal laser scanning microscope (The Biological Imaging Facility, University of California, Berkeley, CA) and were analyzed using either ImageJ Software (NIH) or Imaris (Bitplane, Inc., Saint Paul, MN). Control antibodies and secondary antibodies applied alone were routinely used to assess background fluorescence.
Quantification of 4',6-diamidino-2-phenylindole (DAPI) Staining Intensities
Estimates of relative DNA content were measured by quantification of DAPI staining for individual promastigotes and fusion bodies. Cultures were washed in PBS and methanol/acetone fixed to glass cover slips as previously described (Das et al. 2001) for 5-min on ice and allowed to rehydrate in 2X SSC buffer containing 0.16 µg/ml DAPI for 5-min at room temperature. Stained cover slips were mounted onto standard microscope slides with Fluoro-gel mounting medium and examined by laser scanning confocal and fluorescence microscopy. DAPI staining was quantified by pixel density integration using ImageJ (Abramoff et al. 2004). A total of 10 fusion bodies, 10 dividing cells and 50 individual promastigotes from 10 separate micrographs were examined including the example shown in Fig. 9. Fusion bodies were defined as a single clearly identifiable entity containing multiple foci. Dividing cells were defined as a single cell body containing two clearly identifiable nuclei and kinetoplastids. Fusion body and dividing cell fluorescence measurements were normalized relative to individual promastigote cells located near the fusion body or dividing cell and expressed as a ratio of fusion body to promastigote fluorescence. The distribution of fluorescence intensity within fusion bodies and individual promastigotes in multiple confocal and single fluorescence micrographs was analyzed using ImageJ surface plots.
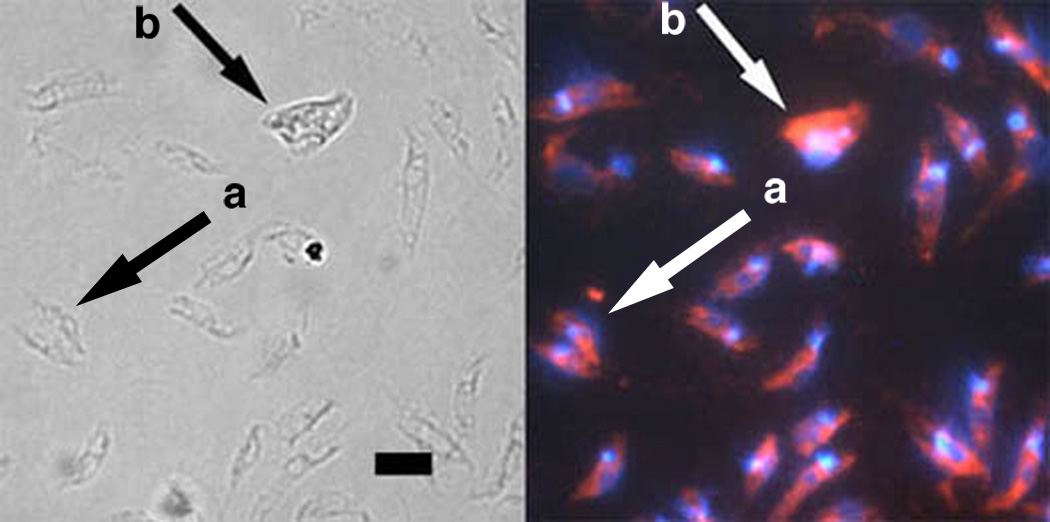
Fig. 9.
Labeling of fusion bodies by Leishmania specific anti–gp63 monoclonal antibody (mAb). Left panel, light microscopy; right panel, fluorescence microscopy. Numerous individual promastigotes are visible and contain a single nucleus and single kinetoplast stained with DAPI. A dividing cell (arrow a) and fusion body (arrow b) are shown along with individual promatigotes. All cells including the fusion body were detected by the Leishmania specific anti–gp63 mAb, demonstrating that fusion bodies are not artifacts or contaminants. Fusion bodies contain multiple DAPI staining foci as expected by the fusion of two or more leishmanial promastigotes. The ratio of the integrated pixel density of DAPI DNA staining in the dividing cell and fusion body compared to five individual promastigotes in the same micrograph were 1.88 and 2.70, respectively. Scale bar, 10 µm.
Rosette Aggegration
Promastigote cultures were grown for 4 weeks at 20 °C. Clusters of ≥4 promastigote cells oriented with their flagella toward the center of the cluster were considered to be rosettes. Two methodologies were employed to demonstrate rosette aggregation. In one approach, a levelled Petri dish containing a mid-log phase culture was photographed at intervals for 72 h to record macroscopic changes in cell density. Changes in cell density were further examined under lower power magnification and observed to involve alterations in the distribution of rosettes in the culture from an initially random arrangement to distinct regions of higher and lower rosette densities. Higher regions of rosette densities initially formed circular to elipsoidal patterns which in later times converged.
Alternatively, 10-fold concentrates of mid-log cultures were videotaped by Audio Visual Consultants, Oakland, CA. A 100x condensed movie of the first 1.5 h of recording was generated using Adobe Premiere Pro. The cell suspension was observed for macroscopic changes in cell density during this period.
Rosette-rosette Adhesion
Live promastigote cultures were placed in a 25 cm2 tissue culture flask and examined on an Olympus IX70 microscope. Exposures were taken every 5 min using a Roper CoolSNAP FX camera and a 1200x movie generated using InVIVO software. Rosettes were considered to be adhered to one another when they attained physical contact and subsequently compressed into a denser mass. Other cells were observed to flow by during this process indicating that the rosette clusters remained attached, in resistance to the flow of the culture medium.
Detergent Extraction and Western Blotting of Poly α 2,8 N-acetyl neuraminic acid (PSA) Bound Protein
To detect PSA/NeuPSA modified proteins, rosette preparations were extracted with Triton X-114 as previously described (Murray et al. 1989). Proteins in the cell pellet, buffer soluble proteins, and detergent soluble proteins were separated by electrophoresis on 4--20% gradient polyacrylamide SDS reducing gels. Western blots were performed after transferring the proteins to a polyvinylidene fluoride (PVDF) membrane. Bound mAbs were detected using horseradish peroxidase-conjugated secondary Abs. To demonstrate specificity of binding in Western blots using SEAM 12, 100 µg/ml of N-Pr PSA was added with the mAb.
RESULTS
Poly α 2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA) expression by Leishmania promastigotes
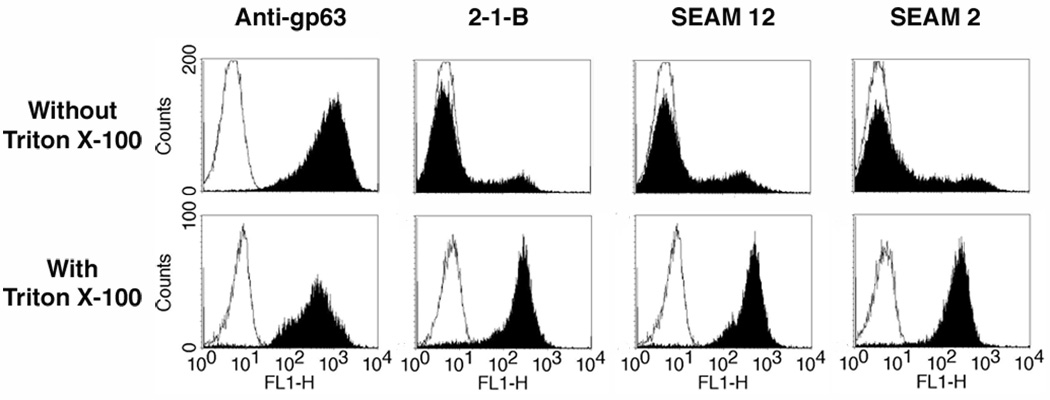
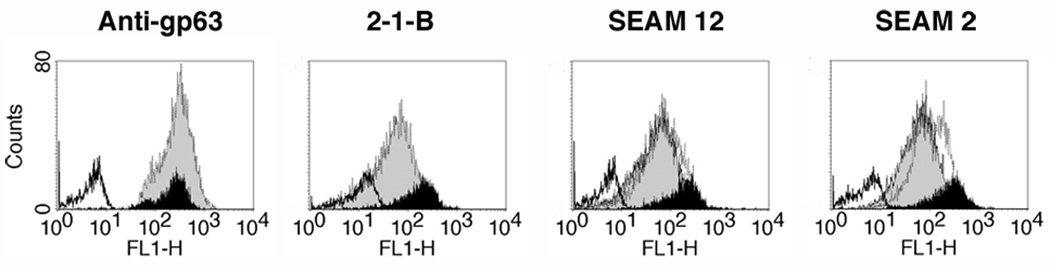
Initial experiments to detect PSA and NeuPSA in L. major were conducted on promastigotes grown at 24--26 °C. Fig. 1 (upper panel) shows the expression of PSA and NeuPSA as detected by flow cytometry in intact cells (i.e. no permeabization with Triton X-100) by reactivity with the anti-PSA mAbs 2-1-B and SEAM 12 and anti-NeuPSA mAb SEAM 2. The PSA/NeuPSA positive promastigote populations represented approximately 11% and 24%, respectively, of the total number of cells compared to nearly 100% of cells that were positive for expression of the cell surface protease gp63. When the cells were permeabilized by brief exposure to Triton X-100 however (Fig. 1, lower panel), the fraction of PSA/NeuPSA-positive cells increased to nearly all cells. The result suggests that all cells contain PSA but only a fraction of them express PSA or NeuPSA antigens on the cell surface. Interestingly, the mAbs SEAM 3 and SEAM 18, which recognize short (Dp~4) NeuPSA and PSA epitopes respectively, were not reactive with Leishmania (data not shown).
Fig. 1.
Detection by monoclonal antibodies (mAb) of Poly α 2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA) in Leishmania major. Promastigote cells were labeled with each mAb indicated and examined by flow cytometry. Upper panel, binding without Triton X-100 exposure to detect only surface-associated antigens; lower panel, mAb binding after a 10 min Triton X-100 exposure to detect both cytoplasmic and surface-associated antigens. Black fill, binding of indicated mAb; white fill, irrelevant isotype matched control mAbs. Results indicate that nearly all leishmanial promastigotes possess PSA/NeuPSA internally, but only a small fraction of the population expresses these antigens on their surface when grown at 24--26 °C. In contrast, nearly all cells express cell surface gp63 under the same culture conditions.
Since only a fraction of intact cells were positive for PSA/NeuPSA expression, we examined cultures of promastigotes that were differentially labeled with anti-PSA (2-1-B) and NeuPSA (SEAM 2) mAbs by confocal microscopy. The expression of PSA/NeuPSA differed within individual rosette-associated promastigotes: some expressed only PSA (Fig. 2B) or NeuPSA (Fig. 2C) and others expressing both (Fig. 2D). Free-swimming promastigotes not associated with rosettes were negative for binding by both mAbs. For example, Fig. 3B–C shows a mixture of rosettes and individual promastigotes where only promastigotes in rosettes were labeled with 2-1-B. Once rosettes were identified as the source of PSA/NeuPSA antigens, cell preparations were enriched for rosettes by differential centrifugation for subsequent binding experiments.
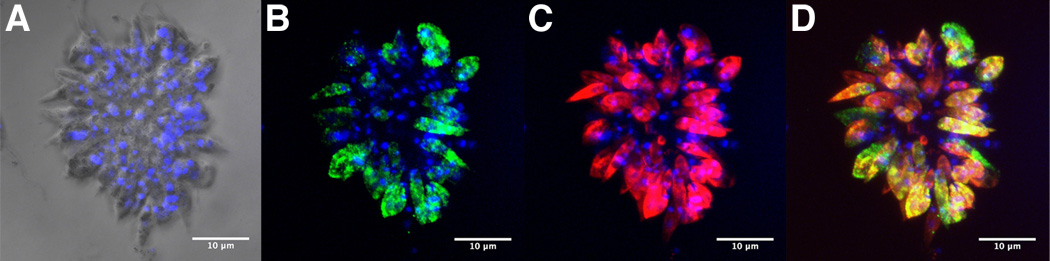
Fig. 2.
Confocal microscopic examination of Leishmania major rosettes labeled with Poly α 2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA). A, light micrograph with DAPI-fluorescent labeling overlay; B, anti-PSA mAb 2-1-B/DAPI labeling; C, anti-NeuPSA mAb SEAM 2/DAPI labeling; D, composite of mAb 2-1-B/mAb SEAM 2/DAPI labeling. Individual promastigote cells within the rosette expressing PSA, NeuPSA or both can be observed. Scale bars, 10 µm.
Fig. 3.
Confocal microscopic examination of Leishmania major culture containing a mixture of rosettes and individual promastigotes labeled with the anti-Poly α 2,8 N-acetyl neuraminic acid (PSA) mAb 2-1-B. A, light micrograph of a mixture of individual promastigotes and promastigotes within rosettes; B, PSA labeling of promastigotes shown in A with 2-1-B; C, composite of A and B. Only promastigote cells within rosettes express PSA. Scale bars, 20 µm.
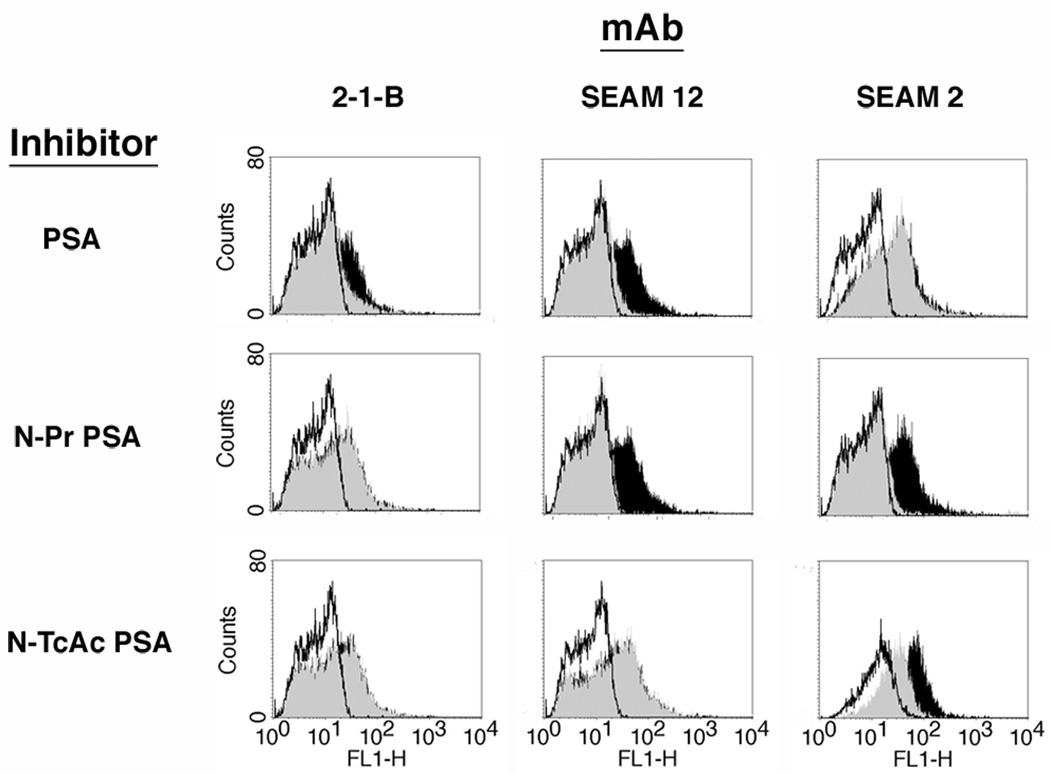
To eliminate the possibility that the observed binding of anti-PSA derivative mAbs to promastigotes was the result of low avidity, non-specific absorption, we compared binding of the mAbs in the absence or presence of specific inhibitors (Fig. 4). Three PSA derivatives - colominic acid, N-Pr PSA, and N-TcAc PSA - were tested. Binding of each mAb to the parasites was inhibited by the specific polysaccharide inhibitor as indicated by the shift in fluorescence in the absence of inhibitor (black fill), compared to in the presence of inhibitor (gray fill) but not by derivatives known to be non-reactive with each particular mAb.
Fig. 4.
Specific inhibition of anti-Poly α 2,8 N-acetyl neuraminic acid (anti-PSA) and anti-PSA containing de-N-acetyl neuraminic acid (anti-NeuPSA) mAb binding to leishmanial rosettes. Monoclonal antibodies (mAbs) were preincubated with each inhibitor for 20 min and subsequently added to rosette preparations. Poly α 2,8 N-acetyl neuraminic acid (PSA) is reactive with mAbs 2-1-B and SEAM 12 but not mAb SEAM 2; N-propionyl PSA (N-Pr PSA) is reactive with mAbs SEAM 12 and SEAM 2 but not mAb 2-1-B; N-trichloroacetyl PSA (N-TcAc PSA) is reactive only with mAb SEAM 2. Labeled cells were examined by flow cytometry. Black fill, indicates mAb binding; gray fill, indicates mAb signal in the presence of specific inhibitor; white fill, irrelevant isotype matched control mAb. Specific inhibition of each anti-PSA and anti-NeuPSA mAb is observed.
Temperature Dependency of Poly α 2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA) Cell Surface Expression
The effect of temperature on the expression of cell surface PSA or NeuPSA was investigated by culturing leishmanial cells at different temperatures, then measuring anti-PSA/NeuPSA mAb binding by flow cytometry. Cultures of promastigotes were maintained at 16 °C, 20 °C, or 24 °C for at least 4 weeks prior to their evaluation. The frequency of rosette formation was observed to be greatly enhanced when leishmanial cells were grown at 20°C. Cell cultures were subsequently harvested, enriched for rosettes by differential centrifugation, and expression of cell surface antigens was measured by flow cytometry using anti-PSA (SEAM 12 and 2-1-B), anti-NeuPSA (SEAM 2), and anti-gp63 primary antibodies. The maximum fluorescence for both anti-PSA and -NeuPSA binding occurs when cells were cultured at 20°C (black fill) and is lower for cells cultured at 16°C (dotted line, white fill) or 24°C (dotted line, gray fill) (Fig. 5). In contrast there is very little variation of gp63 cell surface expression as a function of the cell culture temperature within this temperature range (Fig. 5). When the samples were gated on individual promastigote cells, PSA was undetectable at all temperatures examined, whereas gp63 expression did not differ with growth temperature (data not shown). The addition of exogenous PSA/NeuPSA precursors N-acetyl neuraminic acid and N-acetyl mannosamine to growth media at concentrations up to 10 mM did not influence rosette formation or PSA/NeuPSA expression (data not shown).
Fig. 5.
Temperature dependency of cell surface expression of Poly α 2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA) by leishmanial rosettes. Cells were cultivated at 16 °C (dotted line, white fill), 20 °C (black fill), and 24 °C (dotted line, gray fill) for at least 4 weeks prior to labeling. Irrelevant isotype matched control mAb (solid line, white fill, signal intensity ≤10). Maximal cell surface expression of PSA/NeuPSA occurs when promastigotes were cultured at 20 °C.
Detergent Extraction of Rosettes and Western Blot Analysis
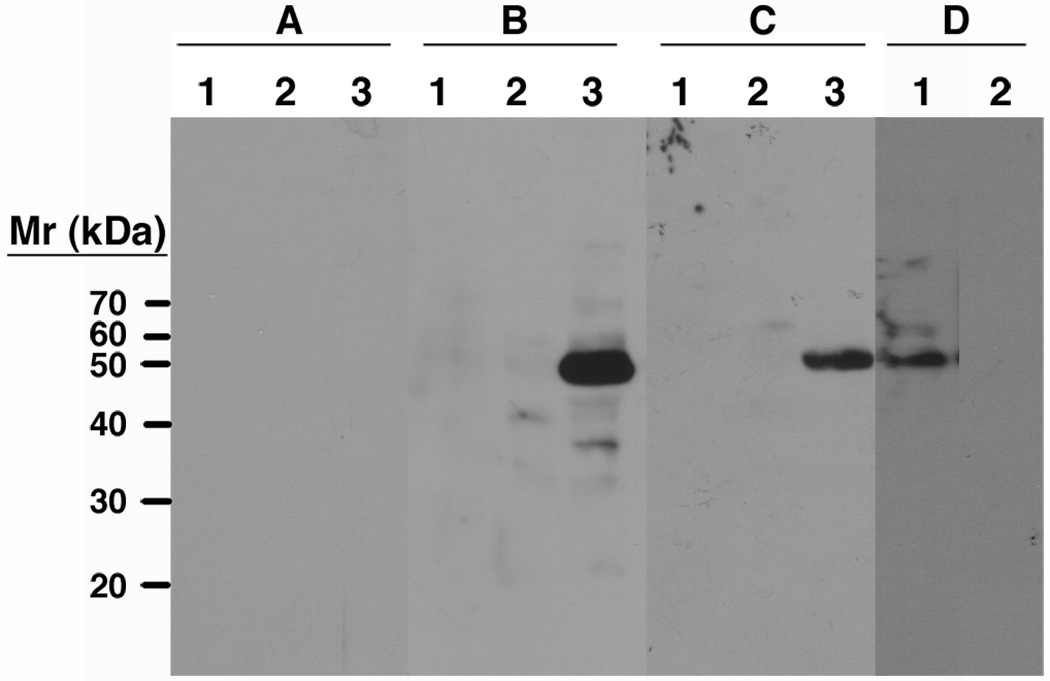
The cell fractions from Leishmania rosettes extracted with Triton X-114 were examined by SDS polyacrylamide gel electrophoresis and Western blot using SEAM 2 and SEAM 12 as primary detection antibodies. A single band with an apparent mass of approximately 50 kDa was identified only in the detergent insoluble cell pellet fraction by SEAM 12 (panel B, lane 3, Fig. 6) and SEAM 2 (panel C, lane 3, Fig. 6), whereas isotype control antibodies were completely negative (panel A). A Western blot of a second cell pellet preparation with detection by SEAM 12 in the absence (lane 1) or presence (lane 2) of soluble polysaccharide inhibitor N-Pr PSA demonstrates specificity of binding (Fig. 6D). Antibody 2-1-B identifies the same 50 kDa band in the detergent insoluble cell pellet fraction as well as higher molecular weight bands in the detergent soluble fraction (data not shown). These results suggest that a single protein associated with the detergent insoluble cell pellet fraction is modified with PSA and that some portion of the PSA is further modified by extensive de-N-acetylation.
Fig. 6.
Western blot of Triton X-114 fractionated leishmanial rosette preparations. A, irrelevant mAb control; B, mAb SEAM 12; C, mAb SEAM 2. Lane 1, Triton X-114 fraction; lane 2, buffer fraction; lane 3, detergent insoluble cell pellet fraction; D, detergent insoluble cell pellet fraction with SEAM 12 in the absence (lane 1) or presence (lane 2) of 100 µg/ml N-Pr PSA as a specific inhibitor of SEAM 12 binding. A single band of approximately 50kDa from the detergent insoluble cell pellet is identified by anti-PSA/NeuPSA and its detection specifically inhibited by N-Pr PSA.
Rosette Formation
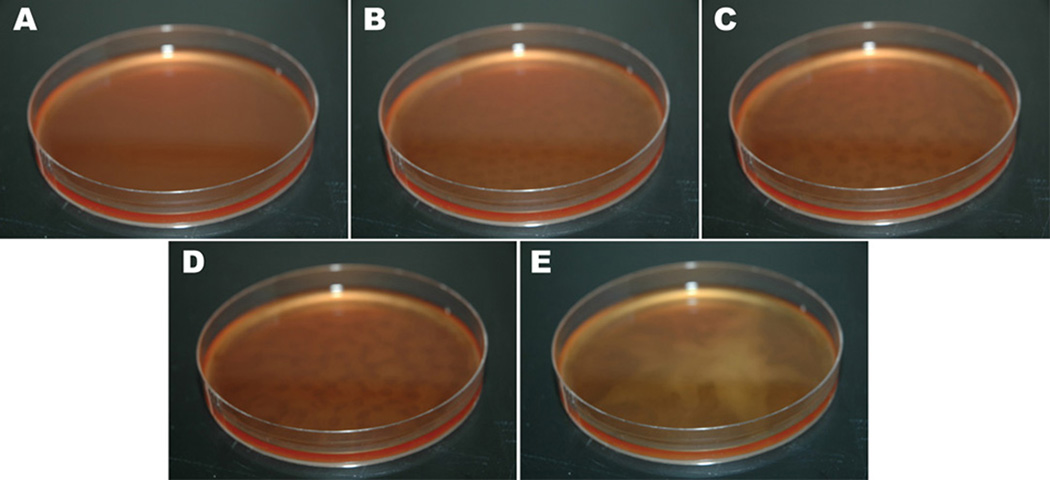
When Leishmania promastigotes were cultured at 20 °C, rosette formation was greatly enhanced compared to higher or lower temperatures. At this cultivation temperature, rosettes frequently formed nearly confluent lawns along the entire bottom of the culture flask and also arranged themselves in meandering chains on the surface of the growth medium. In addition, the rosettes engaged in a swarming behavior. In Fig. 7A, homogeneous suspension of mid-log leishmanial cells is observable at time zero. When left undisturbed, within 2 h the cell suspension no longer had the appearance of confluency. Rather regions of higher and lower cell concentration began to appear, giving the cell suspension a leopard-like speckled appearance (Fig. 7B--D). Examination of the culture under low power magnification revealed that the clear areas were deficient in rosettes, which had concentrated themselves along the perimeters of the cleared regions. By 4 h after plating (Fig. 7C), the cleared regions were seen to increase in diameter and by 8 h coalesced (Fig. 7D). The migrating rosettes ultimately formed a star-shaped aggregate near the center of the plate between 20--30 h after plating (Fig. 7E). This process was accelerated by preparing higher concentrations of cells as described in the Methods section (Supplemental Quicktime® Movie 1).
Fig. 7.
Observation of rosette swarming in Leishmania. A, 0 h; B, 2 h; C, 4 h; D, 8 h; E, 30 h. At 0 h, a confluent cell suspension is observable. At 2 h, a spotty appearance is detectable corresponding to regions of higher and lower cell density. These elongate at 4 h and begin to converge at 8 h. Cells eventually congregate into a star-like appearance in the center of the Petri dish.
During the aggregation process, rosettes were observed under the microscope to be migrating toward one another. The movement of rosettes to form larger clusters appeared to involve active migration, but alternatively may result from random swimming behavior. When rosettes achieved direct contact, they remained attached, becoming part of a larger collective and did not disassociate. A movie documenting rosette-rosette adhesion at the microscopic level has also been generated by time-lapse photography (Supplemental Quicktime® Movie 2).
Rosettes cultured at 20 °C also gave rise to unusual structures resembling the fusion of 2 or more promastigotes within a single rosette (Fig. 8). They ranged in size corresponding to the fusion of 2, 3 or more promastigotes, and thus suggest a mechanism for the generation of recombinant cells of varying ploidy levels. The unique cellular structures are morphologically distinct from individual promastigotes, amastigotes or rosettes, and have not been previously described. Often, one to several flagella can be detected in the early stages of these structures. In later stages, the fusion products develop a spherical-like appearance. They do not have the morphology of any common laboratory contaminants, such as bacteria, yeast, filamentous fungi or algae. Furthermore, they are recognized by the leishmanial specific anti-gp63 antibody (Fig. 9B) and are transient, being no longer observable upon passage of the leishmanial culture into fresh growth medium, features inconsistent with contaminating organisms as an explanation.
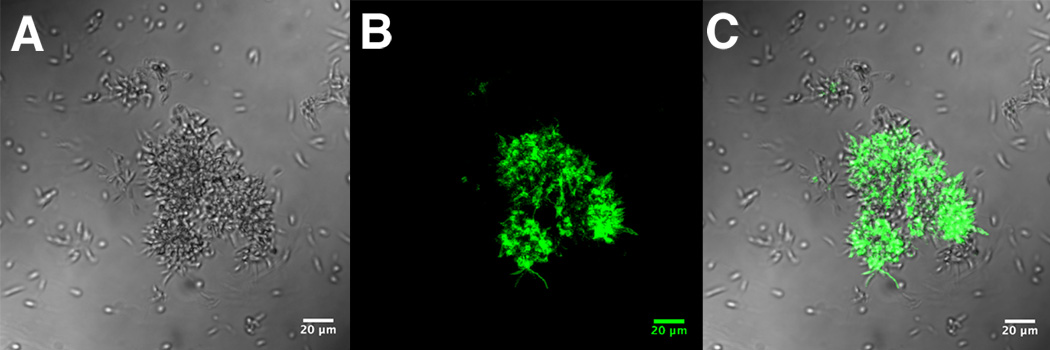
Fig. 8.
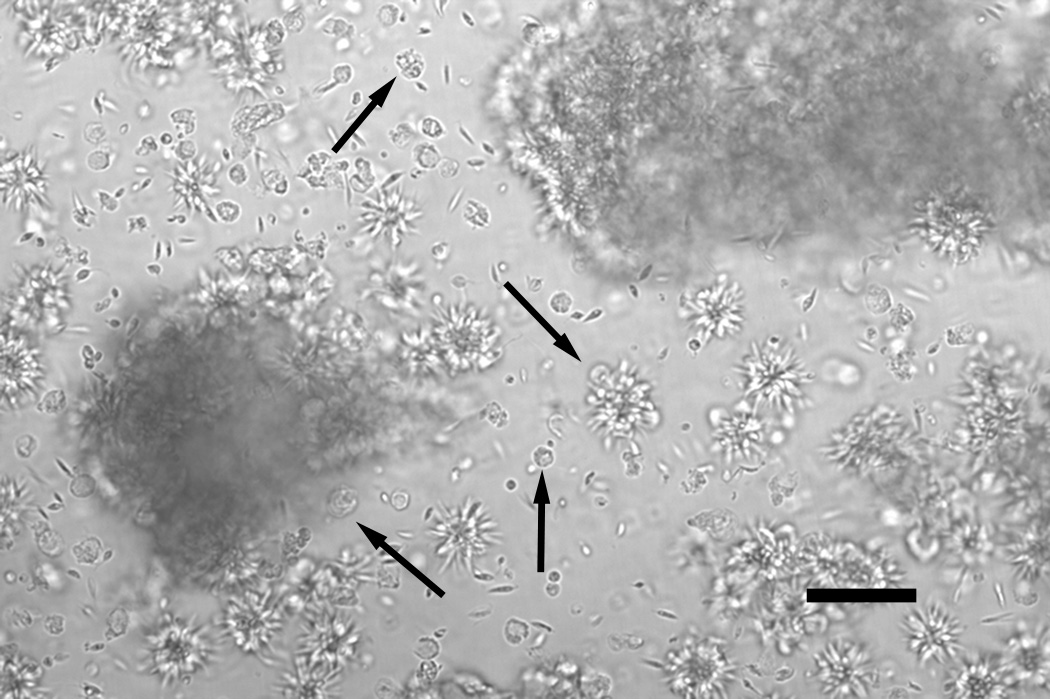
Generation of fusion bodies from leishmanial rosettes. Micrograph taken of a live, unmanipulated culture. Individual as well as large aggregates of rosettes can be seen. Center downward arrow indicates 2 spherical fusion bodies forming on a single rosette. Additional arrows indicate numerous individual fusion bodies that have been released from rosettes. Note the size and morphology compared to nearby promastigote cells. These morphologies are distinct from any previously described leishmanial cellular structures, such as amastigotes, promastigotes or rosettes. Scale bar: 50 µm.
Relative DNA content as measured by integrated pixel density from DAPI fluorescence was used to quantify differences between individual promastigotes, dividing cells, and fusion bodies. Results were normalized relative to individual promastigote cells and expressed as a ratio of average individual promastigote fluorescence to dividing cell or fusion body fluorescence. The ratio of DAPI staining intensity of 10 fusion bodies measured ranged from 2.24 to 2.97 with a mean of 2.70 and a standard deviation of 0.30. In comparison, the ratio of DAPI staining intensity of 10 dividing cells ranged from 1.22 to 1.91 with a mean of 1.57 and a standard deviation of 0.23. The P value calculated by Student’s T Test for two tailed unpaired data sets was less than 0.001 and there was no significant difference in the variance within data sets as determined by an F Test. For example, the ratios for the dividing cell and fusion body shown in Fig. 9B were 1.88 and 2.70, respectively. Surface plots of the integrated fluorescence pixel density typically showed two or more intensely stained foci along the perimeter of the fusion body in addition to a single, diffusely stained, centrally located focus. Foci on the perimeter appeared to be DAPI staining of kinetoplastid DNA (Fig. 9B).
DISCUSSION
Sialylated glycans have an important role in determining immune recognition of self versus non-self antigens in humans (Crocker et al. 2007). Possibly as a consequence, more than 40 human pathogens are known to express sialyated glycans or interact with host sialylated antigens as a means of invasion or evading protective immune responses (Angata and Varki 2002). However, there are only a few human polysialylated glycoproteins and no known PSA-containing glycans in insects (Koles et al. 2009). The most abundant PSA glycan in humans is polysialylated neural cell adhesion molecule (PSA-NCAM), which is highly expressed in brain, kidney, and heart tissues during fetal development (Finne et al. 1983a). The bacterial pathogens Escherichia coli K1 and Neisseria meningitidis group B express PSA capsular polysaccharides that are important virulence factors for evading immune mechanisms of bacterial clearance. Recently, our group showed that N. meningitidis group B and C strains express antigens reactive with NeuPSA antibodies (Moe et al. 2009). Based on reactivity with lectins and sensitivity to exoneuraminidases, it was recently reported that Leishmania also express α 2,3- and α 2,6-sialylated glycans (Mukhopadhyay and Mandal 2006). We now report that Leishmania express both PSA- and NeuPSA-containing glycans and demonstrate their specific association with promastigote cells arranged in rosettes, structures that have previously been considered to be artifacts and have therefore have been universally ignored. The presence of cell surface PSA/NeuPSA was demonstrated to occur in a temperature-dependent manner that corresponds to maximal rosette formation. The specificity of mAbs identifying the PSA/NeuPSA antigens was demonstrated by inhibition of binding by specific PSA derivatives. Furthermore, analysis of Triton X-114-extracted rosettes by SDS-PAGE and Western blot showed that the anti-PSA and NeuPSA mAbs were reactive with a specific protein having an apparent mass of 50 kDa. Identification of this protein by 2D gel electrophoresis and Matrix Assisted Laser Desorption-Ionization Time of Flight (MALDI-TOF) mass fingerprint analysis is currently in progress.
The temperature at which the insect vector phase of Leishmania is cultivated was found to have a significant effect on both the frequency of rosette formation and PSA/NeuPSA cell surface expression. Rosette formation and cell surface PSA/NeuPSA detection were enhanced when promastigotes were cultured at 20°C. Most laboratories routinely propagate the insect vector phase of these organisms at or above 25 °C, and ranging as high as 28--30°C (Ozgoztasi and Baydar 1997; Trager 1953; Vieira et al. 1994; Vieira et al. 2002). At these higher temperatures, rosette formation and PSA/NeuPSA cell surface expression were greatly reduced.
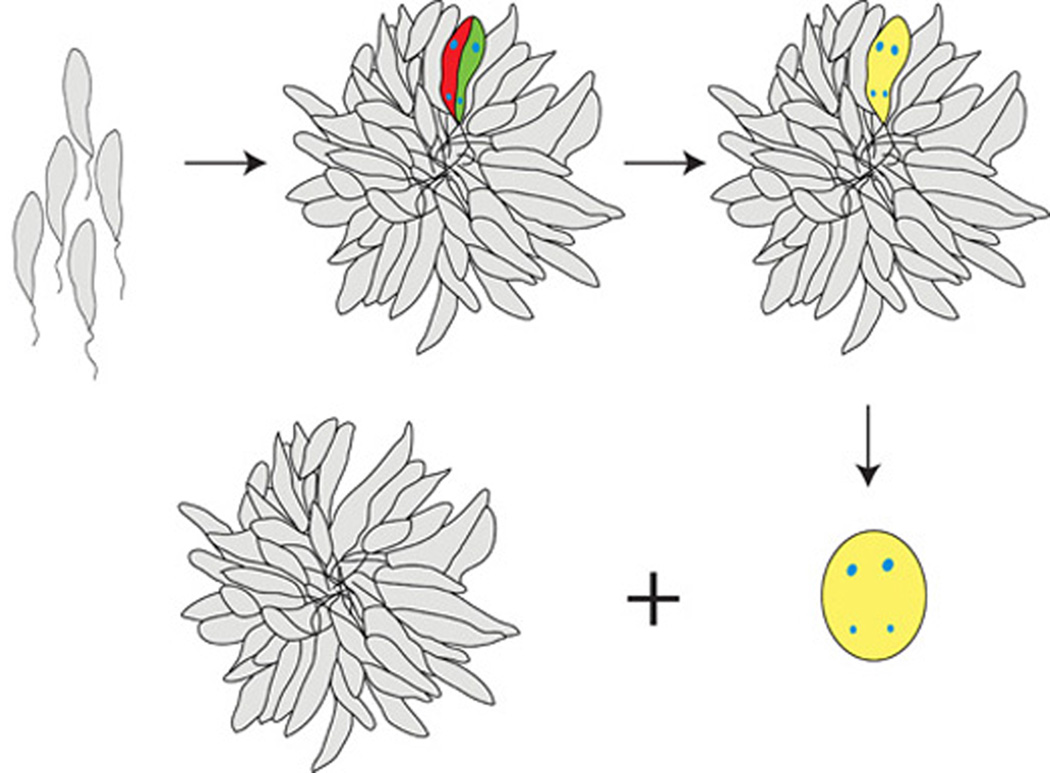
Cultivation of the organisms at the lower temperature also revealed a previously unreported phenomenon - rosette motility, in which rosettes over time form larger and larger aggregates. Under the microscope, individual rosettes were observed in real time to irreversibly adhere to one another once in contact, and so form extended clusters. Individual rosettes and extended clusters progressed in later stages to give rise to cellular fusion structures. Since the fused promastigotes apparently lack subcellular structures to isolate each cytoplasm, we hypothesize that DNA could commingle in this syncytium and give rise to genetic recombinants (Fig. 10).
Fig. 10.
Proposed model for the role of rosette formation, and of Poly α 2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA) expression in the leishmanial life cycle. Under the appropriate growth conditions, promastigotes of Leishmania form rosettes in significant numbers and begin to express cell surface PSA and NeuPSA. For simplicity only two cells within a single rosette are shown expressing PSA or NeuPSA (labeled red and green, respectively), each containing a single nucleus and kinetoplast labeled in blue. Cell surface PSA/NeuPSA expression promotes the fusion of two or more neighboring promastigotes (labeled yellow), allowing the potential co-mingling of their kinetoplast and nuclear genomes. Fusion bodies, subsequently exhibit a distinct morphology, and are released from the rosettes.
The differences in the physical and chemical properties of PSA versus NeuPSA are consistent with a role for PSA/NeuPSA in promastigote fusion and rosette-rosette adherence. PSA is a polyanion that, for example with PSA-NCAM, prevents adhesive interactions (Muhlenhoff et al. 2009). However, removing as few as 10--20% of N-acetyl groups on PSA produces a dramatic change of physical properties where the presence of zwitterionic neuraminic acid residues results in aggregation of NeuPSA derivatives (GM., unpublished). Thus due to these aggregative properties, PSA/NeuPSA expression may also have a role in the formation of bio-films or gels in the sand fly gut that are essential for parasite transmission to its human host (Rogers and Bates 2007). PSA/NeuPSA expression, however, does not appear to be essential for rosette formation as some promastigotes present in rosettes did not express either PSA or NeuPSA.
To our knowledge this is the first report of three observations: the presence of a stage-specific expression of PSA/NeuPSA in any microorganism; predictable rosette swarming in Leishmania; and the development of unique cellular fusion bodies among Leishmania or other kinetoplastids. Therefore, this represents the first molecular, physiological, and morphological evidence that rosettes represent a genuine stage in the life cycle of these parasites.
Of the various morphological forms of leishmanial cells that have been reported, rosettes have largely been ignored, being considered artifacts (Mehlhorn and Armstrong 2001; Schuster and Sullivan 2002) despite their repeated observation within the midgut of sandfly and flea insect vectors (Feliciangeli et al. 1988; Gontijo et al. 1995; Lainson and Shaw 1973; Leishman 1911), and the precise orientation of promastigote cells with their flagella directed toward the center of the rosette, an arrangement with obvious resemblance to the pairing of Chlamydomonas cells during the mating process (Pan and Snell 2000). Recently, genetic recombination in Leishmania has been demonstrated, providing evidence that a sexual cycle does exist for these organisms (Akopyants et al. 2009). However, these authors indicated that passage of parental strains through the sandfly insect vector was necessary in order to achieve genetic exchange. From our studies we hypothesize that conditions promoting the formation of rosette structures are a necessary component for successful in vitro activation of the sexual stage in these organisms and that genetic recombination experiments may now be possible under laboratory conditions without the need for sandfly passage.
In addition, we have recently shown that unlike PSA, NeuPSA is immunogenic and elicits antibodies that are protective against meningococcal group B and C strains by mediating complement activation (Moe et al. 2009). Since we have shown that NeuPSA antigens are also expressed during a previously unrecognized stage in the life cycle of Leishmania, the possibility arises that NeuPSA-based vaccines or drugs that inhibit NeuPSA synthesis may also be useful in preventing or treating leishmanial disease.
Supplementary Material
Video recording of rosette swarming in Leishmania major. Cell cultures were concentrated as described under MATERIALS AND METHODS and videotaped in real time for 4 h. A condensed 100x time lapse movie was generated corresponding to the first 1.5 h of recording.
Demonstration of rosette-rosette adhesion in Leishmania major. When rosettes come into physical contact they remain adhered to one another and do not separate.
ACKNOWLEDGEMENTS
We wish to acknowledge Shane Oram for helpful suggestions concerning Western blot experiments and Jennifer Beckstead for the graphic designs in Fig. 10. DMI wishes to thank Marjorie Crandall, Ph.D. for exceptional graduate instruction in microbial mating systems. This work was supported by grants RO1 AI46464 from the National Institute of Allergy and Infectious Disease of the National Institutes of Health and the family of Jennifer Leigh Wells to G.R.M. The research was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number CO6 RR-16226 from the National Center for Research Resources, National Institutes of Health.
Literature Cited
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Internl. 2004;11:36–42. [Google Scholar]
- Adair WS, Monk BC, Cohen R, Hwang C, Goodenough UW. Sexual agglutinins from the Chlamydomonas flagellar membrane. Partial purification and characterization. J. Biol. Chem. 1982;257:4593–4602. [PubMed] [Google Scholar]
- Akopyants NS, Kimblin N, Secundino N, Patrick R, Peters N, Lawyer P, Dobson DE, Beverley SM, Sacks DL. Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science. 2009;324:265–268. doi: 10.1126/science.1169464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- Bastien P, Blaineau C, Pages M. Leishmania: sex, lies and karyotype. Parasitol. Today. 1992;8:174–177. doi: 10.1016/0169-4758(92)90016-u. [DOI] [PubMed] [Google Scholar]
- Chargui N, Amro A, Haouas N, Schonian G, Babba H, Schmidt S, Ravel C, Lefebvre M, Bastien P, Chaker E, Aoun K, Zribi M, Kuhls K. Population structure of Tunisian Leishmania infantum and evidence for the existence of hybrids and gene flow between genetically different populations. Int. J. Parasitol. 2009;39:801–811. doi: 10.1016/j.ijpara.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Chava AK, Kohla G, Pal S, Merling A, Hinderlich S, Unger U, Strasser P, Gerwig GJ, Kamerling JP, Vlasak R, Crocker PR, Schauer R, Schwartz-Albiez R, Mandal C. Identification and characterization of adsorbed serum sialoglycans on Leishmania donovani promastigotes. Glycobiology. 2003;13:351–361. doi: 10.1093/glycob/cwg027. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Das A, Dasgupta A, Sharma S, Ghosh M, Sengupta T, Bandopadhyay S, Majumder HK. Characterisation of the gene encoding type II DNA topoisomerase from Leishmania donovani: A key molecular target in antileishmanial therapy. Nucleic Acids Res. 2001;29:1844–1851. doi: 10.1093/nar/29.9.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciangeli MD, Reyes RM, Limongi JE. Natural infection of Lutzomyia ovallesi (diptera: Psychodidae) with parasites of the Leishmania braziliensis complex in a restricted focus of cutaneous leishmaniasis in northern Venezuela. Mem. Inst. Oswaldo Cruz. 1988;83:393–394. doi: 10.1590/s0074-02761988000300019. [DOI] [PubMed] [Google Scholar]
- Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem. Biophys. Res. Commun. 1983a;112:482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983b;2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Gontijo CMF, Falca AR, Falcao AL, Coelho Md. The development of species of Leishmania ross, 1903 in Lutzomyia longipalpis (lutz & neiva, 1912) Memórias do Instituto Oswaldo Cruz. 1995;90:367–373. doi: 10.1590/s0074-02761995000300011. [DOI] [PubMed] [Google Scholar]
- Granoff DM, Bartoloni A, Ricci S, Gallo E, Rosa D, Ravenscroft N, Guarnieri V, Seid RC, Shan A, Usinger WR, Tan S, McHugh YE, Moe GR. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J. Immunol. 1998;160:5028–5036. [PubMed] [Google Scholar]
- Greenblatt CL, Handman E, Mitchell GF, Battye FL, Schnur LF, Snary D. Phenotypic diversity of cloned lines of Leishmania major promastigotes. Z. Parasitenkd. 1985;71:141–157. doi: 10.1007/BF00926265. [DOI] [PubMed] [Google Scholar]
- Kamhawi S. Phlebotomine sand flies and Leishmania parasites: Friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kelly JM, Law JM, Chapman CJ, Van Eys GJ, Evans DA. Evidence of genetic recombination in Leishmania. Mol. Biochem. Parasitol. 1991;46:253–263. doi: 10.1016/0166-6851(91)90049-c. [DOI] [PubMed] [Google Scholar]
- Koles K, Repnikova E, Pavlova G, Korochkin LI, Panin VM. Sialylation in protostomes: A perspective from Drosophila genetics and biochemistry. Glycoconj. J. 2009;26:313–324. doi: 10.1007/s10719-008-9154-4. [DOI] [PubMed] [Google Scholar]
- Lainson R, Shaw JJ. Leishmanias and leishmaniasis of the new world, with particular reference to Brazil. Bull. PAHO Bulletin. 1973;VII:1–19. [PubMed] [Google Scholar]
- Leishman WB. Critical review: Kala azar and tropical sore. Quart. J. Med. 1911;os5:109–152. [Google Scholar]
- Mandrell RE, Zollinger WD. Measurement of antibodies to meningococcal group B polysaccharide: Low avidity binding and equilibrium binding constants. J. Immunol. 1982;129:2172–2178. [PubMed] [Google Scholar]
- Mehlhorn H, Armstrong PM. Encyclopedic reference of parasitology. Berlin ; New York: Springer; 2001. p. 108. [Google Scholar]
- Moe GR, Bhandari TS, Flitter BA. Vaccines containing de-N-acetyl sialic acid elicit antibodies protective against Neisseria meningitidis groups B and C. J. Immunol. 2009;182:6610–6617. doi: 10.4049/jimmunol.0803677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe GR, Dave A, Granoff DM. Epitopes recognized by a nonautoreactive murine anti-N-propionyl meningococcal group B polysaccharide monoclonal antibody. Infect. Immun. 2005;73:2123–2128. doi: 10.1128/IAI.73.4.2123-2128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe GR, Dave A, Granoff DM. Molecular analysis of anti-N-propionyl Neisseria meningitidis group B polysaccharide monoclonal antibodies. Mol. Immunol. 2006;43:1424–1431. doi: 10.1016/j.molimm.2005.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff M, Oltmann-Norden I, Weinhold B, Hildebrandt H, Gerardy-Schahn R. Brain development needs sugar: The role of polysialic acid in controlling NCAM functions. Biol. Chem. 2009;390:567–574. doi: 10.1515/BC.2009.078. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Mandal C. Glycobiology of Leishmania donovani. Indian J. Med. Res. 2006;123:203–220. [PubMed] [Google Scholar]
- Murray PJ, Spithill TW, Handman E. Characterization of integral membrane proteins of Leishmania major by Triton X-114 fractionation and analysis of vaccination effects in mice. Infect. Immun. 1989;57:2203–2209. doi: 10.1128/iai.57.7.2203-2209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal RA. Chemotherapy of cutaneous leishmaniasis: Leishmania tropica infections in mice. Ann. Trop. Med. Parasitol. 1964;58:420–430. doi: 10.1080/00034983.1964.11686264. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Tilden EB. Comparative studies of herpetomonads and leishmanias: I. Cultivation of herpetomonads from insects and plants. J. Exp. Med. 1926;44:307–325. doi: 10.1084/jem.44.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgoztasi O, Baydar I. A randomized clinical trial of topical paromomycin versus oral ketoconazole for treating cutaneous leishmaniasis in Turkey. Int. J. Dermatol. 1997;36:61–63. doi: 10.1046/j.1365-4362.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Signal transduction during fertilization in the unicellular green alga, Chlamydomonas. Curr. Opin. Microbiol. 2000;3:596–602. doi: 10.1016/s1369-5274(00)00146-6. [DOI] [PubMed] [Google Scholar]
- Pommerville JC, Alcamo IE. Alcamo's fundamentals of Microbiology. Sudbury, Mass: Jones and Bartlett Publishers; 2004. p. 613. [Google Scholar]
- Rogers ME, Bates PA. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog. 2007;3:e91. doi: 10.1371/journal.ppat.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster FL, Sullivan JJ. Cultivation of clinically significant hemoflagellates. Clin. Microbiol. Rev. 2002;15:374–389. doi: 10.1128/CMR.15.3.374-389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W. The development of Leishmania donovani in vitro at 37 degree C; effects of the kind of serum. J. Exp. Med. 1953;97:177–188. doi: 10.1084/jem.97.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira L, Lavan A, Dagger F, Cabantchik ZI. The role of anions in pH regulation of Leishmania major promastigotes. J. Biol. Chem. 1994;269:16254–16259. [PubMed] [Google Scholar]
- Vieira LL, Sacerdoti-Sierra N, Jaffe CL. Effect of pH and temperature on protein kinase release by Leishmania donovani. Int. J. Parasitol. 2002;32:1085–1093. doi: 10.1016/s0020-7519(02)00067-x. [DOI] [PubMed] [Google Scholar]
- Wilson NF. Gametic cell adhesion and fusion in the unicellular alga Chlamydomonas. Methods Mol. Biol. 2008;475:39–51. doi: 10.1007/978-1-59745-250-2_3. [DOI] [PubMed] [Google Scholar]
- Yahiaoui B, Taibi A, Ouaissi A. A Leishmania major protein with extensive homology to Silent Information Regulator 2 of Saccharomyces cerevisiae. Gene. 1996;169:115–118. doi: 10.1016/0378-1119(95)00785-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video recording of rosette swarming in Leishmania major. Cell cultures were concentrated as described under MATERIALS AND METHODS and videotaped in real time for 4 h. A condensed 100x time lapse movie was generated corresponding to the first 1.5 h of recording.
Demonstration of rosette-rosette adhesion in Leishmania major. When rosettes come into physical contact they remain adhered to one another and do not separate.