Abstract
Rationale: Surfactant protein D (SP-D), a 43-kD collectin, is synthesized and secreted by airway epithelia as a dodecamer formed by assembly of four trimeric subunits. We have previously shown that the quaternary structure of SP-D can be altered during inflammatory lung injury through its modification by S-nitrosylation, which in turn alters its functional behavior producing a proinflammatory response in effector cells.
Objectives: We hypothesized that alterations in structure and function of SP-D may occur in humans with acute allergic inflammation.
Methods: Bronchoalveolar lavage (BAL) fluid was collected from 15 nonsmoking patients with mild intermittent allergic asthma before and 24 hours after segmental provocation with saline, allergen, LPS, and mixtures of allergen and LPS. Structural modifications of SP-D were analyzed by native and sodium dodecyl sulfate gel electrophoresis.
Measurements and Main Results: The multimeric structure of native SP-D was found to be disrupted after provocation with allergen or a mixture of allergen and LPS. Interestingly, under reducing conditions, sodium dodecyl sulfate–polyacrylamide gel electrophoresis demonstrated that 7 of 15 patients with asthma developed an abnormal cross-linked SP-D band after segmental challenge with either allergen or a mixture of allergen with LPS but not LPS alone. Importantly, patients with asthma with cross-linked SP-D demonstrated significantly higher levels of BAL eosinophils, nitrogen oxides, IL-4, IL-5, IL-13, and S-nitrosothiol–SP-D compared with patients without cross-linked SP-D.
Conclusions: We conclude that segmental allergen challenge results in changes of SP-D multimeric structure and that these modifications are associated with an altered local inflammatory response in the distal airways.
Keywords: human asthma, pulmonary collectins, nitric oxide, surfactant proteins, biomarker
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Segmental allergen challenge in patients with asthma induces alterations of expression, structure, and function of surfactant protein D (SP-D). Cross-linked forms of SP-D can potentially serve as a biomarker in allergic asthma because they appear in patients with more severe allergic inflammation and correlate with markers of allergic airway inflammation. S-Nitrosylated SP-D occurs with airway inflammation and is associated with a loss of antiinflammatory function.
What This Study Adds to the Field
SP-D is post-translationally modified in patients with asthma. Although S-nitrosylated SP-D is associated with a loss of antiinflammatory function, cross-linked SP-D is correlated with disease severity and suggests a potential role for this isoform as a biomarker.
Pulmonary surfactant protein D (SP-D) is a calcium-dependent lectin (1). Primarily expressed and secreted by Clara cells and type II cells of the airway (2, 3), SP-D modulates the function of a variety of inflammatory cells including lymphocytes, macrophages, neutrophils, and eosinophils (4). The secreted form of SP-D is assembled into a large cruciform dodecamer (12 subunits) made up of a tetramer of trimers, one of the largest and most flexible molecules found in the innate immune system (5).
Oligomerization is critical to collectin function because it increases affinity for both pathogens and immune cells (6). Multimeric SP-D is capable of aggregation of allergens (7), lysis of microbes, enhancement of phagocytosis (8–11), and modulation of cytokines and reactive oxygen species (12, 13). SP-D is a regulator of pulmonary inflammation because it can activate macrophages and protect them from oxidative stress (14), and it is also able to inhibit allergen-induced activation of mast cells and basophils (15).
Nitric oxide (NO) affects SP-D by several mechanisms and with varying effects on the structural organization of this critical regulator (16). NO can modify SP-D by nitrosylating two critical cysteines in its tail domain. This results in disruption of oligmerization and the release of S-nitrosothiol (SNO) containing SP-D trimers. SNO–SP-D trimers stimulate macrophage migration and lung chemokine production through calreticulin/CD91 binding and p38 activation (17). Higher oxides of nitrogen, such as peroxynitrite, can nitrate and cross-link SP-D in vitro, which results in a significant decrease in SP-D–dependent aggregating activity in mice acutely exposed to nitrogen dioxide (18).
Allergic asthma is a chronic disease characterized by bronchial hyperresponsiveness, episodic airway obstruction, and eosinophilic airway inflammation associated with excess production of NO (19). There is also a large increase in reactive oxygen species production, which is associated with shift in NO metabolism toward higher oxide formation (20, 21). Thus, during pulmonary inflammation, there is a dual effect of an increased concentration of toxic oxygen-nitrogen species and an impairment of normal NO signaling. Because SP-D function has been shown to be regulated by NO-mediated post-translational modifications, we hypothesized that acute allergic inflammation would alter SP-D structure and function. Therefore, we examined the effect of segmental challenge with antigen or endotoxin in subjects with mild allergic asthma (22–24). Here we show that acute segmental allergen challenge alone, or in combination with LPS, induces changes in SP-D multimeric structure in vivo that are associated with the loss of the ability of SP-D to inhibit eosinophil chemotaxis. Some of the results of these studies have been previously reported in the form of an abstract (25).
METHODS
A polyclonal antibody against SP-D (Ab 1754) was produced in rabbits using two synthetic peptides, as described previously (see online supplement) (8). A polyclonal antiserum against recombinant mouse SP-D was purchased from Chemicon, Inc. (Temecula, CA). Recombinant rat SP-D (rrSP-D) was expressed in CHO cells and purified, as described previously (26). A monoclonal antiserum against 3-nitrotyrosine was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mice overexpressing rat SP-D (SP-D OE) were provided by Dr. Frank McCormack and have been described previously (27).
Study Subjects and Design
The study population consisted of 15 nonsmoking subjects (mean age, 28 ± 5.2 yr) (Table 1) with mild intermittent allergic asthma (as defined in the GINA Report 2006, revised). Subjects who were skin prick test positive for house dust mite allergen, airway hyperresponsive to methacholine provocation (PC20 <8 mg/mL), and had a positive response to inhaled allergen, defined as a 20% fall in FEV1 on a standard graded-dose bronchoprovocation test, were eligible for the study. Subjects underwent segmental challenges with allergen (Dermatophagiodes pteronyssinus [ALK-Scherax, Wedel, Germany]), LPS, and the combination of both during a first bronchoscopy. During a second bronchoscopy 24 hours later, BAL of the challenged segments was performed, as previously described (see online supplement) (23).
TABLE 1.
PATIENT DEMOGRAPHICS
| Age (yr) | Sex | FEV1 (% pred) | PD20 (SQE) | Late phase | IgE | Allergen Provocation SQ-U | |
|---|---|---|---|---|---|---|---|
| Patients without cross-linked SP-D (n = 8) | |||||||
| 35 | F | 101 | 745 | − | 20 | 1,000 | |
| 32 | M | 87 | 1,372 | + | 289 | 1,000 | |
| 23 | F | 101 | 149 | − | 173 | 1,000 | |
| 21 | F | 97 | 1,124 | − | 186 | 100,000 | |
| 22 | F | 101 | 87 | − | 353 | 100,000 | |
| 27 | M | 90 | 268 | − | 233 | 1,000 | |
| 25 | F | 79 | 234 | + | 767 | 10,000 | |
| 29 | F | 94 | 52 | + | 183 | 1,000 | |
| Median | 26 | 96 | 251 | 210 | |||
| Range | 21–35 | 6F/2M | 79–101 | 52–1,372 | 20–767 | ||
| Patients with cross-linked SP-D (n = 7) | |||||||
| 34 | F | 109 | 49 | − | 444 | 100 | |
| 37 | M | 98 | 90 | + | 246 | 100 | |
| 26 | M | 107 | 485 | + | 15 | 1,000 | |
| 24 | F | 104 | 9 | + | 495 | 10,000 | |
| 32 | M | 80 | 175 | + | 130 | 10,000 | |
| 31 | M | 111 | 240 | − | 24 | 100,000 | |
| 23 | F | 128 | 898 | − | 161 | 100,000 | |
| Median | 31 | 107 | 175 | 161 | |||
| Range | 23–37 | 3F/4M | 80–128 | 9–898 | 15–495 | ||
Definition of abbreviation: SP-D = surfactant protein D.
All study subjects were nonsmokers. None suffered from acute bronchitis or received treatment with corticosteroids, sodium cromoglycate, theophylline, or leucotriene modifiers within 4 weeks of bronchoscopy. Patients used β2-agonists only when required for relief of symptoms. FEV1 is given as percent of predicted normal. PD20 provocation dose of allergen causing a 20% drop in FEV1. Allergen sensitivity was determined by skin-prick test. Allergen provocation was done with the indicated amount (SQ-U) of Dermatophagoides pteronyssinus mix diluted in 10 ml of saline solution.
The Hannover Medical School ethics committee approved the study protocol. All study subjects gave written informed consent after being fully informed about the purpose of the study.
Control BAL was obtained from two groups: healthy subjects with no asthma with normal FEV1 and no allergy, and subjects with mild intermittent asthma who were challenged with a different allergen (grass pollen extract). Details of these study populations have been published elsewhere (28).
Polyacrylamide Gel Electrophoresis and Immunoblotting for SP-D or Nitrotyrosine
BAL proteins were separated and analyzed by two methods. Denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using NuPAGE 10% or 12% bis-tris gels for SP-D or nitrotyrosine (Invitrogen, Inc., Carlsbad, CA). Native gel electrophoresis for detection of SP-D quaternary structure was performed using NuPAGE 3–8% tris-acetate gels (Invitrogen), as previously described (see online supplement) (17, 29).
Cross-link Modification of SP-D In Vitro
BAL from SP-D OE mice (27) or rrSP-D (1.6 × 10−13 moles) was preincubated with increasing molar ratios of NaOCl for 30 minutes at 37°C in a 5% CO2 atmosphere. Molar ratio was calculated based on the amount of tyrosine in the molecular structure of rrSP-D (18). Reaction product of cross-linked SP-D was evaluated by SDS-PAGE with β-mercaptoethanol followed by immunoblotting with polyclonal SP-D antiserum (Ab 1754), as described previously.
Chemotaxis Assay with Human Eosinophils from Atopic Donors
Eosinophils were isolated from peripheral blood of allergic patients and chemotaxis was measured with a modified Boyden chamber assay with human eosinophils, as described (30, 31). Suspensions of human eosinophils were allowed to migrate toward eotaxin-containing medium in the presence or absence of either native rrSP-D (0.1 μg/ml) or S-nitrosylated rrSP-D (0.1 μg/ml). To generate SNO–SP-D, rrSP-D (10 μg/ml) was incubated with SNOC (200 μM) at 37°C for 30 minutes in the dark, as previously described (17). All assays were performed in triplicate (see online supplement).
Statistical Analysis
Data analyses were performed using GraphPad InStat v 3.06 for Windows (GraphPad Software, San Diego, CA). Parametric data were analyzed with analysis of variance or Student t test assuming equal variances to test differences between groups. Correlations were calculated using Spearman rank correlation coefficients. Data were expressed as mean ± SEM. In all cases a P value of less than 0.05 was considered significant.
RESULTS
Challenge with Allergen Causes Cross-linking of Human SP-D and Alters its Quaternary Structure
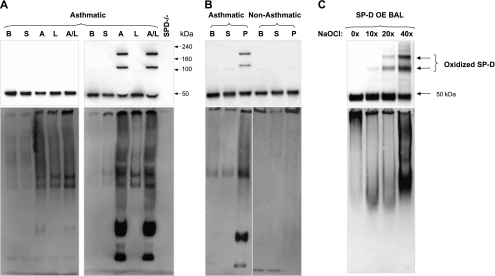
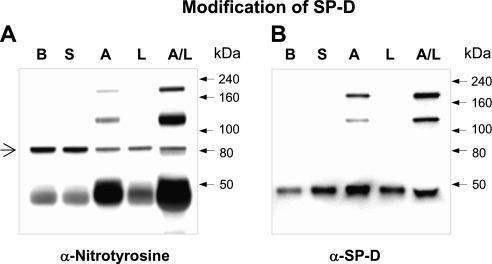
We used segmental challenge with LPS and allergen (23) to investigate the effect of acute allergic inflammation on the oligomeric state of SP-D. SP-D was examined by native and reducing gel electrophoresis of BAL fluid 24 hours after challenges with saline, allergen, LPS, or a combination of allergen with LPS (Figure 1A). Seven out of 15 patients with asthma displayed higher molecular weight (multimeric) forms of SP-D in BAL post-challenge with allergen and LPS despite reducing conditions (quantity of cross-linked SP-D as a percentage of total SP-D in BAL after allergen and LPS challenge was 45 ± 11%; n = 7). Four of the seven subjects additionally showed higher molecular weight forms of SP-D after challenge with allergen alone (quantity of cross-linked SP-D as a percentage of total SP-D in BAL after allergen challenge was 40 ± 13%; n = 4). In Figure 1A, blots from one representative subject out of seven who developed cross-linked SP-D (top right) and from one subject out of eight who did not develop cross-linked SP-D (top left) are shown.
Figure 1.
Challenge with allergen causes cross-linking of human surfactant protein D (SP-D) and alters its quaternary structure. (A) Blots from two subjects of the entire study group (n = 15) who underwent segmental challenges are shown. The left blots are from a representative subject who did not develop cross-linked SP-D (n = 8) and the right blots are from a representative subject who developed cross-linked SP-D (n = 7). Bronchoalveolar lavage (BAL) fluid from patients with asthma at baseline (B), and after challenge with either saline (S), house dust mite allergen (A), LPS (L), or a combination of allergen with LPS (A/L) was analyzed for total SP-D level by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reduced conditions (top) or by native gel electrophoresis to determine quaternary structure of SP-D (bottom). BAL from SP-D–deficient mice (SP-D−/−) was run as a negative control for nonspecific bands. Quantity of cross-linked SP-D as a percentage of total SP-D per group (A and A/L) is given in the result section and individual data are provided in Figure 4. (B) Blots from two representative subjects from a different challenge study are shown. BAL fluid from one subject with asthma (left lanes) and one healthy subject (right lanes) at baseline (B), and after challenge with saline (S) or mixed grass pollen allergen (P) were analyzed as in A. (C) Oxidative cross-linking of SP-D in vitro. BAL from mice overexpressing rat SP-D (OE BAL) was treated with various doses of NaOCl and reaction products were resolved by SDS-PAGE under reducing conditions (top) or by native gel (bottom) followed by immunoblotting with anti–SP-D antibody. Shown data are representative of five independent experiments.
In addition, these same patients demonstrated abnormalities in quaternary structure of SP-D. On native gels, oligomeric forms of SP-D are normally so large that they fail to enter the gel (17) as seen with the baseline and saline-challenged BAL samples (Figure 1A, bottom panels). In contrast, after challenge, the SP-D complex is disassembled and lower molecular weight forms become readily visible. Patients without asthma developed neither cross-linked SP-D nor disrupted multimers after challenge (Figure 1B, right lanes). Patients with asthma challenged with an alternate antigen (grass pollen) (28) displayed cross-linked SP-D and a disrupted multimeric state (Figure 1B, left lanes). Preincubation with 50 mM ethylenediaminetetraacetic acid did not reduce the size of SP-D on reductive electrophoresis (data not shown), indicating that the appearance of these higher molecular weight forms of SP-D is not a result of a direct interaction or binding of SP-D with allergen or mixture of allergen with LPS.
Multimeric SP-D could be generated in vitro. BAL from SP-D OE mice was chemically modified using NaOCl as an oxidative cross-linking agent. This treatment dose-dependently produced nondisulfide-mediated cross-linking of SP-D, which was insensitive to reducing agents, and this treatment elicited a banding pattern that was similar to that which appeared in the BAL of challenged patients with asthma (Figure 1C, top). A similar pattern of cross-linked SP-D was observed for chemically modified rrSP-D (data not shown). In vitro cross-linking of SP-D was associated with disruption of its multimeric structure (Figure 1C, bottom).
Patients with Cross-linked SP-D Exhibit an Increase in Total BAL SP-D Levels after Allergen Challenge
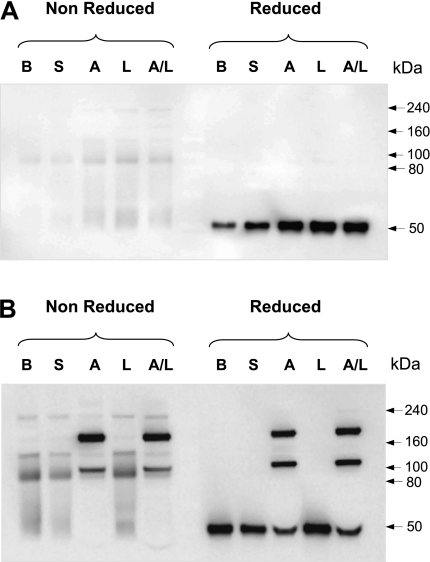
BAL fluids from patients with asthma at baseline and after challenge were subject to SDS-PAGE in the absence and presence of thiol reductants. Those patients whose BAL contained a single band under reducing conditions exhibited a variety of higher molecular weight bands corresponding to dimers, trimers, and higher-order multimers under nonreducing conditions (Figure 2A). In a subset of patients displaying multimeric SP-D under nonreducing conditions, a portion of monomeric SP-D was observed on thiol reduction (Figure 2B); however, a considerable portion of SP-D was resistant to reduction (cross-linked).
Figure 2.
A subset of patients displays a novel form of cross-linked surfactant protein D (SP-D). Representative blots of patients (A) who did not develop and (B) who developed abnormal cross-linked SP-D bands after segmental challenge. Bronchoalveolar lavage (BAL) from patients with asthma was denatured under reducing and nonreducing conditions and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblotting with anti–SP-D antibody. B = baseline; S = saline; A = allergen; L = LPS; A/L = combination of allergen with LPS. Shown data are representative of six independent experiments.
On the basis of this biochemical behavior we next divided patients with asthma into those who exhibited cross-linked SP-D (cross-linked group) and those whose SP-D was monomeric (monomeric group) on reducing gel electrophoresis.
Cross-linked SP-D as a Marker of Asthma Severity
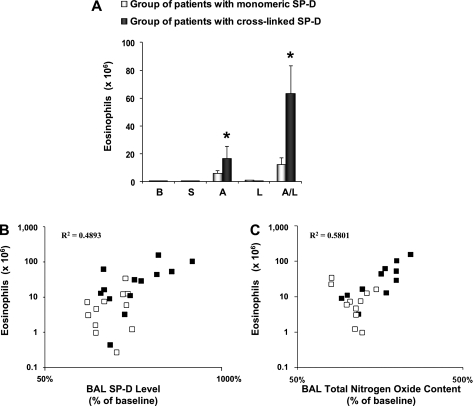
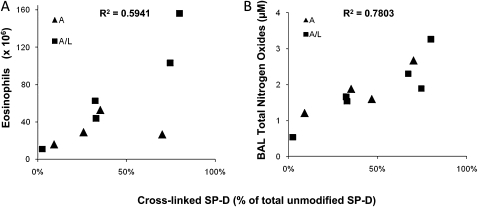
BAL eosinophilia indicates the degree of allergic airway inflammation after segmental challenge. Figure 3A shows that after challenge with allergen or the combination allergen and LPS there was a significant increase in eosinophil number only in patients from the cross-linked group. No differences in other inflammatory cells (macrophages, lymphocytes, and neutrophils) were observed (data not shown). Patients in the cross-linked group exhibited a significant increase in total BAL SP-D levels after segmental challenge compared with baseline and with patients in the monomeric group. SP-D levels in BAL from segments challenged with allergen and allergen plus LPS showed a significant, positive correlation with the numbers of eosinophils across all patients in the study (R2 = 0.49) (Figure 3B). Additionally, patients in the cross-linked group are clustered toward the top right of the distribution, with higher SP-D levels and higher eosinophil numbers. Furthermore, the quantity of cross-linked SP-D (as a percentage of total SP-D) is closely correlated with BAL eosinophil numbers (R2 = 0.59) (Figure 4A), suggesting an association between severity of airway inflammation and the formation of cross-linked SP-D.
Figure 3.
Patients with asthma with cross-linked surfactant protein D (SP-D) exhibit increased bronchoalveolar lavage (BAL) eosinophil numbers, SP-D, and nitrogen oxides. (A) Eosinophil cell counts after segmental challenge. The data are expressed as cell numbers × 106. Values are shown as mean ± SEM (n = 7). B = baseline; S = saline; A = allergen; L = LPS; A/L = combination of allergen with LPS. BAL eosinophils number (log converted values of the absolute number of cells, y axis) after segmental challenge with allergen and combination of allergen with LPS were correlated with (B) BAL SP-D levels (log converted values of the percent of baseline, x axis) and with (C) BAL total nitrogen oxides (percent of baseline, x axis). Mean baseline level for total nitrogen oxides was 1.41 ± 0.29 μM and 1.25 ± 0.11 μM for patients in the monomeric and cross-linked SP-D group, respectively.
Figure 4.
Cross-linked surfactant protein D (SP-D) as a marker of disease severity. For the seven patients with higher molecular weight forms of SP-D, cross-linked SP-D was quantified as a percentage of total SP-D (x axis) in bronchoalveolar lavage (BAL) from segments challenged with either allergen alone (triangles, group mean 40 ± 13%) or allergen plus LPS (boxes, group mean 45 ± 11%) and was correlated with (A) BAL eosinophils number (absolute number of cells, y axis) or (B) BAL total nitrogen oxides (micromolar, y axis).
BAL fluids from both groups of patients with asthma were examined for total NO content after segmental challenge. NO metabolites in BAL from segments challenged with allergen and allergen plus LPS showed a significant, positive correlation with the numbers of eosinophils (R2 = 0.58) (Figure 3C). As in Figure 3B, this statistical relationship is also dominated by the effect of patients within the cross-linked group, because these samples are consistently grouped to the right of the distribution. This observation, in conjunction with Figure 3B, suggests that the presence of cross-linked SP-D may be a valuable biomarker for disease severity. Indeed the quantity of cross-linked SP-D (as a percentage of total SP-D) is closely correlated to both eosinophil number and NO metabolites within the BAL (Figures 4A and 4B).
Cross-linked SP-D Formed by Segmental Challenge Is Nitrated In Vivo
One potential mechanism of reductant-resistant cross-linking of SP-D is the formation of dityrosine. Higher oxides of nitrogen, such as peroxynitrite and nitrogen dioxide, are capable of generating tyrosyl radicals that can be intermediates in dityrosine or nitrotyrosine formation. Therefore, we examined whether the formation of cross-linked SP-D was accompanied by nitration. BAL from patients in the cross-linked group was immunoprecipitated with polyclonal anti–SP-D antibodies followed by immunoblotting for nitrotyrosine (Figure 5A). Nitrotyrosine is observed in both monomeric and cross-linked SP-D (Figure 5B), and is increased by challenge.
Figure 5.
Cross-linked surfactant protein D (SP-D) formed by segmental challenge is nitrated in vivo. (A) Bronchoalveolar lavage (BAL) collected from patients with cross-linked SP-D was immunoprecipitated with rabbit anti–SP-D serum followed by immunoblotting for nitrotyrosine. (B) BAL from the same patient was subject to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by immunoblotting with rabbit anti–SP-D serum. B = baseline; S = saline; A = allergen; L = LPS; A/L = combination of allergen with LPS. The arrow indicates a protein band that is not related to SP-D because it is not present in B. Shown data are representative of three independent experiments.
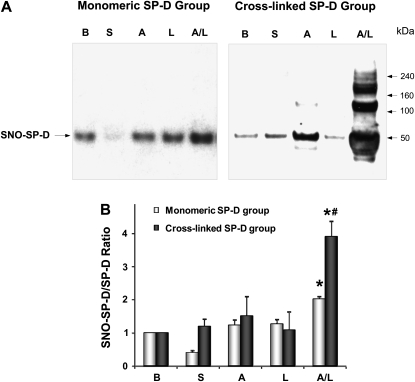
Patients with Cross-linked SP-D Exhibit Significantly Higher SNO–SP-D/SP-D Ratio after Segmental Challenge
NO can also directly alter SP-D structure and function by S-nitrosylation of cysteine residues in its NH2 tail region (17). BAL from patients after segmental challenge contains SNO-modified SP-D as demonstrated in Figure 6. Challenge with combined allergen and LPS results in an increase SNO–SP-D in the BAL from patients in the monomeric group (Figure 6A). Within the cross-linked group there is a significant increase in SNO–SP-D after allergen challenge compared with baseline and the monomeric group. Cross-linked SP-D was also S-nitrosylated after combined challenge with allergen and LPS (Figure 6A). Densitometric scanning revealed significant elevation in the SNO–SP-D/total SP-D ratio after allergen plus LPS challenge, which is significantly higher in the group of patients with cross-linked SP-D (Figure 6B).
Figure 6.
Patients with cross-linked surfactant protein D (SP-D) exhibit significantly higher S-nitrosothiol (SNO)–SP-D/SP-D ratio after segmental challenge. (A) Biotin-switch assay for SNO–SP-D content. Samples from one representative subject per group are shown. (B) Densitometric quantification of SNO–SP-D content. The data are expressed as a ratio SNO–SP-D over total SP-D (n = 7–8 in each group). *P < 0.05 from baseline level. #P < 0.05 from the corresponding challenge group. B = baseline; S = saline; A = allergen; L = LPS; A/L = combination of allergen with LPS.
Cross-linked SP-D in BAL Was Associated with Increased Levels of Cytokines
We determined the BAL cytokine profile induced in patients with asthma by allergen challenge. As shown in Table 2, patients in the cross-linked group exhibited markedly enhanced IL-4, IL-5, and IL-13 levels after challenge with allergen or a combination allergen and LPS. Importantly, the increase in T-helper cell type 2 (Th2) cytokine levels in the monomeric group was not altered after similar challenges, suggesting a lower level of release or reduced synthesis in the presence of intact SP-D. Notably, LPS challenge alone did not induce the release of Th2 cytokines in either group of patients.
TABLE 2.
CROSS-LINKED SP-D IN BRONCHOALVEOLAR LAVAGE WAS ASSOCIATED WITH INCREASED LEVELS OF CYTOKINES
| Challenges | IL-4 pg/ml | IL-5 pg/ml | IL-13 pg/ml |
|---|---|---|---|
| Patients without cross-linked SP-D | |||
| Baseline | 0 ± 0 | 0.9 ± 0.2 | 0.5 ± 0.1 |
| Saline | 0 ± 0 | 1.3 ± 0.5 | 0.8 ± 0.4 |
| Allergen | 6.8 ± 4.7 | 13.6 ± 7.3 | 3.3 ± 1.8 |
| LPS | 0 ± 0 | 1 ± 0.3 | 0.7 ± 0.3 |
| Allergen + LPS | 0.9 ± 0.9 | 5.9 ± 3.3 | 0.7 ± 0.2 |
| Patients with cross-linked SP-D | |||
| Baseline | 0 ± 0 | 0.6 ± 0.3 | 0.8 ± 0.3 |
| Saline | 0 ± 0 | 1.3 ± 0 | 0.3 ± 0.1 |
| Allergen | 35.3 ± 26.4 | 123.6 ± 90.9 | 76.6 ± 69.4 |
| LPS | 1.6 ± 1.6 | 4.3 ± 1.5 | 1.1 ± 0.3 |
| Allergen + LPS | 44 ± 20.6* | 286.2 ± 134.9* | 116.9 ± 70* |
Definition of abbreviation: SP-D = surfactant protein D.
Bronchoalveolar lavage from patients with asthma was collected at baseline or 24 hours after challenges with saline, allergen, LPS, and the combination of allergen with LPS, and cytokines and chemokines were determined simultaneously with a multiplex assay kit. The data are expressed as picogram per milliliter, and values are shown as mean ± SEM (n = 7).
P < 0.05 compared with the corresponding challenge group.
The Inhibitory Effect of SP-D on Eosinophil Chemotaxis Can Be Modulated by S-Nitrosylation
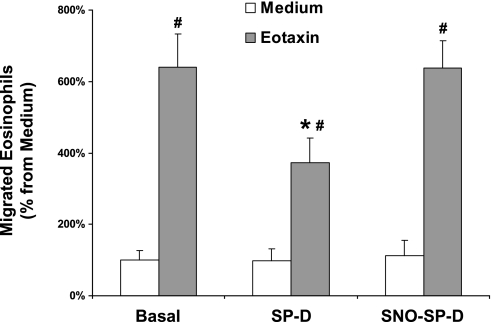
Recently, it has been shown that multimeric SP-D can inhibit eotaxin-induced chemotaxis and degranulation of eosinophils (32). To investigate the effect of modified SP-D on eotaxin-triggered chemotaxis of eosinophils, eosinophils isolated from peripheral blood of allergic patients were allowed to migrate toward 0.1 μg/ml eotaxin (positive controls), medium alone (negative control), 0.1 μg/ml of multimeric native rrSP-D, or 0.1 μg/ml of S-nitrosylated rrSP-D. Eotaxin-mediated eosinophil chemotaxis was significantly inhibited by the addition of native form of rrSP-D (Figure 7). However, S-nitrosylation of SP-D by SNOC abolished this inhibition. Notably, neither native SP-D nor SNO–SP-D induced eosinophil chemotaxis alone.
Figure 7.
The inhibitory effect of surfactant protein D (SP-D) on eosinophil chemotaxis can be modulated by S-nitrosylation. Eosinophils isolated from peripheral blood of allergic patients were assayed for migration toward eotaxin (positive controls), medium alone (negative control), oligomeric (native) rrSP-D, or S-nitrosylated rrSP-D combined with medium or eotaxin. The data are expressed as a percent of migrated eosinophils toward testing solutions, where 100% was calculated as the number of cells migrating toward medium only. #P < 0.05, basal migration toward eotaxin control compared with the same treatment; *P < 0.05, migration toward eotaxin control compared with rrSP-D or S-nitrosothiol–SP-D treatment. All test solutions were used in triplicate, n = 5 individual experiments.
DISCUSSION
In the present study we have identified a number of post-translational modifications of SP-D including oxidative cross-linking, S-nitrosylation, and nitration that occur in humans with acute allergic inflammation. We have demonstrated that segmental challenge with allergen or a combination of allergen and LPS causes covalent cross-linking of SP-D and alters its quaternary structure. The formation of covalent cross-linked SP-D in patients with asthma was associated with increased eosinophil number in the BAL, and increased NO metabolite formation. Furthermore, patients from the cross-linked group showed a significant increase in the level of SNO–SP-D and markedly enhanced Th2 cytokine levels after challenge. These observations suggest a strong relationship between cross-linked SP-D and the severity of allergic inflammation, indicating that it may represent a valuable biomarker for disease. However, accessibility of BAL might limit its clinical usefulness.
Griese and coworkers (33) demonstrated differences in multimeric organization of SP-D between BAL of healthy children and those suffering from gastroesophageal reflux disease. The quaternary structure of SP-D is altered toward an increase in monomers or trimers. Interestingly, gastroesophageal reflux disease is also associated with the formation of nonreducible, covalently linked forms of SP-D (33). In the present study, cross-linked SP-D and disruption of its multimeric structure after segmental challenge are not allergen-specific. When patients with asthma were challenged with another antigen, namely grass pollen (28), cross-linked SP-D was still formed and the multimeric state was disrupted (Figure 1B). Therefore, it is unlikely that the enzymatic activity of house dust mite allergen causes the modification. Importantly, healthy subjects did not develop cross-linked SP-D or disrupted multimeric structure after the same procedure (Figure 1B), which proves that the observed SP-D modifications are clearly related to the inflammatory response.
Studies with murine models of Pneumocystis pneumonia (8) and bleomycin-induced lung injury (34) have shown that enhanced NO production correlates with 3-nitrotyrosine formation, which has also been found in the BAL of subjects with asthma (20). Here we show that SP-D in both its monomeric and cross-linked form is nitrated after challenge. Similar to our findings, Matalon and coworkers (18) demonstrated that peroxynitrite causes nitration and cross-linking of native SP-D and a significant decrease in SP-D–dependent aggregation of LPS in vitro. Recently it has been demonstrated that the myeloperoxidase-derived oxidant hypochlorous acid also causes a loss of SP-D antimicrobial-dependent aggregating activity (35). This loss of function is associated with a variety of oxidations, especially the formation of oxidized methionine. These studies establish the possibility that SP-D oxidation can modify its function. The observation of nonreducible cross-linking of SP-D in our human model demonstrates that oxidative processes have occurred in vivo, if by an alternate pathway, and these processes may result in altered SP-D function.
In addition to mediating nitration and cross-linking, NO can directly modify SP-D by S-nitrosylation, which disrupts its multimeric structure and activates its proinflammatory functions in vivo and in vitro (17, 29). In this study, we demonstrate that SNO–SP-D is formed during the inflammatory response associated with combined allergen and LPS challenge. Patients in the cross-linked group exhibit significantly greater formation of SNO–SP-D (as shown by the SNO–SP-D/SP-D ratio), indicating that nitrosylation occurs at a greater rate than increased SP-D production. Patients in the cross-linked group, and therefore with a high SNO–SP-D level, exhibited markedly enhanced Th2 cytokine levels and airway eosinophilia after challenge with allergen or combination of allergen with LPS. These data suggest that post-translational modifications of SP-D, which trigger its proinflammatory function, amplify the severity of allergic inflammation.
Eosinophilia is a key factor in acute allergic asthma, because eosinophils are a major effector cell in allergic inflammation. The multimeric form of SP-D binds to eosinophils in a saturable, calcium- and carbohydrate-dependent manner and increases apoptosis (32, 36). However, there is an association between SP-D expression and eosinophilia (37) and SP-D–deficient mice display reduced symptoms in an Aspergillus-mediated model of asthma (38). In the present study we also found that increased BAL eosinophilia was positively correlated with BAL SP-D level within challenged patients with asthma. Mechanistically, this may result from a loss of the ability of SP-D to bind to and promote apoptosis of eosinophils (36), because both the increase in eosinophil number and SP-D level was greatest in those patients whose SP-D had become oxidatively cross-linked. In this regard it is interesting to note that SNO–SP-D has a reduced ability to inhibit eotaxin-mediated eosinophil chemotaxis (Figure 7). Taken together, these data provide new evidence for a role of SP-D in eosinophil-mediated inflammatory reactions.
Both SP-D nitration and S-nitrosylation are induced within those with asthma on allergen exposure; however, they are also detected at baseline. In contrast, oxidative cross-linking only occurs after segmental challenge, and then only in half the patients. It is important to note that these patients have the most severe eosinophilia and NO metabolite production in response to challenge. It remains to be determined whether the presence of the oxidatively cross-linked SP-D is a mediator of allergen-induced inflammation or only a biomarker. It is possible that those individuals in whom SP-D did become cross-linked were in some way “sensitive” to such oxidation. In this regard it is interesting to note that a polymorphic variation in the N-terminal domain of the SP-D molecule influences oligomerization, function, and the concentration in serum (39). Polymorphic variations, such as the Thr/Thr11 identified by Hartshorn and coworkers (40), could alter susceptibility to post-translational modification and inflammatory signaling capacity.
In conclusion, the present study demonstrates that SP-D is a target for post-translational modification after segmental challenge within those with asthma resulting in disruption of its multimeric structure. In particular, a novel modification, the formation of covalent cross-links that are not mediated by disulfide bridges, seems to occur in a subset of patients. The formation of covalent cross-links is associated with the degree of inflammatory response as assessed by eosinophil number and NO metabolite production. In addition, it seems that disruption of the native multimeric state of SP-D results in a loss of its antiinflammatory capability.
Supplementary Material
Acknowledgments
The authors thank Anthony Suffredini, National Institutes of Health Clinical Center, for his support to obtain CCRE. Transfected CHO cells for the production of rat recombinant SP-D were kindly provided by JoRae Wright, Duke, North Carolina. The technical assistance of Britta Reubke-Gothe is gratefully acknowledged. Authors' contributions were as follows: conception and design, E.N.A.-V., N.K., A.J.G., M.F.B., J.M.H.; acquisition of data, C.W., H.A., F.S.; analysis and interpretation, E.N.A.-V., C.W., A.J.G., M.F.B., J.M.H.; drafting the manuscript for important intellectual content, E.N.A.-V., C.W., J.M.H.; revision of the manuscript for important intellectual content, H.A., F.S., N.K., A.J.G., M.F.B; and final approval of the manuscript, all authors.
Supported by HL-086621 (A.J.G.), NIH HL-64520, P30-ES013508 (M.F.B.), Deutsche Forschungsgemeinschaft SFB 587/B8, GRK1441 (J.M.H.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201004-0654OC on December 3, 2010
Author Disclosure: E.N.A.-V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.F.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.M.H. received consultancy fees from Roche Diagnostics and Boehringer Ingelheim, and sponsored grants from Boehringer Ingelheim, Allergopharma, Bayer Healthcare, and AstraZeneca.
References
- 1.Drickamer K. Ca2+-dependent carbohydrate-recognition domains in animal proteins. Curr Opin Struct Biol 1993;3:393–400. [Google Scholar]
- 2.Voorhout WF, Veenendaal T, Kuroki Y, Ogasawara Y, Vangolde LMG, Geuze HJ. Immunocytochemical localization of surfactant protein-d (SP-D) in type-II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem 1992;40:1589–1597. [DOI] [PubMed] [Google Scholar]
- 3.Wong CJ, Akiyama J, Allen L, Hawgood S. Localization and developmental expression of surfactant proteins D and A in the respiratory tract of the mouse. Pediatr Res 1996;39:930–937. [DOI] [PubMed] [Google Scholar]
- 4.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005;5:58–68. [DOI] [PubMed] [Google Scholar]
- 5.Crouch E, Persson A, Chang D, Heuser J. Molecular-structure of pulmonary surfactant protein-D (SP-D). J Biol Chem 1994;269:17311–17319. [PubMed] [Google Scholar]
- 6.BrownAugsburger P, Hartshorn K, Chang D, Rust K, Fliszar C, Welgus HG, Crouch EC. Site-directed mutagenesis of Cys-15 and Cys-20 of pulmonary surfactant protein D: expression of a trimeric protein with altered anti-viral properties. J Biol Chem 1996;271:13724–13730. [DOI] [PubMed] [Google Scholar]
- 7.Erpenbeck VJ, Malherbe DC, Sommer S, Schmiedl A, Steinhilber W, Ghio AJ, Krug N, Wright JR, Hohlfeld JM. Surfactant protein D increases phagocytosis and aggregation of pollen-allergen starch granules. Am J Physiol Lung Cell Mol Physiol 2005;288:L692–L698. [DOI] [PubMed] [Google Scholar]
- 8.Atochina EN, Beck JM, Preston AM, Haczku A, Tomer Y, Scanlon ST, Fusaro T, Casey J, Hawgood S, Gow AJ, et al. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect Immun 2004;72:6002–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein-D (SP-D) against influenza-A viruses. J Clin Invest 1994;94:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JC, Crouch EC. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol Lung Cell Mol Physiol 1997;17:L1156–L1166. [DOI] [PubMed] [Google Scholar]
- 11.Levine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001;167:5868–5873. [DOI] [PubMed] [Google Scholar]
- 12.Wert SE, Yoshida M, Levine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA 2000;97:5972–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman-Davis JM, Gibbs-Erwin J, Lindsey JR, Matalon S. Role of surfactant protein-A in nitric oxide production and mycoplasma killing in congenic C57BL/6 mice. Am J Respir Cell Mol Biol 2004;30:319–325. [DOI] [PubMed] [Google Scholar]
- 14.Kingma PS, Zhang LQ, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. Correction of pulmonary abnormalities in Sftpd(−/−) mice requires the collagenous domain of surfactant protein D. J Biol Chem 2006;281:24496–24505. [DOI] [PubMed] [Google Scholar]
- 15.Malherbe DC, Erpenbeck VJ, Abraham SN, Crouch EC, Hohlfeld JM, Wright JR. Surfactant protein D decreases pollen-induced IgE-dependent mast cell degranulation. Am J Physiol Lung Cell Mol Physiol 2005;289:L856–L866. [DOI] [PubMed] [Google Scholar]
- 16.Atochina-Vasserman EN, Beers MF, Gow AJ. Review: chemical and structural modifications of pulmonary collectins and their functional consequences. Innate Immunity 2010;16:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo CJ, Atochina-Vasserman EN, Abramova E, Foley JP, Zaman A, Crouch E, Beers MF, Savani RC, Gow AJ. S-Nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol 2008;6:2414–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matalon S, Shrestha K, Kirk M, Waldheuser S, McDonald B, Smith K, Gao ZQ, Belaaouaj A, Crouch EC. Modification of surfactant protein D by reactive oxygen-nitrogen intermediates is accompanied by loss of aggregating activity, in vitro and in vivo. FASEB J 2009;23:1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson BV, Sears S, Woods J, Ling CY, Hunt J, Clapper LM, Gaston B. Expired nitric oxide as a marker for childhood asthma. J Pediatr 1997;130:423–427. [DOI] [PubMed] [Google Scholar]
- 20.MacPherson JC, Comhair SAA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol 2001;166:5763–5772. [DOI] [PubMed] [Google Scholar]
- 21.Reynaert NL, Ckless K, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide and redox signaling in allergic airway inflammation. Antioxid Redox Signal 2005;7:129–143. [DOI] [PubMed] [Google Scholar]
- 22.Erpenbeck VJ, Schmidt R, Gunther A, Krug N, Hohlfeld JM. Surfactant protein levels in bronchoalveolar lavage after segmental allergen challenge in patients with asthma. Allergy 2006;61:598–604. [DOI] [PubMed] [Google Scholar]
- 23.Schaumann F, Mueller M, Braun A, Luettig B, Peden DB, Hohifeld JM, Krug N. Endotoxin augments myeloid dendritic cell influx into the airways in patients with allergic asthma. Am J Respir Crit Care Med 2008;177:1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Grady NP, Preas HL, Pugin J, Fiuza C, Tropea M, Reda D, Banks SM, Suffredini AF. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med 2001;163:1591–1598. [DOI] [PubMed] [Google Scholar]
- 25.Atochina-Vasserman EN, Winkler C, Abramova H, Beers MF, Schaumann F, Krug N, Gow AJ, Hohlfeld JM. Segmental antigen challenge produces alterations in surfactant protein D expression and multimeric structure in humans. Am J Respir Crit Care Med 2009;179:A6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Q, Wright JR. Degradation of surfactant protein D by alveolar macrophages. Am J Physiol 1998;274:L97–L105. [DOI] [PubMed] [Google Scholar]
- 27.Fisher JH, Sheftelyevich V, Ho YS, Fligiel S, McCormack FX, Korfhagen TR, Whitsett JA, Ikegami M. Pulmonary-specific expression of SP-D corrects pulmonary lipid accumulation in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L365–L373. [DOI] [PubMed] [Google Scholar]
- 28.Erpenbeck VJ, Hagenberg A, Dulkys Y, Elsner J, Balder R, Krentel H, Discher M, Braun A, Krug N, Hohlfeld JM. Natural porcine surfactant augments airway inflammation after allergen challenge in patients with asthma. Am J Respir Crit Care Med 2004;169:578–586. [DOI] [PubMed] [Google Scholar]
- 29.Atochina-Vasserman EN, Gow AJ, Abramova H, Guo CJ, Tomer Y, Preston AM, Beck JM, Beers MF. Immune reconstitution during Pneumocystis lung infection: disruption of surfactant component expression and function by S-nitrosylation. J Immunol 2009;182:2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dulkys Y, Kluthe C, Buschermohle T, Barg I, Knoss S, Kapp A, Proudfoot AE, Elsner J. IL-3 induces down-regulation of CCR3 protein and mRNA in human eosinophils. J Immunol 2001;167:3443–3453. [DOI] [PubMed] [Google Scholar]
- 31.Erpenbeck VJ, Fischer I, Wiese K, Schaumann F, Schmiedl A, Nassenstein C, Krug N, Hohlfeld JM. Therapeutic surfactants modulate the viability of eosinophils and induce inflammatory mediator release. Int Arch Allergy Immunol 2009;149:333–342. [DOI] [PubMed] [Google Scholar]
- 32.von Bredow C, Harti D, Schmid K, Schabaz F, Brack E, Reinhardt D, Griese M. Surfactant protein D regulates chemotaxis and degranulation of human eosinophils. Clin Exp Allergy 2006;36:1566–1574. [DOI] [PubMed] [Google Scholar]
- 33.Griese M, Maderlechner N, Ahrens P, Kitz R. Surfactant proteins A and D in children with pulmonary disease due to gastroesophageal reflux. Am J Respir Crit Care Med 2002;165:1546–1550. [DOI] [PubMed] [Google Scholar]
- 34.Casey J, Kaplan J, Atochina-Vasserman EN, Gow AJ, Kadire H, Tomer Y, Fisher JH, Hawgood S, Savani RC, Beers MF. Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am J Respir Crit Care Med 2005;172:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crouch EC, Hirche TO, Shao B, Boxio R, Wartelle J, Benabid R, McDonald B, Heinecke J, Matalon S, Belaaouaj A. Myeloperoxidase-dependent inactivation of surfactant protein D in vitro and in vivo. J.Biol.Chem 2010;285:16757–16770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahajan L, Madan T, Kamal N, Singh VK, Sim RB, Telang SD, Ramchand CN, Waters P, Kishore U, Sarma PU. Recombinant surfactant protein-D selectively increases apoptosis in eosinophils of allergic asthmatics and enhances uptake of apoptotic eosinophils by macrophages. Int Immunol 2008;20:993–1007. [DOI] [PubMed] [Google Scholar]
- 37.Atochina EN, Beers MF, Tomer Y, Scanlon ST, Russo SJ, Panettieri RA, Haczku A. Attenuated allergic airway hyperresponsiveness in C57BL/6 mice is associated with enhanced surfactant protein (SP)-D production following allergic sensitization. Respir Res 2003;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandt EB, Mingler MK, Stevenson MD, Wang N, Hershey GKK, Whitsett JA, Rothenberg ME. Surfactant protein D alters allergic lung responses in mice and human subjects. J Allergy Clin Immunol 2008;121:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leth-Larsen R, Garred P, Jensenius H, Meschi J, Hartshorn K, Madsen J, Tornoe I, Madsen HO, Sorensen G, Crouch E, et al. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. J Immunol 2005;174:1532–1538. [DOI] [PubMed] [Google Scholar]
- 40.Hartshorn KL, White MR, Tecle T, Tornoe I, Sorensen GL, Crouch EC, Holmskov U. Reduced influenza viral neutralizing activity of natural human trimers of surfactant protein D. Respir Res 2007;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.