Abstract
Rationale: Acute lung injury and the acute respiratory distress syndrome are characterized by increased lung oxidant stress and apoptotic cell death. The contribution of epithelial cell apoptosis to the development of lung injury is unknown.
Objectives: To determine whether oxidant-mediated activation of the intrinsic or extrinsic apoptotic pathway contributes to the development of acute lung injury.
Methods: Exposure of tissue-specific or global knockout mice or cells lacking critical components of the apoptotic pathway to hyperoxia, a well-established mouse model of oxidant-induced lung injury, for measurement of cell death, lung injury, and survival.
Measurements and Main Results: We found that the overexpression of SOD2 prevents hyperoxia-induced BAX activation and cell death in primary alveolar epithelial cells and prolongs the survival of mice exposed to hyperoxia. The conditional loss of BAX and BAK in the lung epithelium prevented hyperoxia-induced cell death in alveolar epithelial cells, ameliorated hyperoxia-induced lung injury, and prolonged survival in mice. By contrast, Cyclophilin D–deficient mice were not protected from hyperoxia, indicating that opening of the mitochondrial permeability transition pore is dispensable for hyperoxia-induced lung injury. Mice globally deficient in the BH3-only proteins BIM, BID, PUMA, or NOXA, which are proximal upstream regulators of BAX and BAK, were not protected against hyperoxia-induced lung injury suggesting redundancy of these proteins in the activation of BAX or BAK.
Conclusions: Mitochondrial oxidant generation initiates BAX- or BAK-dependent alveolar epithelial cell death, which contributes to hyperoxia-induced lung injury.
Keywords: cell death, epithelium, Bcl-2 proteins, acute respiratory distress syndrome
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
High levels of oxidant stress have been observed in the injured lung and have been shown to worsen the injury. Apoptotic cell death is observed in patients with acute respiratory distress syndrome and in animal models of oxidant stress. Whether this cell death contributes to lung injury or is a marker for the injury is not known.
What This Study Adds to the Field
Using nine strains of lung-specific and global knockout mice deficient in or overexpressing proteins required for apoptotic cell death, we show that activation of the intrinsic apoptotic pathway contributes to oxidant-mediated lung injury. These results suggest that therapies preventing alveolar epithelial apoptosis may be beneficial in patients with acute lung injury.
Approximately 200,000 people in the United States develop acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS) annually (1). These clinical syndromes are characterized by diffuse injury to the alveolar epithelium and endothelium allowing the exudation of protein-rich fluid from the blood into the alveolar space, which impairs gas exchange and causes oxygen-refractory arterial hypoxemia (2). Despite two decades of research-driven improvements in the care of these patients, the mortality from ARDS remains unacceptably high (30–40%) (3).
In the lungs of patients with ALI-ARDS, investigators have consistently observed increases in markers of oxidant stress and evidence of apoptotic and necrotic cell death (4–6). Exposure of rodents to hyperoxia causes oxidant-dependent respiratory death of the animal and mimics many of the clinical and pathologic features observed in patients with ALI-ARDS including the development of lung injury, pulmonary edema, and hypoxemic respiratory failure (7, 8). Pathologic evaluation of the lungs from these animals shows evidence of apoptotic and necrotic cell death (8, 9), and exposure of isolated lung epithelial cells to hyperoxia in vitro causes oxidant-dependent cell death (10). However, the molecular links between oxidant production and cell death and the contribution of cell death to the development of the injury remain unclear.
Exposure to a death stimulus triggers activation of the extrinsic or intrinsic apoptotic pathways, both of which culminate in the activation of caspases, which are responsible for all of the morphologic features of apoptosis (11). We and others have proposed that oxidants generated in response to hyperoxia might activate the intrinsic cell death pathway to induce mitochondrial outer membrane permeabilization (12–15). Activation of this pathway requires oligomerization of the proapoptotic Bcl-2 family members BAX or BAK on the outer mitochondrial membrane, allowing the release of cytochrome c and other proapoptotic proteins from the mitochondrial-intermembrane space into the cytosol (16). Mitochondrial membrane permeabilization can also result from opening of the mitochondrial permeability transition pore (MPTP) (17). On opening, this macromolecular complex forms a pore in the outer mitochondrial membrane sufficiently large to allow the release of cytochrome c and other macromolecules into the cytosol (18). Opening of the MPTP has been implicated in apoptotic and necrotic cell death induced by other forms of oxidant stress, such as ischemia–reperfusion injury in the heart and stroke (19–21).
In the present study, we used genetic mouse models to address the following questions: (1) Do mitochondrial oxidants generated in the alveolar epithelium contribute to cell death or lung injury in response to hyperoxia? (2) Are mitochondrial-derived oxidants required for the activation of BAX or BAK and cell death in alveolar epithelial cells? (3) Does cell death mediated by activation of the intrinsic apoptotic pathway or opening of the MPTP contribute to the development of hyperoxia-induced lung injury? Some of these data have been presented previously in abstract form (22).
METHODS
Mice and Exposure to Hyperoxia
All experiments were approved by the Northwestern University Animal Care and Use Committee. The Cyclophilin D−/− (21), Bak−/−/Baxfl/fl (23), and Bid−/− (24) mice were kindly provided by the late Dr. Stanley Korsmeyer and Dr. Nika Danial. The vav-Bcl-2 mice were a kind gift of Dr. Jerry Adams (25). The Bim−/− (26), Puma−/−, and Noxa−/− (27) mice were kindly provided by Dr. Andreas Strasser. Rosa 26R LacZ mice were purchased from Jackson Laboratories (Bar Harbor, ME). All of the mice except the CyclophilinD−/− and Mclk1+/− mice are on the C57BL/6 background. Wild-type littermates were used as controls for the CypD−/−, Mclk1+/−, and Puma−/− mice. Age- and sex-matched wild-type control mice were purchased from Jackson Laboratories for the Noxa −/−, Bim −/−, Bid−/−, and vav-Bcl-2 mice. Male Sprague-Dawley rats used for the isolation of alveolar type II cells were purchased from Charles River (Wilmington, MA). Mice were exposed to hyperoxia in a Kirschner chamber with constant temperature and humidity maintained with 10 L/min O2 (>95% O2 except briefly [<5 min] after cage changes). The chamber is equipped with Dry-Rite scrubbers (Dryrite, Nashville, TN) for the removal of CO2. The oxygen concentration in the chamber was continuously monitored with a MiniOx O2 sensor (MSA Instrument Division, Pittsburgh, PA).
Adenoviral Infection of Cells and Mice
Primary rat alveolar epithelial cells were infected with adenoviral vectors as previously described and used 48 hours after infection (28). Mice were infected with adenoviral vectors in 50% surfactant vehicle, balance TE buffer as previously described, and were exposed to hyperoxia 7 days later (adenoviral SOD2) or 30 days later (adenoviral Cre) (29). Localization of the Ad-SOD2 is shown in Figure E1 in the online supplement.
Isolation and Culture of Alveolar Epithelial Type II Cells
Alveolar epithelial type II cells were isolated from mice and rats as previously described (10, 30). Cells were exposed to hyperoxia (95% O2, 5% CO2) in Oxycycler (BioSperix, Ltd., Redfield, NY) chambers or normoxia (room air) at 37°C, as previously described (10). Glucose concentrations were estimated (Bayer Multistix; Bayer, Leverkusen, Germany) at the end of the exposure to hyperoxia and were always in excess of 200 mg/dl.
Measurement of Reactive Oxygen Species
Mitochondrially generated reactive oxygen species were measured using an oxidant-sensitive green fluorescent protein probe containing a mitochondrial localization sequence delivered to cells in an adenoviral vector, as previously described. Oxidation of the probe was by flow cytometry using a DakoCytomation CyAn high speed multilaser droplet cell sorter (DakoCytomation, Glostrup, Denmark), as previously described (28).
Histology, lung wet-to-dry weight ratios, and bronchoalveolar lavage analysis were measured as previously reported (29).
Cell Death Assays
Cell death was assessed using a commercially available photometric immunoassay that detects histone-associated DNA fragments (Roche Diagnostics, Indianapolis, IN) and using a commercially available assay that measures lactate dehydrogenase, as previously described (10, 31).
Measurement of Bax Activation and Localization
Measurement of Bax activation and localization was performed using a modification of a previously described method (10, 32).
Immunoblotting of Lung Homogenates
Animals were hemorrhaged by opening the abdomen and cutting the renal artery. The heart and lungs were then removed en bloc and the right lung cut from the hilum and placed into 1 ml of mild RIPA buffer with protease and phosphatase inhibitors (Mini EDTA-Free Tablet [Roche] and 1 mM sodium vanadate) in a 1.5-ml Eppendorf tube. The lungs were then homogenized on ice for three cycles, 2 minutes each. After each cycle, the homogenates were centrifuged (4°C, 10 min, 200 × g) and the lysate between the floating and sedimented debris was stored on ice. Immunoblotting was performed as previously described (10, 31).
Lentiviral shRNA Knockdown of Bid
The pLKO.1 vector was used to express shRNA targeting Bid. Constructs were ordered from Open Biosystems (Huntsville, AL) with the following hairpin sequences. Sequence #1, 5′-CCGGGCTCCTTCAACCAAGGAAGAACTCGACTTCCTTGGTTGAAGGAGCTTTTT; Sequence #2, 5′-CCGGCCACACGACTGTAACTTTATCTCGAGATAAAGTTGACAGTCGGTGGTTTTT. The nonsilencing shRNA was ordered from Addgene (Boston, MA) (plasmid 1864), 5′-CCTAAGGTTAAGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTTAGG-3′. Stable cell lines were generated using lentiviral infection using the 293FT packaging cell line and puromycin selection. Knockdown of Bid in these cells has been previously described (33).
Statistical Analysis
Kaplan-Meier survival curves were used to explore differences in survival. For all other experiments, differences between groups were explored using analysis of variance. When the analysis of variance indicated a significant difference, individual differences were explored using t tests with a Dunnett correction for multiple comparisons against control conditions. All analyses were performed using Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Mitochondrial Matrix-generated Superoxide Contributes to Hyperoxia-induced Mortality
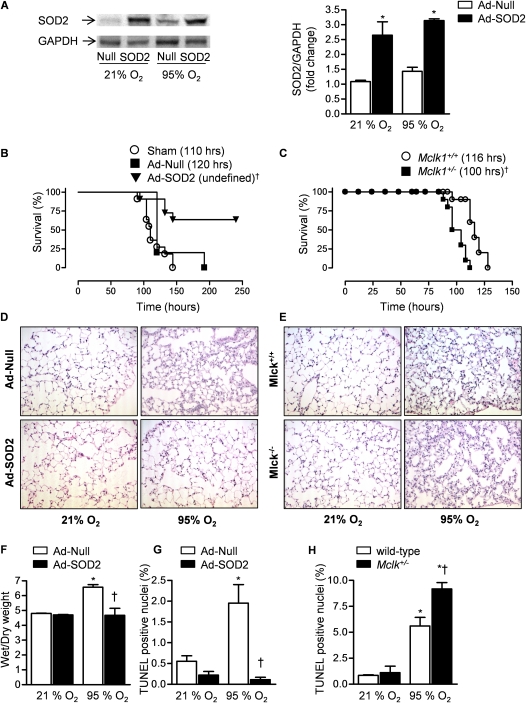
Mitochondrial electron transport complexes I, II, and III can generate superoxide and release it into the mitochondrial matrix (34). In the matrix, SOD2 catalyzes the conversion of superoxide to hydrogen peroxide, which is further metabolized to oxygen and water by catalase or glutathione peroxidase, both of which are present in high concentrations in the mitochondrial matrix (35). If the rate of mitochondrial matrix superoxide generation exceeded the capacity of the SOD2/catalase system, oxidation of matrix proteins and lipids could occur, potentially contributing to activation of the intrinsic apoptotic pathway. To determine the role played by mitochondrially generated oxidants in the development of hyperoxic lung injury and mortality in vivo, mice were sham infected (50% surfactant vehicle alone) or infected with an adenovirus encoding SOD2 (Ad-SOD2) or no transgene (Ad-Null). After 7 days, the animals were exposed to normoxia (21% O2) or hyperoxia (>95% O2) for 84 hours and the lungs were harvested and immunoblotted using an antibody against SOD2 (Figure 1A). Identically treated animals were exposed to hyperoxia for up to 10 days for measurement of survival. The LD50 for hyperoxia for sham and Ad-Null treated animals were 110 and 120 hours, respectively. Because more than 50% of the mice survived more than the 10 days allowed under our animal protocol, the LD50 for hyperoxia was undefined in mice infected with Ad-SOD2 and significantly longer than that observed in sham or Ad-Null treated mice (110 and 120 h, respectively) (Figure 1B). To examine the effect of SOD2 overexpression on the development of hyperoxic lung injury, mice treated with Ad-Null or Ad-Cre 7 days earlier were exposed to normoxia or sublethal hyperoxia (84 h) and lung histology, wet-to-dry lung weight ratios, and the percentage of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive nuclei were measured. Compared with Ad-Null treated mice, mice treated with Ad-SOD2 demonstrated significant improvements in all of these parameters (Figures 1D, 1F, and 1G).
Figure 1.
The generation of mitochondrial superoxide during exposure to hyperoxia contributes to mortality. Mice were intratracheally transfected with 1 × 109 pfu/animal of Ad-Null or Ad-SOD2 in a 50% surfactant vehicle or sham infected (Sham). (A) After 7 days the mice were exposed to sublethal hyperoxia (84 h, >95% O2) or maintained at normoxia (21% O2) after which the lungs were harvested and immunoblotted for SOD2, a representative immunoblot and densitometry (n = 3) is shown. (B) Mice transfected with Ad-Null or Ad-SOD2 7 days earlier were exposed to hyperoxia for measurement of survival (n = 6 sham; n = 5 null; n = 11 SOD2). (C) Mice heterozygous for Mclk, which is required for synthesis of the mitochondrial antioxidant ubiquinone, were exposed to hyperoxia for measurement of survival (n = 10 each group). The median survival (LD50) is in parentheses. Mice infected with Ad-Null or Ad-SOD2 were exposed to sublethal hyperoxia or maintained in normoxia for assessment of lung injury. Hematoxylin and eosin stained lung sections (×100) (D), wet-to dry lung weight ratios (E), and the percentage of TUNEL-positive nuclei (F) (n = 3 animals per group). Mclk+/− or wild-type mice were exposed to sublethal hyperoxia or maintained in normoxia for measurement of lung injury. Hematoxylin and eosin stained lung sections (original magnification ×100) (E) and the percentage of TUNEL-positive nuclei (H). *P < 0.05 for comparison with normoxic controls. †P < 0.05 for comparison of difference between Ad-SOD2 and Ad-SOD null transfected animals or Mclk1+/+ compared with Mclk1+/− animals after exposure to hyperoxia.
To genetically test whether mitochondrial oxidant generation contributes to the development of hyperoxia-induced mortality, we used mice partially deficient in the murine homolog of the Caenorhabditis elegans gene clk1 (Mclk1) (Mclk1+/−). MCLK1 is a mitochondrial hydroxylase that is required for the biosynthesis of ubiquinone (coenzyme Q). Ubiquinone plays a critical role in mitochondrial electron transport and serves as an important lipid-soluble antioxidant (36). In young adult mice (<12 wk) lacking a single allele of this gene (Mclk-1+/−), Lapointe and Hekimi have observed increased levels of mitochondrial oxidant stress including a reduction in aconitase activity, a marker of increased superoxide production in the mitochondrial matrix (37). Compared with their wild-type littermate controls, these mice demonstrated a significantly shortened survival during exposure to hyperoxia (LD50 100 and 116 h in the Mclk-1+/+ and Mclk-1+/− animals, respectively) (Figure 1C). Compared with wild-type mice, Mclk-1+/− demonstrated more histologic evidence of lung injury and more TUNEL-positive nuclei after exposure to sublethal hyperoxia (84 h) (Figures 1E and 1H).
Cyclophilin D–dependent Opening of the MPTP is Dispensable for Hyperoxia-induced Lung Injury
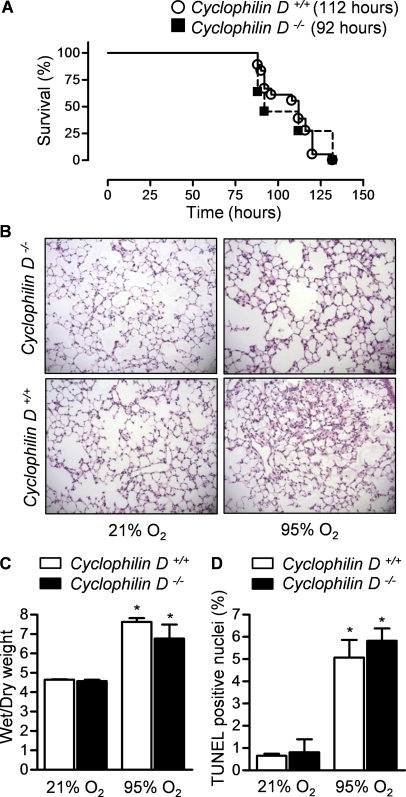
In mouse models of stroke and ischemia reperfusion in the heart, investigators have reported that mitochondrial membrane permeabilization and cell death require formation of the MPTP (19–21, 38). The MPTP is a multiprotein complex that can form in the mitochondrial membrane and includes the voltage-dependent ion channel located in the outer mitochondrial membrane, the adenine nucleotide translocator located in the inner mitochondrial membrane, and cyclophilin D located in the mitochondrial intermembrane space (17). On its assembly, a pore of sufficient size to allow the release of cytochrome c from the intermembrane space to the cytosol is transiently formed. To determine whether MPTP opening might contribute to the development of hyperoxia-induced lung injury, we exposed mice deficient in cyclophilin D (Cyclophilin D−/−) to hyperoxia and measured survival. Wild-type mice and Cyclophilin D−/− had similar survival times during exposure to hyperoxia (LD50 112 and 92 h for Cyclophilin D−/− compared with Cyclophilin D +/+ littermate controls, respectively) (Figure 2A). To examine the effect of the loss of cyclophilin D on the development of lung injury, wild-type and CyclophilinD−/− mice were exposed to hyperoxia and harvested before the onset of mortality (84 h). Compared with wild-type mice, Cyclophilin D−/− mice had similar increases in TUNEL-positive nuclei in the lung (Figure 2B); lung edema (wet-to-dry) weight (Figure 2C); and changes in lung histology (Figure 2D).
Figure 2.
The mitochondrial permeability transition pore is not required for hyperoxia-induced mortality. (A) Mice lacking cyclophilin D, a critical component of the mitochondrial permeability transition pore, or wild-type mice on an identical background were exposed to hyperoxia for measurement of survival. The median survival (LD50) is in parentheses (n = 18 for Cyclophilin D+/+; n = 12 for Cyclophilin D−/−). The difference between wild-type and knockout mice was not significant (P = 0.79). The same mouse strains were exposed to hyperoxia for 84 hours for measurement of lung injury. Hematoxylin and eosin stained lung sections (original magnification ×100) (B), wet-to-dry weight ratios of the lung (C), and the percentage of TUNEL positive nuclei (D) (n >4 animals per group). *P < 0.05 for comparison with nomoxic controls. No significant differences were observed between the Cyclophilin D+/+ and Cyclophilin D−/− animals.
Mitochondrial Oxidant Production is Associated with the Activation of BAX and Cell Death in Alveolar Epithelial Cells
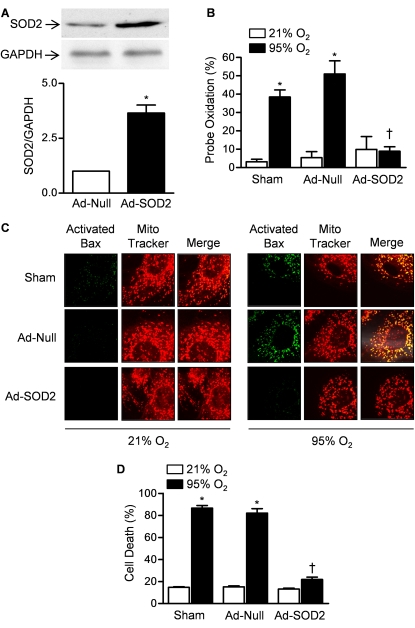
Activation of the intrinsic apoptotic pathway to induce the oligomerization of BAX or BAK in the mitochondrial outer membrane is an alternative mechanism by which mitochondrially generated oxidants might cause cell death (16). To examine the role of BAX in hyperoxia-induced cell death in vitro, we transfected primary rat alveolar type II cells with Ad-SOD2 and measured mitochondrial oxidant production, the activation of BAX at the mitochondrial membrane, and cell death during exposure to hyperoxia. Compared with Ad-Null transfected cells, cells transfected with the Ad-SOD2 48 hours earlier showed significantly higher levels of SOD2 as assessed by immunoblotting (Figure 3A). We then directly measured the effect of SOD2 overexpression on mitochondrial oxidant generation during hyperoxia using an oxidant-sensitive GFP probe containing a mitochondrial localization sequence (mito-Ro-GFP) (localization of the probe and SOD2 vector is shown in Figure E1). Cells were then cotransfected with an adenovirus encoding mito-Ro-GFP and Ad-Null or Ad-SOD2 and 48 hours later placed in an environmentally controlled chamber for exposure to hyperoxia (95% O2, 5% CO2) or normoxia (21% O2, 5% CO2). After 4 hours, oxidation of the probe was measured using flow cytometry (Figure 3B) (28). Also 48 hours after infection, identically treated cells were exposed to hyperoxia for 24 hours and the cells were immunostained using an antibody that recognizes a N-terminal epitope on the Bax molecule that is exposed on its activation (32). Significant staining for activated Bax was observed in sham and Ad-Null–infected cells but not Ad-SOD2–infected cells after exposure to hyperoxia (Figure 3C). The staining for activated Bax colocalized with the mitochondrial marker MitoTracker Red. Identically treated cells were also exposed to hyperoxia for 48 hours and cell death was measured (LDH release) (Figure 3D). Cells overexpressing SOD2 were protected against hyperoxia-induced cell death.
Figure 3.
Mitochondrial superoxide production results in the activation of BAX and cell death. (A) Primary rat alveolar epithelial type II cells were infected with an adenovirus expressing no transgene (Ad-Null) or SOD2 (Ad-SOD2) and the relative abundance of SOD2 was measured by immunoblotting 48 hours later. (B) Cells were cotransfected with an adenovirus encoding an oxidant-sensitive GFP targeted to the mitochondrial matrix (mito-Ro-GFP) and either Ad-Null or Ad-SOD2 48 hours before exposure to hyperoxia or normoxia for 4 hours. The oxidation of mito-Ro-GFP was then assessed using flow cytometry. (C) Sham transfected cells or cells transfected with Ad-Null or Ad-SOD2 were exposed to hyperoxia for 16 hours after which the cells were immunostained with an antibody that recognizes an activated form of Bax (green, left column) and the mitochondrial marker MitoTracker Red (red, middle column). The right column is a merged image. Each column is a representative image sampled from three separate isolations. (D) Identically infected cells were exposed to hyperoxia for 48 hours and cell death was measured (LDH release). *P < 0.05 compared with normoxic controls. †P < 0.05 for comparison of difference between Ad-SOD2 and Ad-SOD null transfected cells.
Conditional Loss of BAX and BAK in the Alveolar Epithelium Prolongs Survival during Hyperoxia
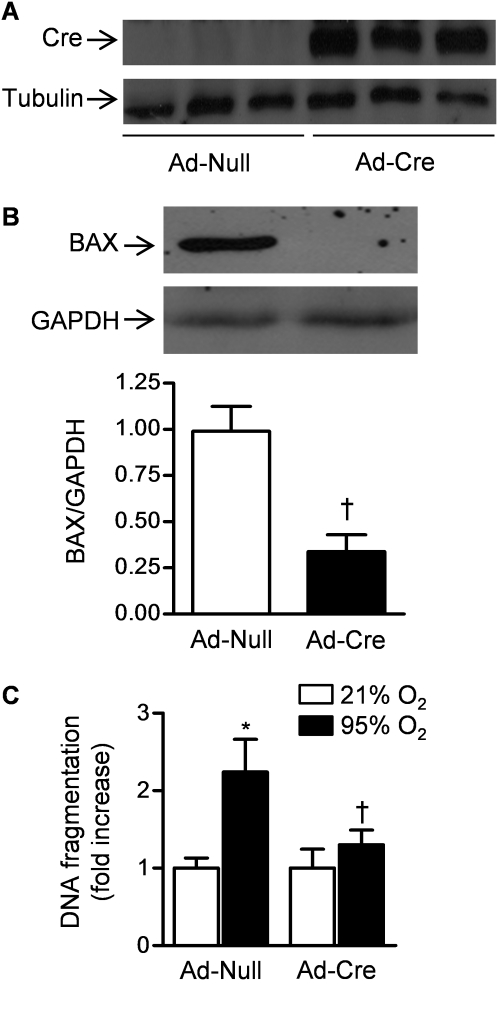
BAX and BAK are Bcl-2 proteins that differ from other members of the Bcl-2 family because they each contain three Bcl-2 homology (BH) domains (11). These proteins are ubiquitously expressed, are functionally redundant, and are required for activation of the intrinsic apoptotic pathway in most cells. To determine whether the activation of BAX or BAK is required for hyperoxia-induced cell death in alveolar epithelial cells in vitro, mice floxed for the Bax gene and null for Bak (Bak−/− Bax fl/fl) were infected with an adenovirus encoding Cre recombinase (Ad-Cre) or Ad-Null. Seven days later, expression of Cre recombinase was present only in whole lung homogenates from Cre-infected mice as assessed by immunoblotting (Figure 4A). Furthermore, identically treated LacZ reporter mice (Rosa 26R LacZ) showed diffuse LacZ staining in intact lungs and in most alveolar cells identified on frozen sections (Figure E2). To confirm that the Cre recombinase was able to excise the gene for Bax in the lung epithelium, primary alveolar epithelial type II cells were isolated from animals 30 days after intratracheal infection with Ad-Cre and Ad-Null. The cells from three animals in each group were pooled and lysed and the lysate was then immunoblotted using an antibody against BAX. The levels of BAX protein were substantially reduced in the primary alveolar type II cells freshly isolated from mice treated 30 days earlier with Ad-Cre compared with those infected with Ad-Null (Figure 4B), providing direct evidence of Cre recombinase-mediated removal of Bax in the alveolar epithelium in vivo. To determine the effect of loss of BAX on hyperoxia-induced cell death, primary mouse alveolar epithelial type II cells were isolated from mice infected 30 days earlier with Ad-Null or Ad-Cre and grown at air liquid interfaces for 72 hours before exposure to hyperoxia (95% O2, 5% CO2) for 48 hours, after which cell death was measured using an ELISA to detect fragmented DNA. Hyperoxia-induced cell death was significantly attenuated in alveolar epithelial cells from Ad-Cre–infected mice compared with Ad-Null–infected mice (Figure 4C).
Figure 4.
BAX or BAK are required for hyperoxia-induced cell death in primary mouse alveolar epithelial cells. (A) Mice deficient in Bak with loxP sites flanking the Bax gene Baxfl/flBak−/− were intratracheally infected with an adenovirus encoding no transgene (Ad-Null) or one encoding Cre recombinase (Ad-Cre) (1 × 109 pfu/animal) and 7 days later Cre abundance in lung homogenates was measured by immunoblotting. (B) Thirty days after infection with Ad-Null or Ad-Cre, primary ATII cells were harvested from mice and immunoblotted for the abundance of BAX (each lane represents pooled samples from four animals, densitometry represents three replicates). (C) These cells were exposed to hyperoxia for 48 hours and cell death was measured using an ELISA that detects DNA fragmentation (n = 3). *P < 0.05 for comparison with normoxic controls. †P < 0.05 for comparison of difference between Ad-Null and Ad-Cre transfected cells after exposure to hyperoxia.
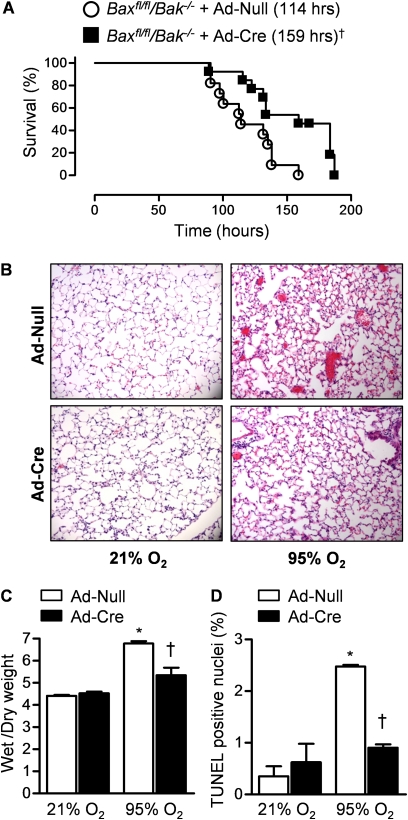
To determine whether alveolar epithelial cell death induced by the activation of BAX or BAK contributes to hyperoxia-induced mortality in vivo, we exposed Bak−/− Bax fl/fl mice infected with Ad-Null or Ad-Cre 30 days earlier to hyperoxia (>95% O2) for measurement of survival. Mice infected with Ad-Cre had significantly longer survival during hyperoxia than mice infected with Ad-Null (LD50 159 h and 114 h in Ad-Cre– and Ad-Null–infected Bak−/− Bax fl/fl animals, respectively) (Figure 5A). We then compared the levels of apoptosis and lung injury in Bak−/− Bax fl/fl mice infected 30 days earlier with Ad-Null or Ad-Cre after exposure to sublethal hyperoxia (84 h). The fraction of TUNEL-positive nuclei after exposure to hyperoxia was significantly reduced in lung sections obtained from mice infected with Ad-Cre compared with those infected with Ad-Null (Figure 5B). Consistent with the survival data, mice infected with Ad-Cre had significantly less lung edema (wet-to-dry ratios) and attenuation of lung injury on histologic examination (Figures 5C and 5D).
Figure 5.
BAX or BAK contribute to hyperoxia-induced mortality in mice. (A) Baxfl/flBak−/− mice were intratracheally infected with an adenovirus encoding no transgene (Ad-Null) or one encoding Cre recombinase (Ad-Cre) (1 × 109 pfu/animal) 30 days before exposure to hyperoxia (≥ 95% O2) for measurement of survival (n = 11 for Ad-Null; n = 13 for Ad-Cre; †indicates P < 0.001 for comparison between Ad-Null and Ad-Cre infected mice after exposure to hyperoxia). Identically treated Baxfl/flBak−/− mice were exposed to hyperoxia or room air for 84 hours for assessment of lung injury. (B) Hematoxylin and eosin stained lung sections (original magnification ×100). (C) Wet-to-dry weight ratios of the lung. (D) Percentage of TUNEL-positive nuclei. n ≥ 4 for all measures. *P < 0.05 for comparison with normoxic controls. †P < 0.05 for comparison of difference between Ad-Null and Ad-Cre transfected animals after exposure to hyperoxia.
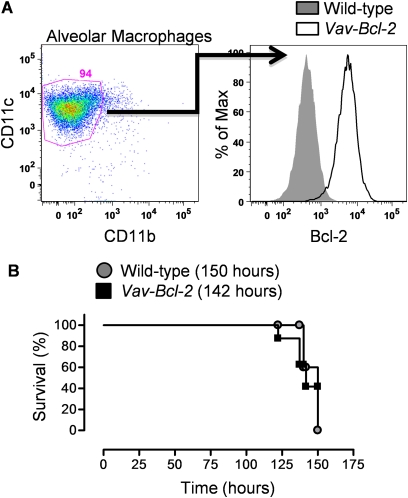
Depletion of hematopoietic cells before hyperoxic exposure does not alter the severity of hyperoxia-induced injury or prolong the survival of hyperoxia-exposed animals, suggesting that inflammation is dispensable in this model. Nevertheless, we wondered whether the loss of BAX or BAK in inflammatory cells within the lung might be responsible for the observed protection against hyperoxia observed in the Bak−/− Bax fl/fl mice treated 30 days earlier with adenoviral Cre. To test this hypothesis, we exposed transgenic mice in which the antiapoptotic protein Bcl-2 is overexpressed in all cells of hematopoietic origin under control of the vav promoter (vav-Bcl-2 mice) (25). Although overexpression of Bcl-2 has been reported in circulating inflammatory cells from these mice and other cells of hematopoietic origin, it has not been confirmed in alveolar macrophages, the predominant cell in the mouse lung (25). We isolated alveolar macrophages (CD11B[−], CD11c [+]) from bronchoalveolar lavage fluid and assessed intracellular levels of Bcl-2 using flow cytometry. Alveolar macrophages comprised 94% of the bronchoalveolar lavage cells. Alveolar macrophages from vav-Bcl-2 mice had approximately 100-fold higher levels of Bcl-2 than wild-type control mice (Figure 6A). Despite this, hyperoxia-induced mortality was similar in wild-type and vav-Bcl-2 mice (Figure 6B).
Figure 6.
Resistance of inflammatory cells to apoptosis does not alter hyperoxia-induced mortality. (A) Alveolar macrophages were identified in bronchoalveolar lavage fluid from wild-type mice and transgenic mice overexpressing Bcl-2 in cells of hematopoietic origin (vav-Bcl-2) as CD11c (+), CD 11b (−) cells and then intracellularly stained using an antibody against Bcl-2 . (B) Wild-type and vav-Bcl-2 mice were exposed to hyperoxia for measurement of survival (n = 8 each group). P = 1.
The Extrinsic Apoptotic Pathway Is Not Required for Hyperoxia-induced Mortality
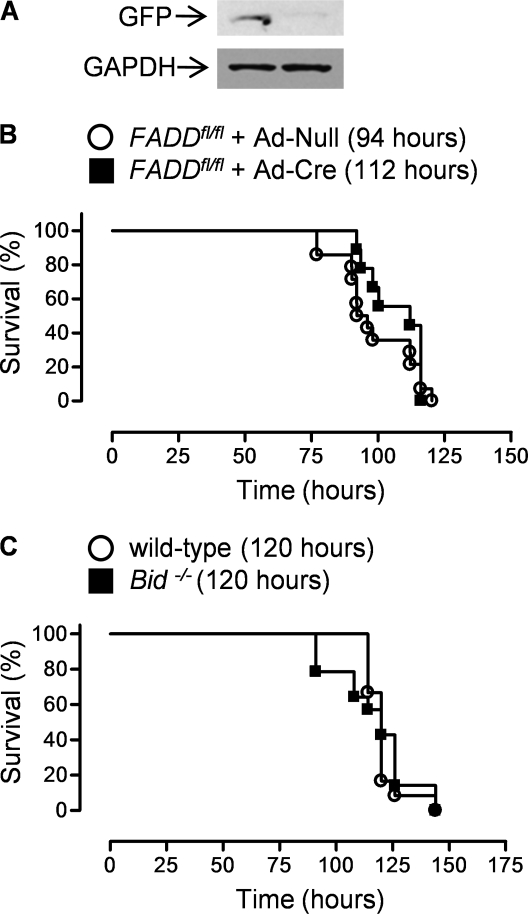
On binding of death ligands to their receptors, a multiprotein complex is assembled in the plasma membrane. This death-inducing signaling complex (DISC), which includes the death ligand, its receptor, caspase-8, and a protein containing a “death domain,” is responsible for cleavage of procaspase-8 to its active form (39). For example, binding of FasL with its cell surface receptor Fas induces the formation of a DISC containing the Fas-Associated Death Domain (FADD) protein, which is required for FasL mediated caspase-8 activation and cell death (39). To determine if binding of FasL with Fas was required for hyperoxia-induced cell death, we infected a mouse expressing FADD:GFP flanked by loxP sites in a FADD−/− background (FADD:GFPfl/fl) with either Ad-Null or Ad-Cre (40). Thirty days later, we isolated alveolar type II cells from these mice and immunoblotted cell lysates using an antibody against the GFP tag (Figure 7A). A significant loss of GFP staining was observed in alveolar type II cells from the Ad-Cre– compared with Ad-Null–infected animals. These mice were then exposed to hyperoxia for measurement of survival (LD50 94 and 112 h for Ad-Null and Ad-Cre, respectively; P = 0.3) (Figure 7B).
Figure 7.
The extrinsic apoptotic pathway is not required for hyperoxia-induced mortality in mice. (A) FADDfl/fl mice were intratracheally infected with an adenovirus encoding no transgene (Ad-Null) or one encoding Cre recombinase (Ad-Cre) (1 × 109 pfu/animal), and 30 days later alveolar type II cell lysates from four mice in each group were pooled and immunoblotted for the presence of the GFP-labeled Fas-Associated Death Domain (FADD). (B) Identically treated mice were exposed to hyperoxia (95% O2) for measurement of survival (n = 14 for Ad-Null; n = 9 for Ad-Cre; P = 0.28 for comparison between FADDfl/fl mice treated with Ad-Null or Ad-Cre). (C) Mice globally deficient in the proapoptotic BH3 protein Bid were exposed to hyperoxia for measurement of survival (n = 12 for wild-type; n = 14 for Bid−/−; P = 0.8).
In most cells, the activation of caspase-8 is insufficient to induce apoptosis in the absence of BAX- or BAK-dependent mitochondrial outer membrane permeabilization (41). This linkage between the extrinsic and intrinsic apoptotic pathways results from cleavage of the proapoptotic Bcl-2 protein BID into an active form by caspase-8. Truncated BID can directly activate BAX or BAK or negate the function of one or more of the antiapoptotic Bcl-2 proteins (e.g., Bcl-XL) (42). We have reported BID is required for the development of pulmonary fibrosis and others have suggested it is required for hyperoxia-induced lung injury (30, 43). We exposed mice globally deficient in Bid or wild-type C57Bl/6 mice to hyperoxia for measurement of survival. No significant difference was noted between the two strains (LD50 120 h for both wild-type and Bid−/− mice) (Figure 7C).
A Single BH3 Protein Is Unlikely to Be Required for the Hyperoxic Activation of BAX or BAK
The activation of BAX and BAK is regulated by members of the Bcl-2 family of proteins that contain only a single Bcl-2 homology domain (BH3 domain–only proteins) (44). When activated by a death stimulus, the BH3-only proteins BIM, PUMA, and BID can directly induce BAX- or BAK-dependent cell death, whereas other BH3 domain–only proteins (e.g., NOXA) negate the function of antiapoptotic proteins (45, 46). To determine whether activation BIM or PUMA was required for hyperoxia-induced lung injury, we exposed Bim−/− and Puma−/− mice to hyperoxia. These mice had similar rates of survival during hyperoxia as their appropriate controls (Figures 8A and 8B). Increased expression of the antiapoptotic protein A1 has been reported as a potential mechanism by which mice overexpressing IL-11 are protected against hyperoxia-induced lung injury (13). Because the antiapoptotic function of A1 can be inhibited by the BH3-only protein NOXA, we also examined survival in Noxa−/− mice during exposure to hyperoxia (45). These mice had similar survival during hyperoxia as wild-type controls (Figure 8C). Based on these observations, we conclude that the activation of a single BH3-only protein is not sufficient to explain the hyperoxic activation of BAX or BAK.
Figure 8.
The loss of an individual BH3 protein is not sufficient to prevent hyperoxia-induced mortality. Mice globally deficient in the proapoptotic BH3 proteins (A) Bim, (B) Puma, or (C) Noxa were exposed to hyperoxia for measurement of survival. Bim: n = 5 for wild-type and Bim−/−, P = 0.8. Puma: n = 5 for wild-type and Puma−/−, P = 0.87. Noxa: n = 21 for wild-type, n = 23 for Noxa−/−, P = 0.87.
Combined Loss of the Proapoptotic Bcl-2 Proteins BID, BIM, and PUMA Does Not Protect against Hyperoxia-induced Cell Death In Vitro
Among the described BH3 domain–only proteins, only BID, BIM, or PUMA are capable of directly activating BAX or BAK; the remaining BH3 domain–only proteins act to negate the function of antiapoptotic Bcl-2 proteins (47). To determine whether a combined loss of these three activator proteins was responsible for the hyperoxic activation of BAX or BAK and the resulting cell death, we exposed murine embryonic fibroblasts (MEF) from mice deficient in Bax and Bak and mice deficient in Bim and Puma to hyperoxia and measured cell death after 48 hours. MEFs that were deficient in both Bax and Bak were protected against hyperoxia-induced cell death (Figure 9A). By contrast, cell death was similar in wild-type MEFs and MEFs from mice doubly deficient in Bim and Puma (Figure 9B). To determine whether the combined loss of the activating BH3 domain–only proteins BID, BIM, and PUMA prevented cell death, we exposed MEFs doubly deficient in Bim and Puma that had been stably transfected with two lentiviral shRNA constructs against Bid or control transfected to hyperoxia for measurement of survival. These cell lines were described and the knockdown efficacy demonstrated in our previous publication (33). The levels of hyperoxia-induced cell death were similar in control transfected MEFs from mice doubly deficient in Bim and Puma and the same cells in which BID was stably knocked down (Figure 9C). These results suggest that redundant activation of these BH3-only proteins is unlikely to contribute to hyperoxia-induced cell death. Another possibility is that endoplasmic reticulum stress during hyperoxia results in BAX/BAK-dependent cell death. To test this possibility, we infected BAX/BAK null MEFs with retroviruses containing GFP alone; wild-type BAK; and BAK targeted either to the mitochondria (BAK-ActA) or ER (BAK-cb5) (48). Cells deficient in BAX/BAK were resistant to hyperoxia-induced cell death. The reconstitution of BAX/BAK null cells with wild-type BAK or mitochondrial targeted BAK-ActA restored their sensitivity to hyperoxia-induced cell death. By contrast, reconstitution of BAX/BAK null cells with ER-directed BAK-cb5 failed to restore the sensitivity to hyperoxia-induced cell death. These results suggest that the BAX/BAK pool in the ER is unlikely to contribute to hyperoxia-induced cell death.
Figure 9.
Neither the BH3 domain only proteins Bim, Bid, and Puma alone or in combination nor ER localized BAK are required for BAX or BAK dependent cell death. (A and B) murine embryonic fibroblasts (MEFs) from Bax−/− Bak−/− and Bim−/−Puma−/− mice or from their wild-type controls were exposed to hyperoxia for measurement of cell death (LDH release). (C) MEFs from Bim−/− Puma−/− double knockout mice were stably transfected with two shRNA constructs against Bid (33) and exposed to hyperoxia for measurement of cell death (LDH release). (D) MEFs from Bax−/− Bak−/−mice were stably transfected with a control retrovirus (GFP) or retroviruses encoding wild-type BAK, a mitochondrially localized BAK (BAK-ActA) or an ER localized BAK (BAK-cb5). n = 3 for all measures, * indicates P < 0.05 for comparison with wild-type air exposed cells, †P < 0.05 between wild-type and mutant cells exposed to hyperoxia.
DISCUSSION
Apoptosis of alveolar epithelial cells is present in biopsy specimens from patients with ARDS and in the lungs of animals exposed to hyperoxia; however, it is not clear whether apoptosis is a contributor to or a marker of the injury (6, 8, 49). Exposure of mice to hyperoxia is a well-established model of ALI (50). We found that inhibiting mitochondrial matrix oxidant production significantly prolonged the survival of mice exposed to hyperoxia. Conversely, mice prone to excess mitochondrial matrix oxidant production had shortened survival during exposure to hyperoxia. In other models of mitochondrial oxidant-mediated cell death (e.g., ischemia reperfusion in the heart and stroke) mitochondrial outer membrane permeabilization and cell death requires assembly of the MPTP (19–21). Surprisingly, we observed that mice lacking a critical component of the MPTP, cyclophilin D, were not protected against hyperoxia-induced mortality or lung injury. Instead, mitochondrial oxidant production in the lung epithelium resulted in the activation of BAX or BAK, key effectors of the intrinsic apoptotic pathway (51). The importance of BAX- or BAK-dependent death in this pathway was confirmed by the protection from death observed in alveolar epithelial cells isolated from mice deficient in BAX and BAK and the protection against hyperoxia-induced lung injury conferred by the conditional knockdown of BAX and BAK in the lung. The functional specificity of BAX and BAK allows us to conclude from the protection we observed in mice with a conditional knockdown of Bax and Bak in the lung that activation of the intrinsic apoptotic pathway directly contributes to hyperoxia-induced lung injury.
The Bcl-2 family comprises a diverse set of proteins that contain one or more conserved BH3 domains (52). BAX and BAK contain three BH3 domains and function to create channels in the mitochondrial outer membrane, allowing the cytosolic release of cytochrome c and other proapoptotic molecules normally sequestered in the mitochondrial intermembrane space (52). Caspase-8–mediated cleavage of the BH3-only protein BID is one mechanism by which BAX or BAK are activated. This extrinsic apoptotic pathway is initiated when death ligands, such as FasL, bind to receptors on the surface of cells to recruit FADD and caspase-8 to form a DISC. In the absence of FADD, the FasL-induced DISC fails to activate caspase-8 (40). We found that mice conditionally deficient in FADD in epithelial cells and mice globally deficient in BID were not protected against hyperoxia-induced lung injury, suggesting that the extrinsic apoptotic pathway does not contribute to hyperoxia-induced lung injury. Our observation that the overexpression of Bcl-2 in inflammatory cells did not alter the sensitivity of animals to hyperoxia-induced lung injury further suggests that apoptotic cell death in inflammatory cells plays little role in the lung injury and mortality induced by hyperoxia.
A second mechanism by which BAX or BAK are activated is through activation of other BH3-only proteins, such as BIM, PUMA, and NOXA. However, we observed no protection against hyperoxia in mice globally deficient in Bim, Puma, or Noxa. Furthermore, our observation that the loss of BAX and BAK, but not the combined loss of BIM, PUMA, and BID, prevented hyperoxia-induced cell death in MEFs suggests that redundant activation of these BH3-only proteins is unlikely to be required for hyperoxia-induced lung injury and mortality. The activation of BAX or BAK in the ER has also been shown to be required for some cell death stimuli that cause ER stress. However, the reconstitution of MEFs doubly deficient in BAX and BAK with BAK localized exclusively to the ER failed to restore their sensitivity to hyperoxia-induced cell death. In contrast, MEFs reconstituted with a mitochondrially targeted BAK showed a similar sensitivity to hyperoxia-induced cell death because MEFS reconstituted with a wild-type BAK, suggesting that the activation of BAX or BAK in the ER does not play a major role in hyperoxia-induced cell death. Although these results were generated in MEFS, they suggest that the most likely mechanism by which BAX/BAK are activated during hyperoxia is via the negation of the prosurvival Bcl-2 proteins, which are normally present in excess and bind to BAX and BAK to prevent their activation. Consistent with this hypothesis, Vitiello and colleagues (53) recently reported that the overexpression of p21 diminished hyperoxia-induced cell death by preventing the hyperoxia-induced fall in Mcl-1 and Bcl-XL.
Our results strongly implicate mitochondrial matrix oxidant production in the lung injury and mortality induced by exposure to hyperoxia. Similar to our findings, Ilizarov and colleagues (54) observed in a mouse lung epithelial cell line that SOD2, but not SOD1 or catalase prevented hyperoxia-induced cell death. By contrast, other investigators have observed that strategies that prevent the assembly of the NAD(P)H oxidase on the plasma membrane of endothelial cells prevent hyperoxia-induced oxidant generation and cell death (55–57). Several possibilities might explain these results. First, it is possible that different types of cells within the lung generate oxidants from different sources during hyperoxia. Second, it may be that a “threshold” of oxidant generation is required to trigger cell death and both NAD(P)H and mitochondrially generated oxidants are required without being sufficient to activate BAX or BAK and induce cell death. Finally, mitochondrially generated oxidants might provide a signal that induces assembly of the NAD(P)H complex on the plasma membrane to amplify the reactive oxygen species signal.
Mice deficient in BAX and BAK in the lung had significantly longer survival and less lung injury than control mice; however, the protection was incomplete. One of several nonexclusive mechanisms might explain these results. First, knockdown of Bax in the lung epithelium after adenoviral Cre infection might have been incomplete and progressive BAX- or BAK-dependent apoptosis of the uninfected epithelium could have allowed for the development of lung injury and mortality. Second, BAX- or BAK-dependent apoptosis in the endothelium, which is relatively resistant to adenoviral infection, may have resulted in lung injury and mortality. Third, the oxidants generated during hyperoxia might have activated inflammatory pathways or produced sufficient metabolic stress to induce lung injury and mortality. Finally, the progressive accumulation of oxidants in the cells might have caused BAX- or BAK-independent cell necrosis in the lung epithelium.
In conclusion, we found that mitochondrial matrix–generated oxidants contribute to the development of hyperoxia-induced mortality in mice. In the lung epithelium, these oxidants activate the intrinsic apoptotic pathway through BAX or BAK to induce cell death. The resulting cell death contributes to the development of lung injury and worsens survival in mice. Neither the formation of the MPTP nor activation of the extrinsic apoptotic pathway plays an important role in the mortality induced by exposure to hyperoxia. Loss of the activator BH3 domain–only proteins BID, BIM, and PUMA alone or in combination does not seem to protect cells against cell death. Our results provide a potential mechanism underlying the protective effects of other interventions that have been shown to attenuate hyperoxia-induced lung injury. For example, growth factors (keratinocyte growth factor and vascular endothelial growth factor) and myristilated Akt have been shown to directly inhibit activation of the intrinsic apoptotic pathway and to provide protection against hyperoxia-induced lung injury (58–60). Similarly, the protection against hyperoxia-induced lung injury conferred by overexpression of the cytokines IL-6, IL-11, and IL-13 has been attributed to their induction of antiapoptotic genes including BCL-2 and A1 (12–14, 61). We speculate that strategies that prevent oxidant-mediated activation of the intrinsic apoptotic pathway might limit lung injury severity in patients with ARDS.
Supplementary Material
Acknowledgments
The authors are grateful to late Dr. Stanley Korsmeyer and Dr. Nika Danial (Dana-Farber Cancer Institute, Boston, MA) for providing the CypD−/−, Bak−/−BaxloxP/loxP, and Bid−/− mice and Dr. Andreas Strasser (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for providing the Puma−/−, Noxa−/−, and Bim−/− mice. The authors thank Dr. Craig Thompson (University of Pennsylvania, Philadelphia, PA) for providing the Bax/Bak null MEFs and Dr. Andreas Villunger (Innsbruck Medical University) for providing the Bim/Puma double knockout MEFs. The adenoviral vector encoding SOD2 was a kind gift of Dr. James Engelhardt (University of Iowa, Iowa City, IA). The authors are grateful to Dr. Danijela Dokic for performing the immunohistochemical analysis.
Supported by National Institutes of Health Grants HL071643–07 (N.S.C), ES013995 (G.R.S.B.), and ES015024 (G.M.M.). This study was supported in part by National Institutes of Health Grants HL071643 (N.S.C., G.M.M., and G.R.S.B.); ES013995 (G.R.S.B); ES015024 (G.M.M.); and AR054796, AR050250, and AI067590 (H.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0181OC on October 19, 2010
Author Disclosure: G.R.S.B. received more than $100,001 from the NIH in research grants. G.M.M. received more than $100,001 from the NIH in grant funding. G.M.M.'s spouse/life partner received more than $100,001 from the NIH in grant funding. D.U. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.J.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.E.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.A.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.P. received more than $100,001 from the NIH in sponsored grants. P.T.S. received more than $100,001 in NIH research grants, more than $100,001 from the Chicago Biomed Consort in research grants, $10,001–$50,000 from the American Academy of Pediatrics as a research grant, and $50,001–$100,000 from the American Heart Association in fellowship grants. M.J. received $,1001–$5,000 from Genentech in advisory board fees, more than $100,001 from Millennium Pharmaceuticals in industry-sponsored grants, and $1,001–$5,000 from the CFF for serving on the Center Committee. N.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 4.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 2002;122:314S–320S. [DOI] [PubMed] [Google Scholar]
- 5.Guinee D Jr, Brambilla E, Fleming M, Hayashi T, Rahn M, Koss M, Ferrans V, Travis W. The potential role of bax and bcl-2 expression in diffuse alveolar damage. Am J Pathol 1997;151:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 6.Bardales RH, Schaefer RF, Hsu SM. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol 1996;149:845–852. [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 1981;256:10986–10992. [PubMed] [Google Scholar]
- 8.Kistler GS, Caldwell PRB, Weibel ER. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol 1967;32:605–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35–56. [PubMed] [Google Scholar]
- 10.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced bax activation and cell death in alveolar epithelial cells. J Biol Chem 2004;279:6753–6760. [DOI] [PubMed] [Google Scholar]
- 11.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004;116:205–219. [DOI] [PubMed] [Google Scholar]
- 12.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 2000;22:535–542. [DOI] [PubMed] [Google Scholar]
- 13.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, et al. Bcl-2-related protein a1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 2005;115:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 2006;12:1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budinger GR, Tso M, McClintock DS, Dean DA, Sznajder JI, Chandel NS. Hyperoxia induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by bcl-2 proteins. J Biol Chem 2002;277:15654–15660. [DOI] [PubMed] [Google Scholar]
- 16.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305:626–629. [DOI] [PubMed] [Google Scholar]
- 17.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol 2001;2:67–71. [DOI] [PubMed] [Google Scholar]
- 18.Forte M, Bernardi P. Genetic dissection of the permeability transition pore. J Bioenerg Biomembr 2005;37:121–128. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005;434:652–658. [DOI] [PubMed] [Google Scholar]
- 20.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434:658–662. [DOI] [PubMed] [Google Scholar]
- 21.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 2005;102:12005–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urich D, Buccellato LJ, Chiarella SE, Eisenbart J, Soberanes S, Hawkins KA, Schumacker PT, Jain M, Chandel NS, Mutlu GM, et al. BAX or BAK mediate mitochondrial superoxide dependent acute lung injury. Am J Respir Crit Care Med 2010;181:A2718. [Google Scholar]
- 23.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in b cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA 2005;102:11272–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to fas-induced hepatocellular apoptosis. Nature 1999;400:886–891. [DOI] [PubMed] [Google Scholar]
- 25.Ogilvy S, Metcalf D, Gibson L, Bath ML, Harris AW, Adams JM. Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood 1999;94:1855–1863. [PubMed] [Google Scholar]
- 26.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic bcl-2 relative bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999;286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 27.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. P53- and drug-induced apoptotic responses mediated by bh3-only proteins puma and noxa. Science 2003;302:1036–1038. [DOI] [PubMed] [Google Scholar]
- 28.Soberanes S, Urich D, Baker CM, Burgess Z, Chiarella SE, Bell EL, Ghio AJ, De Vizcaya-Ruiz A, Liu J, Ridge KM, et al. Mitochondrial complex III-generated oxidants activate ask1 and jnk to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem 2009;284:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res 2004;94:1091–1100. [DOI] [PubMed] [Google Scholar]
- 30.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, et al. Proapoptotic bid is required for pulmonary fibrosis. Proc Natl Acad Sci USA 2006;103:4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soberanes S, Panduri V, Mutlu GM, Ghio A, Budinger GR, Kamp DW. P53 mediates particulate matter-induced alveolar epithelial cell mitochondria-regulated apoptosis. Am J Respir Crit Care Med 2006;174:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of BAX with mitochondrial fission sites, drp1, and mfn2 during apoptosis. J Cell Biol 2002;159:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic bcl-2 family members. PLoS ONE 2009;4:e7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol 2005;37:2478–2503. [DOI] [PubMed] [Google Scholar]
- 35.Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med 2007;42:165–174. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev 2005;19:2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived mclk1+/− mice. J Biol Chem 2008;283:26217–26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 2005;280:18558–18561. [DOI] [PubMed] [Google Scholar]
- 39.Strasser A, Jost PJ, Nagata S. The many roles of fas receptor signaling in the immune system. Immunity 2009;30:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Rosenberg S, Wang H, Imtiyaz HZ, Hou Y-J, Zhang J. Conditional fas-associated death domain protein (FADD):Gfp knockout mice reveal FADD is dispensable in thymic development but essential in peripheral t cell homeostasis. J Immunol 2005;175:3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin X-M. Bid, a bh3-only multi-functional molecule, is at the cross road of life and death. Gene 2006;369:7–19. [DOI] [PubMed] [Google Scholar]
- 42.Green DR, Ferguson TA. The role of fas ligand in immune privilege. Nat Rev Mol Cell Biol 2001;2:917–924. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Ryter SW, Dai C, Tang ZL, Watkins SC, Yin XM, Song R, Choi AM. Necrotic cell death in response to oxidant stress involves the activation of the apoptogenic caspase-8/bid pathway. J Biol Chem 2003;278:29184–29191. [DOI] [PubMed] [Google Scholar]
- 44.Youle RJ, Strasser A. The bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008;9:47–59. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of prosurvival bcl-2 proteins by their bh3-only ligands allows complementary apoptotic function. Mol Cell 2005;17:393–403. [DOI] [PubMed] [Google Scholar]
- 46.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by bcl-2 subfamilies. Nat Cell Biol 2006;8:1348–1358. [DOI] [PubMed] [Google Scholar]
- 47.Galonek HL, Hardwick JM. Upgrading the bcl-2 network. Nat Cell Biol 2006;8:1317–1319. [DOI] [PubMed] [Google Scholar]
- 48.Zong W-X, Li C, Hatzivassiliou G, Lindsten T, Yu Q-C, Yuan J, Thompson CB. BAX and BAK can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 2003;162:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med 2003;35:341–350. [DOI] [PubMed] [Google Scholar]
- 50.Budinger GRS, Sznajder JI. To live or die: a critical decision for the lung. J Clin Invest 2005;115:828–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001;292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational. Cell Death Differ 2002;9:505–512. [DOI] [PubMed] [Google Scholar]
- 53.Vitiello PF, Wu Y-CM, Staversky RJ, O'Reilly MA. P21cip1 protects against oxidative stress by suppressing ER-dependent activation of mitochondrial death pathways. Free Radic Biol Med 2009;46:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, Pollack S, Horowitz S, Davis JM. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol 2001;24:436–441. [DOI] [PubMed] [Google Scholar]
- 55.Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JGN, Natarajan V. Dynamin 2 and c-abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and ROS production in caveolin-enriched microdomains of the endothelium. J Biol Chem 2009;284:34964–34975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AMK. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 2007;282:1718–1726. [DOI] [PubMed] [Google Scholar]
- 57.Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause K-H, Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 2009;180:972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y, Parkyn L, Otterbein LE, Kureishi Y, Walsh K, Ray A, Ray P. Activated akt protects the lung from oxidant-induced injury and delays death of mice. J Exp Med 2001;193:545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang XF, Ray A. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci USA 2003;100:6098–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siner JM, Jiang G, Cohen ZI, Shan P, Zhang X, Lee CG, Elias JA, Lee PJ. VEGF-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J 2007;21:1422–1432. [DOI] [PubMed] [Google Scholar]
- 61.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. Il-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.