Abstract
Tuberculosis (TB) disease remains one of the highest causes of mortality in HIV-infected individuals, and HIV–TB coinfection continues to grow at alarming rates, especially in sub-Saharan Africa. Surprisingly, a number of important areas regarding coinfection remain unclear. For example, increased risk of TB disease begins early in the course of HIV infection; however, the mechanism by which HIV increases this risk is not well understood. In addition, there is lack of consensus on the optimal way to diagnose latent TB infection and to manage active disease in those who are HIV infected. Furthermore, effective point-of-care testing for TB disease remains elusive. This review discusses key areas in the epidemiology, pathogenesis, diagnosis, and management of active and latent TB in those infected with HIV, focusing attention on issues related to high- and low-burden areas. Particular emphasis is placed on controversial areas where there are gaps in knowledge and on future directions of study.
Keywords: tuberculosis, HIV, diagnosis, management, epidemiology
Concurrent infection with HIV and Mycobacterium tuberculosis (MTb) remains a serious and evolving global health crisis. There are 34 million persons infected with HIV worldwide and 15 million are also infected with MTb (1). Tuberculosis disease (TB) is a leading cause of death among HIV-infected persons, and diagnosis of TB remains challenging in HIV-infected persons because of limited resources and atypical presentations. Alarmingly, early reports suggested mortality approached 100% in HIV-infected persons infected with multidrug-resistant (MDR) or extensively drug-resistant (XDR) MTb (2, 3), although more recent reports suggest mortality may not be as high (4). Despite the enormity of the crisis, there remains limited understanding of the underlying mechanisms driving high susceptibility to TB in HIV-infected patients and incomplete and sometimes conflicting clinical data to direct diagnosis and management in coinfected patients.
This review focuses on adult HIV–TB coinfection and emphasizes the current unique and expansive challenges facing this highly vulnerable and expanding population. Particular emphasis is placed on identifying select gaps in knowledge in the understanding of HIV–TB coinfection in the areas of global epidemiological trends, cellular responses, latent infection, diagnosis, and management.
EPIDEMIOLOGY OF HIV–TB COINFECTION
Although the global incidence of TB has stabilized since 2004, data from the World Health Organization (WHO, Geneva, Switzerland) indicate that the percentage of HIV-associated TB is significantly greater than previously estimated, with disease burden in Africa responsible for most of this increase (1). In 2008, there were 9.4 million new cases of TB and 1.78 million deaths from TB worldwide; of these, 1.4 million cases (15%) occurred in HIV-infected individuals, resulting in 0.5 million deaths (28% of total deaths from TB) (5). This estimate, double the 2006 estimate of HIV-associated TB (0.7 million), is the result of increased reporting of HIV prevalence in TB cases, suggesting significant deficiencies in surveillance that may result in further increases in the future, particularly with newer active case-finding approaches (6). The relative risk of developing TB in HIV-positive individuals, compared with HIV-negative individuals, is 21 in high HIV prevalence countries and 37 in low HIV prevalence countries (1). Geographically, sub-Saharan Africa continues to shoulder the vast majority of disease burden. In 2008, 78% of HIV-associated TB cases occurred in Africa, with the highest incidence in South Africa, and 13% of cases occurred in the Southeast Asia region (mainly India) (5).
Despite the global rise in TB incidence in the 1990s attributable to the HIV epidemic and the rapid progression to active TB disease in patients with HIV (7), the overall prevalence of TB has been declining since 1990 (1). This paradox may be explained, in part, by the relatively shorter duration of disease in HIV-infected individuals seen in some communities, with increased mortality (8). Because prevalence is the greatest factor in disease transmission rates, HIV may not be a significant factor contributing to the increase in global transmission rates. However, individual cohort studies have shown HIV-driven increases in TB transmission in some communities (9). Another important factor affecting the impact of HIV on TB disease transmission is the relative infectiousness of coinfected patients. HIV-infected patients have a lower rate of sputum smear positivity, which is the strongest predictor of infectivity (10). However, several reports of nosocomial outbreaks of TB have been reported among HIV-infected individuals (11). Studies of this topic are conflicting; a meta-analysis from 2001 concluded that HIV has no impact on the infectiousness of TB, both in the nosocomial and community settings (11). A study of guinea pigs exposed to air from a TB ward showed that patients coinfected with HIV and TB demonstrated marked variability in infectiousness, and 90% of transmission in this case resulted from a few suboptimally treated patients with MDR TB (12).
Because HIV is associated with both malabsorption of TB drugs (3) and higher rates of TB treatment failure (3), HIV may be a risk factor for TB drug resistance. Although institutional outbreaks of drug-resistant TB have affected primarily HIV-infected patients, including the first report of XDR TB in South Africa (2), whether HIV is an independent risk factor for drug-resistant TB in the community remains unclear. This may be due primarily to the lack of available drug susceptibility testing in most of the world (13). The limited available data, summarized in a meta-analysis review (14), thus far have shown that HIV is a risk factor for primary (transmitted), but not acquired, drug-resistant TB. However, small studies on this topic in various settings have suggested that HIV is a risk for rifampin resistance (15, 16). The most recent analysis from the Global Project on Anti-tuberculosis Drug Resistance reported drug resistance stratified by HIV status from only 7 of 83 countries. Of these, five countries (Cuba, Honduras, Russia [17], Spain, Uruguay) showed no association between MDR TB and HIV. However, two countries—Latvia and Ukraine—reported a significant association, with odds ratios of 2.1 and 1.5, respectively (17). Significantly, the area of greatest burden, Africa, did not report resistance according to HIV status, thus leaving the association between HIV and MDR TB unclear.
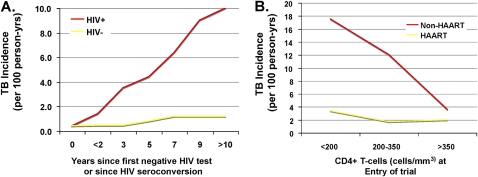
Although the risk of TB disease increases as CD4+ T-cell counts decline (18), TB still occurs at a higher rate in HIV-infected persons with preserved CD4+ T-cell counts. A longitudinal study of South African gold miners who received regular HIV and TB testing showed that within 1 year of HIV seroconversion and in the absence of highly active antiretroviral therapy (HAART) or TB preventive therapy, the incidence of TB doubled compared with HIV-negative peers (19). Although limited in generalizability given the unique susceptibility of this population to inhalational disease, this study nonetheless may provide some important insights into the effects of HIV on TB incidence and transmission in a high-prevalence community. Restricted fragment length polymorphism analysis suggests that the early cases of active TB are due to reactivation and the later cases to primary infection (19). Extension of the study to 11 years of follow-up revealed a persistent, linear increase in TB risk over time and, importantly, that onward transmission, measured by the doubling of TB incidence in HIV-negative workers, contributes to this risk (9) (see Figure 1A).
Figure 1.
Increased incidence of tuberculosis (TB) early in HIV and incomplete protection from TB after HAART. (A) Incidence of TB in South African gold miners who are HIV negative or who have seroconverted to HIV-infected as a function of years since negative HIV test or date of seroconversion. Data show early doubling in incidence of TB among HIV seroconverters after first year and continued rise thereafter. Adapted from Reference 9. (B) Incidence of TB among South African cohorts divided who are HIV-infected and taking or not taking highly active antiretroviral therapy (HAART) therapy, as a function of initial CD4+ T-cell count at commencement of study. Data show that although HAART significantly reduced TB incidence especially among those with low CD4+ T-cells counts, protection is incomplete, and among those with relatively high CD4+ T-cell count, protection is minimal during study period (approximate follow-up, 16 mo). Adapted from Reference 20.
HAART reduces the incidence of TB in HIV-infected individuals by up to 90% (20–22) in studies with follow-up averaging 5 years. Despite this efficacy, there seems to remain a persistently elevated risk of TB above population baseline levels (23) (see Figure 1B). This risk is greatest in those who have the lowest CD4+ T-cell counts at HAART initiation (21, 24). Furthermore, several studies have noted an unchanged or temporary increase in TB incidence in the first 3 months after HAART initiation (24, 25), possibly due to increased case detection and the impact of immune reconstitution. Finally, because HAART increases the life expectancy of HIV-infected persons without eliminating the increased risk of TB, limited data suggest that it is unclear whether HAART alone will significantly decrease the overall community burden of TB, particularly without simultaneous active case finding and widespread institution of isoniazid preventative therapy (IPT) for latent tuberculosis infection (LTBI) (26, 27).
PATHOGENESIS OF HIV–TB COINFECTION
Although the biological synergy between HIV and MTb is well described, a number of aspects concerning the pathogenesis of HIV–TB coinfection are not well understood. It is clear that TB infection greatly impacts the course of HIV disease in several ways. TB disease is associated with a 10-fold increase in serum viral load for a given CD4+ T-cell count (28), and HIV mRNA levels are highest in lung areas with active TB infection (29). MTb infection directly increases viral production in both lymphocytes (30) and alveolar macrophages (31). Severe pulmonary TB infection is also associated with a fall in the CD4+ T-cell count (32). Consequently, TB infection in HIV-infected persons is associated with an almost twofold increase in the risk of death at 1 year (33), with a threefold increase in death for subjects with a CD4+ T-cell count greater than 200 cells/μl (33). Why TB in particular increases the risk of death in early HIV infection, more so than bacterial pneumonias that also occur early in the course of HIV infection and have similar effects on viral replication, is incompletely understood.
It is also well known that the risk of TB is greatly increased in HIV-infected persons, and some of the underlying mechanisms are being elucidated. Effective immunity to TB involves coordination of responses between the innate and adaptive immune systems, both of which are altered by HIV (34). The strongest risk factor for developing TB disease in HIV lies in helper T-cell type 1 (Th1) adaptive immunity, specifically the progressive decline in CD4+ T-cell count associated with advanced HIV (26). In patients with prior TB exposure as assessed by a positive PPD response, the incidence of TB is 2.6%/year for those with a CD4+ T-cell count greater than 350/μl, 6.5%/year for those with a CD4+ T-cell count from 200 to 350/μl, and 13.3%/year for those with a CD4+ T-cell count less than 200/μl (35). With decline of the CD4+ T-cell count, there is also a higher risk of anergy to skin test reactions, suggesting dysfunction of delayed-type hypersensitivity dependent on Th1-type immunity (36). There is also in vitro evidence for qualitative dysfunction of CD4+ T cells in HIV. Compared with TB-infected patients without HIV infection, peripheral blood mononuclear cells from patients coinfected with HIV and TB have decreased proliferative T-cell responses and reduced IFN-γ production to MTb in vitro, whereas antiinflammatory IL-10 production is preserved (37).
However, the observation that TB incidence increases shortly after HIV seroconversion, and before reduction in peripheral blood CD4+ T-cell counts (19), suggests that HIV confers additional mechanisms of susceptibility to TB infection. Investigations into the progression of primary HIV infection to AIDS suggest that primary HIV infection is associated with a precipitous decrease in mucosal CD4+ memory T cells (38), which may set the stage for chronic immune activation and CD4+ T-cell depletion through mucosal translocation of bacteria through the gut (39). Thus, mucosal CD4+ memory T-cell depletion may provide a potential mechanism to account for disrupted T-cell function in early HIV infection, although whether similar events occur in the lung mucosa has not yet been established (40). Indeed, primary HIV infection is associated with decreased PPD-specific IFN-secreting T cells (41, 42) and ESAT (early secreted antigenic target)-6–specific T cells (42) in the blood, suggesting that early depletion of memory T cells may affect specific immunity to TB. Lung lavage enzyme-linked immunospot (ELISPOT) studies also suggest decreased bacillus Calmette-Guérin (BCG)– or PPD-specific pulmonary CD4+ T cells in asymptomatic HIV-infected persons compared with HIV-negative persons (43). HIV–TB coinfection may also be associated with increased serum levels of IL-4, an anti-Th1 type cytokine that hinders immune response to MTb (44). Interestingly, alveolar lavage cells from coinfected individuals may have intact ability to secrete IFN-γ in response to MTb antigens in vitro (45), although this may not translate to equivalent cell function and cell numbers in vivo.
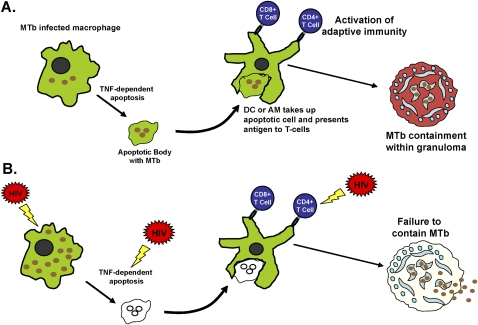
Independent of CD4+ T-cell count, HIV also affects the function of innate immune cells, especially alveolar macrophages (AMs), which serve as the main reservoir for MTb infection (46, 47). MTb has evolved to persist within macrophages in part through prevention of MTb phagosomal fusion with lysosomes, thus preventing intracellular killing of MTb (48, 49). AMs can combat intracellular parasitization by releasing immune-activating cytokines or chemokines, and by programmed cell death or apoptosis (50, 51). Apoptosis benefits the host by promoting intracellular killing of MTb (50, 51) and improving antigen presentation by additional phagocytes to activate adaptive immunity (52, 53) (see Figure 2). Whereas asymptomatic HIV infection does not affect the intracellular growth of MTb (43, 54), AMs from asymptomatic HIV-infected subjects have increased phagocytosis of MTb (54, 55), decreased release of specific cytokines and chemokines (56), and similarly impaired MTb phagosomal maturation (49) compared with AMs from healthy subjects. AMs from HIV-infected subjects also have decreased apoptosis in response to MTb (55) (see Figure 2); the mechanism may involve increased lung levels of IL-10 in HIV, which up-regulates BCL-3 (B-cell lymphoma 3–encoded protein), an apoptosis inhibitor (57). HIV infection of macrophages also inhibits autophagy (58), another cellular process that may be critical for macrophage intracellular killing of MTb (59).
Figure 2.
Immunity against Mycobacterium tuberculosis (MTb) and the effects of HIV. (A) Alveolar macrophages (AMs) are the first cells to encounter and engulf MTb bacteria when they are inhaled deeply into the lungs. MTb bacteria have evolved to escape intracellular killing by AMs by arresting phagosomal maturation and possibly escaping the phagosome to allow for persistence and growth within AMs. The defense mechanisms against this include chemokines/cytokine secretion which activate antimycobacterial defenses and adaptive immunity (56, 142, 143), autophagy (59), and apoptosis (51) among others. (B) HIV is known to affect a number of these steps, including increased phagocytosis of MTb to allow access to intracellular environment (54, 55), decreased AM apoptosis (55) in response to MTb, decreased autophagy (58), and decreased chemokine/cytokine production (56). HIV also affects function and numbers of CD4+ T cells, leading to increased bacillary loads, inadequate granuloma formation, and dissemination (144). DC = dendritic cell; TNF = tumor necrosis factor.
As stated previously, HAART reduces the risk of TB in HIV (20–22), but not to the level of non–HIV-infected subjects (23). In vitro studies have found qualitative impairment in the T-cell response to PPD in patients receiving HAART therapy (undetectable viral load with CD4+ T-cell count > 300 cells/μl), with decreased percentages of PPD-specific IFN-γ–producing T cells when compared with HIV-negative patients (41). This provides further evidence that immune recovery with HAART is incomplete.
LATENT TB INFECTION IN HIV
In patients with HIV and latent TB infection (LTBI), the rate of progression to active tuberculosis disease is 5–8%/year, compared with a 10% lifetime risk in the general population (7). Among risk factors for progression, HIV (relative risk [RR], 9.9) ranks the highest compared with old healed tuberculosis (RR, 5.2), chronic renal failure (RR, 2.4), or infliximab therapy (RR, 2.0) (60). HIV is also associated with higher rates of extrapulmonary and disseminated TB disease. For these reasons, LTBI testing and treatment in HIV-infected patients is a priority, and this is apparent in the available treatment guidelines from low-burden countries, as reflected by Centers for Disease Control and Prevention (CDC, Atlanta, GA) recommendations (61), and in high-burden countries, as reflected by WHO recommendations in the “Three I's” strategy (62). The “Three I's” of Isoniazid Preventive Treatment, Intensified case finding for active TB, and TB Infection control are key public strategies focused on decreasing the impact of TB on people living with HIV (62).
The diagnosis of LTBI requires a positive tuberculin skin test (TST) or IFN-γ release assay (IGRA), or a compelling history of likely infection such as recent close contact (61). In HIV-infected individuals, a positive TST is defined as an induration of at least 5 mm in response to intradermal placement of PPD. IGRAs are more recently developed assays that detect in vitro IFN-γ release by peripheral blood monocytes in response to MTb-specific peptides (61). However, controversy exists as to the equivalence of a positive IGRA and TST. Given the T-cell defects of HIV-infected patients, both of these tests underperform in this setting, and render false negative results, but this should not preclude routine screening (61, 63). In addition, retesting after reconstitution of the immune system in patients receiving HAART has merit as LTBI test performance improves as CD4+ T-cell counts recover to greater than 200 cells/mm3 (61).
IGRAs, the QuantiFERON-TB Gold In-Tube (QFT; Cellestis Limited, Carnegie, Victoria, Australia) and T-SPOT.TB (Oxford Immunotec Ltd, Abingdon, UK) tests, are commercially available worldwide. Compared with TST, IGRAs offer the advantage of a lower false positive rate due to BCG vaccination, better standardization of testing, and lack of need for a repeat visit (63). However, sensitivity may be similar to TST (64). Studies generally suffer from the lack of a “gold standard” in the diagnosis of LTBI, and therefore data are extrapolated from results in patients with active TB. In this regard, the T-SPOT.TB, an ELISPOT assay, may have improved sensitivity compared with the QFT (64), and in one small series from Zambia, T-SPOT.TB had 90% sensitivity in HIV-infected patients with active TB (65). Of major concern, however, is that there are few longitudinal studies of patients with positive IGRA assays, and therefore, it is unclear how these sensitivities predict the risk of developing TB disease with LTBI. One study from Austria found QFT to have a 90.9% rate of predicting active TB disease in HIV-infected subjects. However, this study was limited by its low-prevalence setting, as active TB disease occurred in only 3 of 37 QFT-positive subjects (a rate of 8.1%) (66). A comparison of the tests for LTBI is presented in Table 1.
TABLE 1.
COMPARISON OF TESTS FOR LATENT TUBERCULOSIS INFECTION
| TST | QFT | T-SPOT.TB | |
|---|---|---|---|
| Relative sensitivity (drops with decreasing CD4+ T-cell count) | ++ | ++* | +++* |
| Specificity | + (for BCG vaccinated); +++ (for non-BCG) | +++ | +++ |
| Benefit of treating positives by IPT | Yes† | Unclear† | Unclear† |
| Reproducibility | + | +++ | +++ |
| Costs | + | +++ | +++ |
| Laboratory infrastructure required | No | Yes | Yes |
| Need for repeat visit | Yes | No | No |
| Trained personnel required | + | ++ | +++ |
Definition of abbreviations: IGRA = IFN-γ release assay; IPT = isoniazid preventive therapy; LTBI = latent tuberculosis infection; QFT = QuantiFERON-TB Gold In-Tube test; TST = tuberculin skin test.
Adapted from Reference 61.
+, indicates a comparison with other tests in Table 1.
Data are based on studies of persons with active TB disease, which may or may not correlate with persons having LTBI. Data are suggestive that QFT has a sensitivity similar to TST, while T-SPOT.TB may have increased sensitivity compared to TST, and the sensitivity of all three tests decreases with decreasing CD4+ T-cell count. However, prospective studies are needed in subjects with HIV to confirm that these findings can be applied to evaluating risk and initiating IPT.
There are no prospective trials of IGRAs evaluating the benefit of IPT.
There are few studies that specifically compare the accuracy of TST and IGRA for screening for LTBI in HIV-infected populations. These studies show a number of discordant results, where one test is positive and the other negative (65, 67, 68). TST-positive/IGRA-negative results may be explained, in part, by BCG vaccination although the rate of false negatives is unclear. It is similarly unclear whether TST-negative/IGRA-positive tests are true positives due to better IGRA sensitivity or false positives. For example, one study in a low TB prevalence country (the United States), performed by Luetkemeyer and colleagues, found that the QFT and TST were concordant in only 28% of patients with a positive result of either test (67). In addition, 16% of subjects from this study with a CD4+ T-cell count less than 100 cells/mm3 had an indeterminant QFT result (defined as a lack of interferon response to the assay's positive control, making the test uninterpretable).
A study in a high TB prevalence area (South Africa), performed by Rangaka and colleagues, found that the IGRA tests had a higher rate of positivity in HIV-infected persons compared with TST (69), and that the T-SPOT.TB had a significantly higher positivity rate than QFT or TST among subjects with CD4+ T-cell counts less than 250 cells/mm3. Also observed was a poor correlation between the IGRA and the TST in HIV-uninfected persons (69). Similar results were obtained by Dheda and colleagues (70) and Hoffman and colleagues (71) in low TB prevalence areas in HIV-infected subjects. These data suggest that the T-SPOT.TB ELISPOT may have the highest sensitivity for LTBI in HIV-infected subjects and therefore may be the best option for screening in this population. However, more studies with longitudinal follow-up are needed to determine how predictive T-SPOT.TB ELISPOT testing is for the development of active TB. In addition, expense, need for trained operators and specific laboratory equipment, may limit its utility in resource-poor settings. Because of incomplete and sometimes conflicting data on the IGRAs, national guidelines differ on their use. Some recommend using either TST or IGRA (61), whereas others suggest use of the IGRA as an adjunct to TST (72–74).
Before initiating treatment of LTBI, it is important to first rule out active TB disease, which can be difficult in HIV-infected patients because of atypical presentations (61). A large study of HIV-infected subjects in Southeast Asia suggests that a screening strategy focusing on three questions (cough for any duration, fever, and night sweats lasting 3 wk or more) is adequate. Absence of these symptoms accurately rules out TB in the vast majority of patients (75) and this may in turn allow for safe initiation of IPT. Similar results were obtained in a study from South Africa emphasizing that absence of cough alone is inadequate for ruling out TB in HIV (76). The current recommendations for LTBI treatment in HIV-infected patients are isoniazid prophylaxis therapy (IPT) for 9 months; 6 months has reduced efficacy (61). Several groups have shown that treatment of LTBI in HIV-infected patients is both safe and effective in preventing tuberculosis reactivation in both low TB prevalence areas (77) and high TB prevalence areas (78), without evidence of increased drug resistance (79–81). However, in a study by Grant and colleagues, overall TB incidence in the population remained high despite prophylactic therapy (79). Data also suggest that primary prophylaxis of LTBI coupled with secondary prophylaxis of previously infected individuals may provide an additional benefit in high-prevalence areas (82, 83). However, few high-prevalence countries follow these practices because of concerns regarding drug toxicity and resistance, nonadherence, poor coordination of HIV and TB programs, and reinfection issues (84). Optimal management of LTBI likely involves concurrent initiation of HAART and IPT (83). Studies suggest that concurrent HAART with IPT carries no increase in drug toxicity (85). Therefore, HIV antiretroviral therapy clinics may represent an ideal setting to provide IPT (83). However, there are clear concerns in having patients potentially coinfected with HIV and TB in the same area as other HIV-infected individuals.
ACTIVE TB DISEASE DIAGNOSIS IN HIV
The diagnostic imperative for HIV-infected patients regarding active tuberculosis remains early and accurate detection of TB and drug-resistant TB. This task demands sensitive, specific, and relatively rapid testing algorithms and tools, and is made more challenging by the altered clinicopathological presentation of HIV-associated TB (76, 86, 87).
In the presence of HIV coinfection, the clinical presentation of active TB is increasingly modified as the CD4+ T-cell count declines (88). Clinicians evaluating patients with HIV should recognize the importance of testing for active TB in patients presenting with nonspecific symptoms. An especially challenging aspect of this is in recognizing atypical presentations in patients with undiagnosed HIV presenting with TB as their first opportunistic infection. Not only are HIV-associated TB symptoms more diverse than in non-HIV TB, but the differential diagnosis of conventional cough, fever, and malaise is much more extensive in the presence of HIV coinfection (89). For this reason, if an HIV-infected patient suspected of having TB is not shown to have active TB disease, that patient should not necessarily proceed straight to IPT, but rather remain under evaluation until the cause of their symptoms is revealed (which could still include active TB) or the symptoms resolve. Conventional radiological hallmarks of pulmonary TB such as cavitation and apical localization are also less common (88). Sputum production is often attenuated, which compromises collection of adequate diagnostic specimens, and culture-positive pulmonary TB is more frequently smear negative (88, 90, 91). For example, 69% of HIV-infected persons diagnosed with active TB were smear negative in one study (92). HIV-infected patients also more commonly contract extrapulmonary TB (EPTB) (50 vs. 15%), which evades or delays diagnosis, and are more likely to have disseminated disease (10 vs. 1%) (93).
Although smear microscopy is less frequently positive in patients infected with HIV and TB, advances in fluorescence microscopy (94–97) and optimization of smear preparation (98–101), coupled with important steps toward so-called front-loaded microscopy, in which two samples are taken on the same day (102), ensure that smear microscopy remains the mainstay of diagnosis of TB globally. However, new diagnostic tools for the detection of TB and drug-resistant TB have been developed on the basis of both phenotypic and genotypic methods. Liquid culture, which is more sensitive and rapid than TB culture on solid media (making it particularly useful in HIV coinfection) (103–105), has been endorsed by the WHO (62) and there are both commercial and noncommercial methods available. Culture requires a greater incubation time compared with that for non–HIV-infected patients, consistent with lower bacillary load of sputum (106). The study from Southeast Asia suggests that in HIV-infected subjects with a positive screen for TB (presence of cough, fever, or night sweats), TB could be reliably ruled out only with a negative sputum culture, although TB was relatively uncommon in patients with two negative smears, negative chest radiograph, and a CD4+ T-cell count equal to or exceeding 350 cells/mm3 (75). Molecular (polymerase chain reaction [PCR]– or gene probe–based) tests generally perform less well on lower bacillary load smear-negative samples (107), suggesting lower sensitivity with HIV. However, one study showed high sensitivity for detecting TB in smear-positive sputum from HIV-infected subjects, using the AMPLICOR PCR (Roche Diagnostic Systems, Branchburg, NJ) (99.7%), and no significant difference in sensitivity compared with smear-negative culture-positive specimens from HIV-negative subjects (89 vs. 95%) (108). Urine detection of the MTb cell wall component lipoarabinomannan may improve detection in smear-negative HIV-infected patients with advanced immunosuppression in a high-prevalence setting (109, 110). Serological tests currently have no proven role in the diagnosis of active TB with or without HIV (111) and IFN-γ release assays (IGRAs) do not help to distinguish latent from active TB (63).
Large studies comparing diagnostic approaches to HIV-associated EPTB are limited. Because most of this burden falls on resource-limited settings, there are significant obstacles in the diagnosis of EPTB. These include limited imaging capacity, limited ability to obtain adequate tissue specimens (which could potentially require laparoscopic peritoneal biopsy, computed tomography–guided biopsy, or mediastinoscopy), and limited availability of DNA amplification technology.
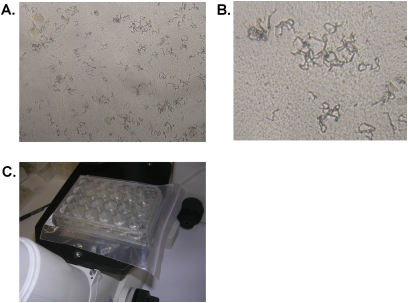
Although it is controversial whether HIV per se is associated with drug resistance, the potential for rapid progression of untreated or inadequately treated HIV-associated TB is clear, and thus there is a need for rapid detection and treatment of drug-resistant TB in HIV-infected patients (33). Drug resistance can be identified phenotypically by culture-based methods or (for some agents) genotypically by molecular methods to determine the presence or absence of resistance-conferring gene mutations. The performance of drug susceptibility–testing methods in patients infected with HIV and TB does not differ significantly from that for non-HIV populations, although methods such as the microscopic observation drug susceptibility (MODS) assay demonstrated to be effective when performed directly on smear-negative, culture-positive sputum samples do confer a time advantage (see Figure 3) (112). The MODS assay may also be effective as part of a screening strategy to rule out active MTb disease before initiating IPT (113) and has been recommended by the WHO for interim use until capacity for genotypic or automated liquid culture susceptibility testing is available (114, 115). Molecular methods, such as line probe assays (116), can offer rapid detection of isoniazid and rifampicin resistance from smear-positive sputum samples and culture isolates, and are now recommended by the WHO (117). Amplification of DNA from paraffin-embedded biopsy samples can also identify MTb and drug-resistant EPTB if cultures are not helpful (118).
Figure 3.
The microscopic-observation drug susceptibility (MODS) assay. (A) Typical cording formation characterized by M. tuberculosis growth in liquid medium visualized by an inverted light microscope at an original magnification of ×400. (B) Magnification of typical cords of MTb. (C) Picture of MODS culture plate. Cultures are prepared in a 24-well tissue culture plate in six columns of four wells each. Each column of four wells is used for a single sample–two wells are drug free and one each contains rifampicin and isoniazid. Six columns allow for five samples per plate and one negative control column. Plates are permanently sealed inside Ziploc bags after inoculation to avoid cross-contamination and for safety and are examined within bags daily for 15 days and then on alternate days until Day 21. Nontuberculous mycobacteria do not form cords, except for M. chelonae, which can be identified by rapid growth.
Inexpensive and easily administered point-of-care tests remain a desirable goal but are not currently available. All existing diagnostic tests and strategies require infrastructure (with varying safety, maintenance, and cost implications) and personnel (who need to be trained and retained) and a strong quality assurance system. These remain huge challenges common to all diagnostic test implementation programs even before consideration of unit costs for tests, and whom to target for testing. However, developments using gene amplification technology (Xpert MTB/RIF; Cepheid, Sunnyvale, CA) that can detect MTb and rifampin resistance simultaneously from sputum samples in a rapid assay (90 min), with minimal training required, with high sensitivity in smear-negative samples (70% when using one Xpert cartridge), and with minimal requirement for biosafety facilities represent a significant advance, although expense may still be too great for resource-poor settings (119).
MANAGEMENT OF HIV–TB COINFECTION
The standard treatment of drug-susceptible active TB in HIV-infected patients does not differ significantly from the standard regimen for HIV-uninfected patients. It consists of an initial phase of four drugs (isoniazid, rifampin, ethambutol, and pyrazinamide) for 2 months, followed by a continuation phase of isoniazid and rifampin for 4 to 6 months (120). However, there are several considerations in administering this regimen to HIV-infected persons. Several aspects of this topic have been reviewed in detail elsewhere (120, 121). In resource-poor settings, because of cost constraints, rifampin is sometimes omitted in the continuation phase. However, there is clear evidence that continuation phase non–rifampin-based regimens are associated with higher relapse rates (122–124).
The optimal duration of anti-TB treatment in patients coinfected with HIV and TB is controversial. There is some evidence to suggest that longer treatment regimens reduce relapse rates (125, 126) but not survival (127, 128) and a meta-analysis suggests no statistically significant benefit beyond 6 months of therapy (123). However, more studies are required to resolve this controversy, particularly because the influence of early HAART on treatment duration is not well understood. Because of the concerns of added complexity in treating HIV-infected patients differently from non–HIV-infected patients, WHO and CDC guidelines recommend at least 6 months of standard daily short-course therapy and suggest that therapy should be similar regardless of HIV infection status (61, 129). Longer courses may be used in those with extensive disease, sputum positivity at 2 months with confirmed drug-sensitive TB, and in high-burden settings in retreatment cases as part of an 8-month regimen with a 5-month continuation phase. It should also be noted that adverse events in response to antituberculous drugs are more common in HIV-coinfected patients compared with uninfected persons and in those HIV-infected persons with lower CD4+ T-cell counts (130), which may contribute to high rates of nonadherence in high-prevalence settings (131).
There is some controversy as to the timing of HAART and TB therapy in coinfected patients. The three main concerns for concurrent HAART and anti-TB treatment include overlapping toxicities, drug interactions, and immune reconstitution inflammatory syndrome (IRIS). The main overlapping toxicities are skin reactions, hepatitis, peripheral neuropathy, and gastrointestinal side effects. An important strategy for managing toxicities includes initiating anti-TB treatment shortly before initiating HAART, thus ensuring tolerability. The interactions between HAART and anti-TB treatment are complex and reviewed in detail elsewhere (132). Drug interactions arise from rifampin and, to a lesser extent, rifabutin-associated induction of several enzyme systems including the P450 cytochrome system (132) (see Table 2). This results in a variable reduction of protease inhibitor (PI), nonnucleoside reverse transcription inhibitor (NNRTI), integrase inhibitor, and fusion inhibitor levels, but not levels of nucleoside reverse transcriptase inhibitors (NRTIs). Hence the WHO recommends that the first-line regimen should be two NRTIs plus one NNRTI (typically efavirenz). Furthermore, when a PI is required and rifabutin is not available (often due to cost in high-prevalence settings), ritonavir-boosted lopinavir or saquinavir is recommended in conjunction with rifampicin-based anti-TB treatment. This regimen should be closely monitored for treatment failure (129). Significantly, work by the Clinton Foundation has lowered the price of rifabutin, which may make this drug more accessible in resource-limited settings (133).
TABLE 2.
SUMMARY OF INTERACTIONS BETWEEN RIFAMYCINS AND ANTIRETROVIRALS
| Rifampin | Rifabutin | |
|---|---|---|
| Cytochrome P450 interaction | +++ | + |
| Interactions with PIs | ++++, recommended for use only with a regimen that includes two PIs | ++, PIs boost Rif levels requiring reduction of dose |
| Interactions with NNRTIs | +, slightly decreases levels of efavirenz, but is favored regimen | ++, decreased levels of Rif with efavirenz requiring increased Rif dose. Should not be used with delaviridine |
| Interactions with CCR-5 receptor antagonists | + | + |
| Interactions with integrase inhibitors | + | + |
| Cost and availability | ++ | ++++, expensive and less available in resource-poor settings |
| Efficacy | ++++ | ++++ |
Definition of abbreviations: NNRTIs = nonnucleoside reverse transcription inhibitors; PIs = protease inhibitors; Rif = Rifabutin.
Paradoxical IRIS occurs when clinical deterioration, not due to other opportunistic infection, drug resistance, or relapse, occurs in individuals who commence HAART while already undergoing antituberculous therapy (134, 135). By contrast, unmasking IRIS occurs when paucisymptomatic individuals who commence HAART develop clinical features of active TB, although it may be impossible to determine whether the event is due to recent infection or subclinical disease merely unmasked by immune reconstitution. In either case, and by definition, it is imperative to exclude nonadherence, drug-resistant TB, and other opportunistic infections (134–136). The median duration from treatment initiation to the development of paradoxical IRIS is about 4 to 6 weeks. It is associated with a short interval between TB treatment initiation and HAART, low CD4+ T-cell counts, rapid reduction in viral load, and disseminated TB and may be life-threatening (134). Most cases, however, can be managed symptomatically (antipyretics and antiinflammatory agents), although in a minority of cases, aspiration of sterile abscesses, discontinuation of HAART, or initiation of corticosteroids may be required. A randomized controlled trial by Meintjes and colleagues suggested that corticosteroids reduce the need for hospitalization and procedures, and resulted in symptom improvement in patients with IRIS (137).
Given these considerations, the psychological burden of dealing with two diseases, and the high associated pill burden, initiation of HAART has been delayed in some patients coinfected with TB and HIV, particularly those with higher CD4+ T-cell counts. However, in high-burden countries, mortality is high in coinfected individuals immediately after TB diagnosis, suggesting specific HIV therapy may be of further benefit (138). Hence, the WHO recommends that HAART be commenced as soon as possible after initiation of TB treatment (and within the first 8 wk) in all HIV-infected individuals with active TB, regardless of CD4+ T-cell count (139). These guidelines are based, in part, on a randomized controlled trial of HIV-infected individuals with CD4+ T-cell counts not exceeding 500 cells/ml, which showed a mortality benefit of integrated HAART and TB therapy (average HAART commenced about 70 d after anti-TB therapy) compared with sequential therapy (HAART commenced after TB therapy was completed) in patients both below and above the cutoff of 200 cells/μl (140). Although not published, preliminary results of the CAMELIA (Cambodian Early versus Late Introduction of Antiretroviral Drugs) trial (available at www.nih.gov/news/health/jul2010/niaid-22.htm) showed a 33% reduction in mortality in coinfected subjects with a CD4+ T-cell count less than 200 cells/mm3 begun on HAART 2 weeks after anti-TB therapy compared with 8 weeks after anti-TB therapy. This study and additional prospective studies, such as the A5221, of the Adult AIDS Clinical Trials Group of the Division of AIDS (141), may further refine these guidelines, and further study is needed to determine whether they are applicable to high- and low-resource settings. Last, data are required to guide the initiation of HAART in patients who have MDR and XDR TB, although preliminary data indicate that HAART is relatively well tolerated with second-line anti-TB drugs (4).
CONCLUSION
Increasing rates of HIV–MTb coinfection worldwide pose not only an enormous threat to the HIV-infected population, but may also pose a threat to the non–HIV-infected population especially in resource-limited settings. Despite the magnitude of the problem, our understanding of the underlying causes of coinfection and our ability to combat it remain limited. This review highlights a number of areas where further study may greatly improve efforts to prevent and treat coinfection. In particular, several key areas such as impact of HAART on TB risk, diagnosis and treatment of LTBI in resource-poor settings, and rapid point-of-care diagnosis for active TB disease with susceptibility testing, optimal timing of treatment of coinfection, and management of IRIS are areas of critical need and the subjects of ongoing research. Significant progress has been made, but much remains to be done. Given the enormity of the problem, it is possible that targeted study and implementation of simple interventions have the potential to provide tremendous benefit in the care of these patients, especially in resource-poor settings.
Supplementary Material
Supported by NIH T32-HL007874 (A.A.), NIH K08AI064014 and an ALA biomedical research grant (N.R.P.), and NIH R01 HL063655 (H.K.).
All authors contributed to the research, writing and preparation of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201008-1246CI on December 22, 2010
Author Disclosure: J.K. has received lecture fees from Abbot and an industry-sponsored from Wyeth. A.A. has received a training grant from the NIH (2008–2010). N.P. has received consultancy fees from Cubist Pharmaceuticals, and holds stock in Pfizer. K.D., H.K., and D.M. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.World Health Organization. Global tuberculosis control: epidemiology, strategy, financing. WHO report 2009. Geneva, Switzerland: WHO; 2009.
- 2.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–1580. [DOI] [PubMed] [Google Scholar]
- 3.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis 2007;196:S86–S107. [DOI] [PubMed] [Google Scholar]
- 4.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, Willcox P, John MA, Reubenson G, Govindasamy D, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010;375:1798–1807. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global tuberculosis control: a short update to the 2009. report. Geneva, Switzerland: WHO; 2009.
- 6.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, Williams BG, Munyati SS, Butterworth AE, Mason PR, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010;376:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 1989;320:545–550. [DOI] [PubMed] [Google Scholar]
- 8.Elliott AM, Halwiindi B, Hayes RJ, Luo N, Mwinga AG, Tembo G, Machiels L, Steenbergen G, Pobee JO, Nunn PP, et al. The impact of human immunodeficiency virus on response to treatment and recurrence rate in patients treated for tuberculosis: two-year follow-up of a cohort in Lusaka, Zambia. J Trop Med Hyg 1995;98:9–21. [PubMed] [Google Scholar]
- 9.Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS 2008;22:1859–1867. [DOI] [PubMed] [Google Scholar]
- 10.Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: the effects of chemotherapy. Tubercle 1976;57:275–299. [DOI] [PubMed] [Google Scholar]
- 11.Cruciani M, Malena M, Bosco O, Gatti G, Serpelloni G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis 2001;33:1922–1930. [DOI] [PubMed] [Google Scholar]
- 12.Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, Noakes C, Martinez C, Sheen P, Ramirez R, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med 2009;6:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Anti-tuberculous drug resistance in the world. Report No. 4. Geneva, Switzerland: WHO; 2008.
- 14.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS One 2009;4:e5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernon A, Burman W, Benator D, Khan A, Bozeman L; Tuberculosis Trials Consortium. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Lancet 1999;353:1843–1847. [DOI] [PubMed] [Google Scholar]
- 16.Sandman L, Schluger NW, Davidow AL, Bonk S. Risk factors for rifampin-monoresistant tuberculosis: a case–control study. Am J Respir Crit Care Med 1999;159:468–472. [DOI] [PubMed] [Google Scholar]
- 17.Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, Hoffner S, Drobniewski F, Barrera L, van Soolingen D, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the global project on anti-tuberculosis drug resistance surveillance. Lancet 2009;373:1861–1873. [DOI] [PubMed] [Google Scholar]
- 18.van der Sande MA, Schim van der Loeff MF, Bennett RC, Dowling M, Aveika AA, Togun TO, Sabally S, Jeffries D, Adegbola RA, Sarge-Njie R, et al. Incidence of tuberculosis and survival after its diagnosis in patients infected with HIV-1 and HIV-2. AIDS 2004;18:1933–1941. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005;191:150–158. [DOI] [PubMed] [Google Scholar]
- 20.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002;359:2059–2064. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS 2005;19:2109–2116. [DOI] [PubMed] [Google Scholar]
- 22.Miranda A, Morgan M, Jamal L, Laserson K, Barreira D, Silva G, Santos J, Wells C, Paine P, Garrett D. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One 2007;2:e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawn SD, Bekker LG, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS 2005;19:1113–1124. [DOI] [PubMed] [Google Scholar]
- 24.Girardi E, Sabin CA, d'Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, Dabis F, Reiss P, Kirk O, Bernasconi E, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 2005;41:1772–1782. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet MM, Pinoges LL, Varaine FF, Oberhauser BB, O'Brien DD, Kebede YY, Hewison CC, Zachariah RR, Ferradini LL. Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS 2006;20:1275–1279. [DOI] [PubMed] [Google Scholar]
- 26.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science 2003;301:1535–1537. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Wood R. Tuberculosis control in South Africa—will HAART help? S Afr Med J 2006;96:502–504. [PubMed] [Google Scholar]
- 28.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis 2003;188:1146–1155. [DOI] [PubMed] [Google Scholar]
- 29.Nakata K, Rom WN, Honda Y, Condos R, Kanegasaki S, Cao Y, Weiden M. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med 1997;155:996–1003. [DOI] [PubMed] [Google Scholar]
- 30.Goletti D, Weissman D, Jackson RW, Graham NM, Vlahov D, Klein RS, Munsiff SS, Ortona L, Cauda R, Fauci AS. Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol 1996;157:1271–1278. [PubMed] [Google Scholar]
- 31.Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse DB, Shioda T, Rom WN, Weiden M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med 2002;195:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilheu JA, De Salvo MC, Gonzalez J, Rey D, Elias MC, Ruppi MC. Cd4+ T-lymphocytopenia in severe pulmonary tuberculosis without evidence of human immunodeficiency virus infection. Int J Tuberc Lung Dis 1997;1:422–426. [PubMed] [Google Scholar]
- 33.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, Mugerwa RD, Ellner JJ. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 2000;14:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel NR, Koziel H. Lung defenses in the immunosuppressed patient. In: Agusti C, Torres A, editors. Pulmonary infection in the immunocompromised patient. Oxford: Wiley-Blackwell; 2009.
- 35.Antonucci G, Girardi E, Raviglione MC, Ippolito G; Gruppo Italiano di Studio Tubercolosie AIDS (GISTA). Risk factors for tuberculosis in HIV-infected persons: a prospective cohort study. JAMA 1995;274:143–148. [DOI] [PubMed] [Google Scholar]
- 36.Markowitz N, Hansen NI, Wilcosky TC, Hopewell PC, Glassroth J, Kvale PA, Mangura BT, Osmond D, Wallace JM, Rosen MJ, et al. Tuberculin and anergy testing in HIV-seropositive and HIV-seronegative persons: pulmonary complications of HIV infection study group. Ann Intern Med 1993;119:185–193. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest 1994;94:2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol 2006;7:235–239. [DOI] [PubMed] [Google Scholar]
- 39.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 40.Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, Hill BJ, Hage CA, Brahmi Z, Khoruts A, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol 2008;1:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland R, Yang H, Scriba TJ, Ondondo B, Robinson N, Conlon C, Suttill A, McShane H, Fidler S, McMichael A, et al. Impaired IFN-γ-secreting capacity in mycobacterial antigen–specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS 2006;20:821–829. [DOI] [PubMed] [Google Scholar]
- 42.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M, et al. Early depletion of Mycobacterium tuberculosis–specific T helper 1 cell responses after HIV-1 infection. J Infect Dis 2008;198:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med 2009;180:1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dheda K, Chang JS, Breen RA, Haddock JA, Lipman MC, Kim LU, Huggett JF, Johnson MA, Rook GA, Zumla A. Expression of a novel cytokine, IL-4δ2, in HIV and HIV–tuberculosis co-infection. AIDS 2005;19:1601–1606. [DOI] [PubMed] [Google Scholar]
- 45.Dheda K, van Zyl-Smit RN, Meldau R, Meldau S, Symons G, Khalfey H, Govender N, Rosu V, Sechi LA, Maredza A, et al. Quantitative lung T cell responses aid the rapid diagnosis of pulmonary tuberculosis. Thorax 2009;64:847–853. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol 2001;1:20–30. [DOI] [PubMed] [Google Scholar]
- 47.Russell DG. Mycobacterium tuberculosis: Here today, and here tomorrow. Nat Rev Mol Cell Biol 2001;2:569–577. [DOI] [PubMed] [Google Scholar]
- 48.Brown CA, Draper P, Hart PD. Mycobacteria and lysosomes: a paradox. Nature 1969;221:658–660. [DOI] [PubMed] [Google Scholar]
- 49.Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, Molyneux ME, Squire SB. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol 2004;172:4592–4598. [DOI] [PubMed] [Google Scholar]
- 50.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol 2000;164:2016–2020. [DOI] [PubMed] [Google Scholar]
- 51.Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand–induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol 1998;160:5448–5454. [PubMed] [Google Scholar]
- 52.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 2003;9:1039–1046. [DOI] [PubMed] [Google Scholar]
- 53.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 2006;24:105–117. [DOI] [PubMed] [Google Scholar]
- 54.Day RB, Wang Y, Knox KS, Pasula R, Martin WJ II, Twigg HL III. Alveolar macrophages from HIV-infected subjects are resistant to Mycobacterium tuberculosis in vitro. Am J Respir Cell Mol Biol 2004;30:403–410. [DOI] [PubMed] [Google Scholar]
- 55.Patel NR, Zhu J, Tachado SD, Zhang J, Wan Z, Saukkonen J, Koziel H. HIV impairs TNF-α mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol 2007;179:6973–6980. [DOI] [PubMed] [Google Scholar]
- 56.Saukkonen JJ, Bazydlo B, Thomas M, Strieter RM, Keane J, Kornfeld H. β-Chemokines are induced by Mycobacterium tuberculosis and inhibit its growth. Infect Immun 2002;70:1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel NR, Swan K, Li X, Tachado SD, Koziel H. Impaired M. tuberculosis–mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J Leukoc Biol 2009;86:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 2009;186:255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004;119:753–766. [DOI] [PubMed] [Google Scholar]
- 60.Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004;350:2060–2067. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009;58:1–207; quiz CE201–204. [PubMed] [Google Scholar]
- 62.World Health Organization. Report of a WHO joint HIV and TB department meeting. Report from the WHO's three I's meeting: Intensified case finding (ICF), isoniazid preventative therapy (IPT) and TB infection control (IC) for people living with HIV. Geneva, Switzerland: WHO; 2008.
- 63.Mazurek M, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon γ release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010;59:1–25. [PubMed] [Google Scholar]
- 64.Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, Reece WH, Mwinga A, Godfrey-Faussett P, Lalvani A. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis–specific T cells. AIDS 2002;16:2285–2293. [DOI] [PubMed] [Google Scholar]
- 66.Aichelburg MC, Rieger A, Breitenecker F, Pfistershammer K, Tittes J, Eltz S, Aichelburg AC, Stingl G, Makristathis A, Kohrgruber N. Detection and prediction of active tuberculosis disease by a whole-blood interferon-γ release assay in HIV-1–infected individuals. Clin Infect Dis 2009;48:954–962. [DOI] [PubMed] [Google Scholar]
- 67.Luetkemeyer AF, Charlebois ED, Flores LL, Bangsberg DR, Deeks SG, Martin JN, Havlir DV. Comparison of an interferon-γ release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med 2007;175:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talati NJ, Seybold U, Humphrey B, Aina A, Tapia J, Weinfurter P, Albalak R, Blumberg HM. Poor concordance between interferon-γ release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis 2009;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, Mouton P, Diwakar L, Connell TG, Maartens G, et al. Effect of HIV-1 infection on T-cell–based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med 2007;175:514–520. [DOI] [PubMed] [Google Scholar]
- 70.Dheda K, Lalvani A, Miller RF, Scott G, Booth H, Johnson MA, Zumla A, Rook GA. Performance of a T-cell–based diagnostic test for tuberculosis infection in HIV-infected individuals is independent of CD4 cell count. AIDS 2005;19:2038–2041. [DOI] [PubMed] [Google Scholar]
- 71.Hoffmann M, Reichmuth M, Fantelli K, Schoch OD, Fierz W, Furrer H, Vernazza P. Conventional tuberculin skin testing versus T-cell–based assays in the diagnosis of latent tuberculosis infection in HIV-positive patients. AIDS 2007;21:390–392. [DOI] [PubMed] [Google Scholar]
- 72.Canadian Tuberculosis Committee. Updated recommendations on interferon γ release assays for latent tuberculosis infection: an Advisory Committee Statement (ACS). Can Commun Dis Rep 2008;34:1–13. [PubMed] [Google Scholar]
- 73.National Institute for Health and Clinical Excellence. Tuberculosis: Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London: Royal College of Physicians; 2006. [PubMed]
- 74.League SL. Detection of tuberculous infection by a blood-test (interferon-γ). Bern: Lungeglia Schwiez; 2005.
- 75.Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, Kimerling ME, Chheng P, Thai S, Sar B, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med 2010;362:707–716. [DOI] [PubMed] [Google Scholar]
- 76.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, Bearnot B, Allen J, Walensky RP, Freedberg KA. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 2010;51:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elzi L, Schlegel M, Weber R, Hirschel B, Cavassini M, Schmid P, Bernasconi E, Rickenbach M, Furrer H. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin Infect Dis 2007;44:94–102. [DOI] [PubMed] [Google Scholar]
- 78.Mosimaneotsile B, Mathoma A, Chengeta B, Nyirenda S, Agizew TB, Tedla Z, Motsamai OI, Kilmarx PH, Wells CD, Samandari T. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: a Botswana experience. J Acquir Immune Defic Syndr 2004–2006;54:71–77. [DOI] [PubMed] [Google Scholar]
- 79.Grant AD, Charalambous S, Fielding KL, Day JH, Corbett EL, Chaisson RE, De Cock KM, Hayes RJ, Churchyard GJ. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA 2005;293:2719–2725. [DOI] [PubMed] [Google Scholar]
- 80.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, Gray GE, McIntyre JA, Chaisson RE, Martinson NA. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS 2009;23:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinson NA. Novel regimens for treating latent TB in HIV-infected adults in South Africa: a randomized clinical trial. Presented at the 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, February 8–11, 2009.
- 82.Churchyard GJ, Fielding K, Charalambous S, Day JH, Corbett EL, Hayes RJ, Chaisson RE, De Cock KM, Samb B, Grant AD. Efficacy of secondary isoniazid preventive therapy among HIV-infected southern Africans: time to change policy? AIDS 2003;17:2063–2070. [DOI] [PubMed] [Google Scholar]
- 83.Basu S, Maru D, Poolman E, Galvani A. Primary and secondary tuberculosis preventive treatment in HIV clinics: simulating alternative strategies. Int J Tuberc Lung Dis 2009;13:652–658. [PubMed] [Google Scholar]
- 84.Hamlyn E, Childs K, Post FA. Reducing tuberculosis incidence in HIV-infected patients by tuberculin skin testing, preventive treatment, and antiretroviral therapy. Clin Infect Dis 2007;44:1393–1394; author reply 1394–1395. [DOI] [PubMed] [Google Scholar]
- 85.Tedla Z, Nyirenda S, Peeler C, Agizew T, Sibanda T, Motsamai O, Vernon A, Wells CD, Samandari T. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med 2010;182:278–285. [DOI] [PubMed] [Google Scholar]
- 86.Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985;131:393–396. [DOI] [PubMed] [Google Scholar]
- 87.Lee MP, Chan JW, Ng KK, Li PC. Clinical manifestations of tuberculosis in HIV-infected patients. Respirology 2000;5:423–426. [PubMed] [Google Scholar]
- 88.Huebner RE, Castro KG. The changing face of tuberculosis. Annu Rev Med 1995;46:47–55. [DOI] [PubMed] [Google Scholar]
- 89.Huang L, Crothers K. HIV-associated opportunistic pneumonias. Respirology 2009;14:474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goodman PC. Tuberculosis and AIDS. Radiol Clin North Am 1995;33:707–717. [PubMed] [Google Scholar]
- 91.Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis 2007;196:S15–S27. [DOI] [PubMed] [Google Scholar]
- 92.Monkongdee P, McCarthy KD, Cain KP, Tasaneeyapan T, Nguyen HD, Nguyen TN, Nguyen TB, Teeratakulpisarn N, Udomsantisuk N, Heilig C, et al. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am J Respir Crit Care Med 2009;180:903–908. [DOI] [PubMed] [Google Scholar]
- 93.Whalen C, Horsburgh CR Jr, Hom D, Lahart C, Simberkoff M, Ellner J. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS 1997;11:455–460. [DOI] [PubMed] [Google Scholar]
- 94.Kivihya-Ndugga LE, van Cleeff MR, Githui WA, Nganga LW, Kibuga DK, Odhiambo JA, Klatser PR. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource-poor urban setting. Int J Tuberc Lung Dis 2003;7:1163–1171. [PubMed] [Google Scholar]
- 95.Prasanthi K, Kumari AR. Efficacy of fluorochrome stain in the diagnosis of pulmonary tuberculosis co-infected with HIV. Indian J Med Microbiol 2005;23:179–181. [DOI] [PubMed] [Google Scholar]
- 96.Singh NP, Parija SC. The value of fluorescence microscopy of auramine stained sputum smears for the diagnosis of pulmonary tuberculosis. Southeast Asian J Trop Med Public Health 1998;29:860–863. [PubMed] [Google Scholar]
- 97.Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins M, Aziz MA, Pai M. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006;6:570–581. [DOI] [PubMed] [Google Scholar]
- 98.Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006;6:664–674. [DOI] [PubMed] [Google Scholar]
- 99.Bonnet M, Ramsay A, Githui W, Gagnidze L, Varaine F, Guerin PJ. Bleach sedimentation: an opportunity to optimize smear microscopy for tuberculosis diagnosis in settings of high prevalence of HIV. Clin Infect Dis 2008;46:1710–1716. [DOI] [PubMed] [Google Scholar]
- 100.Eyangoh SI, Torrea G, Tejiokem MC, Kamdem Y, Piam FF, Noeske J, Van Deun A. HIV-related incremental yield of bleach sputum concentration and fluorescence technique for the microscopic detection of tuberculosis. Eur J Clin Microbiol Infect Dis 2008;27:849–855. [DOI] [PubMed] [Google Scholar]
- 101.Lawson L, Yassin MA, Ramsay A, Emenyonu EN, Thacher TD, Davies PD, Squire SB, Cuevas LE. Short-term bleach digestion of sputum in the diagnosis of pulmonary tuberculosis in patients co-infected with HIV. Tuberculosis (Edinb) 2007;87:368–372. [DOI] [PubMed] [Google Scholar]
- 102.Ramsay A, Yassin MA, Cambanis A, Hirao S, Ahmad A, Mohamed G, Lovett L, Arbide I, Al-Aghbari N, Al-Sonboli N, et al. Front-loading sputum microscopy services: an opportunity to optimise smear-based case detection of tuberculosis in high prevalence countries. J Trop Med 2009;2009:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diraa O, Fdany K, Boudouma M, Elmdaghri N, Benbachir M. Assessment of the mycobacteria growth indicator tube for the bacteriological diagnosis of tuberculosis. Int J Tuberc Lung Dis 2003;7:1010–1012. [PubMed] [Google Scholar]
- 104.Tortoli E, Cichero P, Piersimoni C, Simonetti MT, Gesu G, Nista D. Use of BACTEC MGIT 960 for recovery of mycobacteria from clinical specimens: multicenter study. J Clin Microbiol 1999;37:3578–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Venkataraman P, Herbert D, Paramasivan CN. Evaluation of the BACTEC radiometric method in the early diagnosis of tuberculosis. Indian J Med Res 1998;108:120–127. [PubMed] [Google Scholar]
- 106.Johnson JL, Vjecha MJ, Okwera A, Hatanga E, Byekwaso F, Wolski K, Aisu T, Whalen CC, Huebner R, Mugerwa RD, et al. Impact of human immunodeficiency virus type-1 infection on the initial bacteriologic and radiographic manifestations of pulmonary tuberculosis in Uganda: Makerere University–Case Western Reserve University research collaboration. Int J Tuberc Lung Dis 1998;2:397–404. [PubMed] [Google Scholar]
- 107.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, Drobniewski F, Lalvani A. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007;11:1–196. [DOI] [PubMed] [Google Scholar]
- 108.Kivihya-Ndugga L, van Cleeff M, Juma E, Kimwomi J, Githui W, Oskam L, Schuitema A, van Soolingen D, Nganga L, Kibuga D, et al. Comparison of PCR with the routine procedure for diagnosis of tuberculosis in a population with high prevalences of tuberculosis and human immunodeficiency virus. J Clin Microbiol 2004;42:1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shah M, Variava E, Holmes CB, Coppin A, Golub JE, McCallum J, Wong M, Luke B, Martin DJ, Chaisson RE, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a high HIV prevalence setting. J Acquir Immune Defic Syndr 2009;52:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dheda K, Davids V, Lenders L, Roberts T, Meldau R, Ling D, Brunet L, van Zyl Smit R, Peter J, Green C, et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One 2010;5:e9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, Cunningham J, Weldingh K, Pai M. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med 2007;4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moore DA, Evans CA, Gilman RH, Caviedes L, Coronel J, Vivar A, Sanchez E, Pinedo Y, Saravia JC, Salazar C, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 2006;355:1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reddy KP, Brady MF, Gilman RH, Coronel J, Navincopa M, Ticona E, Chavez G, Sanchez E, Rojas C, Solari L, et al. Microscopic observation drug susceptibility assay for tuberculosis screening before isoniazid preventive therapy in HIV-infected persons. Clin Infect Dis 2010;50:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minion J, Leung E, Menzies D, Pai M. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:688–698. [DOI] [PubMed] [Google Scholar]
- 115.World Health Organization. Non-commercial culture and drug-susceptibility testing methods for screening of patients at risk of multi-drug resistant tuberculosis. Geneva, Switzerland: WHO; 2010. [PubMed]
- 116.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis. Geneva, Switzerland: WHO; 2009.
- 117.Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med 2008;177:787–792. [DOI] [PubMed] [Google Scholar]
- 118.Luo RF, Scahill MD, Banaei N. Comparison of single-copy and multicopy real-time PCR targets for detection of Mycobacterium tuberculosis in paraffin-embedded tissue. J Clin Microbiol 2010;48:2569–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nachega JB, Maartens G. Clinical aspects of tuberculosis in HIV-infected adults. In: Schaaf HS, Zumla AI, editors. Tuberculosis—a comprehensive clinical reference, 1st ed. St. Louis, MO: Elsevier; 2009.
- 121.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367:926–937. [DOI] [PubMed] [Google Scholar]
- 122.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis 2003;37:101–112. [DOI] [PubMed] [Google Scholar]
- 123.Khan FA, Minion J, Pai M, Royce S, Burman W, Harries AD, Menzies D. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis 2010;50:1288–1299. [DOI] [PubMed] [Google Scholar]
- 124.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet 2004;364:1244–1251. [DOI] [PubMed] [Google Scholar]
- 125.Driver CR, Munsiff SS, Li J, Kundamal N, Osahan SS. Relapse in persons treated for drug-susceptible tuberculosis in a population with high coinfection with human immunodeficiency virus in New York City. Clin Infect Dis 2001;33:1762–1769. [DOI] [PubMed] [Google Scholar]
- 126.Nahid P, Gonzalez LC, Rudoy I, de Jong BC, Unger A, Kawamura LM, Osmond DH, Hopewell PC, Daley CL. Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med 2007;175:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Perriens JH, St Louis ME, Mukadi YB, Brown C, Prignot J, Pouthier F, Portaels F, Willame JC, Mandala JK, Kaboto M, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire: a controlled trial of treatment for either 6 or 12 months. N Engl J Med 1995;332:779–784. [DOI] [PubMed] [Google Scholar]
- 128.Swaminathan S, Narendran G, Venkatesan P, Iliayas S, Santhanakrishnan R, Menon PA, Padmapriyadarsini C, Ramachandran R, Chinnaiyan P, Suhadev M, et al. Efficacy of a 6-month versus 9-month intermittent treatment regimen in HIV-infected patients with tuberculosis: a randomized clinical trial. Am J Respir Crit Care Med 2010;181:743–751. [DOI] [PubMed] [Google Scholar]
- 129.World Health Organization. Treatment of tuberculosis guidelines. Geneva, Switzerland: WHO; 2010. [PubMed]
- 130.Marks DJ, Dheda K, Dawson R, Ainslie G, Miller RF. Adverse events to antituberculosis therapy: influence of HIV and antiretroviral drugs. Int J STD AIDS 2009;20:339–345. [DOI] [PubMed] [Google Scholar]
- 131.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007;4:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burman WJ. Drug interactions in the treatment of HIV-related tuberculosis. In: Schaaf HS, Zumla AI, editors. Tuberculosis—a comprehensive clinical reference, 1st ed. St. Louis, MO: Elsevier; 2009.
- 133.Alcorn K. Second-line treatment: Clinton Foundation announces new price cut [Internet; accessed January 2011]. Available from: http://www.aidsmap.com/news/Second-line-treatment-Clinton-Foundation-announces-new-price-cut/page/1435524/.
- 134.Dhasmana DJ, Dheda K, Ravn P, Wilkinson RJ, Meintjes G. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and management. Drugs 2008;68:191–208. [DOI] [PubMed] [Google Scholar]
- 135.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008;8:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meintjes G, Rangaka MX, Maartens G, Rebe K, Morroni C, Pepper DJ, Wilkinson KA, Wilkinson RJ. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis 2009;48:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010;24:2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis 2009;13:6–16. [PubMed] [Google Scholar]
- 139.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendation for a public health approach. Geneva, Switzerland: WHO; 2010. [PubMed]
- 140.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010;362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blanc FX, Havlir DV, Onyebujoh PC, Thim S, Goldfeld AE, Delfraissy JF. Treatment strategies for HIV-infected patients with tuberculosis: ongoing and planned clinical trials. J Infect Dis 2007;196:S46–S51. [DOI] [PubMed] [Google Scholar]
- 142.Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor–dependent interleukin-12 p40 production in macrophages. J Biol Chem 2005;280:42794–42800. [DOI] [PubMed] [Google Scholar]
- 143.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci 1998;111:897–905. [DOI] [PubMed] [Google Scholar]
- 144.Lalvani A, Hingley-Wilson S. Editorial: Live or let die—does HIV exacerbate tuberculosis by attenuating M. tuberculosis–induced apoptosis? J Leukoc Biol 2009;86:9–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.