Abstract
Sensory transduction in the cochlea depends on regulated ion secretion and absorption. Results of whole-organ experiments suggested that Reissner’s membrane may play a role in the control of luminal Cl−. We tested for the presence of Cl− transport pathways in isolated mouse Reissner’s membrane using whole-cell patch clamp recording and gene transcript analyses using RT-PCR. The current-voltage (I-V) relationship in the presence of symmetrical NMDG-Cl was strongly inward-rectifying at negative voltages, with a small outward current at positive voltages. The inward-rectifying component of the I-V curve had several properties similar to those of the ClC-2 Cl− channel. It was stimulated by extracellular acidity and inhibited by extracellular Cd2+, Zn2+, and intracellular ClC-2 antibody. Channel transcripts expressed include ClC-2, Slc26a7 and ClC-Ka, but not Cftr, ClC-1, ClCa1, ClCa2, ClCa3, ClCa4, Slc26a9, ClC-Kb, Best1, Best2, Best3 or the beta-subunit of ClC-K, barttin. ClC-2 is the only molecularly-identified channel present that is a strong inward rectifier. This study is the first report of conductive Cl− transport in epithelial cells of Reissner’s membrane and is consistent with an important role in endolymph anion homeostasis.
Keywords: Cl− channel, epithelial transport, cochlea
Introduction
The transduction of sound into neural activity depends on the creation and maintenance of a luminal fluid, endolymph, in the inner ear that is high in K+ concentration ([K+]) and low in both [Na+ ] and [Ca2+] [21]. However, there is little difference in [Cl−] (~120 to 130 mM) between endolymph and the basolateral fluid, perilymph, in spite of the large transepithelial endocochlear potential (EP) of +80 to +100 mV [21]. The EP and perilymphatic [Cl−] predict (via the Nernst equation) an extremely high endolymphatic [Cl−] of ~2600 mM based on simple passive electrochemical diffusion. Dysfunction of Cl− regulation would be expected to lead to large osmotic disturbances that would result in luminal volume changes and the consequent disruption of normal hearing. Gross volume changes have been associated with pathological states such as Meniere’s syndrome (swelling) and Schiebe’s deformity (shrinking).
On that basis, it has long been thought that some epithelial cells lining the cochlear duct may actively absorb Cl− from endolymph to maintain its [Cl−] near that of perilymph, and radiotracer experiments in the intact cochlea point to Reissner’s membrane as a mediator of Cl− transport [19]. Reissner’s membrane is an epithelial monolayer (with a discontinuous mesothelial layer on the basolateral side) that forms much of the boundary of the cochlear lumen. The present study was undertaken to resolve at the single cell level whether there are significant Cl− conductive pathways in Reissner’s membrane epithelial cells that could support its putative role in endolymph Cl− homeostasis.
Methods
Tissues were obtained for RNA isolation and for electrophysiology following protocols approved by the Institutional Animal Care and Use Committee of Kansas State University. as described earlier [17]. The compositions of the solutions for electrophysiological recordings were (in mM) pipette 150 NMDG-Cl, 1 MgCl2, 0.273 CaCl2, 1 EGTA, 10 Hepes, pH 7.3, ~300 mOsm, 100 nM free Ca2+ [29] and bath 150 NMDG-Cl, 1 MgCl2, 0.7 CaCl2, 10 Hepes, 5 glucose, pH 7.3, ~300 mOsm. All solutions for patch clamp were passed through 0.22 µm cellulose acetate filters (Corning). ClC-2 antibody against an intracellular domain was obtained from Alomone Labs. Other chemicals were purchased from Sigma Chemical Co. (St Louis, MO).
Currents were recorded using the whole-cell configuration of the patch clamp technique, similar to our previous study [3]. Patch pipettes were made from borosilicate glass capillaries (1B150F; World Precision Instruments, Sarasota, FL), pulled in three stages. Inner diameter of the tip was approximately 2 µm and after heat polishing the pipettes had resistances of 3.6 – 5.2 MΩ (n=46) in NMDG-Cl solutions.
Currents were recorded with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) and low-pass filtered at 1 kHz. Current signals were digitized at 5 kHz using a computer with a Digidata 1322A (Axon Instruments) and pCLAMP 9 software (clampex9, Axon Instruments). In addition, AxoScope software (Axon Instruments) with MiniDigi 1A (Axon Instruments) data acquisition hardware was simultaneously used for continuous trace recordings and current signals were digitized at 1 kHz. The temperature was maintained at 37°C on a glass-bottomed bath chamber by a continuous, warmed perfusion with supplemental chamber heater. Liquid junction potentials in symmetric NMDG-Cl were near zero. Voltage protocols were used as described in the figures. Data were plotted with Origin software, version 7 (OriginLab Software, Northampton, MA).
Real-time RT-PCR experiments were performed on total RNA using QuantiTect SYBR Green RT-PCR Kit (Qiagen) and an iQ5 Real-Time PCR Detection System (Bio-Rad). Primers were designed using Primer3 (http://frodo.wi.mit.edu/primer3) and produced by Integrated DNA Technologies (Table 1). Reverse transcription for 30 min at 50°C was followed by 15 min at 95°C and 40 PCR cycles. Each PCR cycle consisted of 94°C for 20 sec, 56°C for 30 sec, and 72°C for 30 sec and readings of fluorescence were made at 78 °C. PCR products were analyzed by Bioanalyzer, purified with a PCR purification kit (Qiagen) and sequenced to validate the identity of the RT-PCR products.
Table 1.
Primer sequences for RT-PCR and expression of gene transcripts.
| Gene | Sequence (5´-3´) | Product Size (bp) Exp. /Meas.a |
GenBank | P / Ab | |
|---|---|---|---|---|---|
| Slc26a7 | S | GGAAAAAGAGAAGCGTGCTG | 309 / 317 | NM_145947 | P |
| AS | AGGATGTCAAGGCAAAGGTG | ||||
| Slc26a9 | S | CCTGACTGCTGTCATCCAGA | 324/323 | NM_177243 | A |
| AS | GTAGGGATGGGGAAGTGGAT | ||||
| ClC-1 | S | CTGGGTCACCTTCCCACTTA | 292 / 284 | NM_013491 | A |
| AS | TGGCTGCTCATAGACACCAG | ||||
| ClC-2 | S | CTGGATGTCTGCACTGGCTA | 271 / 271 | NM_009900 | P |
| AS | AGGCAGAATGTGAGCGATCT | ||||
| ClC-Ka | S | ACTCCCAGAGCTGAAGACCA | 337 / 337 | NM_024412 | P |
| AS | CCAGACGGAGAAGTGAGAGG | ||||
| Barttin | S | CAGAGCCTCCCAGACTTCAC | 399 / 387 | NM_080458 | A |
| AS | TGTAGGGGTGTCGTCAATCA | ||||
| Best1 | S | TACAAGCGCTTTCCCACTCT | 366/376 | NM_011913 | A |
| AS | CATCTCATGGCCTGGGTAGT | ||||
| Best3 | S | GCTGCCGACTACTGCATACC | 368/362 | NM_001007583 | A |
| AS | GTCTCCCTGATGGTGGACAG | ||||
| ClCa1 | S | CTACAAGTGGCAGCGTCTCC | 367/358 | NM_009899 | A |
| AS | GCAGTAGCCAGGAGTGGTTC | ||||
S, sense primer; AS, antisense primer; Product Size, length in the base pairs (bp) of RT-PCR product including primers.
Expected/Measured product size;
Present/Absent.
Data were expressed as the mean ± S.E.M. (n=number of whole cell patches). Increases and decreases in current and conductance were determined by Student’s paired or unpaired t-test and correlation coefficients were calculated and tested for significance. Differences were considered statistically significant at a level of P < 0.05.
Results
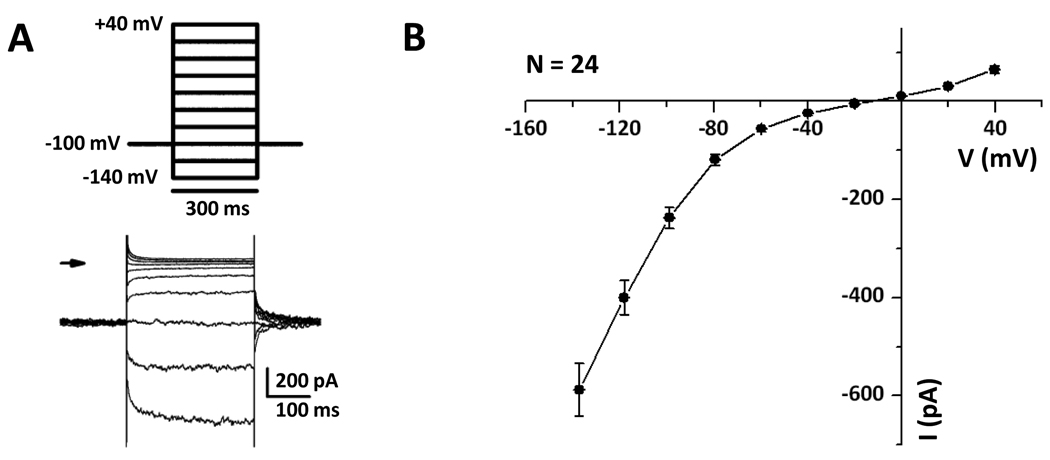
Whole cell patch clamp recordings from Reissner’s membrane epithelial cells were made under conditions where Cl− was the only major permeable ion. The Cl− currents were characterized by a) strongly inward-rectifying currents with slow activation at negative voltages and b) weakly outward-rectifying currents (Fig. 1). The prominent inward-rectifying currents were similar to those described for ClC-2 and were investigated further in more detail.
Figure 1. Strong inward-rectifier and smaller outward Cl− currents.
A, Step pulses were applied from −140 mV to +40 mV, returning to the holding voltage −100 mV and repeated every 10 s. B, The mean current voltage relationship was obtained from 24 cells.
We tested the effects of agents (external pH, Cd2+, Zn2+ and intracellular ClC-2 antibody) known to stimulate and inhibit ClC-2 Cl− channels on the Cl− currents in Reissner’s membrane epithelial cells (Figs. 2, 3, 4).
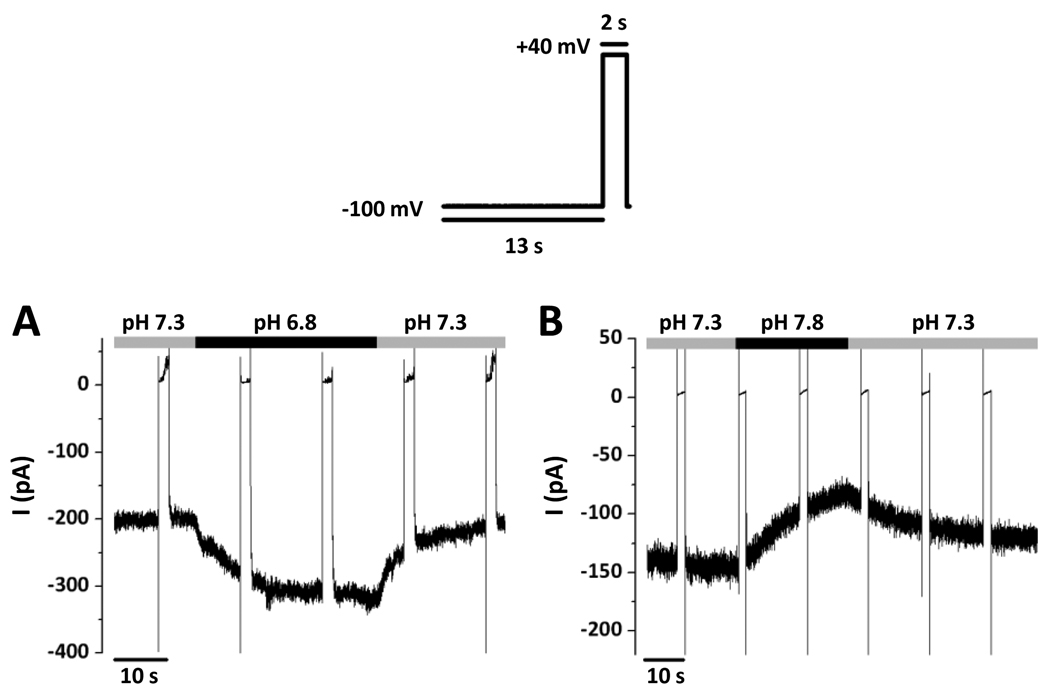
Figure 2. Dependence of inward-rectifier Cl− currents on pH.
The voltage protocol consisted of holding at −100 mV for 13 s with a 2 s pulse at +40 mV. All effects of pH were reversible. A, The activation of the current at −100 mV by external acidification from pH 7.3 to pH 6.8. B, The inhibition of the current at −100 mV by external alkalinization from pH 7.3 to pH 7.8.
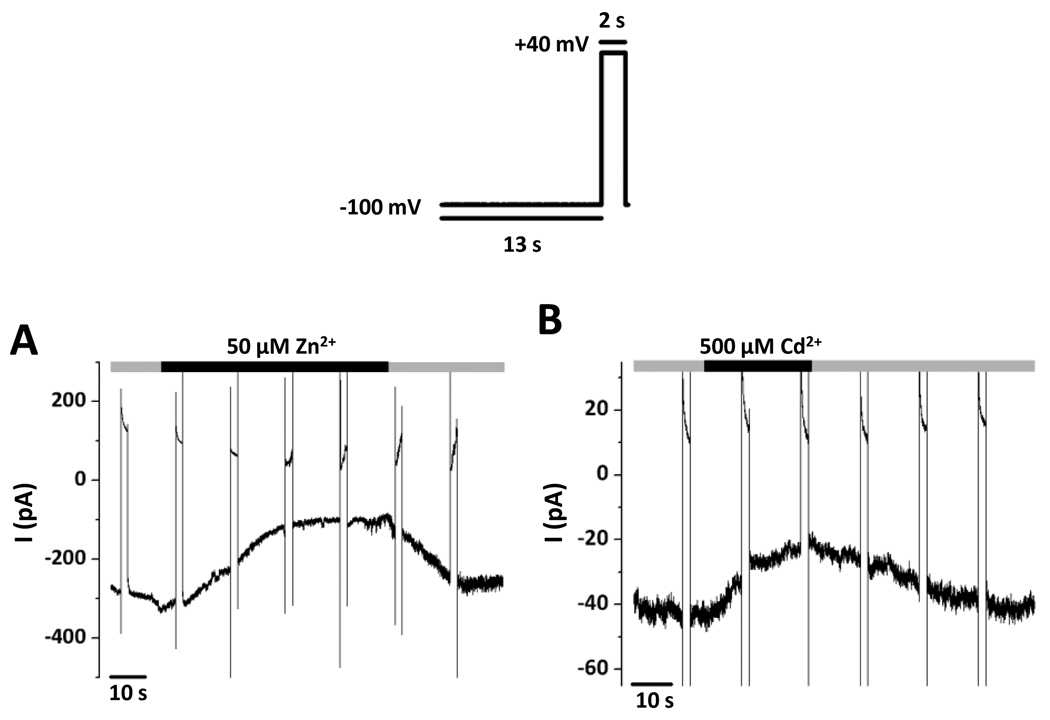
Figure 3. Dependence of inward-rectifier Cl− currents on inhibition by Zn2+ and Cd2+.
Representative recordings; voltage protocol as in Figure 2. All effects of Zn2+ and Cd2+ were reversible. A, The inhibition of the current at −100 mV by 50 µM Zn2+. B, The inhibition of the current at −100 mV by 500 µM Cd2+.
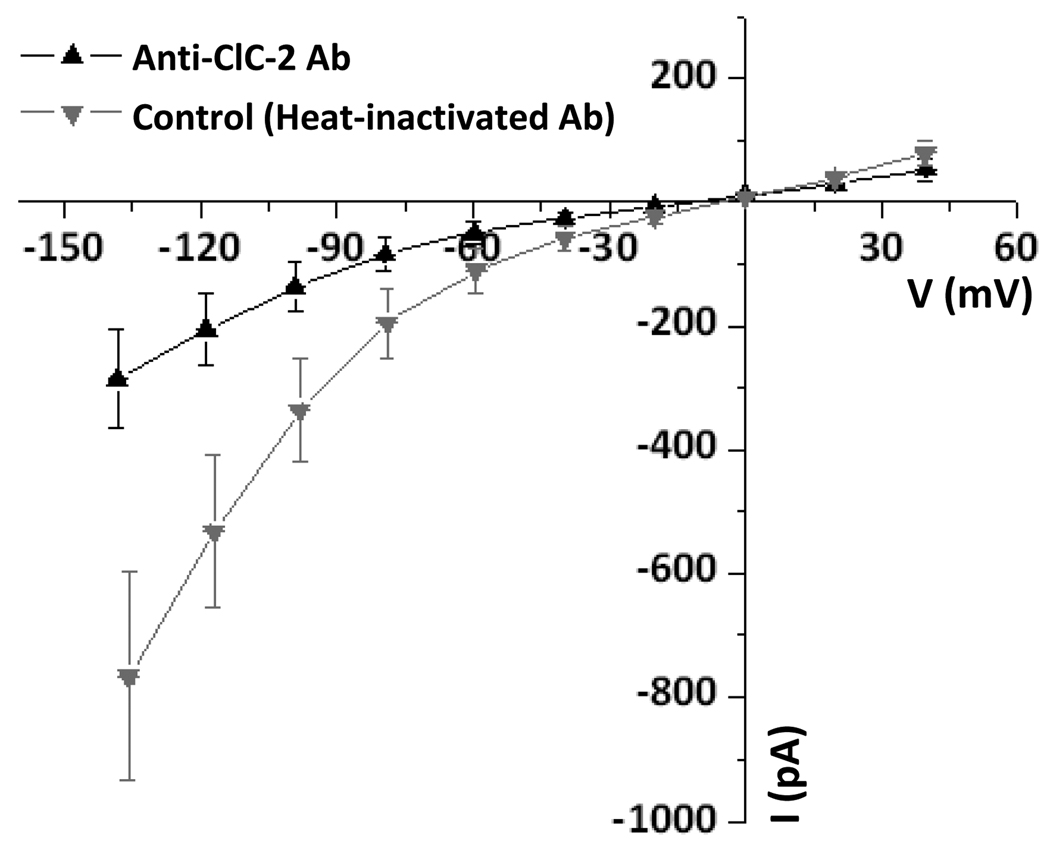
Figure 4. Inhibition of inward-rectifier Cl− currents by ClC-2 antibody.
Summary I-V relationships; voltage protocol as in Figure 1. Currents recorded with antibody (3 µg/ml) raised against an intracellular epitope of ClC-2 added to the pipette solution (Anti-ClC-2 Ab; up triangles) were significantly reduced at negative membrane voltages compared to those serving as “Control” with heat-inactivated antibody (down triangles).
Acidifying the bath pH from 7.3 to 6.8 caused a reversible increase in I−100 by 79.4 ± 11.1 % (from −104 ± 26 pA to −181 ± 37 pA, n=5) (Fig. 2A). By contrast, alkalinizing the bath pH from 7.3 to 7.8 caused a reversible decrease in I−100 by 37.9 ± 3.7 % (from −106 ± 37 pA to −69 ± 28 pA, n=4) (Fig. 2B). These pH changes are in the monophasic pH response region of inward-rectifier Cl− channels in mouse parotid acinar cells [1].
Similar experiments were performed with Zn2+ (Fig. 3A) and Cd2+ (Fig. 3B) at concentrations known to inhibit ClC-2 channels [12;36]. I−100 was reversibly decreased by 50 µM Zn2+ by 45.6 ± 7.5 % (from −248 ± 24 pA to −132 ± 15 pA, n=4) and by 500 µM Cd2+ by 45.3 ± 7.1 % (from −138 ± 28 pA to −79 ± 23 pA, n=5).
Antibodies against intracellular epitopes of ClC-2 have been reported to block inward-rectifier Cl− currents in native cells [9;26]. Intracellular ClC-2 antibody (3 µg/ml) [9] significantly reduced the conductance at −120 mV from 11.5 ± 2.5 nS (control with heat-inactivated antibody) to 3.8 ± 1.1 nS, n=5 (Fig. 4).
Candidate anion channel genes were determined by their presence call in our gene array database (GEO accession number GSE6196 [17]), compared to expression levels in the neighboring tissue, stria vascularis (GSE4749 [11]). Genes related to Na+ absorption and its regulation in Reissner’s membrane were previously reported [17]. Several Cl− channels were found to be present (Table S1). ClCa1 was called ‘present’ by the gene array, but the signal strength was near the background level of the chips or less.
On the basis of those results, RT-PCR experiments were conducted to validate the presence or absence of selected genes (Table 1, Fig. S1). Cl− channels that were found to be expressed and are known to be located in the plasma membrane were ClC-2, Slc26a7 and ClC-Ka. Interestingly, the beta-subunit of ClC-K (barttin) was not expressed in Reissner’s membrane. Cl− channels that were absent include Cftr, ClC-1, ClCa1, ClCa2, ClCa3, ClCa4, Slc26a9, ClC-Kb, Best1, Best2, Best3.
These results are not specific to the epithelial cells since Reissner’s membrane also consists of a discontinuous subepithelial layer of mesothelial cells. Whole cell currents, however, originated solely from the epithelial cells.
DISCUSSION
The contribution of Cl− transporters to the support of auditory and vestibular neural processes has recently been reviewed [21]. However, the present paper is the first report of a significant involvement of conductive Cl− pathways in Reissner’s membrane epithelium. We identified by means of gene array, RT-PCR and electrophysiology several channels that carry Cl−. The molecular identities of the channels that carry the observed currents were not unambiguously determined, but candidate genes were identified.
The voltage-dependence of the current under symmetrical Cl− conditions has some similarities to channels reported in the literature. The strong inward rectification has been observed in expression systems and native cells. The strongest candidate for a molecularly-identified, inward-rectifier Cl− channel in Reissner’s membrane is ClC-2, although many Cl− channels have been functionally demonstrated whose molecular identity remains unknown [12;36]. Few plasma membrane Cl− channels are known to be inward-rectifying [12] and of those that are molecularly identified, the only candidate channel transcript in Reissner’s membrane was ClC-2.
ClC-2 has an established electrophysiological and pharmacological fingerprint [13;31]. Salient features include whole-cell currents that a) are slowly activated by negative voltages; b) sensitive to extracellular pH (activated by acid); c) inhibited by Cd 2+ and Zn2+ [4;27;36]; d) inhibited by antibodies directed against intracellular epitopes of the ClC-2 channel [9;26]. All of these characteristics were observed for currents in Reissner’s membrane epithelial cells and transcripts for ClC-2 were present in the tissue.
ClC-2 was earlier said to be ‘broadly’ or ‘ubiquitously expressed’, although many studies have since shown a more specific distribution [12]. The view of cell-specific distribution is supported by our finding in the cochlea that Reissner’s membrane expresses over 3 times as much transcript for ClC-2 as the neighboring tissue, the stria vascularis (Table S1). The stria is composed of numerous types of cells, including surface epithelial cells, intermediate cells of neural crest origin, basal cells, capillary endothelial cells and pericytes.
Nonetheless, Cl− currents have been found in mouse choroid plexus epithelial cells that have many of the characteristics of ClC-2 but also display some differences, such as dependence on intracellular ATP [14]; in fact, it was found that those currents persisted in ClC-2 knockout mice, pointing to an unidentified channel with characteristics that overlap those of ClC-2 [30]. The lack of an antibody with convincing specificity for ClC-2 in fixed tissues [35] precluded localization of the protein to the apical or basolateral membrane in Reissner’s membrane, although the effective inhibition of the inward current by ClC-2 antibody supports a similar epitope on the underlying channel or an associated protein.
Cellular functions ascribed to ClC-2 include Cl− absorption in the colon, volume activation, volume inhibition, regulation of cardiac pacemaker activity and maintaining Cl− homeostasis in rat rod bipolar cells of the retina [2;4;9;25], but the physiological function in mouse salivary gland epithelium is unknown [27]. The inward rectifier may participate in transepithelial Cl− transport across Reissner’s membrane, but a possible alternative or additional function includes regulation of cell volume [8].
Transcripts of additional Cl− channels identified by gene array and/or RT-PCR in Reissner’s membrane are Slc26a7 and ClC-Ka. Slc26a7 is a Cl− channel with nearly linear I-V relationship [16] that remains a candidate for the channel mediating the outward current in Reissner’s membrane. Studies of inward-rectifier currents in other native cells (e.g., rat parotid acinar cells and both rat and mouse choroid plexus epithelial cells) have also noted an additional minor outward current [14;15;24], even though heterologously expressed ClC-2 and the inward rectifier conductance of rat neocortical cultured astrocytes are nearly perfect inward-rectifiers [6;24]. ClC-K alpha-subunits require the presence of the beta-subunit, barttin, in order to be functional channels [5]. Barttin, however, was found to be absent by RT-PCR, suggesting that ClC-Ka does not form a functional channel in Reissner’s membrane.
The Ca2+-activated Cl− channels (ClCa isoforms), bestrophin isoforms and Tmem16a were either absent or had weak gene array signal strength (Table S1). Clns1a is a putative Cl− channel that was present at very low signal strength in the gene array. However, the protein is ubiquitously expressed and has been reported to have diverse functions that make it essential for cell viability, making it impossible to unambiguously determine whether it is indeed a Cl− channel [7].
Previous reports of ion transport by Reissner’s membrane epithelium have focused predominantly on cation transport. Observations include demonstrations of electrogenic transepithelial absorption of Na+ from endolymph via Na+-permeable, amiloride-sensitive channels in the apical membrane [17;20]. Na+/K+-ATPase in the basolateral membrane and Ca2+-ATPase in the apical membrane [10;34] were found by histochemistry. Several patch-clamp studies have demonstrated the presence of ATP-gated cation channels [18], stretch and voltage-sensitive nonselective cation channels and potassium channels in the apical membrane [32;33]. Single-channel recordings of voltage-sensitive chloride channels were obtained from the apical membrane [33], but these channels had the opposite voltage sensitivity to those reported here and therefore may not play a significant role under physiologic conditions.
Conclusion
In summary, we have identified a complex Cl− current in Reissner’s membrane epithelial cells that may be carried by multiple transport proteins. Cl− is known to play a critical role in sensory outer hair cell tuning and amplification through its involvement with the motor protein, prestin [22;23;28], although the influence of luminal (endolymphatic) [Cl−] is not known. Our findings support a possible role of Reissner’s membrane in Cl− homeostasis of endolymph in the support of hearing. Dysfunctions of Cl− transport may contribute to pathological states such as Meniere’s syndrome and Schiebe’s deformity.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants R01-DC-00212 and P20-RR-017686. We thank Dr. Philine Wangemann for advice and helpful discussions. The advice and assistance of Donald Harbidge, Joel Sanneman, Dr. Hiromitsu Miyazaki, Dr. Takayuki Kudo, Dr. Hyoung-mi Kim, and Dr. Kalidou Ndaiye are greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Arreola J, Begenisich T, Melvin JE. Conformation-dependent regulation of inward rectifier chloride channel gating by extracellular protons. J.Physiol.(Lond.) 2002;541:103–112. doi: 10.1113/jphysiol.2002.016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology. 2004;126:1104–1114. doi: 10.1053/j.gastro.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Chiba T, Marcus DC. Basolateral K+ conductance establishes driving force for cation absorption by outer sulcus epithelial cells. J.Membr.Biol. 2001;184:101–112. doi: 10.1007/s00232-001-0079-0. [DOI] [PubMed] [Google Scholar]
- 4.Enz R, Ross BJ, Cutting GR. Expression of the voltage-gated chloride channel ClC-2 in rod bipolar cells of the rat retina. J.Neurosci. 1999;19:9841–9847. doi: 10.1523/JNEUROSCI.19-22-09841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ. Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 6.Ferroni S, Marchini C, Nobile M, Rapisarda C. Characterization of an inwardly rectifying chloride conductance expressed by cultured rat cortical astrocytes. Glia. 1997;21:217–227. doi: 10.1002/(sici)1098-1136(199710)21:2<217::aid-glia5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Furst J, Botta G, Saino S, Dopinto S, Gandini R, Dossena S, Vezzoli V, Rodighiero S, Bazzini C, Garavaglia ML, Meyer G, Jakab M, Ritter M, Wappl-Kornherr E, Paulmichl M. The ICln interactome. Acta Physiol.(Oxf) 2006;187:43–49. doi: 10.1111/j.1748-1716.2006.01549.x. [DOI] [PubMed] [Google Scholar]
- 8.Gründer S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- 9.Huang ZM, Prasad C, Britton FC, Ye LL, Hatton WJ, Duan D. Functional role of CLC-2 chloride inward rectifier channels in cardiac sinoatrial nodal pacemaker cells. J.Mol.Cell.Cardiol. 2009;47:121–132. doi: 10.1016/j.yjmcc.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwano T, Yamamoto A, Omori K, Akayama M, Kumazawa T, Tashiro Y. Quantitative immunocytochemical localization of Na+,K+-ATPase alpha-subunit in the lateral wall of rat cochlear duct. J.Histochem.Cytochem. 1989;37:353–363. doi: 10.1177/37.3.2537354. [DOI] [PubMed] [Google Scholar]
- 11.Jabba SV, Oelke A, Singh R, Maganti RJ, Fleming SD, Wall SM, Green ED, Wangemann P. Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC Med. 2006;4:37. doi: 10.1186/1741-7015-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol.Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 13.Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kibble JD, Garner C, Colledge WH, Brown S, Kajita H, Evans M, Brown PD. Whole cell Cl− conductances in mouse choroid plexus epithelial cells do not require CFTR expression. Am.J.Physiol. 1997;272:C1899–C1907. doi: 10.1152/ajpcell.1997.272.6.C1899. [DOI] [PubMed] [Google Scholar]
- 15.Kibble JD, Trezise AE, Brown PD. Properties of the cAMP-activated Cl− current in choroid plexus epithelial cells isolated from the rat. J.Physiol.(Lond.) 1996;496(Pt 1):69–80. doi: 10.1113/jphysiol.1996.sp021666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J.Biol.Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Kim KX, Raveendran NN, Wu T, Pondugula SR, Marcus DC. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner's membrane epithelium. Am.J.Physiol., Cell Physiol. 2009;296:C544–C557. doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King M, Housley GD, Raybould NP, Greenwood D, Salih SG. Expression of ATP-gated ion channels by Reissner's membrane epithelial cells. Neuroreport. 1998;9:2467–2474. doi: 10.1097/00001756-199808030-00008. [DOI] [PubMed] [Google Scholar]
- 19.Konishi T, Hamrick PE. Ion transport in the cochlea of guinea pig. II. Chloride transport. Acta Otolaryngol. 1978;86:176–184. doi: 10.3109/00016487809124734. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Marcus DC. Endolymphatic sodium homeostasis by Reissner's membrane. Neuroscience. 2003;119:3–8. doi: 10.1016/s0306-4522(03)00104-0. [DOI] [PubMed] [Google Scholar]
- 21.Marcus DC, Wangemann P. Cochlear and Vestibular Function and Dysfunction. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System--From molecules to diseases. Elsevier; 2009. pp. 421–433. [Google Scholar]
- 22.Muallem D, Ashmore J. An anion antiporter model of prestin, the outer hair cell motor protein. Biophys.J. 2006;90:4035–4045. doi: 10.1529/biophysj.105.073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver D, He DZ, Klocker N, Ludw J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- 24.Park K, Arreola J, Begenisich T, Melvin JE. Comparison of voltage-activated Cl− channels in rat parotid acinar cells with ClC-2 in a mammalian expression system. J.Membr.Biol. 1998;163:87–95. doi: 10.1007/s002329900373. [DOI] [PubMed] [Google Scholar]
- 25.Park K, Begenisich T, Melvin JE. Protein kinase A activation phosphorylates the rat ClC-2 Cl− channel but does not change activity. J.Membr.Biol. 2001;182:31–37. doi: 10.1007/s00232-001-0026-0. [DOI] [PubMed] [Google Scholar]
- 26.Roman RM, Smith RL, Feranchak AP, Clayton GH, Doctor RB, Fitz JG. ClC-2 chloride channels contribute to HTC cell volume homeostasis. Am.J.Physiol.Gastrointest.Liver Physiol. 2001;280:G344–G353. doi: 10.1152/ajpgi.2001.280.3.G344. [DOI] [PubMed] [Google Scholar]
- 27.Romanenko VG, Nakamoto T, Catalan MA, Gonzalez-Begne M, Schwartz GJ, Jaramillo Y, Sepulveda FV, Figueroa CD, Melvin JE. Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am.J.Physiol.Gastrointest.Liver Physiol. 2008;295:G1058–G1067. doi: 10.1152/ajpgi.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rybalchenko V, Santos-Sacchi J. Cl− flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J.Physiol.(Lond.) 2003;547:873–891. doi: 10.1113/jphysiol.2002.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. BioTechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- 30.Speake T, Kajita H, Smith CP, Brown PD. Inward-rectifying anion channels are expressed in the epithelial cells of choroid plexus isolated from ClC-2 'knock-out' mice. J.Physiol.(Lond.) 2002;539:385–390. doi: 10.1113/jphysiol.2001.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 32.Yeh TH, Herman P, Tsai MC, Tran Ba Huy P, Van den Abbeele T. A cationic nonselective stretch-activated channel in the Reissner's membrane of the guinea pig cochlea. Am.J.Physiol. 1998;274:C566–C576. doi: 10.1152/ajpcell.1998.274.3.C566. [DOI] [PubMed] [Google Scholar]
- 33.Yeh TH, Tsai MC, Lee SY, Hsu MM, Tran Ba Huy P. Stretch-activated nonselective cation, Cl− and K+ channels in apical membrane of epithelial cells of Reissner's membrane. Hear.Res. 1997;109:1–10. doi: 10.1016/s0378-5955(97)00030-0. [DOI] [PubMed] [Google Scholar]
- 34.Yoshihara T, Igarashi M. Cytochemical localization of Ca++-ATPase activity in the lateral cochlear wall of the guinea pig. Arch.Otorhinolaryngol. 1987;243:395–400. doi: 10.1007/BF00464650. [DOI] [PubMed] [Google Scholar]
- 35.Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, Jentsch TJ. Additional disruption of the ClC-2 Cl− channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J.Biol.Chem. 2004;279:22276–22283. doi: 10.1074/jbc.M309899200. [DOI] [PubMed] [Google Scholar]
- 36.Zifarelli G, Pusch M. CLC chloride channels and transporters: a biophysical and physiological perspective. Rev.Physiol.Biochem.Pharmacol. 2007;158:23–76. doi: 10.1007/112_2006_0605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.