Abstract
Rationale: Asthma prevalence and morbidity are especially elevated in adolescents, yet few interventions target this population.
Objectives: To test the efficacy of Asthma Self-Management for Adolescents (ASMA), a school-based intervention for adolescents and medical providers.
Methods: Three hundred forty-five primarily Latino/a (46%) and African American (31%) high school students (mean age = 15.1 yr; 70% female) reporting an asthma diagnosis, symptoms of moderate to severe persistent asthma, and asthma medication use in the last 12 months were randomized to ASMA, an 8-week school-based intervention, or a wait-list control group. They were followed for 12 months.
Measurements and Main Results: Students completed bimonthly assessments. Baseline, 6-month, and 12-month assessments were comprehensive; the others assessed interim health outcomes and urgent health care use. Primary outcomes were asthma self-management, symptom frequency, and quality of life (QOL); secondary outcomes were asthma medical management, school absences, days with activity limitations, and urgent health care use. Relative to control subjects, ASMA students reported significantly: more confidence to manage their asthma; taking more steps to prevent symptoms; greater use of controller medication and written treatment plans; fewer night awakenings, days with activity limitation, and school absences due to asthma; improved QOL; and fewer acute care visits, emergency department visits, and hospitalizations. In contrast, steps to manage asthma episodes, daytime symptom frequency, and school-reported absences did not differentiate the two groups. Most results were sustained over the 12 months.
Conclusions: ASMA is efficacious in improving asthma self-management and reducing asthma morbidity and urgent health care use in low-income urban minority adolescents.
Keywords: asthma, urban, adolescents, school-based, intervention
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Ethnic minority adolescents are disproportionately impacted by asthma, yet there is a dearth of interventions targeting this population.
What This Study Adds to the Field
We demonstrate that an intervention designed for adolescents and their medical providers significantly improves asthma management, reduces asthma morbidity and urgent health care use, and improves quality of life.
Despite advances in medical management, asthma remains the most common chronic illness in youth, with prevalence, morbidity, and mortality relatively high among adolescents (1–4). For example, 11- to 17-year-olds have more asthma exacerbations (4, 5); more exacerbations that require hospitalization, intubation, and cardiopulmonary resuscitation (6); and higher asthma-related mortality rates than younger children (3). In childhood, boys have higher rates of asthma than girls, but this trend reverses itself in early to mid-teenagers with female adolescents having higher rates of asthma (7, 8). Asthma prevalence is especially elevated in urban, minority youth (2, 9, 10), who also experience relatively greater asthma morbidity (4, 11), even when controlling for ethnic/racial differences in prevalence (3). One possible reason for the additional burden on adolescents and minorities is related to differences in medical care. Adolescents are less likely to receive regular medical care compared with younger children (4), and relative to white, non-Hispanic youth, minorities make fewer nonemergency ambulatory care visits (3) and are less likely to use preventive medication (12, 13). Therefore, there is a great need for addressing asthma management in these disadvantaged groups.
Another possible reason for the increased burden during adolescence may be related to the transfer of responsibility to manage asthma from parent to child, and adolescents may be ill-equipped to manage the illness independently (14). Nonetheless, adolescence is an ideal time to teach asthma management because cognitive gains and psychosocial developments made during this developmental period should make it possible for adolescents to assume management of their illness (14). Yet, few interventions targeting adolescents have been evaluated systematically (15, 16).
Additionally, asthma morbidity may be attributed in part to clinicians' nonadherence to National Heart, Lung, and Blood Institute (NHLBI) practice guidelines, including not prescribing controller medication, not recommending daily peak flow monitoring, not screening and counseling parents and adolescents regarding smoking cessation, and not counseling regarding allergen exposure (17–21). Barriers contributing to medical providers' nonadherence include lack of familiarity and agreement with the guideline recommendations, not believing that recommendations are effective, limited confidence in their ability to implement the guidelines, and inadequate training (17, 18, 22, 23). Interventions with clinicians have been shown to improve prescribing and communication behaviors, which leads to improved pediatric patient outcomes and reductions in acute medical visits, emergency department (ED) visits, and hospitalizations (24–27). Concurrent provider and patient interventions may have synergistic effects on patient outcomes; this dual approach has yet to be tested.
Group interventions offer the advantage of reaching many individuals at one time, and providing a supportive peer environment, which may allow participants to identify with their similarly affected peers, which is especially important for adolescents (14). Simultaneously, tailoring of asthma education to individuals is essential (14) because asthma is a dynamic disease with intraindividual variations in symptom presentation and management based on lifestyles and disease characteristics (28). Despite this, few interventions tailor educational messages and medical management (15, 29).
To address these gaps, we developed Asthma Self-Management for Adolescents (ASMA), a school-based intervention for adolescents that uses both group and tailored individual sessions and includes education for their medical providers (14). This article describes a randomized trial of the efficacy of ASMA. The primary hypothesis was that, relative to wait-list control subjects, students who participated in ASMA would have improved asthma self-management, symptom frequency, and quality of life. Secondary hypotheses were that ASMA would lead to relatively better medical management of asthma, fewer days with activity restriction and school absences, and less urgent health care use. Some of the results of these studies have been previously reported in the form of abstracts (30, 31).
METHODS
Participants
Students were drawn from five participating high schools with a high proportion of Latino/a and African American students. Eligible students included 9th and 10th graders with moderate to severe persistent asthma. Students reported having a diagnosis of asthma by a medical provider, as well as experiencing symptoms of moderate persistent or severe persistent asthma as defined by NHLBI guidelines (32) and taking asthma medication prescribed by a medical provider in the last 12 months.
Identifying and enrolling eligible students.
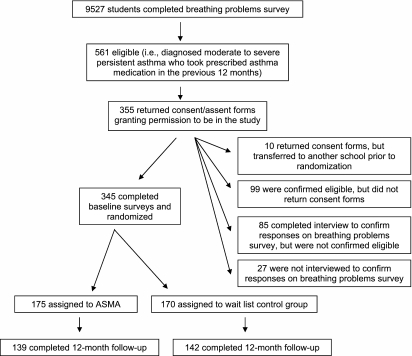
To identify eligible students, 9th and 10th grade students completed a case detection survey. Parents of all 9th and 10th grade students were informed by letter that their child would complete a survey on breathing problems at school. Using a passive consent procedure, parents had the option of not allowing their child to participate by signing and returning the letter with the nonparticipation box checked. Students could also choose to refuse to complete the survey. Students indicated if they had: (1) ever been diagnosed with asthma, and the frequency in the last 12 months of (2) asthma symptoms, including items from the International Survey of Asthma and Allergies in Childhood (ISAAC) questionnaire (1), and (3) if they had taken asthma medication prescribed by a medical provider. Together with the primary investigator and a pediatric pulmonologist, study personnel reviewed the case detection surveys to determine eligibility; study personnel interviewed eligible students to confirm their responses on the case detection survey before distributing consent/assent forms. Figure 1 details the study flow. Enrollment took place over four consecutive school years from 2001 to 2004. Case identification took place in the fall semester, and parent consent/student assent and randomization in the winter semester. The institutional review boards of Columbia University College of Physicians and Surgeons, New York University School of Medicine, and the New York City Department of Education (DOE) approved all study procedures.
Figure 1.
Flowchart of participants through the study.
Data Collection and Study Outcomes
Trained staff interviewed students every 2 months for 12 months. Students self-identified as: Hispanic/Latino/a or Hispanic American; African American or African; Caribbean or Caribbean American; White, Non-Hispanic/European or European American, Asian or Asian American; Native American; Mixed Ethnicity; or Other. Because relatively few students self-identified as White, Non-Hispanic/European or European American, Asian or Asian American, Native American, or Other, we collapsed these groups into “Other.” Baseline, 6-month and 12-month surveys were comprehensive, assessing: (1) asthma self-management—strategies to prevent and to manage symptoms and self-efficacy; (2) asthma medical management—use of controller medication and written treatment plans (WTP); (3) health outcomes—(a) the number of symptom days, nights awakenings, days with activity restrictions due to asthma, and asthma-related school absences in the last 2 weeks; and (b) asthma-related quality of life (QOL); and (4) urgent health care use in the last 2 months—acute medical visits, ED visits, and hospitalizations. The asthma management indices were adapted from the investigators' prior research (33); factor analyses confirmed their factor structure in this sample. Students were asked if they took each of 10 steps to prevent symptoms (α = 0.62) and 5 steps to manage symptoms when they occurred (α = 0.52). Using a seven-point self-efficacy scale (1 = not at all; 7 = completely), students rated their confidence to implement 15 actions to care for their asthma (α = 0.81). QOL was assessed using the Pediatric Asthma Quality of Life Questionnaire (34). The brief 2-, 4-, 8-, and 10-month surveys included only the health outcomes and urgent health care use items, which were written and used by the investigators in prior research (35, 36).
Baseline data were collected during the winter of the enrollment year. In addition to self-reported school absences, the New York City DOE provided de-identified school attendance data for the full sample by cohort (i.e., the students enrolled at a school during a particular study year), and identified data only for those students whose parents gave permission. Data were calculated for the two semesters before the intervention and the two semesters corresponding to the study's 12-month assessment interval.
Primary outcomes were symptom frequency (number of symptom days and nights awoken in the last 2 weeks), QOL, and asthma self-management (number of prevention steps taken, number of steps taken to manage asthma episodes, and self-efficacy). Secondary outcomes were number of days with activity restriction in last 2 weeks, school absences, asthma medical management, and urgent health care use.
Randomization
On completion of baseline surveys, students were randomized to ASMA or a wait-list control group. Within each school, we stratified students by asthma severity (moderate vs. severe persistent asthma). Within each stratum, we randomized students to control or intervention using computerized randomization lists generated in advance by the data manager who concealed them until randomization. Interviewers were blind to group assignment.
Intervention
ASMA, a developmentally appropriate intervention for adolescents that is grounded in social cognitive theory, has been described in detail elsewhere (14). Briefly, ASMA consists of two complementary components: (1) an 8-week intensive program for the students, and (2) academic detailing for the adolescents' medical providers. The student intervention consists of three 45- to 60-minute group sessions, and individual tailored coaching sessions held at least once per week for 5 weeks. Sessions are delivered by trained health educators during the school day. In addition to teaching asthma management skills and ways to cope with asthma, the health educators encourage students to see their medical provider for a clinical evaluation and treatment. The individual sessions reinforce the educational messages taught in the group, help students identify and overcome barriers to managing their asthma, and coach students regarding their medical visits. The health educator offers to accompany the student to the medical visit to provide moral support, coaching, or advocacy when coaching fails.
Academic detailing is an educational technique whereby experts make a presentation in person or by telephone to medical providers about a recommended change in therapy (37). In ASMA, the medical providers were first mailed a packet containing: a letter informing them that one of their patients was in the study and would be referred to him/her for a clinical evaluation; a blank asthma checklist the students complete throughout the intervention and bring to the visit with the provider; and a blank asthma action plan the provider is asked to complete. Within 2 weeks, a pediatric pulmonologist or adolescent medicine specialist called the students' medical providers to discuss the concepts presented in the program and to answer any questions regarding NHLBI Institute criteria for treating asthma. The aim of this part of the intervention is to increase the chances that students will receive appropriate medical care for asthma and that efforts by students to control their asthma through self-monitoring and working with a medical provider are met with a positive response. The academic detailing was completed in January of the school year in which students were enrolled; the student program began in February of that school year.
Students without a medical provider were given referrals to a primary care provider in their community, or if available, to the on-campus school-based health center. Make-up sessions were offered to students who missed the group sessions; health educators were at schools daily to conduct individual sessions and therefore were able to reach out multiple times to students who may have been absent on a given day. Wait-list control students received ASMA upon completion of 12-month interviews.
Sample Size
Testing a priori hypotheses with two-sided α = 0.05, with no additional control for comparisons across hypotheses, and using an estimated intracluster correlation of 0.60 based on data from our prior research in elementary schools (36), a sample of 240 students (120 per group) was calculated to have 80% power to detect an effect size of 0.23 SD. This sample size could tolerate up to 54% contamination of control subjects and still have 80% power to detect a treatment effect of 0.50 SD. A 25% attrition was assumed, requiring enrollment of 320 students.
Statistical Analyses
All analyses were conducted following the intention-to-treat principle. We compared all baseline, demographic, clinical, and behavioral characteristics of students who completed the study with those who dropped out. In addition, we explored if there were differences with respect to the pattern of change of the outcomes during follow-up that would suggest that missing data were not random. The only discernible difference was with respect to age at baseline, with older students more likely to miss follow-up assessments; this difference was similar across treatment groups. In multivariate analyses, we adjusted for cohort, baseline values, and age. All analyses were performed using the SAS statistical software (SAS Institute Inc, Cary, NC).
Repeated measures of symptom days, night awakenings, school absences, and days with restricted activity were analyzed using normal regression, binomial, or Poisson models depending on the distribution of the outcome variable (38). Generalized estimating equations were used to account for possible correlation between repeated observations over time and for nesting of students within cohorts (39). Square root transformations were made to normalize the distribution of school absence data provided by the DOE; data for students enrolled fewer than 30 days a semester were excluded from these analyses. Results were similar for the de-identified and identified data; therefore, we present only the findings for the de-identified data for the full sample.
Because urgent health care use (i.e., ED visits, hospitalizations, and acute visits to a medical provider) are rare events, to improve interpretability, responses were averaged over all available follow-up assessments and multiplied by six to obtain a yearly count. Urgent health care use variables over the post-treatment follow-up period were analyzed using zero-inflated Poisson models (ZIP) (40, 41), because their distributions had an excess of zeros over those allowed by the Poisson model. The ZIP model postulates that the study population is a mixture of two subpopulations. One consists of individuals who will not use the urgent care of interest (e.g., hospitalizations). For them the probability of making an urgent care visit is zero; this subpopulation is referred to as “sure zero population.” The other subpopulation consists of individuals who will use the urgent care with a nonzero probability; their distribution is Poisson with a nonzero mean. This subpopulation is called “nonsure zero population” or “Poisson population.” The ZIP distribution is characterized by two parameters: (1) the prevalence of the sure zero population, and (2) the mean number of visits for the nonsure zero population. We tested for treatment effects on both parameters by modeling the probability of belonging to the sure zero population and the mean number of visits in the nonsure zero population as functions of treatment, adjusting for baseline values and age.
RESULTS
Five schools participated with a total of 12 cohorts enrolled over 4 years (2 to 3 cohorts per school). Three of the schools, or seven cohorts, had school-based health centers. On average at each school, most students were eligible for free (73.7%) or reduced price (7.9%) lunch. Over the 4 years, 9,527 students completed the breathing problems survey (Figure 1), and 561 (6%) were eligible. Of these, 355 (64%) returned signed consent/assent forms and 345 (61%) completed baseline surveys and were randomly assigned; the 10 students (2%) who returned consent forms but were not enrolled transferred to other schools before randomization. Other reasons for nonenrollment are detailed in Figure 1. There was no significant difference between those who enrolled or did not enroll by NHLBI asthma classification (i.e., moderate persistent vs. severe persistent), but there was a significantly greater proportion of females among enrolled students (68%) than among nonenrolled students (58%; P < 0.01).
Table 1 shows student demographic characteristics by treatment group. There were no clinically meaningful differences between the treatment and control students on any of the demographic or baseline asthma characteristics, except for self-reported school absences. Attrition, which was primarily attributable to truancy or no longer being enrolled in the school, did not differ by group assignment at any follow-up time point (P = 0.26–0.99); 12-month retention was 81% (Figure 1).
TABLE 1.
BASELINE DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS BY TREATMENT GROUP
| ASMA (n = 175) | Wait-list Control (n = 170) | Total (N = 345) | |
|---|---|---|---|
| Age, yr, mean (SD) | 15.10 (0.84) | 15.10 (0.88) | 15.10 (0.86) |
| Male, % (n) | 29.14 (51) | 30.00 (51) | 29.56 (102) |
| Race/ethnicity, % (n) | |||
| Hispanic/Latino/a or Hispanic American | 44.57 (78) | 46.47 (79) | 45.51 (157) |
| African American/African or Caribbean American/Caribbean | 38.86 (68) | 36.47 (62) | 37.68 (130) |
| Mixed ethnicity | 10.29 (18) | 12.94 (22) | 11.59 (40) |
| Other | 6.29 (11) | 4.12 (7) | 5.22 (18) |
| NHLBI asthma classification, % (n) | |||
| Moderate persistent | 66.29 (116) | 71.18 (121) | 68.70 (237) |
| Severe persistent | 33.71 (59) | 28.82 (49) | 31.30 (108) |
| Has a medical provider, % (n) | 83.42 (146) | 88.82 (151) | 86.09 (297) |
Definition of abbreviations: ASMA = Asthma Self-Management for Adolescents; NHLBI = National Heart, Lung, and Blood Institute.
Intervention Reach
Intervention students attended on average 2.8 group workshops (SD = 0.8). The vast majority (90%; 157/175) attended all three groups; only 6% (11/175) attended no group sessions. On average, students met with a health educator for individual coaching sessions 4.9 times (SD = 1.8; range = 0–9). The majority (78%; 137/175) met four to six times with a health educator; only 7% (13/175) received no individual coaching sessions. Most (79%; 138/175) saw their medical providers for a clinical evaluation, with 14% (25/175) going twice during the intervention phase. A health educator accompanied 21% (34/164) on their first appointment. Academic detailing packets were mailed to all of the students' medical providers, and the pulmonologists and adolescent medicine specialists spoke with 81% (142/175) of the students' medical providers by telephone.
Study Outcomes
Asthma self-management.
Table 2 shows treatment differences for prevention steps taken, management steps taken, and self-efficacy, and the adjusted analyses. At each follow-up assessment, ASMA students took significantly more steps to prevent asthma from starting than control students, with the difference being substantially larger at 6 months than 12 months. There was no significant treatment difference in the number of steps taken to manage asthma symptoms once symptoms began at the 6- or 12-month follow-up. ASMA students felt more confident to manage asthma at each time point, with the effect being relatively stable over the follow-up year. In the treatment group, confidence (self-efficacy) improved by 10% from baseline to follow-up at 6 and 12 months, but in control subjects it increased only 2 and 4% at 6 months and 12 months, respectively.
TABLE 2.
TREATMENT EFFECTS OF ASTHMA SELF-MANAGEMENT FOR ADOLESCENTS ON ASTHMA MANAGEMENT
| ASMA |
Wait-list Control |
|||||
|---|---|---|---|---|---|---|
| N | M (SD) | N | M (SD) | Adjusted Mean Difference (95% CI) | P Value | |
| Asthma self-management | ||||||
| Prevention steps* | ||||||
| Baseline | 175 | 5.59 (2.26) | 170 | 6.06 (2.52) | ||
| 6 mo | 150 | 6.91 (2.88) | 139 | 5.83 (2.92) | 1.34 (0.93 to 1.76) | <0.0001† |
| 12 mo | 139 | 6.63 (2.92) | 141 | 6.38 (2.65) | 0.50 (0.02 to 0.98) | 0.04† |
| Management steps | ||||||
| Baseline | 175 | 5.71 (1.92) | 170 | 5.90 (1.72) | ||
| 6 mo | 150 | 6.93 (1.90) | 139 | 6.64 (2.12) | 0.27 (−0.19 to 0.74) | 0.24 |
| 12 mo | 139 | 6.52 (2.05) | 141 | 6.54 (2.33) | 0.01 (−0.59 to 0.62) | 0.96 |
| Self-efficacy | ||||||
| Baseline | 175 | 4.59 (0.83) | 170 | 4.72 (0.91) | ||
| 6 mo | 150 | 5.09 (0.93) | 139 | 4.80 (1.05) | 0.37 (0.17 to 0.56) | <0.0001† |
| 12 mo | 139 | 5.10 (0.91) | 141 | 4.94 (0.91) | 0.19 (0.07 to 0.32) | 0.003† |
| Rates of asthma medical management by medical providers | ||||||
| Controller medications‡ | N | % (n) | N | % (n) | Adjusted OR (95% CI) | P |
| Baseline | 175 | 28.00 (49) | 169 | 32.54 (55) | ||
| 6 mo | 150 | 50.67 (76) | 139 | 37.41 (52) | 2.25 (1.28 to 3.93) | 0.006§ |
| 12 mo | 139 | 46.04 (76) | 141 | 43.66 (64) | 1.22 (0.71 to 2.08) | 0.47 |
| Use of written treatment plan | ||||||
| Baseline | 175 | 8.00 (14) | 170 | 13.61 (23) | ||
| 6 mo | 148 | 51.35 (76) | 138 | 26.81 (37) | 3.60 (2.25 to 5.77) | <0.0001§ |
| 12 mo | 139 | 57.55 (80) | 138 | 25.32 (80) | 4.57 (2.97 to 7.04) | <0.0001§ |
Definition of abbreviations: ASMA = Asthma Self-Management for Adolescents; GEE = generalized estimating equations; OR = odds ratio.
There was a significant interaction between time and treatment group indicating that the treatment effect was stronger at 6 mo than 12 mo.
Normal regression with GEE adjusting for baseline levels, age, and cohort.
There was a significant interaction between time and treatment group indicating that the treatment effect was significant at 6 mo but not 12 mo.
Binomial regression with GEE adjusting for baseline levels, age, and cohort.
Asthma medical management.
At the 6-month assessment, the odds of using a controller medication among those in the ASMA group were twice as high as control subjects (see Table 2); there was no significant difference at the 12-month assessment due to the greater use by control subjects at this assessment. Relative to control subjects, the odds of ASMA students using a WTP were more than three times as high at the 6-month assessment and more than four times as high at the 12-month assessment.
Health outcomes.
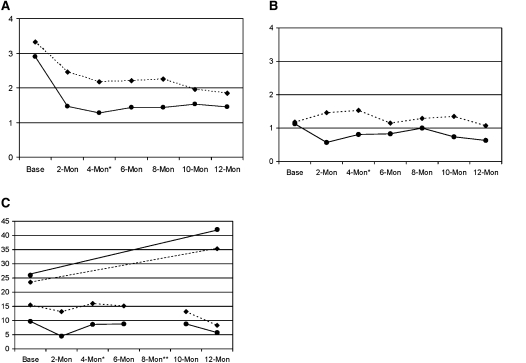
Table 3 presents unadjusted means averaged over the 12-month follow-up and adjusted risk ratios for symptom days, night awakenings, days with activity restriction, and self-reported school absences due to asthma. Figures 2A–2C present outcomes with significant intervention-by-time effects. To compare school absences obtained by self-report to school records, in Figure 2C we converted to annualized rates at each time point.
TABLE 3.
TREATMENT EFFECTS OF ASTHMA SELF-MANAGEMENT FOR ADOLESCENTS ON HEALTH OUTCOMES
| ASMA Mean (SD) | Wait-list Control Mean (SD) | Adjusted RR (95% CI) | P Value | |
|---|---|---|---|---|
| Symptom days in prior 2 wk | ||||
| Baseline | 4.41 (4.45) | 4.91 (4.60) | ||
| 1-yr follow-up* | 2.78 (2.43) | 3.30 (2.51) | 0.88 (0.74–1.04) | 0.12 |
| Night wakening in prior 2 wk | ||||
| Baseline | 2.89 (3.98) | 3.33 (4.45) | ||
| 1-yr follow-up* | 1.42 (1.72) | 2.23 (2.39) | 0.69 (0.60–0.86) | 0.001† |
| Days with activity restrictions in prior 2 wk | ||||
| Baseline | 1.13 (2.08) | 1.18 (2.03) | ||
| 1-yr follow-up* | 0.77 (1.12) | 1.34 (2.01) | 0.58 (0.43–0.78) | 0.003† |
| School absences in prior 2 wk: self-reported | ||||
| Baseline‡ | 0.54 (1.33) | 0.86 (1.62) | ||
| 1-yr follow-up* | 0.43 (0.69) | 0.78 (1.09) | 0.63 (0.46–0.85) | 0.004† |
Definition of abbreviations: ASMA = Asthma Self-Management for Adolescents; GEE = Generalized estimating equations; RR = risk ratio.
Mean number of days/nights in 2 wk over the 12-mo follow-up assessment period.
Poisson regression with GEE adjusting for baseline level, age, and cohort.
Baseline values significantly different, P < 0.0001.
Figure 2.
Descriptive data for significant health outcomes by assessment time point and treatment group. (A) Number of nights wakened in previous 2 weeks. (B) Number of days activity limitation in previous 2 weeks. (C) Number of school absences displayed as annualized rates at each time point. Top two lines show Department of Education de-identified data of all students for the baseline and follow-up years. Bottom two lines show bimonthly self-report data for absences due to asthma over the last 2 weeks, converted to annualized rates (mean 2-wk values at each time point × 18, the number of 2-week periods in a school year. Diamonds, dotted lines: control; circles, solid lines: intervention. *Corresponds roughly to immediate postintervention; **August, no school.
As hypothesized, students who participated in ASMA had significantly less asthma morbidity than control subjects at follow-up. Over the 12-month assessment period, relative to control subjects, ASMA students reported a 31% reduction in night awakenings due to asthma and a 42% reduction in the number of days with activity restriction due to asthma in the previous 2 weeks. This difference translates to a relative reduction of almost 21 night awakenings and 13 days with activity restriction per student over 1 year for the intervention group. There was no significant difference between groups in days with asthma symptoms over the year.
At baseline, both groups had relatively high QOL (treatment: mean = 4.3, SD = 1.2; control: mean = 4.1, SD = 1.3). There was no difference in QOL between groups at the 6-month assessment period (adjusted mean difference = 0.1; 95% CI, −0.1 to 0.3; P = 0.38). However, at 12 months, ASMA students had significantly better QOL than control subjects (adjusted mean difference = 0.3; 95% CI, 0.09–0.5; P = 0.0045).
Both groups had reductions in bimonthly repeated measures of self-reported school absences attributed to asthma over the previous 2 weeks. Over the 12-month assessment period, there were 37% fewer self-reported school absences in the treatment group than in the control subjects (Table 3 and Figure 2C). This translates to almost 7 days per student saved per school year relative to control subjects. School de-identified records of absences for all reasons, however, showed substantial increase at follow-up with no significant differences between groups (P = 0.61) (Figure 2C). ASMA students increased from 26.0 (± 21.5) days absent for the year before the intervention to 42.1 (± 35.5) days absent for the follow-up school year; control subjects increased from 23.5 (± 21.3) days absent to 35.3 (± 39.9) days.
Urgent health care use.
For all three measures, the probability of belonging to the sure zero population depended on baseline levels of urgent health care use. Table 4 summarizes the results for the three urgent care use measures. Over the 12-month assessment period, the treatment did not significantly affect the prevalence of the sure zero population (all P values > 0.2), but it did significantly reduce the rates of urgent health care use in the population of nonsure zero ASMA students relative to control subjects. That is, the sure zero prevalence in the year after treatment was the same for the treatment and control groups, and compared with control subjects, the treatment group experienced a 28% reduction in acute medical visits, a 49% reduction in ED visits, and 76% reduction in hospitalizations. This finding translates to a yearly savings of 625 acute medical visits, 681 ED visits, and 180 fewer hospitalizations per 1,000 students. Or stated differently, there was a per-student savings of one acute medical visit, one ED visit, and one hospitalization in 1.6, 1.5, and 5.6 years, respectively.
TABLE 4.
TREATMENT EFFECTS OF ASTHMA SELF-MANAGEMENT FOR ADOLESCENTS ON URGENT HEALTH CARE USE
| Raw Data from Full Sample |
ZIP Model–Based Inferences |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ASMA |
Wait-list Control |
||||||||
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | ASMA Mean | Wait-list Control Mean | Prevalence Nonsure Zero Population (%) | Adjusted RR (95% CI) | P Value* | |
| Acute medical visits | |||||||||
| Baseline† | 3.18 (7.14) | 0 (0–48) | 4.14 (7.14) | 0 (0–42) | |||||
| 1-yr follow-up total | 1.41 (2.81) | 0 (0–24) | 2.42 (4.16) | 0 (0–30) | 1.58 | 2.20 | 51.01 | 0.72 (0.60–0.85) | 0.0002 |
| ED visits | |||||||||
| Baseline† | 1.80 (5.46) | 0 (0–36) | 1.92 (4.44) | 0 (0–30) | |||||
| 1-yr follow-up total | 0.64 (1.51) | 0 (0–10) | 1.46 (3.69) | 0 (0–30) | 0.69 | 1.38 | 31.85 | 0.52 (0.40–0.68) | <0.0001 |
| Hospitalizations | |||||||||
| Baseline† | 0.24 (1.32) | 0 (0–12) | 0.24 (1.14) | 0 (0–6) | |||||
| 1-yr follow-up total | 0.05 (0.30) | 0 (0–3) | 0.24 (1.18) | 0 (0–13) | 0.06 | 0.24 | 8.96 | 0.24 (0.09–0.66) | 0.0042 |
Definition of abbreviations: ASMA = Asthma Self-Management for Adolescents; ED = emergency department; ZIP = zero-inflated Poisson.
ZIP regression adjusting for baseline level, age, and cohort.
Mean number of visits in last 2 mo prorated to 1 year (i.e., baseline value × 6).
DISCUSSION
Previous school-based asthma interventions have focused primarily on elementary school children, despite adolescents' equal or greater need for such programs. ASMA is among the first interventions designed specifically for adolescents and the first to provide an empirically based intervention combined with an intervention for their medical providers. With few exceptions, the results of the randomized trial support our hypotheses. Relative to control subjects, students who participated in ASMA (1) showed improvements in asthma self-management (prevention steps and self-efficacy); (2) received better medical management from their medical providers (controller medication and WTPs); (3) had reductions in night awakening, days with activity restrictions, and self-reported asthma-related school absences; (4) had improved quality of life; and (5) had reductions in acute care visits, ED visits, and hospitalizations.
Control of asthma symptoms is an important goal. Although we improved students' confidence to manage asthma and the number of steps they took to prevent asthma, we did not improve the number of steps taken to manage symptoms once they occur. This suggests that at baseline adolescents may have skills to manage asthma symptoms but not prevent them, an observation that is consistent with prior research suggesting that families of younger children with uncontrolled asthma manage their asthma reactively, not preventively (42). ASMA resulted in sharp increases in the percentage of students who used controller medication and WTPs by the 6-month assessment relative to control subjects, whose use increased more slowly. At 12 months, the percentage of control students using WTPs remained significantly lower than ASMA students, but the percentage using controller medication was not significantly different. Increases in controller medication and WTP use in the control group most likely reflect secular trends toward wider use of these tools as well as having community-wide interventions in place in New York City. For example, during the study, the New York City Department of Health and Mental Hygiene's Childhood Asthma Initiative was implementing a citywide campaign targeting the public and the medical community that emphasized the use of written treatment plans for all asthma patients and use of controller medication for those with persistent asthma.
Participation in ASMA resulted in improvements in self-reported days with activity limitations and night wakening but not daytime symptoms. These improved outcomes may be associated with a more negative impact on students' experiences than daytime symptoms, and thus may be easier to recall. Prior research suggests that daytime symptoms are ignored by families with poor asthma management skills (43).
Of particular interest is the finding that the intervention reduced urgent medical care, including unscheduled medical visits to a medical provider, ED visits, and hospitalizations. Because direct costs of acute care visits are high, fewer visits will generate significant savings. To the extent that these cost offsets could accrue to a single entity (i.e., a third-party payer), it might provide a mechanism for funding implementation of ASMA. Indirect costs (e.g., school absences, lost wages of students and parents) associated with acute care visits are expensive as well. Because indirect costs are spread among many and savings would not be concentrated, they are more difficult to leverage. Future research should define the economic impact and cost-effectiveness of ASMA.
A recent review of school-based asthma interventions has shown that most improve intermediate outcomes (e.g., knowledge, self-efficacy, and self-management), but fewer impact health outcomes (44). The comprehensive nature of our intervention, including group workshops and tailored individual sessions for students, and academic detailing for their providers, may account for ASMA's ability to improve health outcomes and reduce urgent health care use. Individually, ASMA's intervention elements have been found to be effective. For example, Open Airways for Schools is an efficacious group intervention for elementary schools students (35, 45, 46). Joseph and colleagues found that tailored web-based messages for adolescents reduced asthma morbidity and hospitalizations, but not ED visits (15). Interventions with medical providers have resulted in change in the medical providers' behavior as well as patient health outcomes (24–27). Our study design precluded the exploration of which intervention component contributed to our results or if the components were synergistic. It has been suggested that appropriate medical care and pharmacotherapy are essential elements of successful interventions (47, 48). The academic detailing component of ASMA may have served this purpose. Although not directly assessed, one may assume the intervention had positive effects on medical providers' behavior because ASMA students were more likely to have controller medication and use WTPs than control subjects.
Our results regarding improvement in self-reported asthma-related school absences, but not overall absenteeism as recorded by school data, are consistent with prior studies with elementary school–aged children (49, 50). These discrepant findings may reflect the fact that school absences are due to numerous reasons other than asthma, and the intervention did not impact these other reasons. Prior research in elementary school students with asthma has shown that about 24% of overall school absences are due to the disease (51). In this study, there were also far fewer self-reported absences reported due to asthma than overall absences from school data (42 to 82% fewer depending on the group assignment and assessment period). Because school records do not capture reasons for absences, we could not directly compare self-reported absences to school data on absences due to asthma.
The fact that self-reported improvements in attendance are not supported by school records in three studies (49, 50) suggests that relying on school attendance as an outcome is problematic. Self-report data may have potential errors in student recall and/or could be flawed due to reporting biases. Despite having the advantage of being an objective measure, school records may also be inaccurate (50). For example, students may arrive at school late, and thus may be counted as absent.
The study has several limitations. We relied primarily on self-reported data and did not corroborate self-report data with objective data. Therefore, the potential self-report bias found in our school attendance data may also be present in our other outcome data. Similarly, our case detection was by self-report and thus has the potential for misclassification bias. Because we targeted those with moderate and severe persistent asthma, the chance of enrolling a student who did not have asthma was low. However, we may have had false-negatives, missing students in need. Although recruitment of eligible students exceeded that in other asthma trials with urban adolescents (15), just less than 40% of eligible youth were not enrolled; this limits the extrapolation of study results to a larger population of urban, minority high school students with moderate to severe persistent asthma. We are also unable to extrapolate study results to other populations of high school students with asthma (e.g., white suburban adolescents with mild asthma) because we limited enrollment to minority youth with moderate to severe persistent asthma. The control group was a wait-list control group who received no attention. Although unlikely, it is possible that treatment benefits of ASMA were due to “nonspecific therapeutic factors,” including the effects of attention and positive regard received by those in the intervention condition (52). At the same time, treatment differences may have been mitigated (1) if the consent process and/or surveys fostered attention to treating asthma in the control students; (2) if participants received interim treatments from other sources, which we did not assess; and/or (3) if there was contamination of control subjects by the treated adolescents. However, we had sufficient power to control for contamination, and our informal interviews with control subjects regarding their contact with other students in the program suggest that such contamination did not occur. Although cigarette use is prevalent among adolescents (53), and smoke is an asthma trigger, we did not assess the impact of smoking behavior nor exposure to secondhand smoke.
Despite these limitations, ASMA is one of the first interventions for adolescents with asthma with demonstrated efficacy. Targeting students and their medical providers, ASMA resulted in improvements in asthma management, asthma morbidity, and urgent health care use for urban adolescents with moderate to severe persistent asthma. This extends our earlier work showing that adolescents with persistent asthma can be engaged in ASMA and consider their participation in the intervention helpful (14). ASMA addresses an illness with high public health significance and, as such, can serve as a model for other populations of adolescents with asthma (e.g., suburban and rural settings) or for adolescents with other chronic illnesses. Adoption of ASMA by schools would contribute to reducing the burden of asthma on adolescents. However, this is potentially challenging to schools. In addition to schools' limited resources, including school personnel to deliver the intervention, a unique challenge to disseminating ASMA is the need for a pediatric pulmonologist or allergist to deliver the academic detailing component of ASMA; not all communities may have easy access to such a specialist. A potential dissemination model schools might consider is collaborating with health insurance companies who have health educators as well as pulmonologists on staff who could deliver the intervention. Building on a successful dissemination model used in Australia (54), schools could also partner with universities that have medical schools, as well as health education, public health, nursing, or psychology departments. University students could deliver the student intervention, and medical school faculty could provide the academic detailing.
Acknowledgments
The authors thank Rachel G. Klein, Ph.D. for her careful review of this manuscript and Eva Petkova, Ph.D. for her assistance with statistical analyses.
Originally Published in Press as DOI: 10.1164/rccm.201003-0429OC on December 7, 2010
Supported by National Heart, Lung, and Blood Institute grants R01HL067268 (D.E.) and the NYC Speaker's Fund (J.-M.B.). Preparation of this manuscript was partially supported by National Heart, Lung, and Blood Institute grants R01HL079953 and R01HL089493 (J.-M.B.).
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.ISAAC. Worldwide variations in the prevalence of asthma symptoms: The International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J 1998;12:315–335. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Self-reported asthma among high school students–United States, 2003. MMWR Morb Mortal Wkly Rep 2005;54:765–767. [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States. 1980–2007. Pediatrics 2009;123:S131–S145. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics 2002;110:315–322. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Measuring childhood asthma prevalence before and after the 1997. redesign of the National Health Interview Survey–United States. MMWR Morb Mortal Wkly Rep 2000;49:908–911. [PubMed] [Google Scholar]
- 6.Calmes D, Leake BD, Carlisle DM. Adverse asthma outcomes among children hospitalized with asthma in California. Pediatrics 1998;101:845–850. [DOI] [PubMed] [Google Scholar]
- 7.Venn A, Lewis S, Cooper M, Hill J, Britton J. Questionnaire study of effect of sex and age on the prevalence of wheeze and asthma in adolescence. BMJ 1998;316:1945–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osman M, Hansell AL, Simpson CR, Hollowell J, Helms PJ. Gender-specific presentations for asthma, allergic rhinitis and eczema in primary care. Prim Care Respir J 2007;16:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aligne CA, Auinger P, Byrd RS, Weitzman M. Risk factors for pediatric asthma: contributions of poverty, race, and urban residence. Am J Respir Crit Care Med 2000;162:873–877. [DOI] [PubMed] [Google Scholar]
- 10.Claudio L, Stingone JA, Godbold J. Prevalence of childhood asthma in urban communities: the impact of ethnicity and income. Ann Epidemiol 2006;16:332–340. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RS, Carrion-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol 2006;117:351–358. [DOI] [PubMed] [Google Scholar]
- 12.Lieu TA, Lozano P, Finkelstein JA, Chi FW, Jensvold NG, Capra AM, Quesenberry CP, Selby JV, Farber HJ. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002;109:857–865. [DOI] [PubMed] [Google Scholar]
- 13.Halterman JS, Aligne CA, Auinger P, McBride JT, Szilagyi PG. Inadequate therapy for asthma among children in the United States. Pediatrics 2000;105:272–276. [PubMed] [Google Scholar]
- 14.Bruzzese J-M, Bonner S, Vincent EJ, Sheares BJ, Mellins RB, Levison MJ, Wiesemann S, Du Y, Zimmerman BJ, Evans D. Asthma education: the adolescent experience. Patient Educ Couns 2004;55:396–406. [DOI] [PubMed] [Google Scholar]
- 15.Joseph CLM, Peterson E, Havstad S, Johnson CC, Hoerauf S, Stringer S, Gibson-Scipio W, Ownby DR, Elston-Lafata J, Pallonen U, et al. A web-based, tailored asthma management program for urban African-American high school students. Am J Respir Crit Care Med 2007;175:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah S, Peat JK, Mazurski EJ, Wang H, Sindhusake D, Bruce C, Henry RL, Gibson PG. Effect of peer led programme for asthma education in adolescents: cluster randomised controlled trial. BMJ 2001;322:583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabana MD, Rand CS, Becher OJ, Rubin HR. Reasons for pediatrician nonadherence to asthma guidelines. Arch Pediatr Adolesc Med 2001;155:1057–1062. [DOI] [PubMed] [Google Scholar]
- 18.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein JA, Lozano P, Shulruff R, Inui TS, Soumerai SB, Ng M, Weiss KB. Self-reported physician practices for children with asthma: are national guidelines followed? Pediatrics 2000;106:886–896. [PubMed] [Google Scholar]
- 20.Goodman DC, Lozano P, Stukel TA, Chang C, Hecht J. Has asthma medication use in children become more frequent, more appropriate, or both? Pediatrics 1999;104:187–194. [DOI] [PubMed] [Google Scholar]
- 21.Crim C. Clinical practice guidelines vs actual clinical practice: the asthma paradigm. Chest 2000;118:62S–64S. [DOI] [PubMed] [Google Scholar]
- 22.Cabana MD, Ebel BE, Cooper-Patrick L, Powe NR, Rubin HR, Rand CS. Barriers pediatricians face when using asthma practice guidelines. Arch Pediatr Adolesc Med 2000;154:685–693. [DOI] [PubMed] [Google Scholar]
- 23.Doerschug KC, Peterson MW, Dayton CS, Kline JN. Asthma guidelines: an assessment of physician understanding and practice. Am J Respir Crit Care Med 1999;159:1735–1741. [DOI] [PubMed] [Google Scholar]
- 24.Clark NM, Gong M, Schork MA, Evans D, Roloff D, Hurwitz M, Maiman L, Mellins RB. Impact of education for physicians on patient outcomes. Pediatrics 1998;101:831–836. [DOI] [PubMed] [Google Scholar]
- 25.Cabana MD, Slish KK, Evans D, Mellins RB, Brown RW, Lin X, Kaciroti N, Clark NM. Impact of physician asthma care education on patient outcomes. Pediatrics 2006;117:2149–2157. [DOI] [PubMed] [Google Scholar]
- 26.Cloutier MM, Hall CB, Wakefield DB, Bailit H. Use of asthma guidelines by primary care providers to reduce hospitalizations and emergency department visits in poor, minority, urban children. J Pediatr 2005;146:591–597. [DOI] [PubMed] [Google Scholar]
- 27.Cloutier MM, Jones GA, Hinckson V, Wakefield DB. Effectiveness of an asthma management program in reducing disparities in care in urban children. Ann Allergy Asthma Immunol 2008;100:545–550. [DOI] [PubMed] [Google Scholar]
- 28.Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav 2001;28:769–782. [DOI] [PubMed] [Google Scholar]
- 29.Lancaster T, Stead L, Shepperd S. Helping parents to stop smoking: which interventions are effective? Paediatr Respir Rev 2001;2:222–226. [DOI] [PubMed] [Google Scholar]
- 30.Bruzzese J-M, Evans D, Vincent E, Mellins R, Sheares B. Asthma self-management for adolescents: improving health outcomes [abstract]. Proc Am Thorac Soc 2006;3:A269. [Google Scholar]
- 31.Vincent EJ, Bruzzese JM, Evans D. How to successfully enroll and engage teenagers in an asthma health education intervention: lessons from NYC [abstract]. Am J Respir Crit Care Med 2004;169:A255. [Google Scholar]
- 32.National Heart Lung and Blood Institute. Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97–4051.
- 33.Evans D, Clark NM, Levison MJ, Levin B, Mellins RB. Can children teach their parents about asthma? Health Educ Behav 2001;28:500–511. [DOI] [PubMed] [Google Scholar]
- 34.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res 1996;5:35–46. [DOI] [PubMed] [Google Scholar]
- 35.Evans D, Clark NM, Feldman CH, Rips J, Kaplan D, Levison MJ, Wasilewski Y, Levin B, Mellins RB. A school health education program for children with asthma aged 8–11 years. Health Educ Q 1987;14:267–279. [DOI] [PubMed] [Google Scholar]
- 36.Bruzzese J-M, Evans D, Wiesemann S, Pinkett-Heller M, Levison MJ, Du Y, Fitzpatrick C, Krigsman G, Ramos-Bonoan C, Turner L, et al. Using school staff to establish a preventive network of care to improve elementary school students' control of asthma. J Sch Health 2006;76:307–312. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, Forsetlund L, Bainbridge D, Freemantle N, Davis DA, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007;4:CD000409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullagh P, Nelder JA. Generalized linear models. Boca Raton, FL: Chapman & Hall/CRC; 1999.
- 39.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of longitudinal data. Oxford, UK: Oxford University Press; 2002.
- 40.Lambert D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 1992;34:1–14. [Google Scholar]
- 41.Dietz E, Böhning D. On estimation of the Poisson parameter in zero-modified Poisson models. Comput Stat Data Anal 2000;34:441–459. [Google Scholar]
- 42.Zimmerman BJ, Bonner S, Evans D, Mellins RB. Self-regulating childhood asthma: a developmental model of family change. Health Educ Behav 1999;26:55–71. [DOI] [PubMed] [Google Scholar]
- 43.Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma 2002;39:167–179. [DOI] [PubMed] [Google Scholar]
- 44.Coffman JM, Cabana MD, Yelin EH. Do school-based asthma education programs improve self-management and health outcomes? Pediatrics 2009;124:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruzzese JM, Markman LB, Appel D, Webber M. An evaluation of Open Airways for Schools: using college students as instructors. J Asthma 2001;38:337–342. [DOI] [PubMed] [Google Scholar]
- 46.American Lung Association. Open airways for schools. New York: American Lung Association; 2003.
- 47.Frankowski BL. Asthma education: are pediatricians ready and willing to collaborate with schools? Pediatrics 2009;124:793–795. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler LS, Merkle SL, Gerald LB, Taggart VS. Managing asthma in schools: lessons learned and recommendations. J Sch Health 2006;76:340–344. [DOI] [PubMed] [Google Scholar]
- 49.Clark NM, Brown R, Joseph CL, Anderson EW, Liu M, Valerio MA. Effects of a comprehensive school-based asthma program on symptoms, parent management, grades, and absenteeism. Chest 2004;125:1674–1679. [DOI] [PubMed] [Google Scholar]
- 50.Gerald LB, Redden D, Wittich AR, Hains C, Turner-Henson A, Hemstreet MP, Feinstein R, Erwin S, Bailey WC. Outcomes for a comprehensive school-based asthma management program. J Sch Health 2006;76:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mangan JM, Gerald LB. Asthma agents: monitoring asthma in school. J Sch Health 2006;76:300–302. [DOI] [PubMed] [Google Scholar]
- 52.Jensen PS, Weersing R, Hoagwood KE, Goldman E. What is the evidence for evidence-based treatments? A hard look at our soft underbelly. Ment Health Serv Res 2005;7:53–74. [DOI] [PubMed] [Google Scholar]
- 53.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. 2008. Monitoring the future national results on adolescent drug use: overview of key findings, National Institute on Drug Abuse. Bethesda, MD: National Institutes of Health; 2007. NIH publication 08-6418.
- 54.Shah S, Roydhouse JK, Sawyer SM. Medical students go back to school–the Triple A journey. Aust Fam Physician 2008;37:952–954. [PubMed] [Google Scholar]