Abstract
Rationale: Matrix metalloprotease (MMP)-9 is an elastolytic endopeptidase produced by activated macrophages that may be involved in the development of human pulmonary emphysema and could be inhibited with existing compounds. Mouse models have demonstrated that excess MMP-9 production can result in permanent alveolar destruction.
Objectives: To determine if MMP-9 causes cigarette smoke–induced emphysema using MMP-9 knockout mice and human samples.
Methods: Mouse lungs were analyzed for inflammation and airspace enlargement using a mainstream smoke-exposure model. Human macrophage mRNA was isolated from subjects with emphysema by laser capture microdissection. Human blood monocyte mRNA was isolated from subjects with greater than 30 pack-year smoking history. Human gene expression was determined by quantitative polymerase chain reaction and compared with emphysema severity determined by automated computed tomography analysis. Plasma Clara cell secretory protein and surfactant protein-D were quantified to measure ongoing lung injury.
Measurements and Main Results: Mice deficient in MMP-9 develop the same degree of cigarette smoke–induced inflammation and airspace enlargement as strain-matched controls. Macrophages are the predominant source of MMP-9 production in human emphysema specimens and similar quantities of macrophage MMP-9 mRNA is present in areas of lung with and without emphysema. Circulating monocytes produce more MMP-9 in individuals with advanced emphysema severity despite no correlation of MMP-9 with markers of ongoing lung damage.
Conclusions: These results suggest that MMP-9 in humans who smoke is similar to smoke-exposed mice, where MMP-9 is present in emphysematous lung but not correlated with the emphysema. To the degree that the mechanisms of emphysema in humans who smoke resemble the mouse model, these data suggest specific inhibition of MMP-9 is unlikely to be an effective therapy for cigarette smoke–induced emphysema.
Clinical trial registered with www.clinicaltrials.gov (NCT 00757120).
Keywords: pulmonary disease, chronic obstructive; laser capture microdissection; mice, knockout
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Inflammatory cell production of matrix metalloproteinase (MMP) has a role in chronic obstructive pulmonary disease pathogenesis, and MMP-9 could be inhibited with existing compounds.
What This Study Adds to the Field
Genetic deletion of MMP-9 did not protect mice from smoking-induced alveolar inflammation and airspace enlargement. In human lungs with emphysema and elevated macrophage MMP-9, there was no correlation between macrophage MMP-9 and the extent of emphysema in adjacent lung tissue. These findings indicate that inhibition of MMP-9 is unlikely to prevent progression of smoking-associated emphysema.
Several studies have implicated matrix metalloproteinase-9 (MMP-9, gelatinase B, type IV collagenase B), in emphysema and chronic obstructive pulmonary disease (COPD) pathogenesis (1–5). MMP-9 can be accurately measured and is an elastolytic protease that is produced in large quantities by inflammatory cells, thus making it suitable for investigation in emphysema (5–7). However, because the pathophysiology of emphysema follows such an indolent course, developing over decades in response to cigarette smoke, a link between MMP-9 activity and alveolar destruction is lacking.
Mouse models have identified several MMPs that cause airspace enlargement by overexpression (8, 9), or that prevent airspace enlargement by gene deletion in smoking models (10). The recent discovery that mice transgenically altered to overexpress human MMP-9 in alveolar macrophages develop progressive airspace enlargement (8) adds importance to the existing literature about MMP-9 in COPD. However, whether MMP-9 deletion is protective in nonsmoking mouse models of airspace enlargement depends on the mouse model used (11, 12). We now demonstrate the results of chronic smoke exposure on MMP-9 knockout (KO) mice in a smoking-sensitive strain.
Although MMPs likely have multiple functions in vivo, the destruction of structural lung matrix is clearly one irreversible step in the development of emphysema and may represent a common pathologic finding in both human disease and mouse models of disease. For this reason, we specifically examined the relationship between MMP-9 and alveolar enlargement or emphysema. Conceptually, if MMP-9 is involved in emphysema pathogenesis, the production of MMP-9 in mice, and in humans, should correlate with alveolar destruction. We recognize that MMP-9 production may also be involved in airway remodeling in obstructive lung disease, but for these studies we used a mouse model and lung specimens that are most appropriate for study of emphysema. So, we have confined our investigation to the examination of alveolar destruction.
To examine the possible relationship of MMP-9 with human emphysema, we have quantitatively defined emphysema severity of tissue cores from explanted human lungs, computed tomography (CT) scans of study subjects, and histologic sections of mouse lungs in this study. Because lung destruction is heterogeneous in human emphysema, we examined areas of varying degrees of emphysema severity within the same lung using laser capture microdissection (LCM) to isolate macrophages. This approach allowed us to avoid the averaging artifacts that arise when sputum cells, bronchoalveolar lavage cells, or total lung cells are used for mRNA analysis of small samples originating from heterogeneous areas of disease. LCM allows one to obtain an accurate quantitative value for MMP-9, specifically from macrophages, in emphysema-susceptible individuals from areas with gradations of emphysema severity.
The systemic MMP-9 elevations seen in COPD (13–15) could reflect blood monocyte MMP-9 production, which may be a more sensitive marker of emphysema-mediated inflammation, and could be mechanistically relevant to the role of alveolar macrophages in emphysema pathogenesis. Accordingly, we compared circulating monocyte MMP-9 production with the whole-lung emphysema severity as determined by quantitative CT scan analysis and with two systemic markers of active lung injury (Clara cell secretory protein [CCSP] and surfactant protein-D [SP-D]) (16–18) that are altered in current smokers and subjects with COPD (19–24). Some of the results of this manuscript have been previously published in the form of an abstract (25).
METHODS
Mice
All animal studies were approved by the Washington University Animal Studies Committee. All wild-type (WT) C57Bl/6 mice were purchased from Taconic Farms (Hudson, NY). MMP-9 KO mice were originally generated on a 129SvEv background (26), but have been backcrossed greater than 15 generations to C57Bl/6 strain mice. All smoking experiments were performed using a vented nose exposure chamber previously described using a 6-day a week daily exposure to four 3R4F cigarettes (University of Kentucky) with filters removed (10). Mice were killed by CO2 narcosis always 24 hours after the last cigarette exposure. Histologic assessment was performed only on mice not used for lung lavage, as previously described (27, 28). All lungs were inflation-fixed in formalin at 25 cm H2O pressure and chord length results represent automated measurements from 15 randomly acquired images from three hematoxylin and eosin stained slides at least 50 μm apart averaged for each animal (28). Lung lavage was performed as previously described on mice not used for chord length analysis, with manual assessment of differential counts. Statistical analysis was done on the mean chord lengths of animals from each group (MMP-9 WT smoked and nonsmoked, MMP-9 KO smoked and nonsmoked) using analysis of variance (ANOVA) with Tukey test for post hoc significance (SPSS 16, Chicago, IL).
Subject Recruitment
Human materials were obtained from two sources: tissue cores were obtained from the explanted lungs of GOLD 4 subjects at the time of lung transplantation surgery; and peripheral blood monocytes were obtained from subjects enrolled for a biomarker study (www.clinicaltrials.gov NCT00757120) that consisted of a local cohort of subjects who received chest CT scans as part of the National Lung Screening Trial (NLST, NCT00047385) and had no radiographic lung diseases other than emphysema.
Subjects undergoing lung transplantation for COPD at Washington University Medical Center were consented for sampling of explanted lungs at the time of lung transplantation. Within several hours of surgery an entire explanted lung was inflated over liquid nitrogen vapor (29, 30). From this lung multiple 13-mm diameter cores were acquired from 2-cm thick lung slices using a uniform nonrandom sampling method (29, 30). Ten cores, each randomly chosen from five consecutive subjects, were used for alveolar macrophage analysis. Subject demographic data are included in Table 1. All of the subjects at time of transplantation had stopped smoking for at least 6 months and none had evidence of an acute infection.
TABLE 1.
SUBJECT DEMOGRAPHICS FOR LASER CAPTURE MICRODISSECTION MACROPHAGE ANALYSIS
| Subject | Age | Sex | BMI (kg/m2) | Pack-years | Years Quit | FEV1 %pred | FVC %pred | RV %pred | Mean PAP (mm Hg) | LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | F | 27.6 | 25 | 2 | 15 | 56 | 331 | 34 | 52 |
| 2 | 56 | M | 22.2 | 105 | 2 | 17 | 63 | 289 | 24 | 74 |
| 3 | 51 | F | 22.2 | 30 | 3 | 12 | 63 | 389 | 24 | 58 |
| 4 | 62 | M | 25.1 | 97 | 10 | 15 | 68 | 490 | 20 | 53 |
| 5 | 64 | F | 22.1 | 60 | 9 | 21 | 64 | 196 | 22 | 53 |
Definition of abbreviations: BMI = body mass index; LVEF = left ventricular ejection fraction; PAP = pulmonary artery pressure; %pred = percent predicted; RV = residual volume.
The NLST is an National Cancer Institute–sponsored trial that compares the merits of screening for lung cancer with three annual low-dose chest CT scans as opposed to three annual standard chest radiographs in heavy smokers (subjects between the ages of 55 and 74 at enrollment with at least a 30 pack-year smoking history). At our institution, 879 of the subjects who had been randomized to CT scan screening and who required no further follow-up for lung nodules were identified. Automated image analysis (VIDA diagnostics, Iowa City, IA) was performed on the most recent preexisting CT study for each of these subjects to determine the emphysema index (EI), which was defined as the percentage of total lung with density less than −950 Hounsfield units (HU). Members of this cohort were invited to participate in this study, which was not part of the NLST. Any subject with a known history of solid organ malignancy or inflammatory-immunomodulatory disorder or current oral corticosteroid use was excluded (three subjects: α1-antitrypsin [α1AT] deficiency, rheumatoid arthritis, and hepatitis C). From this cohort we have enrolled 38 subjects with an EI of greater than 10% into our emphysema biomarker study. In this report we refer to this group as “emphysema-sensitive” subjects. We have also enrolled 47 subjects with an EI less than 5% that we refer to as an “emphysema-resistant” control group. All testing was performed within 3 years of the NLST CT scan. Emphysema-sensitive subjects were predominantly male, former smokers with at least moderate airflow obstruction, although several did not meet GOLD criteria for COPD (Table 2). The emphysema-resistant cohort was predominantly female (Fisher exact test P < 0.0005) and nonwhite (Fisher exact test P < 0.001). The emphysema-sensitive cohort included fewer current smokers (Fisher exact test P < 0.003). The resistant current smokers were significantly younger than the sensitive former smokers (ANOVA P < 0.01 by Tukey test). As expected, airflow obstruction as measured by post-bronchodilator FEV1 % predicted was lower in the emphysema-sensitive group compared with resistant former smokers and current smokers (ANOVA P < 0.01 by Tukey test).
TABLE 2.
SUBJECT DEMOGRAPHICS FOR MONOCYTE ANALYSIS
| Sensitive FS (26) | Resistant FS (18) | Sensitive CS (4) | Resistant CS (20) | |
|---|---|---|---|---|
| Age, yr | 68* | 65 | 65 | 63 |
| Female, % | 4*† | 67 | 50 | 68 |
| White, % | 100*† | 67† | 100 | 89 |
| BMI, kg/m2 | 29.5† | 34.2 | 25.6 | 28 |
| Pack-years | 64 | 50 | 43 | 50 |
| FEV1 %pred | 71*† | 91 | 65 | 87 |
| EI, % | 26*† | 3 | 19 | 4 |
Definition of Abbreviations: BMI = body mass index; CS = current smoker; EI = emphysema index; FS = former smoker; %pred = percent predicted.
Statistically significant compared with: * Resistant CS or † Resistant FS (see Methods section).
Subject Testing
All protocols were approved by the human research protection office at Washington University. Permission to recruit and use preexisting CT scans was obtained from the NLST. None of the testing performed for this study is sponsored by or part of the NLST. All subjects performed pulmonary function tests consisting of spirometry prebronchodilator and post-bronchodilator, lung volumes by plethysmography, diffusing capacity, and a 6-minute walk test according to American Thoracic Society consensus statement guidelines (31) on the same day as blood collection at Washington University. A total of 45 ml of blood was drawn from each subject while in a sitting position and collected in K2EDTA-coated tubes (BD Vacutainer, Franklin Lakes, NJ). Tubes were inverted gently six times and transported at room temperature to the laboratory immediately after collection. All blood specimens were processed within 2 hours of collection. Additionally, six spots of blood were placed on cards for testing for mutations that cause α1AT deficiency. α1AT testing was performed anonymously at the University of Florida (Mark Brantly, Gainesville, FL) via a fee-for-service agreement. No subjects had low α1AT protein levels or homozygous mutations in the α1AT gene.
Blood Processing
All plasma samples were separated by centrifugation and immediately frozen in 2-ml aliquots at −80°F for further testing. The cell pellet was resuspended in sterile phosphate-buffered saline (PBS) and subjected to density gradient separation with Histopaque-1077 (Sigma, St. Louis, MO) to remove granulocytes and red blood cells. The remaining cells were processed for monocyte isolation by elutriation.
ELISA for CCSP and SP-D
The plasma samples were thawed once and SP-D (Biovendor, Modrice, Czech Republic), and CCSP levels (Biovendor) were determined using commercially available ELISA kits. All samples were measured in duplicate and the average value was used. The difference in concentration between all duplicate samples was below the detection threshold of the assay. All CCSP samples and standards were diluted 25-fold and all SP-D samples were diluted 10-fold. We did not measure CCSP or SP-D levels in current smokers with emphysema because this group was too small to use in biomarker analysis.
Elutriation
Monocytes were isolated by countercurrent elutriation at 4°C (32). The protocol generally results in 8–12 million monocytes per subject and does not require incubation with antibodies or plating on plastic, which could activate monocytes ex vivo. An aliquot of each sample was examined by modified Wright staining to confirm purity and the predominant contaminating cell type was large granular lymphocytes at less than 10%.
Monocyte mRNA and Quantitative Polymerase Chain Reaction for MMP-9
RNA was isolated from 2 × 106 monocytes using PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA). cDNA was transcribed with high-capacity RNA to cDNA kit (Applied Biosystems, Foster City, CA). Using forward primer gacgcagacatcgtcatccagttt and reverse primer gccgcgccatctgcgttt, relative MMP-9 quantity was assayed using SYBR green (Sigma) and a Stratagene MX300 cycler (Stratagene, LaJolla, CA) and normalized for loading quantity by subtracting the cycle threshold (CT) of the housekeeping gene ribosomal protein L32 (F-cagggttcgtagaagaatcaaggg, R-cttggaggaaacattgtgagcgatc). Because there is no normal MMP-9 value to use for fold change (usually described as delta-delta cycle threshold), mRNA quantity was calculated using the following formula: relative MMP-9 mRNA quantity = 2-(MMP9 CT- L32 CT). Results were multiplied by 1,000 to make all values greater than 1 to improve display on a log scale. All values are normalized to the housekeeping gene so results are expressed in relative units.
CT Analysis
The inflated, frozen lungs explanted from the lung transplant patients were scanned on a Definition 64-multidetector scanner (Siemens AG, Munich, Germany) using 120-kilovolt (peak) (kVp), 0.5-second gantry rotation, and mA/pitch/effective mA of 132/1.2/110 or a Sensation 16-multidetector scanner (Siemens) using 120-kVp, 0.42-second gantry rotation, and mA/pitch/effective mA of 100/1.1/94. These images were reconstructed contiguously at 10-mm section thickness using a medium smooth algorithm (B30f). CT analysis of lung cores was performed as previously described (29). Basically, the CT sections were matched with photographs of the transversely sliced frozen lungs from which cores had been removed, and the mean CT attenuation of the lung in the region of the core was measured using Image J software (available at: http://rsb.info.nih.gov/ij). Based on previous experience a CT density of a lung core that is less than −950 HU reflects mean linear intercepts greater than 0.5 mm on histology. For this study CT density was used because the extra tissue handling adversely alters the histology making histologic measurements less representative of the in vivo state.
The CT scans from the NLST trial were obtained using 120-kVp, 0.5-second gantry rotation, and mA/pitch/effective mA of 60/2.0/30 (Siemens Volume Zoom 4-multidetector CT) or 45/1.5/30 (Siemens Sensation 16-multidetector CT). All images were reconstructed contiguously at 2-mm section thickness using a medium smooth algorithm (B30f). Only the most recent preexisting CT study was used. All scans were performed within 3 years of enrollment in the present study. Quantitative analysis of the in vivo CT scans from the NLST was performed on both lungs using Emphysema Profiler software (VIDA Diagnostics). Emphysema was defined as regions of lung parenchyma with attenuation less than −950 HU. The percentage of total lung with emphysema, defined as the EI, was calculated. Results of analysis at −910 HU, and the HU at the 15th percentile of lung density, were also determined but are not reported because they generally correlated with the EI at −950 HU and did not change the conclusions.
Laser Capture Microdissection
Each lung core was placed in the base of a cryomold carefully overlaid with Tissue-Tek OCT (Sakura Finetek USA, Torrance, CA) and immediately frozen with liquid nitrogen. The pieces of lung were kept flat in the bottom of the cryomold to maximize the area of tissue that was sectioned. Tissues were sectioned at 7 μm in a cryostat (Leica, Nussloch, Germany). Frozen sections were placed on plain glass slides (Matsunami, Osaka, Japan) and were then immediately fixed in 70% ethanol for 5 minutes before being rehydrated in RNase-free water for 30 seconds. To identify macrophages in the lung, sections were stained with mouse monoclonal antihuman cluster of differentiation 68 (CD68) antibodies (Dako, Carpinteria, CA) as previously reported using an EnVision+ kit (Dako) (33). After washing in PBS, sections were exposed to a horseradish peroxidase–labeled polymer conjugated with secondary mouse and rabbit antibodies from the kit for 5 minutes. After washing in RNase-free water and PBS, sections were incubated with diaminobenzidine for 2 minutes and were washed in RNase-free water. Sections were then dehydrated as above and cleaned in xylene for 90 seconds (three times for 30 seconds each) before LCM. Slides were completely air-dried until use to prevent RNA degradation. RNase inhibitor (400 U/ml) was added in all aqueous steps, including color development. Macrophages localized in the alveolar space and in the alveolar walls were harvested using a PixCell II System (Arcturus Engineering, Mountain View, CA) with laser beam diameter of 7.5 μm. A total of 30,000 laser bursts were used to collect cells from each subject.
RNA Purification and Reverse Transcription
Total RNA was extracted from LCM samples and from the serial sections adjacent to the sections used for LCM, referred to as whole sections of lung tissue, using an RNeasy Mini kit (Qiagen, Hilden, Germany), and was eluted in 31 μl of elution buffer included in the kit. RNA was reverse-transcribed using random hexamers from the TaqMan Reverse Transcription Reagents and RT Reaction Mix (Applied Biosystems) in a total volume of 60 μl at 25°C for 10 minutes, at 42°C for 30 minutes, and at 94°C for 5 minutes on the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). The resulting first-strand cDNA was used as a template for reverse transcription polymerase chain reaction (RT-PCR).
5′-Exonuclease-Based Fluorogenic PCR
Quantitative PCR of LCM-captured macrophages was performed using an ABI PRISM 7700 Sequence Detector (Applied Biosystems), as described previously (33). Primers and a labeled probe for MMP-9 and glyceraldehyde phosphate dehydrogenase (GAPDH) cDNA as an endogenous control were purchased from Applied Biosystems (TaqMan human GAPDH control reagents). PCRs for MMP-9 and GAPDH were performed in separate tubes to avoid possible competition or interference. Briefly, a 2-μl aliquot of the RT products (cDNA) from macrophages, or a 0.5-μl aliquot of those from whole-lung sections, was placed into each tube and PCR was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) in a total volume of 25 μl. PCR was performed for 40 cycles, consisting of a denaturation step at 95°C for 15 seconds and a combined annealing and extension step at 60°C for 1 minute. For linear regression analysis of unknown samples in each assay, a standard curve was generated using 8- or 10-fold serial dilutions of cDNA from human alveolar macrophages obtained by bronchoalveolar lavage from a normal control subject, as described previously (33). The relative amount of target mRNA in the samples was assessed by interpolation of their threshold cycles from a standard curve and was then normalized against GAPDH mRNA. PCR assay was independently performed three times to confirm reproducibility, and representative data are shown.
Statistical Analysis
Results are expressed as a mean ± SEM in tables for continuous variables and as a percent of total for categorical variables. Differences in categorical variables between groups were evaluated with a Fisher exact test. Two-way ANOVA followed by a Tukey test was used for multiple comparisons in mouse experiments and one-way ANOVA was used for groups of monocytes subjects. The t test was used for pairwise comparisons of emphysema-sensitive and -resistant subjects. Because of nonnormal distribution of macrophage and monocyte MMP-9 mRNA quantity, Spearman correlation (expressed as ρ) was used for evaluating the relationship of MMP-9 mRNA quantity with emphysema severity and lung injury biomarker quantities. Significance was set at a P value of less than 0.05.
RESULTS
Mice Deficient in MMP-9 Develop Smoke-induced Airspace Enlargement Similar to Strain-matched Control Mice
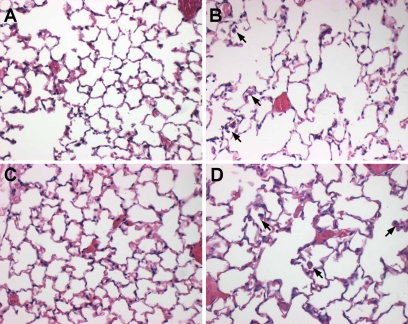
Because mice that overexpress MMP-9 in alveolar macrophages develop spontaneous airspace enlargement (8), we sought to evaluate if MMP-9 deletion in mice reduced susceptibility to cigarette smoke–induced airspace enlargement. MMP-9 KO mice that had been bred onto an emphysema-sensitive C57Bl/6 background were evaluated after 6 months of smoke exposure. MMP-9 KO mice demonstrated a similar degree of airspace enlargement compared with WT mice (Table 3 and Figure 1). Two-way ANOVA analysis with Tukey test HSD analysis reveals significant effects of smoking on chord length (P < 0.01) with no additional effect of MMP-9 deficiency. Consistent with the known effects of chronic smoke exposure, an increase in alveolar macrophages was seen in both strains of smoking mice (see arrows in Figures 1B and 1D).
TABLE 3.
CHORD LENGTH IN MICE
| Genotype/Treatment | Length in μm ± SEM |
|---|---|
| C57 nonsmoking | 27.1 ± 0.7 |
| MMP-9 KO nonsmoking | 28 ± 0.8 |
| C57 6M smoking | 40.2 ± 0.5* |
| MMP-9 KO 6M smoking | 38.9 ± 1.1* |
Definition of Abbreviations: KO = knockout; MMP = matrix metalloproteinase.
P < 0.05 compared with nonsmoking, n = 10 mice per condition.
Figure 1.
Airspace size after 6 months of cigarette smoke exposure. Age-matched nonsmoked matrix metalloproteinase (MMP)-9 wild-type (WT) (A) and knockout (KO) (C) and MMP-9 WT (B) and KO (D) mice after 6 months of cigarette smoke exposure. Inflation-fixed lungs demonstrate similar airspace size in nonsmoked animals (A and C) with similar airspace enlargement in smoked animals (B and D) comparable with mild emphysematous changes in humans. An increase in alveolar macrophages (arrows, B and D) but not neutrophils is seen in both smoke-exposed WT and KO animals.
Mice Deficient in MMP-9 Have Lung Inflammation after Chronic Cigarette Smoke Exposure Similar to Control Mice
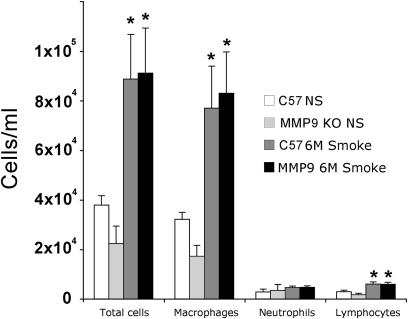
To examine if MMP-9 KO mice had altered lung inflammation, lung lavage cell counts and differentials were performed after 6 months of cigarette smoke exposure on an additional 10 mice of each genotype. The lavage cell counts demonstrated a similar smoke-induced increase in total alveolar macrophages and lymphocytes but no additional alteration in the cell counts because of MMP-9 deficiency (Figure 2). We did not demonstrate any effects on neutrophil accumulation at this time point because of smoking or MMP-9 deficiency. The lung lavage fluid and lung homogenates of smoke-exposed MMP-9 KO mice demonstrate no MMP-9 and the presence of MMP-2 and MMP-12 in quantities that are less than or equal to WT smoked mice (see Figure E1 in the online supplement).
Figure 2.
Mouse lung lavage inflammatory cell profile after smoking. Chronic smoke exposure induces a modest but significant increase in alveolar macrophages at 6 months (*P < 0.05 in both wild-type and knockout (KO) compared with nonsmoked). Matrix metalloproteinase (MMP)-9 KO mice demonstrated no significant difference in number or type of inflammatory cells after smoking compared with wild-type mice.
Alveolar Macrophage MMP-9 Production Does Not Correlate with Local Emphysema Severity in Severe COPD
Because MMP-9 has been associated with emphysema in human subjects (2, 4, 34), we sought to evaluate the association of emphysematous lung destruction with macrophage MMP-9 production. To evaluate MMP-9 production in human alveolar macrophages, we isolated mRNA from macrophages in serial sections by LCM of CD68+ cells in lungs removed at the time of transplantation (49 cores analyzed, only one lung core produced macrophage mRNA of insufficient quality for analysis). Emphysema severity of individual lung cores was determined by evaluating the mean HU of the defined region of interest on CT scan (29).
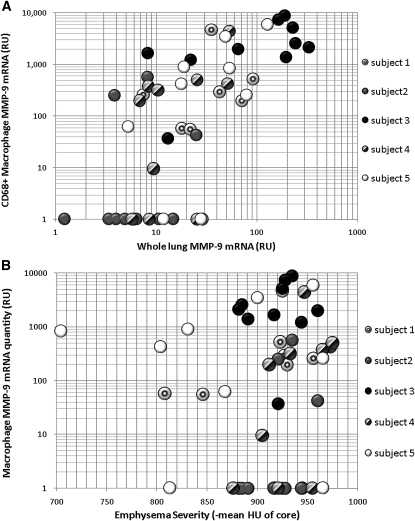
To confirm macrophages were the predominant source of MMP-9 mRNA in lung tissue, mRNA from slides without capture by LCM was also evaluated (whole-lung mRNA). The relative amount of MMP-9 mRNA in the whole-lung samples was on average 20 times less than the MMP-9 mRNA quantity in macrophages (both normalized to a GAPDH loading control). This suggests that increases in MMP-9 production in emphysematous lungs are largely from a macrophage source. As is expected, there was fairly good correlation of whole-lung MMP-9 mRNA quantity with alveolar macrophage MMP-9 quantity (ρ = 0.7; P < 0.01) (Figure 3A), suggesting that the variability of MMP-9 mRNA quantity in different areas of the lung in GOLD 4 subjects is dictated by alveolar macrophage production.
Figure 3.
Matrix metalloproteinase (MMP)-9 gene expression in emphysematous lung. (A) MMP-9 expression in whole-lung compared with CD68+ macrophages. Using mRNA from single slices of lung cores or laser capture isolated alveolar macrophages quantitative polymerase chain reaction was performed to determine mRNA quantity after adjustment for loading by normalizing to the housekeeping gene GAPDH. Ten samples each from five subjects with GOLD 4 chronic obstructive pulmonary disease were evaluated. Gene expression is in relative units based on a simultaneous standard curve. MMP-9 is much more abundant in alveolar macrophages and demonstrates good correlation with whole-lung MMP-9 quantity (ρ = 0.7; P < 0.01). (B) MMP-9 expression in CD68+ alveolar macrophages compared with emphysema severity of the lung core. Emphysema severity is expressed as the mean density of the region of the lung core on computed tomography. MMP-9 mRNA quantity from alveolar macrophages plotted on a log scale (values of 0 converted to 1) demonstrates no correlation with emphysema severity (ρ = 0.14; P = 0.33).
There was significant variability of macrophage MMP-9 production from core to core within the same lung, but no relationship between local emphysema severity and macrophage MMP-9 production was seen (Figure 3B) (ρ = 0.14; P = 0.33). In fact, all subjects except three had at least one core with no measurable macrophage MMP-9 mRNA and several had elevated MMP-9 in areas with minimal emphysema (Figure 3B). We note that the normal quantity of MMP-9 produced by human alveolar macrophages in healthy individuals cannot be determined from this data. We have used specimens described previously (35) to determine the alveolar macrophage production of MMP-9 in nonsmokers without emphysema and former smokers with no radiographic emphysema and found similar macrophage MMP-9 production (Figure E2). This is consistent with the current finding of macrophage MMP-9 elevations in nonemphysematous areas of GOLD 4 lung.
Monocyte MMP-9 mRNA Correlates with Emphysema Severity in Former Heavy Smokers
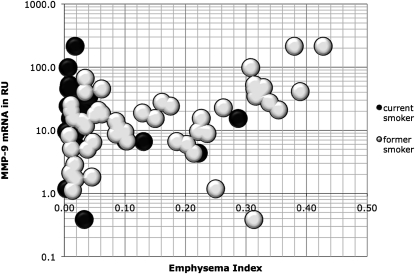
Serum MMP-9 levels have been correlated with COPD severity (13, 15), so we evaluated MMP-9 production in alveolar macrophage precursors, circulating monocytes. Because plating can induce MMP-9 production (approximately 100- to 500-fold induction in our samples, data not shown) monocytes were isolated by cold countercurrent elutriation. Isolated monocyte MMP-9 mRNA quantity correlated modestly with severity of emphysema as measured by CT density in our subjects (ρ = 0.28; P = 0.02) (Figure 4). This correlation was predominantly caused by a strong correlation in the former smokers with emphysema (EI >10%; ρ = 0.49; P = 0.01). MMP-9 mRNA quantity did not separate the emphysema-sensitive subjects from the emphysema-resistant subjects (t test P = 0.295). This suggests that there may be a specific type of systemic inflammation that correlates with emphysema, but monocyte MMP-9 production results from multiple processes that are present in current and former heavy smokers with and without emphysema. The higher correlation of monocyte MMP-9 quantity with emphysema severity in former smokers with at least a 10% EI suggests that production in monocytes may be a systemic response to the ongoing lung damage in emphysema and elevated levels may correlate with the quantity of ongoing lung injury.
Figure 4.
Monocyte matrix metalloproteinase (MMP)-9 expression relative to emphysema index. Monocytes from former and current heavy smokers were evaluated for MMP-9 mRNA by quantitative polymerase chain reaction. MMP-9 quantity is expressed as relative units after normalization to the housekeeping gene L32. Emphysema index is calculated from low-dose computed tomography scan whole-lung density.
Monocyte MMP-9 Levels Do Not Correlate with Systemic Markers of Lung Injury
Both serum CCSP (CCSP, CC16, and CC10) and SP-D have been demonstrated to be markers of lung injury and inflammation with respective decreases and increases with active smoking and lung diseases including COPD (13, 16, 17, 19–24, 36, 37). Neither of these markers has correlated with COPD severity, suggesting that they both represent lung-specific biomarkers of ongoing lung injury (23). It is notable that no studies have examined the relation of either biomarker with emphysema. We tested plasma samples of our subjects for both of these markers to determine if monocyte MMP-9 production was a systemic response to ongoing lung injury.
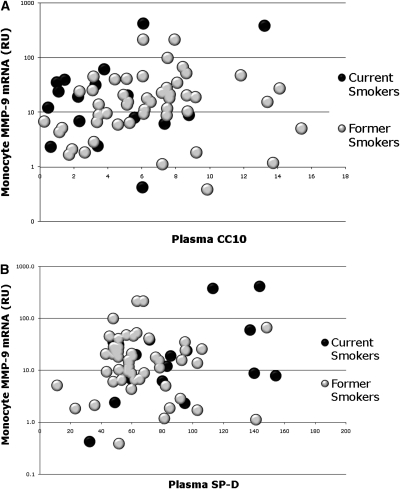
Monocyte MMP-9 mRNA quantity did not correlate with CCSP (Figure 5A) (ρ = 0.15; P = 0.21). CCSP quantity was low in current smokers, as expected (t test vs. former smokers P = 0.02), but was not depressed in former smokers with emphysema (Figure E4, t test vs. resistant former smokers P = 0.6). Theoretically, CCSP levels are low because of smoke-induced toxicity and decrease in or loss of CCSP production. The lack of depression of systemic CCSP levels in our emphysema subjects may reflect that this molecule does not represent emphysematous damage, as might be expected with a protein that is produced in the small airways and not the alveolus.
Figure 5.
Monocyte matrix metalloproteinase (MMP)-9 expression in relation to plasma Clara cell secretory protein (CCSP) and surfactant protein-D (SP-D). (A) Plasma CCSP in current smokers (black circles) and former smokers (gray circles) compared with monocyte MMP-9 mRNA levels (log scale). Current smoking causes a significant decrement in CCSP (t test vs. former smokers, P < 0.05) but no relationship with monocyte MMP-9 mRNA is seen (ρ = 0.17; P = 0.2). (B) Plasma SP-D in current smokers (black circles) and former smokers (gray circles) compared with monocyte MMP-9 mRNA graphed on a log scale. Current smoking causes a significant increase in SP-D (t test vs. former smokers, P = 0.02) but no relationship with monocyte MMP-9 mRNA is seen (ρ = 0.05; P = 0.6).
Unlike CCSP, SP-D is produced by alveolar epithelial cells. Elevation of systemic levels of SP-D with smoking, COPD, or other lung injuries is believed to reflect ongoing damage associated with disruption of the alveolar epithelial integrity (16, 18, 37, 38). In our cohort, plasma SP-D levels did not correlate with monocyte MMP-9 production (Figure 5B) (ρ = 0.08; P = 0.52). This suggests that if ongoing lung damage in subjects with emphysema is reflected by SP-D (or CCSP), then monocyte MMP-9 mRNA elevations are unrelated to active lung damage and therefore likely a nonpathogenic bystander.
DISCUSSION
MMP-9 is easily measured and is a product of several types of inflammatory cells. MMP-9 can cause emphysema when overexpressed in alveolar macrophages of mice (8) and it is present in the lungs of subjects with emphysema (2). Data such as these have implicated MMP-9 in emphysema pathogenesis. However, direct assessment of MMP-9 as a cause of cigarette smoke–induced emphysema has been lacking. Because Food and Drug Administration approved, commercially available compounds (e.g., doxycycline) can decrease MMP-9 activity at achievable systemic concentrations (39, 40), we decided to evaluate MMP-9 in the mouse smoking model and human monocytes and alveolar macrophages. We now demonstrate that MMP-9 deletion in mice does not prevent either cigarette smoke–induced lung inflammation or cigarette smoke–induced airspace enlargement. We believe this is a critical finding because it suggests that although MMP-9 could be involved in emphysema pathogenesis it is not required in mouse smoking-associated airspace enlargement.
MMP-9 as a Cause of Progressive Airspace Enlargement in Mice
Alveolar enlargement in mice can be generated by many different mechanisms (8, 9, 11, 12, 41, 42), but alveolar enlargement from chronic cigarette smoke exposure is an MMP-9–independent process. MMP-9 is an elastolytic enzyme that, if produced in sufficient quantity in the proper location, can generate airspace enlargement in mice. However, our mouse model of chronic smoke exposure is geared toward active lung destruction probably by both macrophage MMP-12 and neutrophil elastase (10, 43, 44). MMP-9 KO mice develop similar inflammatory cell recruitment after cigarette smoke exposure to WT mice, so the absence of MMP-9 has no impact on MMP-12 or neutrophil elastase in this model. MMP-9 is also not important in a chronic LPS administration model where neutrophil elastase is likely the cause of airspace enlargement (12, 45).
MMP-9 in Human Emphysema
Polymorphism studies have not strongly implicated MMP-9 overexpression in the development of COPD (46, 47). However, the presence of classic upper lobe–predominant emphysema was associated with an MMP-9 overexpressing polymorphism in a Japanese cohort (4). Thus, some people may be similar to MMP-9–overexpressing mice, in that under the right conditions, they develop emphysema because of MMP-9 overexpression. We hypothesized that if local macrophage MMP-9 expression causes emphysema, then areas of the lung without significant emphysema should lack MMP-9 expression. Our data suggest macrophages are the major source of MMP-9 in subjects with severe COPD, but there was no evidence of a relationship between macrophage MMP-9 quantity and local emphysema severity.
The cores studied did have sufficient alveolar macrophages for LCM despite the fact that subjects had no active infection and had not smoked for greater than 2 years before transplantation (Table 1). Smoking cessation can alter MMP synthesis, because very few of the cores demonstrated macrophage MMP-12 production (Figure E3), which is highly up-regulated in macrophages of current smokers (48–51). If MMP-9 is a cause of smoking-related emphysema, one would expect regulation similar to that seen for MMP-12 after smoking cessation. The MMP-12 data are notable, in that the best treatment for MMP-12–mediated emphysema may be smoking cessation.
No data exist on the progression of emphysema after smoking cessation but the rate should be diminished based on physiologic data of long-term quitters. In α1AT deficiency disease, emphysema progression is seen in nonsmokers by quantitative CT scan analysis (52, 53), and severe emphysematous lung resected during lung volume reduction clearly demonstrates active inflammation despite smoking cessation (54, 55). Our cohort of GOLD 4 subjects should therefore represent individuals who continue to progress despite smoking cessation given the need for transplant. We have assumed that local progression of already involved areas is the rule and that is a caveat of single time point of human studies. However, based on our mouse data we believe these macrophage data confirm that the human condition is consistent with the mouse findings, in that although elevated MMP-9 production in alveolar macrophages can be seen in emphysematous areas of GOLD 4 lung, it is equally elevated in areas of the same GOLD 4 lungs without emphysematous destruction.
Our sample size (50 cores total from five different subjects) is the largest alveolar macrophage LCM study to date but we cannot comment on the role of many other genes including inhibitors that were not measured. However, the lack of inhibitor data has no bearing on the cores that demonstrate no macrophage MMP-9 expression. We have also measured MMP-9 in banked specimens and confirmed that similar elevations of macrophage MMP-9 expression can be seen in nonsmokers and smokers with no emphysema or lower GOLD class disease severity (Figure E2).
MMP-9 as a Systemic Marker of Emphysema
Because our data suggest that alveolar macrophage MMP-9 production is not directly causal in cigarette smoke–induced airspace enlargement in mice, it is possible that production of MMP-9 is related to human emphysema by a mechanism that does not involve direct alveolar destruction. Consistent with this possibility, blood monocyte MMP-9 production in former smokers with emphysema correlated with emphysema severity. However, elevated MMP-9 production was not specific to subjects with emphysema. For this reason we correlated monocyte MMP-9 production with systemic markers of lung destruction. We could not find any correlation of monocyte MMP-9 levels with CCSP or SP-D. This suggests that monocyte MMP-9 at best may serve as a correlate of severity in subjects who already have emphysema. We should note that in our subjects there was a high inverse correlation of emphysema severity with obstructive lung disease (measured by post-bronchodilator FEV1), so a role of MMP-9 in airway remodeling, which was not evaluated, could also be responsible for this relationship.
For biomarker studies our single-center study is likely too small because the progression of emphysema is likely multifactorial generating a lot of noise in this small sample, but the correlation of monocyte MMP-9 quantity with emphysema severity suggests that further follow-up of our subjects to evaluate the rate of emphysema progression in relation to monocyte MMP-9 production may be of interest. Our emphysema-sensitive group represents subjects where a biomarker of disease activity would be useful given the relatively mild disease and lack of current smokers with emphysema.
We enrolled too few subjects with emphysema that were current smokers to make any comment on this group. In fact, the paucity of current smoking in our emphysema group suggests that it may be a stimulus for smoking cessation by itself. Our emphysema group is mostly male and white. Given we recruited from the NLST study based on CT data, without knowledge of sex or race, likely our results represent a relative resistance of nonwhite and female heavy smokers to emphysema as detected by CT scan analysis. A larger cohort of NLST subjects recruited at a different site demonstrated a similar finding of differential emphysema severity related to sex (56).
MMP-9 Inhibition for Preventing Emphysema Progression
Our study indicates that MMP-9 is not required for air space enlargement in the setting of cigarette smoke exposure in mice. In addition, if progression of emphysema is local and macrophage mediated, MMP-9 quantity is not associated with local emphysema severity in humans. Despite our finding of modest associations of monocytic MMP-9 synthesis with emphysema severity, monocyte MMP-9 synthesis does not relate to biomarkers of active lung destruction or presence of emphysema.
Supplementary Material
Supported by NIH grants P50 HL084922, K08 HL081270, and PO1 HL29594, and the Alan and Edith Wolff Charitable Trust/Barnes-Jewish Hospital Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201005-0718OC on November 5, 2010
Author Disclosure: J.J.A. received grant support from the NIH (more than $100,001) and the Alpha-one Foundation ($50,001–$100,000). B.A.L. has received salary from an NIH grant (less than $50,000/yr). Y.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.M.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G.K. received grant support from the NIH (more than $100,001). D.K.K. received grant support from the NBHL ($50,001–$100,000) and the NIH (more than $100,001). W.G.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.H.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H.C. received grant support from the NIH ($10,001–$50,000). D.S.G. received grant support from Pfizer, the NIH (more than $100,001), and the Barnes-Jewish Hospital Foundation ($50,001–$100,000). R.A.P. received grant support from the NIH (more than $100,001). T.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.S. received grant support from the NIH (more than $100,001) and from the Barnes-Jewish Hospital Foundation ($50,000–$100,000).
References
- 1.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 2003;28:12–24. [DOI] [PubMed] [Google Scholar]
- 2.Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 1999;159:1985–1991. [DOI] [PubMed] [Google Scholar]
- 3.Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax 2006;61:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito I, Nagai S, Handa T, Muro S, Hirai T, Tsukino M, Mishima M. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am J Respir Crit Care Med 2005;172:1378–1382. [DOI] [PubMed] [Google Scholar]
- 5.Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med 2000;162:1355–1360. [DOI] [PubMed] [Google Scholar]
- 6.Russell RE, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, Barnes PJ. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2002;26:602–609. [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 1998;78:1077–1087. [PubMed] [Google Scholar]
- 8.Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, D'Armiento J. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol 2008;294:L1149–L1157. [DOI] [PubMed] [Google Scholar]
- 9.Foronjy RF, Okada Y, Cole R, D'Armiento J. Progressive adult-onset emphysema in transgenic mice expressing human MMP-1 in the lung. Am J Physiol Lung Cell Mol Physiol 2003;284:L727–L737. [DOI] [PubMed] [Google Scholar]
- 10.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 11.Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest 2002;110:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brass DM, Hollingsworth JW, Cinque M, Li Z, Potts E, Toloza E, Foster WM, Schwartz DA. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol 2008;39:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashimoto Y, Iwata T, Okada M, Satoh H, Fukuda K, Tohda Y. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med 2009;103:1231–1238. [DOI] [PubMed] [Google Scholar]
- 14.Mao JT, Tashkin DP, Belloni PN, Baileyhealy I, Baratelli F, Roth MD. All-trans retinoic acid modulates the balance of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with emphysema. Chest 2003;124:1724–1732. [DOI] [PubMed] [Google Scholar]
- 15.Pinto-Plata V, Toso J, Lee K, Bilello J, Mullerova H, De Souza M, Vessey R, Celli B. Use of proteomic patterns of serum biomarkers in patients with chronic obstructive pulmonary disease: correlation with clinical parameters. Proc Am Thorac Soc 2006;3:465–466. [DOI] [PubMed] [Google Scholar]
- 16.Maeda M, Ichiki Y, Aoyama Y, Kitajima Y. Surfactant protein D (SP-D) and systemic scleroderma (SSC). J Dermatol 2001;28:467–474. [DOI] [PubMed] [Google Scholar]
- 17.Haddam N, Samira S, Dumont X, Taleb A, Haufroid V, Lison D, Bernard A. Lung epithelium injury biomarkers in workers exposed to sulphur dioxide in a non-ferrous smelter. Biomarkers 2009;14:292–298. [DOI] [PubMed] [Google Scholar]
- 18.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 2009;137:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomas DA, Silverman EK, Edwards LD, Locantore NW, Miller BE, Horstman DH, Tal-Singer R. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J 2009;34:95–102. [DOI] [PubMed] [Google Scholar]
- 20.Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, York E, Mainra RR, Ramesh W, Melenka LS, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1207–1214. [DOI] [PubMed] [Google Scholar]
- 21.Madsen C, Durand KL, Nafstad P, Schwarze PE, Ronningen KS, Haheim LL. Associations between environmental exposures and serum concentrations of clara cell protein among elderly men in Oslo, Norway. Environ Res 2008;108:354–360. [DOI] [PubMed] [Google Scholar]
- 22.Sin DD, Leung R, Gan WQ, Man SP. Circulating surfactant protein D as a potential lung-specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SX, Liu P, Wei MT, Chen L, Guo Y, Wang RY, Tu ZG, Liang XC. Roles of serum clara cell protein 16 and surfactant protein-D in the early diagnosis and progression of silicosis. J Occup Environ Med 2007;49:834–839. [DOI] [PubMed] [Google Scholar]
- 24.Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the eclipse cohort. Thorax 2008;63:1058–1063. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson JJ, Toennies HM, Kobayashi DK, Shapiro SD, Senior RM. MMP-9 deficiency does not protect mice from cigarette smoke induced emphysema, but does decrease airway collagen synthesis. Am J Respir Crit Care Med 2008;177:A24. [Google Scholar]
- 26.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998;93:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, Mecham RP, Senior RM. A site on laminin alpha 5, aqarsaaskvkvsmkf, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol 2003;171:398–406. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson JJ, Holmbeck K, Yamada S, Birkedal-Hansen H, Parks WC, Senior RM. Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Dev Dyn 2005;232:1079–1090. [DOI] [PubMed] [Google Scholar]
- 29.Deslee G, Woods JC, Moore CM, Liu L, Conradi SH, Milne M, Gierada DS, Pierce J, Patterson A, Lewit RA, et al. Elastin expression in very severe human COPD. Eur Respir J 2009;34:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deslee G, Woods JC, Moore C, Conradi SH, Gierada DS, Atkinson JJ, Battaile JT, Liu L, Patterson GA, Adair-Kirk TL, et al. Oxidative damage to nucleic acids in severe emphysema. Chest 2009;135:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 32.Wahl LM, Wahl SM, Smythies LE, Smith PD. Isolation of human monocyte populations. Curr Protoc Immunol 2006;Chapter 7:Unit 7.6A. [DOI] [PubMed]
- 33.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-e2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2008;39:673–682. [DOI] [PubMed] [Google Scholar]
- 34.Minematsu N, Nakamura H, Tateno H, Nakajima T, Yamaguchi K. Genetic polymorphism in matrix metalloproteinase-9 and pulmonary emphysema. Biochem Biophys Res Commun 2001;289:116–119. [DOI] [PubMed] [Google Scholar]
- 35.Fuke S, Betsuyaku T, Nasuhara Y, Morikawa T, Katoh H, Nishimura M. Chemokines in bronchiolar epithelium in the development of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2004;31:405–412. [DOI] [PubMed] [Google Scholar]
- 36.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003;58:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil HW, Hong JR, Park JH, Seo YS, Yang JO, Lee EY, Hong SY. Plasma surfactant D in patients following acute paraquat intoxication. Clin Toxicol (Phila) 2007;45:463–467. [DOI] [PubMed] [Google Scholar]
- 38.Nakano M, Omae K, Tanaka A, Hirata M, Michikawa T, Kikuchi Y, Yoshioka N, Nishiwaki Y, Chonan T. Causal relationship between indium compound inhalation and effects on the lungs. J Occup Health 2009;51:513–521. [DOI] [PubMed] [Google Scholar]
- 39.Thompson RW, Baxter BT. MMP inhibition in abdominal aortic aneurysms. Rationale for a prospective randomized clinical trial. Ann N Y Acad Sci 1999;878:159–178. [DOI] [PubMed] [Google Scholar]
- 40.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg 2002;35:923–929. [DOI] [PubMed] [Google Scholar]
- 41.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med 2005;171:734–742. [DOI] [PubMed] [Google Scholar]
- 42.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 2008;295:L44–L53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 2003;163:2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med 2003;167:1083–1089. [DOI] [PubMed] [Google Scholar]
- 45.Birrell MA, Wong S, Dekkak A, De Alba J, Haj-Yahia S, Belvisi MG. Role of matrix metalloproteinases in the inflammatory response in human airway cell-based assays and in rodent models of airway disease. J Pharmacol Exp Ther 2006;318:741–750. [DOI] [PubMed] [Google Scholar]
- 46.DeMeo DL, Hersh CP, Hoffman EA, Litonjua AA, Lazarus R, Sparrow D, Benditt JO, Criner G, Make B, Martinez FJ, et al. Genetic determinants of emphysema distribution in the national emphysema treatment trial. Am J Respir Crit Care Med 2007;176:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 2009;5:e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaykhiev R, Krause A, Salit J, Strulovici-Barel Y, Harvey BG, O'Connor TP, Crystal RG. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol 2009;183:2867–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ilumets H, Rytila P, Demedts I, Brusselle GG, Sovijarvi A, Myllarniemi M, Sorsa T, Kinnula VL. Matrix metalloproteinases -8, -9 and -12 in smokers and patients with stage 0 COPD. Int J Chron Obstruct Pulmon Dis 2007;2:369–379. [PMC free article] [PubMed] [Google Scholar]
- 50.Leclerc O, Lagente V, Planquois JM, Berthelier C, Artola M, Eichholtz T, Bertrand CP, Schmidlin F. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J 2006;27:1102–1109. [DOI] [PubMed] [Google Scholar]
- 51.Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res 2004;30:1–15. [DOI] [PubMed] [Google Scholar]
- 52.Parr DG, Dirksen A, Piitulainen E, Deng C, Wencker M, Stockley RA. Exploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiency. Respir Res 2009;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, Stockley RA. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009;33:1345–1353. [DOI] [PubMed] [Google Scholar]
- 54.Spira A, Beane J, Pinto-Plata V, Kadar A, Liu G, Shah V, Celli B, Brody JS. Gene expression profiling of human lung tissue from smokers with severe emphysema. Am J Respir Cell Mol Biol 2004;31:601–610. [DOI] [PubMed] [Google Scholar]
- 55.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 2001;164:469–473. [DOI] [PubMed] [Google Scholar]
- 56.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest 2007;132:464–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.