Abstract
Objective
The purpose of this study was to characterize the human term placental villous tissue explant culture model as a tool to study the formation and efflux of 1-chloro-2,4-dinitrobenzene (CDNB) conjugate 2,4-dinitrophenyl-S-glutathione (DNP-SG) as a model system for phase II metabolism and ATP-binding cassette (ABC) transporter-mediated cellular efflux.
Methods
Placental tissue samples were obtained after cesarean section following normal pregnancies (n=9). Cultured villous tissue was monitored up to 48 h to study the effect of time in culture on biochemical parameters, formation and efflux of DNP-SG in the absence or presence of ATPase inhibitor sodium orthovanadate and the protein expression of ABC transporters - multidrug resistance associated protein 2 (MRP2), P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and enzyme glutathione-S-transferase isoform P1-1 (GSTP1-1).
Results
Villous tissue structure, tissue viability and expression of BCRP, GSTP1-1 remained unchanged, while expression of MRP2, P-gp and total tissue glutathione decreased with time in culture. Tissue integrity was unchanged up to 24 h but declined at 48 h. However, DNP-SG formation, DNP-SG efflux, and the extent of inhibition of DNP-SG efflux by sodium orthovanadate showed only minor changes through 48 h. Sodium orthovanadate decreased DNP-SG efflux, consistent with inhibition of apical ABC transporters.
Conclusion
The results support the use of the cultured human term placental villous tissue explants model to study coordinated function of GSTP1-1 and apical ABC transporters in the formation and efflux of the model substrate DNP-SG.
Keywords: ATP-binding cassette transporters; 1-chloro-2,4-dinitrobenzene; 2,4-Dinitrophenyl-S-glutathione; DNP-SG; glutathione-S-transferase isoform P1-1; placental explants culture
1. Introduction
Pregnant women are often under medication for pre-existing medical conditions such as asthma, epilepsy or hypertension, or for pregnancy related symptoms such as nausea or vomiting. While pharmacological benefit to the mother is desired, potential fetal toxicity is a major concern. The placenta has several active transporters on the apical side of the syncytiotrophoblast or the fetal capillary endothelium that may reduce fetal exposure to xenobiotics. These efflux transporters include ATP-binding cassette (ABC) transporters such as P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance associated proteins (MRP1, 2, 3). These transporters have wide substrate specificity and are involved in the excretion of endogenous compounds in the bile or urine, or provide protection against xenobiotics in other tissues such as the liver, kidney, lung, gastrointestinal tract, blood-brain barrier and blood-testis barrier [1]. Inside the placental syncytiotrophoblast are certain Phase I (oxidative) and Phase II (conjugative) enzymes, similar to those in other organs such as the liver, that detoxify xenobiotics, make them more hydrophilic and facilitate maternal excretion. The coordinated function of the ABC transporters and placental enzymes is believed to play an important role in determining the placental disposition of several endogenous and exogenous substances.
In vivo assessment of placental transfer of substances in humans is difficult due to ethical considerations. Furthermore, extrapolation of functional data regarding placental transfer of substances from animal studies needs to be approached with caution due to significant interspecies differences in placental structure, function and localization of ABC transporters. Species differences in placental anatomy and may lead to differences in the transplacental permeability of large molecules and overall placental handling of endogenous and exogenous substances. Expression, localization and/or directionality as well as effect of gestational age on mRNA/protein expression of ABC transporters on the fetal and/or maternal capillary endothelial layers as well as different trophoblast layers have been reported for a few species and show some differences from the human placenta [2–4]. In addition to differences in placental anatomy and expression of ABC transporters, other differences exist in transport protein activities as well. In view of these differences, animal models have limited use in placental drug disposition studies, especially for studying ABC transporter activity.
The functional activity of human placental ABC transporters has been evaluated in vitro using several techniques such as isolated or dually perfused cotyledons with open or closed recirculating systems, trophoblast cell lines such as BeWo, JEG, Jar, placental tissue slices and plasma membrane vesicles. The advantages and limitations of these methods have been comprehensively reviewed [5]. Of all these models, the perfused placental cotyledon model represents the most well-conserved structural integrity and placental transport function; however, placental perfusion is difficult to set up and has limited duration of viability and allows very limited manipulations on each tissue. The cultured placental villous tissue explants model has been used in several studies to assess the transport of small molecules and amino acids [6, 7] as well as the release of eicosanoids such as thromboxanes and prostacyclins [8] from the trophoblast. However, this model has not been used to study the activity of ABC transporters in the placenta. Though not as intact as the perfused placental cotyledon model, structural integrity of the tissue is maintained in the cultured villous tissue explants and materno-fetal efflux mediated by apical ABC transporters can potentially be studied in this system since the placental capillaries are collapsed and the trophoblast layer is exposed to the medium [5].
Human placental villous tissue can be cultured under several different conditions depending upon the intended application and every model needs to be validated to ensure that it is fit for the purpose. Various conditions such as the choice of culture medium, oxygen concentration and the composition of matrix ions are known to affect the in vitro stability of explant cultures. Validation approaches include monitoring of tissue morphology and viability, secretion of placental hormones in the culture media and functional assessment of transport activity [9]. In this study we describe the characterization and application of human placental villous tissue explants grown in suspension in M199 medium under 95% air/5% CO2 over a short duration of 48 h to study placental metabolism and ABC transport activity.
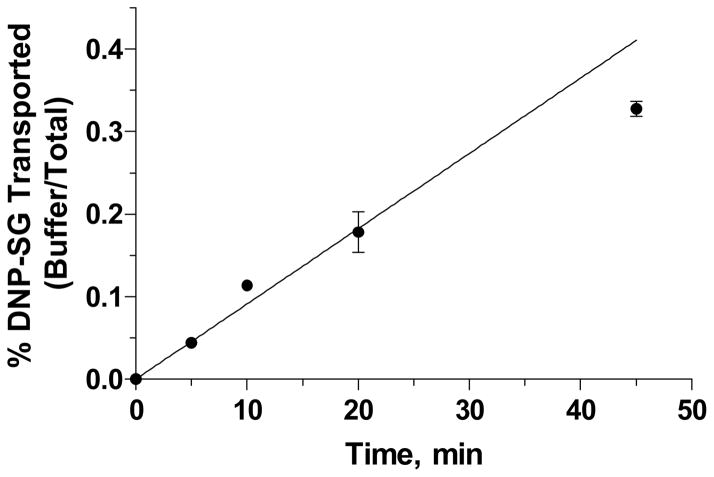
Previously we established that upon exposure to 1-chloro-2,4-dinitrobenzene (CDNB), the placenta forms its glutathione conjugate 2,4-dinitrophenyl-S-glutathione (DNP-SG) by the action of glutathione-S-transferase isoform P1-1 (GSTP1-1). DNP-SG was effluxed from the tissue; the efflux process was found to increase linearly with time up to 45 minutes (Figure 1) and was significantly inhibited upon treatment with ATPase inhibitor sodium orthovanadate suggesting the coordinated role of GSTP1-1 and ABC transporters in the detoxification of CDNB from the human placenta [10]. Furthermore, we also reported the involvement of an ABC transporter such as MRP1 in efflux of DNP-SG from human placental villous tissue fragments [11]. The purpose of the present study was to characterize the human term placental villous tissue explant culture model and assess its utility to study the formation and efflux of DNP-SG as a model system for phase II metabolism and cellular efflux. The current study describes the effect of time in culture on protein expression of ABC transporters- P-gp, MRP2, BCRP and the enzyme GSTP1-1; MTT incorporation – a marker for tissue viability, LDH –a marker for tissue integrity [12] and total tissue glutathione content. Tissue morphology was assessed by histochemical detection and functional activity of the tissue was assessed by studying the effect of time in culture on the formation (metabolic activity) and efflux (transport activity) of DNP-SG in the placental villous tissue fragments. Furthermore, the kinetics of DNP-SG formation and efflux were also characterized in the absence and presence of sodium orthovanadate.
Figure 1. Time Course for DNP-SG Efflux.
Figure 1. DNP-SG Efflux upon brief exposure to 100 μM CDNB followed by incubation with DPBS with shaking at 37°C for 45 minutes. Data represent mean ± SD from triplicate determinations in a representative patient. Similar results were observed in 2 additional patients.
2. Materials and Methods
2.1. Chemicals and Reagents
CDNB (99% purity), and perchloric acid (PCA) reagent (70% in water) were purchased from Acros Organics (Morris Plains, NJ). All other chemicals, solvents or reagents were of analytical grade and were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Chemicals (Fair Lawn, NJ) unless indicated below.
2.2. Human Subjects
Placental tissue samples were obtained from n=9 women (between ages of 18–45 years, gestational length ≥ 36 weeks) within 30 minutes of birth from cesarean section deliveries following normal pregnancies at the VCU Medical Center Hospital. Patients with hypertension, diabetes, preeclampsia, HIV infection or febrile illness; history of smoking, alcohol or drug abuse were excluded. After collection of the placenta, patients’ medical records were accessed to record the age, parity, weight, race, medical history, obstetric course, neonatal birth weight, time of birth, Apgar scores at the time of birth, and gross placental abnormalities. The study was approved by the VCU Institutional Review Board and informed consent was obtained from patients prior to delivery. Data for each of the experiments presented in this manuscript are from n=3 placental tissues each, randomly chosen from the total n=9 placentas as per experimental convenience.
2.3. Placental Villous Tissue Fragments: Culture Conditions
Placental tissue was chilled on ice and processed under sterile conditions at 4°C. Placental weight and gross abnormalities such as placental infarcts, absent cotyledons, discolorations on the maternal and fetal surfaces, and anomalies in cord insertion, if any, were noted. Triangular wedges of placental tissue, extending from the point of cord insertion to the margins were cut, basal and chorionic plates removed, washed with ice cold saline and blotted with sterile gauze. Triangular wedges of tissue (approximately 100 g) were gently cut into small pieces, washed in Dulbecco’s phosphate buffered saline (DPBS) supplemented with antibiotics (200 U/mL penicillin, 200 μg/ml streptomycin, 500 U/ml nystatin), to remove blood from intervillous space, filtered through gauze and then minced to smaller pieces. After several cycles of mincing and washing, 300–400 mg of the resulting placental villous tissue was incubated in suspension in 6-well polystyrene tissue culture dishes in 7 mL of M199 medium with Earle’s salts, L-glutamine, 2.2 g/L sodium bicarbonate, without phenol red (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 2 mM L-glutamine, for up to 48 h at 37°C in a water-jacketed incubator (NuAire, Inc., Plymouth, MN) set to maintain 95% air and 5% CO2. Medium was replaced at 24 h.
2.4. Villous Tissue Culture Model Validation
Human placental villous tissue cultures have been underused in the study of placental drug disposition. Utility of this model was assessed by studying the effect of time in culture on villous tissue morphology, integrity, viability, protein expression and biochemical parameters likely to affect formation and efflux of DNP-SG from cultured human placental villous tissue. Villous tissue (n=3 patients) was cultured in M199 medium up to 48 hours. At 2, 4, 6, 10, 24 and 48 h post culture, DNP-SG formation and efflux at 20 minutes in the absence or presence of sodium orthovanadate were analyzed following brief exposure (5 minutes) to 100 μM CDNB. Changes in expression of MRP2, P-gp, BCRP and GSTP1-1 were assessed by immunoblotting. LDH release into media as an indicator of tissue integrity, MTT incorporation into the tissue as a marker for tissue viability and total glutathione content in the tissue homogenate were monitored up to 48 hours. Villous tissue morphology was assessed by histochemical detection as described below.
2.5. Biochemical Assays
Tissue viability was assessed by the MTT assay as described previously [13]. Tissue samples (200–300 mg, unfrozen) from n=3 patients were collected in triplicate at 2, 4, 6, 10, 24, 32 and 48 h, exposed to MTT and the formation of the formazan product of MTT was measured by monitoring relative absorbance at 595 nm using the Synergy 2 Multi-Detection Microplate Reader (BioTek Instruments, Inc., Winooski, VT).
Tissue morphology was assessed by histochemical detection in placental tissue from a representative patient. Briefly, fresh minced tissue (200–300 mg) was incubated in media with or without 5 mM sodium orthovanadate for 1 hour followed by shaking in DPBS at 37°C for 20 min. Tissue samples thus treated were collected in triplicate at 0, 2, 4, 6, 10, 24 and 48 h, paraffin embedded after formalin fixation and cut in 10 μm sections. Tissue sections were cleared with Histoclear (National Diagnostics, Atlanta, GA) to remove paraffin and hydrated in a graded alcohol series. Tissue sections were incubated with 3% H2O2 in methanol for 30 min to quench endogenous peroxidase activity, stained with hematoxylin, dehydrated in a graded alcohol series and preserved. Slides were viewed using a BH-2 light microscope (Olympus, Center Valley, PA) and an image analysis software (IP Lab, Scanalytics, Inc., Fairfax, VA).
Effect of time in culture on the expression of placental ABC transporters- MRP2, P-gp, BCRP, enzyme GSTP1-1 as well as housekeeping genes actin and β-actin was assessed by immunoblotting as previously described [14] in placental tissue from n=2 patients. Tissue samples (200–300 mg) were collected at 0, 2, 4, 6, 10, 24, 48 h and frozen at −80°C. At a later time, tissue samples were thawed on ice and homogenized in 1:10 w/v buffer (50 mM Tris, 1mM EDTA, 1 mM PMSF, 5 μg/mL aprotinin, 5μg/mL leupeptin, 2 μg/mL pepstatin A, 2 μg/mL antipain, 1 mg/mL soya trypsin inhibitor, pH 7.4 at 4°C) using a Polytron PT 10–35 homogenizer with a PTA 10 TS generator (Kinematica, Lucerne, Switzerland; speed setting 6.5), for 30 seconds on ice. Tissue homogenate samples were centrifuged at 6000×g for 5 min at 4°C, 500 μL aliquots were snap frozen by dropping into liquid nitrogen and stored at −80°C. Protein concentrations were determined by a modification of the Lowry protein assay using bovine serum albumin as a standard [15]. For immunoblotting, proteins were denatured in the presence of sodium dodecyl sulfate at 37°C for 30 min before loading onto 8% Tris/glycine polyacrylamide Novex precast gels (Invitrogen) for MRP2 and P-gp, or 4–20% Precise protein gels (Pierce, Rockford, IL) for all other proteins, separated by standard electrophoresis in the presence or absence of NuPage sample reducing agent (Invitrogen) and transferred onto nitrocellulose membranes (Schleicher and Scheull, Keene, NJ). 0.05 μg Sf9 protein from in-house membrane vesicles individually over-expressing MRP2, P-gp or BCRP were used as positive controls. Membranes were blocked overnight at 4°C using Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE). Binding of the primary antibodies -mouse anti-human BCRP (BXP-21; 1:1000), mouse anti-human MRP2 (M2-III6; 1:4000) (Alexis Biochemicals, San Diego, CA), mouse anti-human P-gp (F4; 1:2000) (Kamiya Biomedical Co., Seattle, WA), mouse anti-β-actin (1:2000), rabbit anti-actin (1:1000) (Sigma), rabbit anti-GSTP1-1 pAb (1:2000) (Calbiochem, La Jolla, CA) and the secondary antibodies - goat anti-mouse IR Dye 800, goat anti-rabbit Alexa Fluor 680 (Li-Cor) was performed in the Odyssey blocking buffer at room temperature for 1 h, in the dark. The resultant fluorescent complexes were detected and the band intensity was quantified using the Odyssey Infrared Imaging System and the Odyssey Application Software, version 2.1 (Li-Cor).
Tissue homogenates prepared in a similar manner as described for immunoblotting were also used to assess the effect of time in culture on total tissue glutathione content (n=3 patients). Total tissue glutathione was measured by the method of Tietze et. al. [16]. Briefly, tissue homogenate samples were incubated with Ellman’s reagent in the presence of β-NADPH and glutathione reductase enzyme at room temperature and the reaction was monitored by measuring the absorbance at 405 nm using the Synergy 2 Multi-Detection Microplate Reader (BioTek). Reduced glutathione was used as a standard.
LDH release into the tissue culture media was measured as a marker for tissue integrity. Media samples (200 μL) were collected in triplicate at 2, 4, 6, 10, 24 and 48 h and frozen at −20°C. At a later time, LDH release was measured using the LDH UV-Rate assay kit (Stanbio Laboratory, Boerne, TX). Reported sensitivity for LDH measurement was 6.6 U/L. Catatrol I serum sample (Catachem, Bridgeport, CO) with a reported LDH content of 146–166 U/L was used as a standard for one-point calibration.
2.6. Kinetics of Formation and Efflux of DNP-SG
To characterize the formation and efflux of DNP-SG, villous tissue (n=3 patients) was cultured overnight in M199 medium, preincubated for 1 h without or with ATPase inhibitor sodium orthovanadate, exposed briefly to 3–1000 μM CDNB, rinsed twice in DPBS to remove extracellular CDNB and incubated in DPBS with shaking at 37°C. Buffer and tissue samples were collected in 10% PCA in water (1:1 v/v) up to 45 minutes; DNP-SG and CDNB were assayed by a sensitive high-performance liquid chromatography method with ultraviolet detection for the simultaneous determination of CDNB, DNP-SG and its metabolites for human placental disposition studies, previously developed and validated in our lab [10]. The lower limit of quantitation (LLOQ) and linear range for this assay were 0.1 μM and 0.1 – 100 μM for DNP-SG; 1 μM and 1–20 μM for CDNB, respectively following a 100 μL sample injection for quantification from both buffer and human placental tissue homogenate.
2.7. Data Analysis
Data were plotted and analyzed using Prism version 5 (GraphPad Software Inc., San Diego, CA). Effects of time in culture on MTT incorporation, LDH release, total tissue glutathione content, DNP-SG formation and efflux in the absence or presence of sodium orthovanadate were analyzed by repeated measures one-way analysis of variance (α =0.05) followed by Tukeys’s multiple comparison test for all possible pair wise comparisons (Prism v5).
Kinetics of DNP-SG formation were analyzed by unweighted nonlinear regression curve fitting to the Michaelis-Menten equation (Prism v5), where Km represents the rate of formation of DNP-SG in terms of tissue CDNB content normalized to tissue weight and vmax represents the maximum rate for saturable DNP-SG formation. Tissue CDNB content was estimated by the summation of the measured tissue CDNB content and the total DNP-SG content at the end of the experiment, assuming a 1:1 stoichiometric ratio for conversion of CDNB to DNP-SG. Parameter estimates of Km and vmax for DNP-SG formation in the absence or presence of sodium orthovanadate in n=3 patients were compared by a paired two-tailed t-test (α =0.05) using Prism v5 (GraphPad Software Inc.). Kinetics of DNP-SG efflux were analyzed by unweighted nonlinear regression curve fitting to equation 1.
| equation 1 |
where J and S represent the DNP-SG transport rate and tissue DNP-SG content normalized to tissue weight, respectively. Jmax, Kt, and kd represent the maximum transport rate for saturable transport, the affinity for saturable transport, and the rate constant for nonsaturable transport respectively (Prism v5).
3. Results
3.1. Human Subjects
Clinical data for patients recruited in this study are summarized in Table 1. The study population was representative of the patient population at the VCU Medical Center Hospital. All patients underwent C-section deliveries following normal pregnancies. Term placental tissues (≥36 weeks of gestation) collected from these patients had no gross abnormalities. Two of the pregnant patients had asthma (patients 4 and 6) and used albuterol on an as needed basis. One patient had hypothyroidism (patient 5) and used synthroid 125 mg qd, and another patient had polycystic ovarian syndrome (patient 1). All infants were healthy and had normal Apgar scores.
3.2. Tissue Morphology
Tissue morphology was assessed by histochemical detection following hematoxylin staining. Figure 2 illustrates a representative section of sodium orthovanadate treated villous tissue collected 48 h post-culture. The structure of the syncytiotrophoblast layer is intact in the microvilli as well as the trunk villi regions. Similar tissue sections collected with or without sodium orthovanadate treatment at 2, 4, 6, 10, 24 and 48 h were also examined. The structure of the syncytiotrophoblast layer was not adversely affected over time in culture or upon treatment with sodium orthovanadate. Maternal blood cells were not observed, suggesting effective removal of maternal blood from the villous tissue during the villous tissue isolation. Red blood cells as well as the fetal capillary endothelium are known to express some efflux transporters. In the absence of red blood cells or exposed fetal capillary endothelium in the villous tissue explants, any observed transport activity could be attributed to efflux form the placental syncytiotrophoblast, suggesting that the tissue explants could be effectively used to study transport mediated by apical ABC transporters.
Figure 2. Representative section of sodium orthovanadate treated villous tissue 48 h post-culture.

Figure 2. Representative section of sodium orthovanadate treated villous tissue (tissue collected from patient 2), 48 h post-culture. Light microscope image (100X magnification) after staining with hematoxylin. V-Villous tree branches, IV-Intervillous space, TV-Villous tree trunk. Scale bar indicates 50 μm.
3.3. Protein Expression
Effect of time in culture on the protein expression of ABC transporters MRP2 (190 kDa), P-gp (180 kDa) and BCRP (70 kDa), enzyme GSTP1-1 (25 kDa), housekeeping proteins actin (42 kDa) and β-actin (42 kDa) was studied by western blotting. Results from a representative patient (patient 1) are illustrated in Figure 3. Integrated intensity values corresponding to the protein bands were compared across different time points for each protein (data not shown). Protein expression of BCRP, GSTP1-1, actin and β-actin remained unchanged over time, while the expression of MRP2 and P-gp decreased slightly between 2–10 h and then decreased more substantially with time in culture up to 48 h. Similar results were also observed in cultured placental tissue from one additional patient (patient 3). These protein expression data suggest that the model can be used to study BCRP transport activity or GSTP1-1 metabolic activity up to 48 h, but studies related to P-gp or MRP2 transport activity should preferably be carried out at earlier time points up to 10 h of time in culture. Ideally, a single time point should consistently be used for inhibition or transport activity studies across different placental tissues, to minimize variability associated with changes in protein expression with time in culture. Further studies need to be carried out to understand the mechanism for down regulation of protein expression of P-gp and MRP2 in villous tissue explants in culture.
Figure 3. Effect of time in culture on protein expression of MRP2, P-gp, BCRP, GSTP1-1.
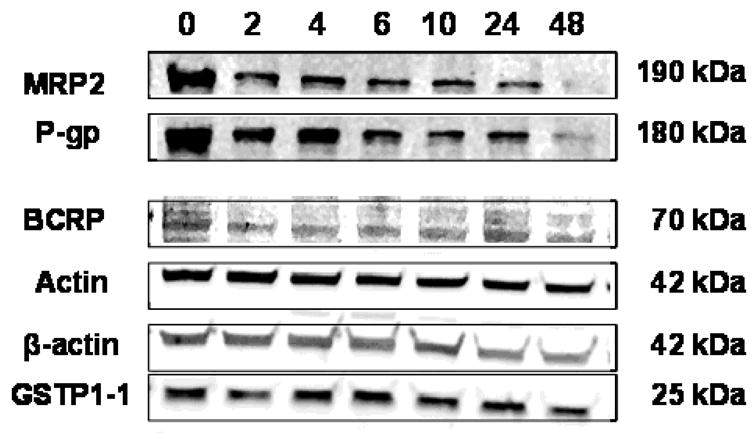
Figure 3. Western blots illustrating the effect of time in culture on protein expression of MRP2, P-gp, BCRP, GSTP1-1, actin and β-actin in tissue homogenates (25 μg homogenate protein) of cultured placental tissue from a representative patient (patient 1). Cultured tissue samples were collected at 0, 2, 4, 6, 10, 24 and 48 h post-culture. Similar results were also obtained for patient 3.
3.4. Biochemical Parameters
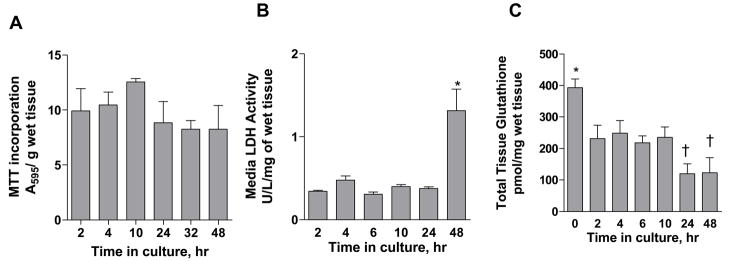
Figure 4 illustrates the effect of time in culture on the biochemical status of the villous tissue. The biochemical status of the tissue was assessed by studying MTT incorporation, LDH release and total tissue glutathione content up to 48 h. MTT incorporation, a measure for mitochondrial dehydrogenase enzymatic activity and tissue viability remained unchanged between 2–48 h, suggesting that the tissue was viable up to 48 h in culture (Figure 4A).
Figure 4. Effect of time in culture on tissue biochemical parameters.
Figure 4. Effect of time in culture on tissue biochemical parameters. A: MTT incorporation; an indicator of tissue viability, B: LDH release; an indicator of tissue integrity, C: total tissue glutathione content as a function of time in culture. Media was replaced at 24 h. Data represent mean ± SEM from triplicate determinations in placental tissue from 3 patients (patients 3, 4, 5 for LDH release and total glutathione measurements; patients 6, 7, 8 for MTT incorporation). * represents p<0.05 vs. each of the other time points and † represents p<0.05 vs. the 4 h time point when compared by repeated measures one-way ANOVA followed by Tukey’s post test for all possible pair wise comparisons; all other comparisons were not significant.
The release of LDH, a cytosolic enzyme, into the culture media was monitored over 48 h as a measure of tissue integrity (Figure 4B). LDH activity remained unchanged up to 24 h (0.380 ± 0.025 U/L/mg wet tissue weight; combined mean ± SEM of measurements over first 24 h) but showed a 3.4 fold increase to 1.31 ± 0.448 U/L/mg wet tissue weight at 48 h (p<0.05). These data indicate that the tissue was intact over the first 24 h in culture but may begin to lose its integrity at further time points. Maintenance of tissue integrity is an important consideration when studying efflux activity mediated by ABC transporters. Observed transport of hydrophilic ionized compounds such as DNP-SG can be attributed to ABC transporter-mediated activity only when the tissue is intact and diffusion or paracellular transport does not overwhelm active transport.
Total glutathione content in the tissue was measured over 48 h (Figure 4C). Tissue glutathione content decreased from 393 ± 48 pmol/mg wet tissue weight at 0 h to a constant value of 234 ± 16 pmol/mg wet tissue weight (combined mean ± SEM of measurements) between 2–10 h (p<0.05) and then further decreased to a mean value of 122 pmol/mg wet tissue weight (combined mean) at 24 and 48 h (p<0.05 vs. glutathione content at the 4 h time point). Tissue glutathione content would be a rate limiting factor in the formation of DNP-SG when tissue CDNB content is greater than 233 ± 12.5 pmol/mg tissue (combined mean ± SEM of measurements between 2–10 h). Assuming a tissue density of 1 g/mL, the tissue glutathione content values were in the range of 0.12–0.39 mM. These values were similar to those previously reported [17–19].
3.5. Formation and Efflux of DNP-SG
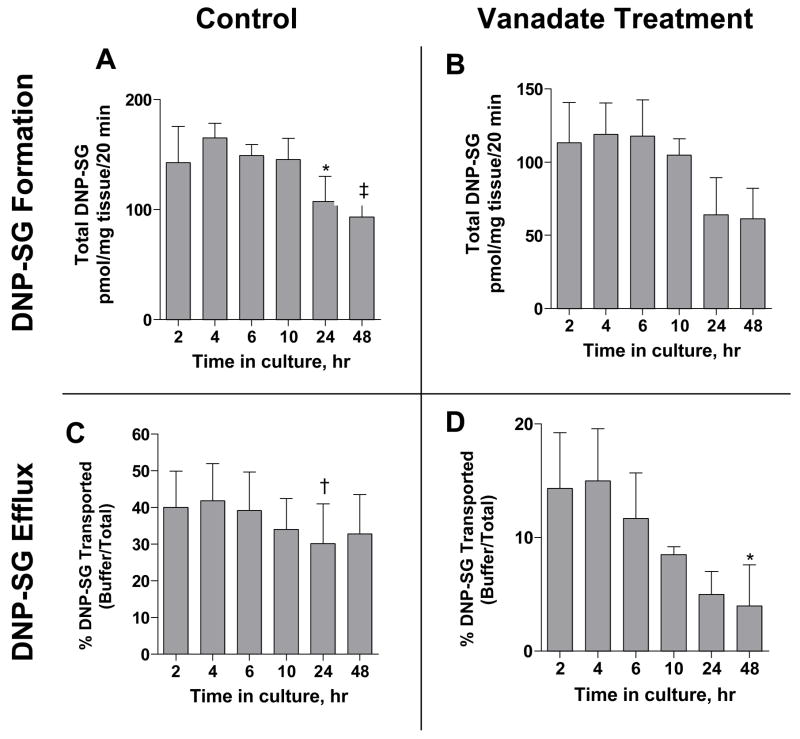
Effect of time in culture on DNP-SG formation and efflux in the absence or presence of sodium orthovanadate, after brief exposure to 100 μM CDNB, was monitored over 48 h as a measure of functional activity of the tissue (Figure 5). DNP-SG formation in the absence of sodium orthovanadate (Figure 5A) remained unchanged at 151 ± 10 pmol DNP-SG/mg tissue/20 min between 2–10 h (combined mean ± SEM of measurements between 2–10 h), decreased slightly to 107 ± 23 pmol DNP-SG/mg tissue/20 min at 24 h (p<0.05 vs. measurement at the 4 h time point) and then further decreased marginally to 93.4 ± 10.8 pmol DNP-SG/mg tissue/20 min at 48 h (p<0.05 vs. measurements between 2–10 h time points). DNP-SG formation in the presence of sodium orthovanadate remained unchanged at 96.7 ± 26.8 (combined mean ± SEM of measurements at 2–48 h) up to 48 h (Figure 5B). DNP-SG efflux (expressed as a percentage of total DNP-SG detected in the efflux buffer), in the absence of sodium orthovanadate (Figure 5C) remained unchanged up to 48 h at 37.5 ± 3.95 % (combined mean ± SEM of measurements at 2, 4, 6, 10 and 48 h) except for a slight decrease to 30.1 ± 10.9 % at 24 h (p<0.05 vs. measurements at the 2 and 4 h time point). DNP-SG efflux in the presence of sodium orthovanadate (Figure 5D) remained unchanged at 10.9 ± 4.18% (combined mean ± SEM of measurements between 2–24 h) up to 24 h and decreased to 4 ± 3.6 % at 48 h (p<0.05 vs. measurement at the 4 h time point). These data suggest that culturing time affected DNP-SG formation and/or efflux only to a minor extent at the 24 and 48 h time points.
Figure 5. Effect of time in culture on DNP-SG formation and DNP-SG efflux.
Figure 5. Effect of time in culture on DNP-SG formation (A & B) and DNP-SG efflux (C & D) measured at 20 min, in the absence (A & C) or presence (B & D) of ATPase inhibitor sodium orthovanadate, following brief exposure to 100 μM CDNB. Media was replaced at 24 h. Data represent mean ± SEM from triplicate determinations in placental tissue samples from n=3 patients (patients 1, 3, 4). * represents p<0.05 vs. 4 h time point, † represents p<0.05 vs. 2, 4 h time points each and ‡ represents p<0.05 vs. 2, 4, 6, 10 h time points each for the individual panel graphs, when compared by repeated measures one-way ANOVA followed by Tukey’s post test for all pair wise comparisons, all other comparisons were not significant.
3.6. Kinetics of DNP-SG Formation and Efflux
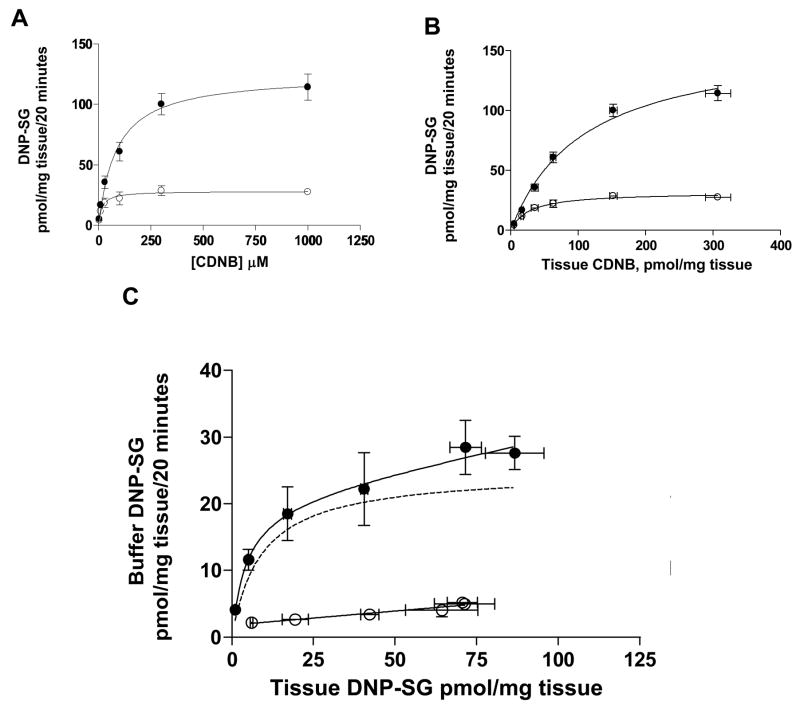
DNP-SG formation and efflux processes were concentration dependent, saturable when assessed at 20 minutes, following brief exposure to 3–1000 μM CDNB. Formation was greater than efflux at CDNB loading concentrations greater than 30 μM (or approximate tissue CDNB content > 35 pmol/mg tissue) as illustrated in data from a representative patient (patient 7) in Figure 6.
Figure 6. Kinetics of DNP-SG formation and efflux.
Figure 6. Kinetics of DNP-SG formation and efflux in placental tissue from a representative patient. A & B. DNP-SG formation (total DNP-SG; closed circles) and efflux (buffer DNP-SG; open circles) as a function of CDNB concentration in the loading buffer (A) or tissue CDNB content (B), measured at 20 min upon exposure to 3–1000 μM CDNB. Data (unweighted) for DNP-SG formation and efflux were fitted to the Michaelis-Menten equation and equation (1), respectively. C. DNP-SG efflux (buffer DNP-SG) in the absence (closed circles) or presence (open circles) of sodium orthovanadate as a function of tissue DNP-SG content, following exposure to 3–1000 μM CDNB. Data for DNP-SG efflux in the absence of sodium orthovanadate were fitted to equation 1 (unweighted), and the data for DNP-SG efflux in the presence of sodium orthovanadate were analyzed by linear regression. Active component of transport (dotted line) was estimated by subtracting the buffer DNP-SG values in the presence of sodium orthovanadate from the buffer DNP-SG values in the absence of sodium orthovanadate at the corresponding CDNB loading concentration, then fitting the resulting values (unweighted) to the Michaelis-Menten equation. Data represent mean ± SD from triplicate determinations. Parameter estimates are summarized in Table 2.
Formation of DNP-SG followed classic Michaelis-Menten kinetics when assessed with respect to CDNB loading concentration (Figure 6A) or tissue CDNB content (Figure 6B). Individual parameter estimates of Km and vmax for DNP-SG formation determined in n=3 patients in the absence or presence of sodium orthovanadate are summarized in Table 2. Km and vmax values were compared between the control and sodium orthovanadate treated tissues by a paired two-tailed t-test. Km for DNP-SG formation in the absence of sodium orthovanadate was significantly greater by 50.0 [95% CI: 23.7–77.3] pmol tissue CDNB/mg tissue (p<0.05) than that in the presence of sodium orthovanadate. Also, the vmax for DNP-SG formation in the absence of sodium orthovanadate was significantly greater by 72.5 [95% CI: 35.5–109] pmol DNP-SG/mg tissue/20 min (p<0.05) than that in the presence of sodium orthovanadate. A decrease in both Km and vmax values suggests that sodium orthovanadate exhibits a mixed mechanism for inhibition of GSTP1-1-mediated DNP-SG formation. However, estimates for vmax/Km, a measure of enzymatic efficiency, were similar in the absence (1.39 ± 0.33 pmol DNP-SG/pmol tissue CDNB/20 min) or presence (1.35 ± 0.59 pmol DNP-SG/pmol tissue CDNB/20 min) of sodium orthovanadate indicating that the metabolic activity of GSTP1-1 towards CDNB was not affected by the presence of sodium orthovanadate.
DNP-SG efflux occurred by a combination of saturable active transport and other non-saturable transport processes (Figure 6C) and data were fit to equation 1. The Kt, Jmax and kd for DNP-SG efflux were 6.60 ± 3.05 pmol DNP-SG/mg tissue, 18.9 ± 8.6 pmol DNP-SG/mg tissue/20 min and 0.070 ± 0.040 (20 min−1) respectively. The individual kinetic parameter estimates from n=3 patients are summarized in Table 2.
CDNB loading concentration of 100 μM was determined to be optimum for further experimentation, based on the kinetics of DNP-SG formation and efflux. At this CDNB loading concentration formation followed first order kinetics, while DNP-SG efflux was saturated and followed zero order kinetics (Figure 6A). At a CDNB loading concentration of 100 μM, the approximate tissue CDNB content was 50 pmol/mg tissue (Figure 6B), and total tissue glutathione which had a value of 233 ± 12.5 pmol/mg tissue (combined mean ± SEM of measurements between 2–10 h) (Figure 4C), was not a rate limiting factor in the formation of DNP-SG. However, at 1000 μM CDNB, when the tissue CDNB content was 307 pmol/mg tissue, tissue glutathione was a rate limiting factor in the formation of DNP-SG and might have led to underestimation of Km and vmax for DNP-SG formation kinetics. As previously established [10], DNP-SG efflux proceeded linearly with time up to 45 min, with minimal further DNP-SG metabolism and was significantly greater in control tissue than sodium orthovanadate treated tissue, upon exposure to 100 μM CDNB. DNP-SG formation remained unchanged in absence or presence of sodium orthovanadate, when exposed to 100 μM CDNB.
4. Comment
We developed and validated a simple in vitro model to study the activity of placental ABC transporters, using short-term cultured human term placental villous tissue fragments. This model has previously been used to study the uptake of amino acids and small molecules but has not been used to study the efflux activity of apical ABC transporters. We characterized the system using several biochemical factors such as MTT incorporation, LDH activity and total tissue glutathione which are markers for tissue viability, tissue integrity and tissue redox status respectively. Tissue viability was maintained over 48 h, tissue integrity was maintained up to 24 h but decreased at 48 h and total tissue glutathione content decreased at 24 and 48 h. Tissue morphology monitored by histochemical detection over 48 h indicated that the syncytiotrophoblast structure was intact and the preparation was not contaminated by presence of RBC’s from the maternal circulation. Furthermore, treatment with sodium orthovanadate had no adverse effect on tissue morphology. Protein expression of P-gp and MRP2 decreased at 24 and 48 h while that of BCRP and GSTP1-1 remained unchanged over time in culture up to 48 h. Functional activity of the tissue was measured in the form of DNP-SG formation (metabolism) and efflux (transport) in the presence or absence of sodium orthovanadate. Both DNP-SG formation and efflux showed very minor changes at the 24 and 48 h time points. These data indicate that change in biochemical parameters did not affect the functional activity of the placental enzymes and transporters.
Furthermore, we characterized the kinetics of DNP-SG formation and efflux in the presence and absence of sodium orthovanadate. DNP-SG formation followed classic Michaelis-Menten kinetics. Although the Km and vmax values for the formation kinetics decreased in the presence of sodium orthovanadate, the enzymatic efficiency (vmax/Km) of GSTP1-1 for conversion of CDNB to DNP-SG was not affected, DNP-SG efflux was a combination of saturable active transport and other non-saturable transport processes. DNP-SG is a prototypical substrate for the MRP’s [20] and has been widely studied in several systems such as human colon carcinoma cells (Caco-2) [21], human erythrocytes [22] as well as rat and guinea pig liver [23–25] and intestine [26, 27]. There is limited data suggesting that DNP-SG may be a substrate for BCRP [28]; since BCRP is highly expressed in the human placenta, it may be involved in maternally directed DNP-SG efflux from the placenta. Transport experiments using placental villous tissue explants, in the presence of ABC transporter inhibitors have suggested the involvement of an ABC transporter such as MRP1 in efflux of DNP-SG from human placental villous tissue fragments [11].
The cultured human term placental villous tissue explants technique offers the advantages of simplicity, greater flexibility in terms of experimental design and an increased period of viability than the placental perfusion model. This technique provides data that is more relevant to the in vivo situation as compared to transport data from trophoblast cell lines. The tissue fragments have a limited viability and time points for experiments need to be chosen carefully with respect to the transporter of interest. Also, tissue biochemical parameters such as glutathione content which may affect the conjugation reaction need to be monitored when studying the interplay between enzymes and transporters. The term placental villous tissue explants model can be applied to study the enzyme-transporter interplay for other substrates, besides DNP-SG, that may follow the mechanism of phase II conjugation followed by ABC transporter mediated efflux of metabolites, where the precursor molecule is different from the molecule that is effluxed from the tissue. Furthermore, this model can also be applied to study the efflux transport of drugs that could be pre-loaded into the tissue, washed to remove extracellular substrate and then efflux of the same substrate could be followed over time.
In summary, we validated the cultured villous tissue explants model to study the coordinated activity of the human placental enzyme GSTP1-1 and ABC transporters. The paradigm of glutathione conjugation of CDNB followed by DNP-SG efflux could be further applied to study the effect of pathophysiologic conditions such as preeclampsia and gestational diabetes that are associated with oxidative stress on placental enzyme and ABC transporter activities, either by retrieving normal placental tissue and inducing oxidative stress in vitro, or by retrieving term placental tissue from patients with these pathophysiologic conditions and then comparing the DNP-SG formation and efflux parameters between the normal placental tissues and placental tissues from patients with different disease states.
Acknowledgments
Financial Support: We acknowledge financial support from NIH MD002256, the A.D. Williams Foundation, The Thomas F. and Kate Miller Jeffress Memorial Trust, VCU School of Pharmacy and the VCU Graduate School.
The authors acknowledge Dr. Susan Lanni, Ms. Deaette Smith, Ms. Sonya Washington, nurses and staff of the Labor & Delivery Unit at the VCU Medical Center Hospital for help in various aspects of this work. We also acknowledge financial support from NIH MD002256, the A.D. Williams Foundation, The Thomas F. and Kate Miller Jeffress Memorial Trust, VCU School of Pharmacy and the VCU Graduate School.
Abbreviations
- ABC
ATP-binding cassette
- BCRP
breast cancer resistance protein
- CDNB
1-chloro-2,4-dinitrobenzene
- DNP-SG
2,4-dinitrophenyl-S-glutathione
- DPBS
Dulbecco’s phosphate buffered saline
- GSTP1-1
glutathione-S-transferase isoform P1-1
- LDH
lactate dehydrogenase
- LLOQ
lower limit of quantitation
- MRP
multidrug resistance associated protein (human isoform)
- MTT
thiazolyl blue tetrazolium bromide
- PCA
perchloric acid
- P-gp
P-glycoprotein
Footnotes
Parts of this work were presented at the Annual Meeting of the Society for Gynecologic Investigation, San Diego, CA, March 26-29, 2008.
References
- 1.St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1495–503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- 2.Aleksunes LM, Cui Y, Klaassen CD. Prominent Expression of Xenobiotic Efflux Transporters in Mouse Extraembryonic Fetal Membranes Compared to Placenta. Drug Metab Dispos. 2008 doi: 10.1124/dmd.108.021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 2003;31:153–67. doi: 10.1124/dmd.31.2.153. [DOI] [PubMed] [Google Scholar]
- 4.Novotna M, Libra A, Kopecky M, Pavek P, Fendrich Z, Semecky V, Staud F. P-glycoprotein expression and distribution in the rat placenta during pregnancy. Reprod Toxicol. 2004;18:785–92. doi: 10.1016/j.reprotox.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Sastry BV. Techniques to study human placental transport. Adv Drug Deliv Rev. 1999;38:17–39. doi: 10.1016/s0169-409x(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 6.Dancis J, Money WL, Springer D, Levitz M. Transport of amino acids by placenta. Am J Obstet Gynecol. 1968;101:820–9. doi: 10.1016/0002-9378(68)90038-0. [DOI] [PubMed] [Google Scholar]
- 7.Miller RK, Berndt WO. Characterization of neutral amino acid accumulation by human term placental slices. Am J Physiol. 1974;227:1236–42. doi: 10.1152/ajplegacy.1974.227.6.1236. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–40. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 9.Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–48. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya SS, Gerk PM. Simultaneous determination of 1-chloro-2,4-dinitrobenzene, 2,4-dinitrophenyl-S-glutathione and its metabolites for human placental disposition studies by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;859:94–102. doi: 10.1016/j.jchromb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya SS, Walsh SW, Gerk PM. Formation and Efflux of ATP-Binding Cassette Transporter Substrate 2,4-Dinitrophenyl-S-Glutathione from Cultured Human Term Placental Villous Tissue Fragments. Mol Pharm. 2009;6:1689–1702. doi: 10.1021/mp900019z. [DOI] [PubMed] [Google Scholar]
- 12.Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1116–22. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya SS, Gerk PM. Lack of interaction between tauroursodeoxycholate and ATP-binding cassette transporter isoform G2 (ABCG2) Mol Pharm. 2006;3:303–6. doi: 10.1021/mp0600079. [DOI] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 17.Perez MJ, Macias RI, Marin JJ. Maternal cholestasis induces placental oxidative stress and apoptosis. Protective effect of ursodeoxycholic acid. Placenta. 2006;27:34–41. doi: 10.1016/j.placenta.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Raijmakers MT, Bruggeman SW, Steegers EA, Peters WH. Distribution of components of the glutathione detoxification system across the human placenta after uncomplicated vaginal deliveries. Placenta. 2002;23:490–6. doi: 10.1053/plac.2002.0832. [DOI] [PubMed] [Google Scholar]
- 19.Wellner VP, Sekura R, Meister A, Larsson A. Glutathione synthetase deficiency, an inborn error of metabolism involving the gamma-glutamyl cycle in patients with 5-oxoprolinuria (pyroglutamic aciduria) Proc Natl Acad Sci U S A. 1974;71:2505–9. doi: 10.1073/pnas.71.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–94. [PubMed] [Google Scholar]
- 21.Oude Elferink RP, Bakker CT, Jansen PL. Glutathione-conjugate transport by human colon adenocarcinoma cells (Caco-2 cells) Biochem J. 1993;290(Pt 3):759–64. doi: 10.1042/bj2900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akerboom TP, Narayanaswami V, Kunst M, Sies H. ATP-dependent S-(2,4-dinitrophenyl)glutathione transport in canalicular plasma membrane vesicles from rat liver. J Biol Chem. 1991;266:13147–52. [PubMed] [Google Scholar]
- 23.Hinchman CA, Matsumoto H, Simmons TW, Ballatori N. Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. Metabolism of 1-chloro-2,4-dinitrobenzene in isolated perfused rat and guinea pig livers. J Biol Chem. 1991;266:22179–85. [PubMed] [Google Scholar]
- 24.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology. 2002;35:1409–19. doi: 10.1053/jhep.2002.33327. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt M, Kubitz R, Wettstein M, vom Dahl S, Haussinger D. Retrieval of the mrp2 gene encoded conjugate export pump from the canalicular membrane contributes to cholestasis induced by tert-butyl hydroperoxide and chloro-dinitrobenzene. Biol Chem. 2000;381:487–95. doi: 10.1515/BC.2000.063. [DOI] [PubMed] [Google Scholar]
- 26.Gotoh Y, Suzuki H, Kinoshita S, Hirohashi T, Kato Y, Sugiyama Y. Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J Pharmacol Exp Ther. 2000;292:433–9. [PubMed] [Google Scholar]
- 27.Yokooji T, Murakami T, Ogawa K, Yumoto R, Nagai J, Takano M. Modulation of intestinal transport of 2,4-dinitrophenyl-S-glutathione, a multidrug resistance-associated protein 2 substrate, by bilirubin treatment in rats. J Pharm Pharmacol. 2005;57:579–85. doi: 10.1211/0022357056019. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278:22644–9. doi: 10.1074/jbc.M212399200. [DOI] [PubMed] [Google Scholar]