Abstract
In addition to their primary role in hemostasis and wound healing, platelets play important roles in a multitude of physiological functions including immune and inflammatory responses. We present data that platelets, by virtue of their expression of the human specific FcγR, FcγRIIA, bind IgG complexes in vivo and that circulating phagocytes from healthy individuals internalize platelets in vivo. Human platelets, as a consequence of their expression of FcγRIIA, may thus contribute to the clearance of IgG-containing complexes from the circulation.
Keywords: platelets, immune-complexes, Fcγ receptor, phagocytosis, phagocytes, transgenic mice
Introduction
Although platelets are best known as the primary mediators of hemostasis in humans and other mammals, they also play important roles in a multitude of other physiological functions (Klinger and Jelkmann, 2002). Platelets are rapidly deployed to sites of injury or infection and release proteins that kill certain bacteria and fungi. Platelets also modulate inflammatory processes by interacting with leukocytes and by secreting cytokines, chemokines, and other inflammatory mediators (Elzey et al., 2005; Semple and Freedman, 2010). In addition, their function has been linked with various pathological conditions, including atherosclerosis, arthritis and immune thrombocytopenic purpura (ITP) (Semple and Freedman, 2010; Cines and Blanchette, 2002; Gasparyan, 2010). Our demonstration that human platelets can bind and endocytose IgG complexes in vitro (Worth et al., 2006) suggested that platelets also participate in the clearance of IgG-containing complexes.
Immune complexes (IC) are present in the circulation of healthy individuals and the formation of such complexes is part of a normal immune process. Efficient clearance of IgG complexes can be critical because their deposition in tissues and organs can set off reactions that lead to inflammation and tissue damage (Jancar and Sánchez Crespo, 2005; Mayadas et al., 2009). For example, in some pathological conditions, including autoimmune diseases such as systemic lupus erythematosus and autoimmune glomerulonephritis significant amounts of immune complexes are formed and deposited in the kidney and other tissues, causing severe injury (Niederer et al., 2010; Bagavant and Fu, 2009).
FcγRIIA is the only Fcγ receptor expressed on platelets (Cassel et al., 1993; King et al., 1990). On professional human phagocytes such as monocytes and neutrophils, FcγRIIA plays an important role in the clearance of IgG immune complexes and the phagocytosis of IgG coated particles (McKenzie and Schreiber, 1994). Apart from the recognition that FcγRIIA is important for platelet activation by von Willebrand factor (Canobbio et al., 2001), the role of FcγRIIA in platelet function has not been well defined.
It is known that platelets from patients suffering from certain autoimmune and thrombocytopenic disorders have high levels of surface bound IgG. Platelets from normal donors also bind IgG immune complexes in vivo, albeit at lower levels (Romero-Guzmán et al., 2000; George, 1990; Christopoulos et al., 1993; Court et al., 1987). We present evidence that platelets of normal individuals can serve as vehicles for delivery of immune complexes for destruction by phagocytes and that circulating neutrophils/ monocytes play a role in this process. We also demonstrate that in addition to the ability to ingest small IgG particulates (heat aggregated IgG, Worth et al., 2006), human platelets can internalize larger IgG coated particles by a process similar in some respects to phagocytosis in leukocytes.
Materials and Methods
Reagents
PE labeled F(ab’)2 fractions of goat anti-rat IgG, goat anti-human IgG, goat anti-mouse IgG and FITC labeled human IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Mouse anti-human CD61 was obtained from BD Biosciences, (San Jose, CA). Fab fractions of mAb anti-human FcγRII (IV.3) were prepared in our laboratory. Monomeric human IgG (mono-IgG) was prepared by ultracentrifugation of human IgG (MP Biomedicals, OH) at 80K for 15 min. Heat aggregated human IgG (HA-IgG) was prepared by heating human IgG (10mg/ml) in PBS at 62°C for 20 min, followed by centrifugation at 12,000 rpm for 10 min to remove insoluble aggregates. The final IgG complex was used at 100 μg/ml as assessed by absorbance at 280 nm.
Animals and cell lines
FcγRIIA transgenic mice were provided by Dr. Steven E. McKenzie (Thomas Jefferson University, Philadelphia, PA). All protocols were performed in accordance with National Institutes of Health guidelines and with the approval by the University of Pennsylvania Animal Use Committee. COS cells stably expressing FcγRIIA cell line (COSIIA) was constructed in our laboratory as previously described (Huang et al., 2004).
Isolation of human and mouse platelets
Platelet-rich plasma was prepared from heparinized venous blood of healthy volunteers by centrifugation of the blood at 900 rpm (175×g) at room temperature. Platelets were isolated by gel filtration on Sepharose 2B. A similar protocol was used to isolate platelets from WT and FcγRIIA TG mice.
Internalization of IgG coated beads
Streptavidin conjugated polystyrene beads (0.1, 0.5 and 1.0 μm) (Bangs Laboratories, Inc, Fishers, IN) were coated with biotin/anti-biotin mAb according to the manufacturer's instructions. The IgG-coated beads were allowed to bind (45 min) to platelets at 4°C. One set of cells was maintained at 4°C as a binding control. Other cells were warmed to 37°C for 20 min to allow internalization of bound IgG-coated beads and then returned to 4°C to stop internalization. Goat anti-mouse IgG conjugated to phycoerythrin (PE) was added to label remaining externally bound IC polystyrene beads and cell fluorescence was analyzed by flow cytometry. The cells maintained at 4°C retain a large amount of IC on the surface and display bright fluorescence. However, cells incubated at 37°C that have internalized some of the surface bound IgG-coated beads display less fluorescence. Internalization is defined as the % loss of surface expression (fluorescent label). For each condition, one set of cells was pre-incubated with cytochalesin D (CytD) and CytD was also present during the uptake assay.
Isolation of human monocytes and neutrophils
Heparinized venous blood collected from healthy volunteers was overlaid on Ficoll-Hypaque (LSM; Organon-Teknika, West Chester, PA) and centrifuged at 2200 rpm for 30 minutes. Pelleted granulocytes were cleared of residual erythrocytes by brief hypertonic lysis and resuspended in RPMI. Mononuclear cells from the interface were washed, suspended in RPMI medium (GIBCO, Grand Island, NY) plus 10% heat inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), and incubated at 37°C on serum-coated flasks for 45 minutes to adhere monocytes. Nonadherent cells were removed by washing with RPMI.
Internalization of human platelets by human monocytes and neutrophils: Analysis by flow cytometry
Gel filtered human platelets were incubated with HA-IgG for 30 min at 0°C. The IgG-coated platelets were washed and then incubated with human monocytes or neutrophils on ice for 45 min. One aliquot was maintained at 0°C for an additional 30 min for use as a binding control. The other aliquot was rapidly warmed and incubated at 37°C for 30 min to allow internalization of the HA-IgG platelet complexes. Both sets of cells were washed with ice-cold PBS and further incubated for 30 min on ice with 3.5 μg/ml phycoerythrin (PE) conjugated F(ab’)2 goat anti-human IgG to label surface bound HA-IgG platelet complexes. Cells were washed again with ice-cold PBS before analysis by flow cytometry. The mean fluorescence (MF) of cells maintained on ice throughout the procedure was used as the binding reference (no phagocytosis) (Van de Winkel and Capel, 1993). In other experiments, monocytes were exposed to platelets labeled with FITC-conjugated HA-IgG. Under these conditions, FITC fluorescence represents both surface-bound and intracellular HA-IgG platelet complexes.
Electron microscopy
Monocytes, isolated as described above, were washed and fixed with 2% gluteraldehyde at 4°C overnight. The samples were then washed extensively with cacodylate buffer, fixed with osmium tetroxide, dehydrated, embedded in Spurr's resin and sectioned (Worth, 2006). The sections were viewed at the EM core facility at University of Pennsylvania.
Results and Discussion
Human platelets from healthy donors bind IgG in vivo
The binding of IgG to the surface of normal human platelets in vivo is here illustrated using flow cytometry (Figure 1A). Gel filtered human platelets were treated with fluorescence-labeled antibody directed to human IgG [PE-labelled F(ab)’2 anti-human IgG]. The large increase in fluorescence intensity of these platelets compared to platelets treated with control antibody (PE labelled F(ab)’2 anti-rat IgG) or saline indicates the presence of surface bound human IgG.
Figure 1.
(A) Human platelets bind IgG in vivo. Gel-filtered human platelets were incubated with PE-labelled F(ab’)2 goat anti-human IgG-PE (grey line), PE-labelled F(ab’)2 goat anti-rabbit IgG (dashed line), or only saline (Solid black line). Binding of IgG complexes on the surface of platelets was detected by flow cytometry.
(B) Human platelets can bind heat aggregated (HA-IgG) in the presence of physiologic concentrations of monomeric IgG. Human platelets were incubated with monomeric IgG (10 mg/ml), mAb IV.3 (25 μg/ml) or saline before exposure to HA human IgG. IgG bound to the platelet surface was detected by flow cytometry using PE-tagged F(ab’)2 goat anti-human IgG.
(C). FcγRIIA binds aggregated HA-IgG but not monomeric IgG. COS cells transfected to stably express FcγRIIA were incubated with monomeric human IgG (dashed line), HA-IgG (grey line) or saline (solid black line). IgG bound to the platelet surface was detected by flow cytometry using PE-tagged F(ab’)2 goat anti-human IgG. Flow cytometry using PE-tagged F(ab’)2 goat anti-human IgG detected only aggregated IgG on the cell surface.
It is known that FcγRIIA, the only FcγR expressed on platelets, binds IgG complexes (McKenzie and Schreiber, 1994). Since platelet FcγRIIA encounters circulating monomeric IgG as well as IgG complexes in vivo, we examined whether platelet FcγRIIA can bind IgG complexes in the presence of physiologic levels of monomeric IgG (Figure 1B). The mean fluorescence intensity of platelets treated with monomeric IgG (10mg/ml) before exposure to heat-aggregated IgG (HA-IgG) is equivalent to that of platelets pretreated with saline, indicating that monomeric IgG does not interfere with the binding of HA-IgG complexes to platelet FcγRIIA. Figure 1B also demonstrates that incubation of the platelets with mAb IV.3 (which binds to the ligand binding site of FcγRIIA) before exposure to human HA-IgG diminishes fluorescence intensity, illustrating that access to FcγRIIA is required for IgG complex binding to platelets.
The inability of monomeric IgG to interfere with platelet FcγRIIA binding to IgG complexes is a consequence of the low affinity of FcγRIIA for monomeric IgG and its high affinity for complexed IgG (Van de Winkel and Capel, 1993). In Figure 1C, we illustrate that FcγRIIA binds only aggregated IgG and not monomeric IgG, using as model COS cells transfected to stably express FcγRIIA (COS IIA). Flow cytometry using PE-tagged F(ab’)2 goat anti-human IgG detected only aggregated IgG on the cell surface; i.e. there was a substantial increase in fluorescence intensity in cells treated with HA-IgG human IgG but the mean fluorescence intensity of cells treated with monomeric human IgG (10mg/ml) followed the same pattern as control cells treated with saline.
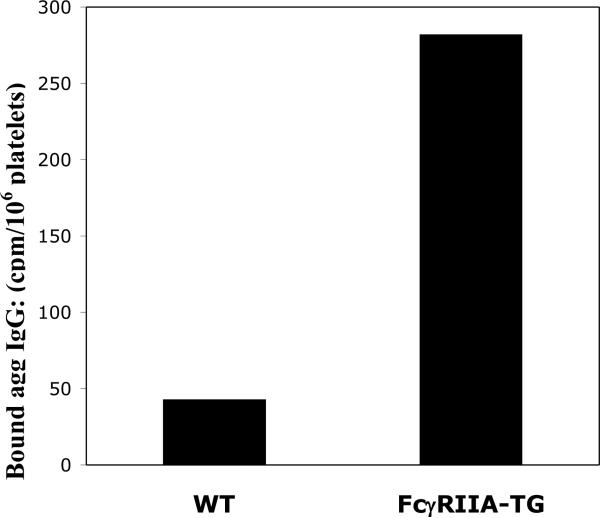
Because mice do not express FcγRIIA (Qiu et al., 1990; Hulett and Hogarth, 1994), the FcγRIIA transgenic mouse provides a useful tool for examination of FcγRIIA function (Taylor et al., 2000). When FcγRIIA TG mice and WT mice were injected via tail vein with 125I -labeled human HA-IgG (200 μl, 100μg/ml), the radioactivity associated with gel filtered platelets isolated from FcγRIIA TG mice was >5 fold greater than that from platelets isolated from WT mice (Figure 2). These data further illustrate that association of platelets with HA-IgG can occur in vivo and that it is dependent on the expression of FcγRIIA.
Figure 2.
Comparison of HA human IgG binding in vivo: Platelets from FcγRIIA TG mice vs wild type (WT) mice. 125I-labeled HA human IgG was injected via tail vein into WT and FcγRIIA TG mice. After 5 min, the animals were sacrificed and platelets isolated. The radioactivity associated with platelets was determined in gel-filtered platelets. Wild type (43 cpm/106 platelets); FcγRIIA TG mice (282 cpm/106 platelets).
Internalization of IgG coated particles by platelets
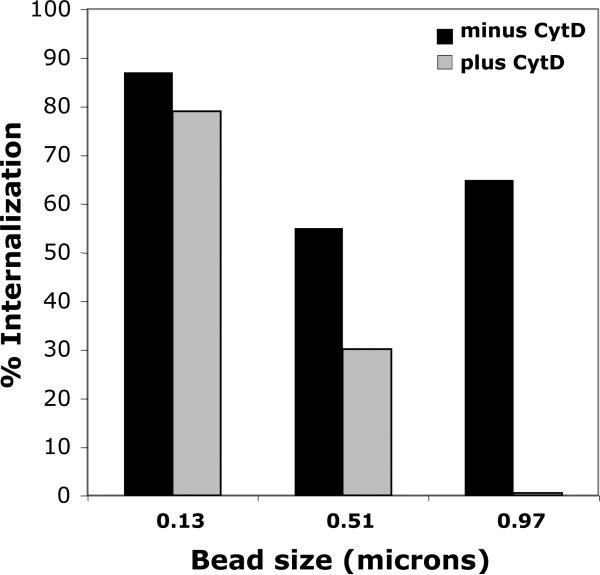
We next turned to examine mechanisms by which platelets could participate in immune complex clearance. In phagocytes, small immune complexes are internalized through the process of endocytosis (actin independent), and larger particulate immune complexes are internalized through the process of phagocytosis (actin dependant). We previously demonstrated that human platelets via FcγRIIA can endocytose small IgG complexes such as heat-aggregated IgG (Worth et al., 2006). Since platelets in vivo may be exposed to IgG immune complexes in the macromolecular size range (e.g. microorganisms in host defense and nucleoprotein complexes in autoimmune disorders), we examined platelet ingestion of IgG coated beads sized from 0.1 to ~ 1.0 μm (Figure 3). We observed that human platelets can readily internalize both small and large IgG-coated beads. After incubation at 37 °C for 20 min, platelet internalization of IgG coated 0.13 micron beads was 87%, and internalization of the IgG coated 0.97 micron beads was 65%.
Figure 3.
Platelets internalize both small and large IgG-coated beads. Streptavidin conjugated polystyrene beads coated with biotin/anti-biotin mAb were incubated with human platelets as described in Methods. Goat anti-mouse IgG conjugated to phycoerythrin (PE) was added to label remaining externally bound IC polystyrene beads and cell fluorescence was analyzed by flow cytometry.
In phagocytes, IgG-coated beads of 1.0 μm and larger trigger the actin cytoskeleton rearrangement required for phagocytosis (Koval et al., 1998) while smaller IgG-coated beads do not trigger actin cytoskeleton rearrangement, or minimally so. The internalization of IgG coated beads by human platelets follows a similar pattern. In the presence of cytochalasin D (10μM), an inhibitor of actin rearrangement and phagocytosis (Casella et al., 1981, May and Machesky, 2001), the internalization of IgG coated 0.97micron beads by human platelets was almost completely abolished, while the ingestion of IgG coated 0.13 micron beads was very minimally affected (Figure 3). Thus, as in professional phagocytes, the internalization of large IgG complexes by human platelets is dependant on actin rearrangement. However, unlike true phagocytes, neither killing nor digestion of microorganisms within platelets has been demonstrated (White, 2006). Nevertheless, the ability of platelets to engulf large IgG coated particulates may provide another mechanism for sequestering bacteria for clearance.
Platelet satellitism
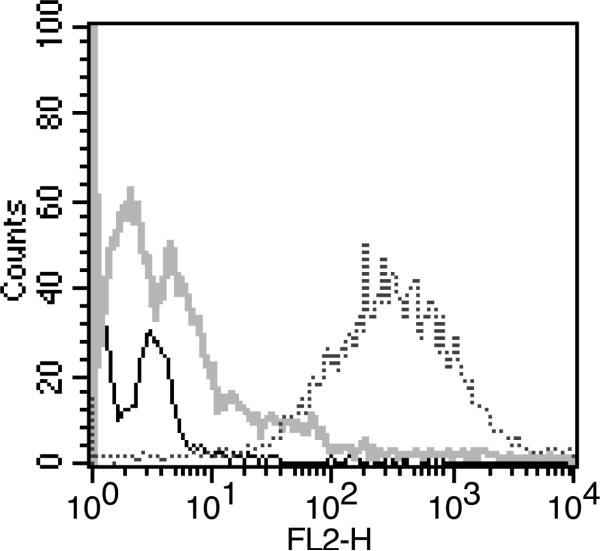
We also investigated the interaction of IgG coated platelets with phagocytes. In examining freshly isolated monocytes from healthy individuals by light microscopy, we noted that many of the monocytes were ringed by small particles whose size appeared consistent with that of platelets. To determine whether these particles were indeed monocyte-bound platelets, we exposed monocytes from normal donors to a monoclonal antibody directed against CD61, a platelet specific surface marker, and, using flow cytometry, analyzed for monocyte-associated CD61 in the cell population gated for monocytes. The large increase in fluorescence intensity for anti-CD61 antibody-treated cells compared to the isotype control and untreated cells (Figure 4) identifies the particles bound to the monocytes in vivo as platelets.
Figure 4.
Detection of platelet-monocyte complexes by flow cytometry. Freshly isolated human monocytes were treated with antibody targetted to CD61, a specific platelet surface marker (dotted line), isotype control IgG (grey line) or saline (solid line). The secondary antibody was PE-labeled goat anti-mouse IgG. The platelet surface marker CD61 was detected on the cell population gated for monocytes.
The rosetting/binding of platelets around leukocytes is termed platelet “satellitism” (Criswell et al., 2001). Platelet satellitism is considered an uncommon phenomenon and has been most often reported for neutrophils in blood that has been anti-coagulated with EDTA (Criswell et al., 2001; Bizzaro, 1991; Morselli et al, 1999). Some studies have suggested that it occurs more frequently in disease states, especially conditions involving thrombotic complications, but others have found no correlation between the presence of disease and platelet satellitism (Criswell et al., 2001). Our results indicate that platelet satellitism also occurs on monocytes isolated from heparinized blood of healthy individuals (Figure 4).
Phagocytosis of platelets by circulating leukocytes
The coating of platelets with IgG complexes renders the platelets susceptible to destruction by phagocytes. The ability of monocytes and neutrophils to phagocytose HA-IgG platelet complexes in vitro is illustrated in Figure 5A. In this experiment, monocytes or neutrophils were bound at 0°C to platelets that had been coated in vitro with HA-IgG. The temperature of one aliquot was then raised to 37°C, the temperature conducive to internalization of IgG complexes, while the other remained at 0°C, as a binding control. The remaining external platelets were then labeled with PE-conjugated F(ab’)2 goat anti-human IgG (see Methods). The cell surface fluorescence of monocytes and neutrophils in cells incubated with platelets at 37°C was greatly reduced relative to cells maintained at 0°C (Figure 5A), suggesting that internalization (phagocytosis) of the HA-IgG coated platelets had occurred.
Figure 5.
Determination of phagocytosis of HA-IgG platelet complexes by leukocytes in vitro. A. Gel filtered human platelets were treated with HA-IgG before incubation at 0°C or 37°C with freshly isolated monocytes or neutrophils (see Methods). PE-labelled goat anti-human IgG was used to label HA-IgG platelet complexes remaining on the surface of the leukocytes. Fluorescence was analyzed in cell populations gated for monocytes or neutrophils. The loss in surface bound HA-IgG platelet complexes at 37°C, as indicated by decreased mean fluorescence (MF) of the cells, suggests their internalization by the phagocytic cells. Using the formula (MF 0°C - MF 37°C)/MF 0°C x100, we estimated the % phagocytosis by neutrophils as 42% and phagocytosis by monocytes as 45%.
B. Monocytes were incubated with FITC-labeled HA-IgG platelet complexes in order to examine total cell associated labeling (i.e. both external and internalized HA-IgG platelet complexes) at 0°C and 37°C. The similarity of fluorescence at 37°C and 0°C suggests that the FITC-IgG platelet complexes are internalized by the monocytes and not shed from the surface.
To determine whether the decrease of HA-IgG platelet complexes on the surface of the phagocytes after incubation at 37°C is due to their internalization or to dissociation from the cell surface, we also examined the fluorescence of monocytes that had been incubated with platelets bound to FITC-labeled human HA-IgG (Figure 5B). The FITC-labeled human HA-IgG allows the detection of both internalized and external platelets on monocytes. Thus, the similarity of fluorescence associated with the monocytes at 37°C and 0°C indicates the maintenance of FITC-HA-IgG levels and strongly suggests that the loss of PE staining from the cell surface shown in Figure 5A is due to the internalization of the HA-IgG platelet complexes (Figure 5B). Similar results were observed for neutrophils (not shown).
The electron micrographs of Figure 6 demonstrate that monocytes from healthy individuals also bind and internalize human platelets in vivo. The phagocytosis of platelets by leukocytes in vivo has been generally associated with disease states (Gasparyan et al., 2010; Lee and Tripathy, 2000; Maugeri et al., 2009). For example, in ITP, anti-platelet IgG antibodies bound to the surface of platelets have been shown to promote the premature destruction of platelets by splenic macrophages (Gasparyan et al., 2010). Further, the internalization of platelets seen in freshly isolated leukocytes has been considered an in vitro phenomenon, related to the effect of anticoagulent EDTA on hidden determinants of platelet membrane glycoproteins (Bizzaro, 1991; White et al., 1978). Our micrographs demonstrate platelet internalization by monocytes from healthy individuals whose blood was anti-coagulated with heparin. Our data also illustrate that although splenic macrophages are considered the major site for destruction of IgG complexes and IgG-coated cells, including IgG-coated platelets, platelets are also internalized by circulating phagocytes in vivo.
Figure 6.
Representative electron micrographs demonstrating the binding and phagocytosis of platelets by monocytes from the same normal donor. Arrows indicate location of platelets. A and B. Binding of platelets to human monocytes. C and D. Phagocytosis of platelets by monocytes. E. Enlarged image of internalized human platelet from panel D. In B, C and D, in vivo binding and phagocytosis of platelets by monocytes is demonstrated using freshly isolated monocytes. In A, monocytes were incubated with platelets pre-coated with HA-IgG before processing for EM. Human monocytes and platelets were prepared from heparinized venous blood.
EDTA dependant platelet satellitism has been shown to be antibody-mediated (Pegels et al., 1982; von dem Borne et al., 1986) and in some cases platelet satellitism has been associated with the ability of leukocytes to phagocytose platelets (Bizzaro, 1991; White et al., 1978). The occurrence of platelet satellitism in our preparations of monocytes (Figure 4), and the observations that platelets from healthy individuals bind IgG complexes in vivo (Figure 1A), suggest that the platelets bound and internalized by the monocytes shown in Figure 6B-6D are platelets to which IgG complexes have been bound in vivo.
We have provided further evidence that human platelets can internalize both small and large IgG coated particles. We have also provided evidence that FcγRIIA, by facilitating the binding of IgG complexes by platelets, provides a mechanism whereby IgG complexes can be sequestered for delivery to and destruction by circulating phagocytes. We propose that because of their large numbers, platelets provide a powerful system for clearance of IgG complexes both in disease states and in healthy individuals.
Acknowledgements
Research supported by NIH research grant AI-22193.
Abbreviations
- HA-IgG
Heat aggregated IgG
- Mono-IgG
monomeric IgG
- MF
mean fluorescence
- TG
transgenic
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Current Opinion in Rheumatology. 2009;21:489–494. doi: 10.1097/BOR.0b013e32832efff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzaro N. Platelet satellitosis to polymorphonuclears: cytochemical, immunological, and ultrastructural characterization of eight cases. Am. J. Hematol. 1991;36:235–342. doi: 10.1002/ajh.2830360403. [DOI] [PubMed] [Google Scholar]
- Canobbio I, Bertoni A, Lova P, Paganini S, Hirsch E, Sinigaglia F, Balduini C, Torti M. Platelet activation by von Willebrand factor requires coordinated signaling through thromboxane A2 and Fc gamma IIA receptor. J. Biol. Chem. 2001;276:26022–26029. doi: 10.1074/jbc.M102639200. [DOI] [PubMed] [Google Scholar]
- Cassel DL, Keller MA, Surrey S, Schwartz E, Schreiber AD, Rappaport EF, McKenzie SE. Differential expression of FcgammaRIIA, FcgammaRIIB and FcgammaRIIC in hematopoietic cells: analysis of transcripts. Mol. Immunol. 1993;30:451–460. doi: 10.1016/0161-5890(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Casella JF, Flanagan MD, Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- Christopoulos CG, Kelsey HC, Machin SJ. A flow-cytometric approach to quantitative estimation of platelet surface immunoglobulin G. Vox Sang. 1993;64:106–115. doi: 10.1111/j.1423-0410.1993.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N. Engl. J. Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- Court WS, Bozeman JM, Soong SJ, Saleh MN, Shaw DR, LoBuglio AF. Platelet surface-bound IgG in patients with immune and nonimmune thrombocytopenia. Blood. 1987;69:278–283. [PubMed] [Google Scholar]
- Criswell KA, Breider MA, Bleavins MR. EDTA-dependent platelet phagocytosis. A cytochemical, ultrastructural, and functional characterization. Am J Clin Pathol. 2001;115:376–384. doi: 10.1309/MG6T-YQJQ-7C74-RE1V. [DOI] [PubMed] [Google Scholar]
- Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell. Immunol. 2005;238:1–9. doi: 10.1016/j.cellimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Douglas KM, Kitas GD. Platelet function in rheumatoid arthritis: arthritic and cardiovascular implications. Rheumatol Int. 2010 doi: 10.1007/s00296-010-1446-x. Epub Apr 14, 2010. [DOI] [PubMed] [Google Scholar]
- George JN. Platelet immunoglobulin G: its significance for the evaluation of thrombocytopenia and for understanding the origin of alpha-granule proteins. Blood. 1990;76:859–870. [PubMed] [Google Scholar]
- Huang ZY, Hunter S, Kim M-K, Chien P, Worth RG, Indik ZK, Schreiber AD. The monocyte Fcgamma receptors FcgammaRI/gamma and FcgammaRIIA differ in their interaction with Syk and with Src-related tyrosine kinases. J. Leukoc. Biol. 2004;7:491–499. doi: 10.1189/jlb.1103562. [DOI] [PubMed] [Google Scholar]
- Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv. Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- Jancar S, Sánchez Crespo M. Immune complex-mediated tissue injury: a multistep paradigm. Trends Immunol. 2005;26:48–55. doi: 10.1016/j.it.2004.11.007. [DOI] [PubMed] [Google Scholar]
- King M, McDermott P, Schreiber AD. Characterization of the Fcgamma receptor in human platelets Cell. Immunol. 1990;128:462–479. doi: 10.1016/0008-8749(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J. Interferon. Cytokine. Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Exp. Cell Res. 1998;242:265–273. doi: 10.1006/excr.1998.4110. [DOI] [PubMed] [Google Scholar]
- Lee JC, Tripathy K. Neutrophilic thrombophagocytosis. Arch Pathol Lab Med. 2000;124:1545–1546. doi: 10.5858/2000-124-1545-NT. [DOI] [PubMed] [Google Scholar]
- May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MTS, Piccoli A, Totani L, Cianflone D, Maseri A, Manfredi AA. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and β2 integrin–dependent cell clearance program. Blood. 2009;113:5254–5265. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex–mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie SE, Schreiber AD. Biological advances and clinical applications of Fc receptors for IgG. Curr. Opin. Hematol. 1994;1:45–52. [PubMed] [Google Scholar]
- Morselli M, Longo G, Bonacorsi G, Potenza L, Emilia G, Torelli G. Anticoagulant pseudothrombocytopenia with platelet satellitism. Haematologica. 1999;84:655. [PubMed] [Google Scholar]
- Niederer HA, Clatworthym MR, Willcocks LC, Smith KG. FcgammaRIIB, FcgammaRIIIB, and systemic lupus erythematosus. Ann N Y Acad Sci. 2010;1183:69–88. doi: 10.1111/j.1749-6632.2009.05132.x. [DOI] [PubMed] [Google Scholar]
- Pegels JG, Bruynes EC, Engelfriet CP, von dem Borne AE. Pseudothrombocytopenia: an immunologic study on platelet antibodies dependent on ethylene diamine tetra-acetate. Blood. 1982;59:157–161. [PubMed] [Google Scholar]
- Qiu WQ, de Bruin D, Brownstein BH, Pearse R, Ravetch JV. Organization of the human and mouse low-affinity Fc gamma R genes: duplication and recombination. Science. 1990;248:732–735. doi: 10.1126/science.2139735. [DOI] [PubMed] [Google Scholar]
- Romero-Guzmán LT, López-Karpovitch X, Paredes R, Barrales-Benitez O, Piedras J. Detection of platelet-associated immunoglobulins by flow cytometry for the diagnosis of immune thrombocytopenia: a prospective study and critical review. Haematologica. 2000;85:627–631. [PubMed] [Google Scholar]
- Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SM, Reilly MP, Schreiber AD, Chien P, Tuckosh JR, McKenzie SE. Thrombosis and shock induced by activating antiplatelet antibodies in human Fcgamma RIIA transgenic mice: the interplay among antibody, spleen, and Fc receptor. Blood. 2000;96:4254–4260. [PubMed] [Google Scholar]
- Van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity: Molecular aspects and clinical implications. Immunol. Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- von dem Borne AE, van der Lelie H, Vos JJ, van der Plas-van Dalen CM, Risseeuw-Bogaert NJ, Ticheler MD, Pegels HG. Antibodies against cryptantigens of platelets: characterization and significance for the serologist. Curr Stud Hematol Blood Transfus. 1986;52:33–46. [PubMed] [Google Scholar]
- White JG. Why human platelets fail to kill bacteria. Platelets. 2006;17:191–200. doi: 10.1080/09537100500441234. [DOI] [PubMed] [Google Scholar]
- Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp. Hematol. 2006;34:1490–1495. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]