Abstract
Necrotizing enterocolitis (NEC) is a devastating gastrointestinal disease predominantly of prematurely born infants, characterized in its severest from by extensive hemorrhagic inflammatory necrosis of the distal ileum and proximal colon. Proinflammatory cytokines have been implicated in the development of NEC, and we have previously shown that IL-18 is significantly elevated in the well-established neonatal rat model of NEC. To determine whether IL-18 contributes to intestinal pathology in NEC, we subjected IL-18 knockout mice to the protocol used to develop experimental NEC in newborn rats. Newborn B6.129P2-Il18tm1Aki/J (NEC IL-18−/−) and wild-type (NEC WT) mice were hand fed every 3 h with cow’s milk-based formula and exposed to asphyxia and cold stress twice daily. After 72 h, animals were killed and distal ileum and liver were removed. Disease development was determined via histological changes in the ileum as scored by a blinded evaluator. The number of TNF-α-, IL-12-, and IL-1β-positive cells and macrophages were determined in both ileum and liver via immunohistology. IκB-α; and IκB-β were determined from protein extracts from both ileum and liver using Western blot analysis. The incidence and severity of NEC was significantly reduced in NEC IL-18−/− mice compared with NEC WT. Furthermore, mean ileal macrophages and hepatic IL-1β were significantly reduced in IL-18−/− mice subjected to the NEC protocol. There were no statistically significant changes in Kupffer cells, hepatic TNF-α, ileal IL-1β, or IL-12. IκB-α; and IκB-β were significantly increased in NEC IL-18−/− mice ileum and liver, respectively. These results confirm that IL-18 plays a crucial role in experimental NEC pathogenesis.
Keywords: interleukin-18, inflammation
Necrotizing Enterocolitis (NEC) is a devastating gastrointestinal disease, predominantly of prematurely born infants, characterized in its severest from by extensive hemorrhagic inflammatory necrosis of the distal ileum and proximal colon. NEC affects thousands of newborns in the U.S. every year, with death occurring in 10–50% of affected individuals (27, 32). Survivors of a severe occurrence frequently suffer the effects of short bowel syndrome, resulting in prolonged medical expenses and chronic gastrointestinal difficulties (24, 25). As more premature infants are born in this country each year (22), the effects of this disease are likely to contribute to greater morbidity and mortality. The pathophysiology of this disease remains poorly understood; however, prematurity, enteral feeding, intestinal hypoxia-ischemia, and bacterial colonization are considered major risk factors (4, 20, 39). A better understanding of the mechanisms involved in the development of NEC and elucidation of early pathological changes in tissues at the molecular level will help to improve therapy and could lead to prevention of this disease.
The major risk factors for NEC likely promote an inflammatory cascade that results in the pathology associated with this disease. Of the many cytokines that play important roles in inflammation, proinflammatory IL-18 has been implicated in inflammatory diseases of the small intestine (23, 30, 34, 43). IL-18 is capable of inducing interferon-γ (IFN-γ) alone, and together with IL-12, it can work synergistically to induce greater quantities of IFN-γ. IL-18 is also capable of promoting an inflammatory cascade by enhancing the release of proinflammatory TNF-α. TNF-α, in turn, can stimulate production of IL-12. IL-18, IL-12, and TNF-α can also activate NF-κB, which initiates transcription of a variety of proinflammatory genes.
We have previously shown that IL-18 is significantly increased in the ileum (16) and liver (15) of neonatal rats with NEC. Furthermore, there is a positive correlation between production of IL-18 and the progression of ileal damage during disease development (16). To determine whether IL-18 contributes to intestinal pathology in experimental NEC, we subjected IL-18−/− mice to the protocol currently utilized in our laboratory and others to develop NEC in neonatal rats: enteral feeding of newborn, never-suckled pups with cow’s milk-based formula coupled with twice daily exposure to asphyxia and cold stress (9, 12, 13, 15–17). We evaluated the incidence and severity of disease as well as specific parameters known to be altered in experimental NEC: proinflammatory cytokine production, infiltration of macrophages, and IκB-α and IκB-β in the ileum and liver.
METHODS
Animal model
This protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801-95081). Neonatal B6.129P2-Il18tm1Aki/J (IL-18−/−) and C57BL/6J wild-type (WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Homozygous breeder pairs were utilized; IL-18−/− pups originating from 12 separate litters and WT mice originating from 10 separate litters were utilized in 3 different experiments. Newborn IL-18−/− (NEC IL-18−/−) and WT (NEC WT) mice were collected immediately after birth to prevent suckling of maternal milk. All pups were hand fed 50 µl of rat milk substitute every 3 h for 3 days using the Hoshiba nipple, Yajima style (19, 44) developed and manufactured by Meiji Dairies (Tokyo, Japan). With the use of this device (Fig. 1), newborn mouse pups could be fed measured amounts of diet without potential physical damage inherent in the gavage method typically utilized in the much larger neonatal rat. All pups were stressed twice daily with asphyxia (breathing 100% nitrogen gas for 60 s) followed by cold (4°C for 5 min). Three-day-old dam-fed wild-type (DF WT; n = 10) and dam-fed IL-18−/− (DF IL-18−/−; n = 15) pups were used for comparison in all evaluations.
Fig. 1.
Feeding method for neonatal mouse pups. A 1-day-old C57BL/6J pup is hand fed formula using the Hoshiba nipple Yajima-style system.
NEC evaluation
Pathological changes in intestinal architecture were evaluated using the NEC scoring system developed for use in neonatal rats. From each animal, 0.5-cm sections of tissue from the distal ileum and proximal and proximal colon were fixed overnight in 70% ethanol, paraffin embedded, microtome sectioned at 4–6 µm, and stained with hematoxylin and eosin for histological evaluation. Histological changes in the ileum were scored by a blinded evaluator on a scale of 0 (normal) to 4 (necrosis), using our previously published ileal damage scoring scale in neonatal rats (12–17).
Immunohistology
Ethanol-fixed, paraffin-embedded liver and ileum were serially sectioned at 4–6 µm. After deparaffinizing, sections were blocked with 1.5% appropriate serum (Vector Laboratories, Burlingame, CA) and then incubated with anti-mouse TNF-α (R&D Systems, Minneapolis, MN), anti-mouse macrophage (clone BM8; Biosource), or anti-mouse IL-1β, followed by biotinylated secondary antibody (Vector Laboratories), Vectastain Elite ABC reagent (Vector Laboratories), and diaminobenzidine (DAB), and counterstained with hematoxylin. No immunostaining was observed in the immunostaining controls. Sections from both experimental groups were stained for a specific primary antibody at the same time so that comparisons between groups could be assessed. Stained slides were evaluated by a blinded observer, and enumeration of positively stained cells was accomplished by counting ten ×40 microscopic fields.
Western blot analysis
Individual frozen ileum or liver samples were homogenized with a hand-held homogenizer (Pellet Pestle; Kimble/Kontes, Vineland, NJ) in a 5× volume of ice-cold homogenization buffer (50 mM Tris•HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Na-deoxycholic acid, 1% Triton X-100, 50 mM DTT, 50 µg/ml aprotinin, 50 µg/ml leupeptin, and 5 mM PMSF). The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected. Total protein concentration was quantified using the Bradford protein assay. For protein analysis, 50 g of protein were added to an equal volume of 2× Laemmli sample buffer and boiled for 5 min. The samples were run on a 10–20% gradient polyacrylamide gel (Bio-Rad, Hercules, CA) at 95 V for 1 h. Protein was transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad) at 15 V for 1 h. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma, St. Louis, MO) for 1 h at room temperature and then incubated with anti-IκB-α or IκB-β antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After being washed, the membranes were incubated for 1 h at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibody. Proteins were visualized with a chemiluminescence system (Pierce, Rockford, IL) and exposed to X-ray film. Membranes were stripped and probed with anti-β-actin to determine equal loading of protein. Densitometry was performed to compare protein expression between groups with Bio-Rad QuantityOne software. IκB-α; and IκB-β optical density (OD) levels from each sample were normalized to the OD of the corresponding β-actin sample.
Statistics
Statistical analyses between groups were performed using ANOVA followed by Fisher’s protected least significant difference. Analysis of NEC score was accomplished using the Mann-Whitney test for nonparametric values, and the χ2 test was used to analyze differences in incidence of disease. All statistical analyses were determined using the statistical program StatView (Abacus Concepts, Berkeley, CA). All numerical data are expressed as means ± SE.
RESULTS
Incidence and severity of NEC in IL-18−/− mice
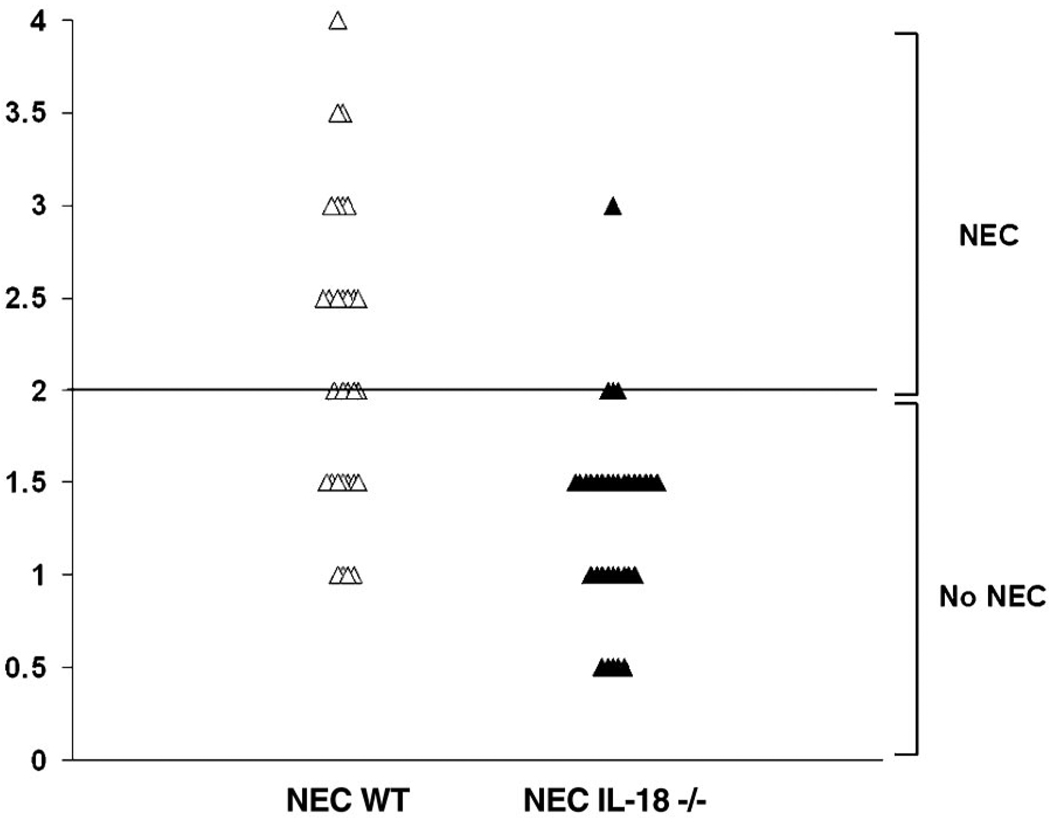
In Fig. 2, normal histology from 3-day-old DF WT (A) and typical ileal damage in 3-day-old formula-fed, asphyxia/cold-stressed NEC WT mice (B–D) is shown. For NEC IL-18−/− mice, the median histological ileal damage score was 1.5 compared with 2.5 for WT NEC WT mice (P ≤ 0.05). Representative histology for both groups is shown in Fig. 3. Any animal with a histological damage score of 2 or greater was considered to have developed disease. We found that 4/34 (11.76%) NEC IL-18−/− mice developed NEC compared with 19/31 (61.29%) NEC WT mice (Fig. 4). The incidence of NEC in both DF WT and DF IL-18−/− groups was 0%; 0/10 and 0/15, respectively (data not shown).
Fig. 2.
Histological damage in control mice. Representative hematoxylin and eosin (H&E)-stained, ethanol-fixed distal ileal tissue is shown from 3-day-old dam-fed, asphyxia/cold-stressed (DF WT) histologically normal mice (A), formula-fed, asphyxia/cold-stressed [necrotizing enterocolitis wild-type (NEC WT)] mice with a histology score of 2.0 (B), NEC WT with a histology score 3.0 (C), and NEC WT mice with a histology score of 4.0 (D). Magnification, ×200.
Fig. 3.
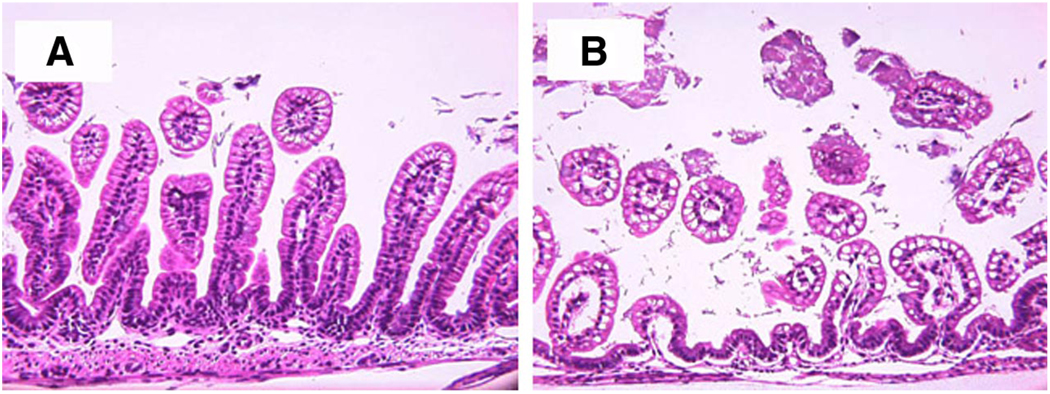
Ileal structure of NEC IL-18−/− and NEC WT mice. Representative images show NEC IL-18−/− (A) and NEC WT mice (B) that were fed formula and asphyxia/cold-stressed for 3 days. Magnification, ×200.
Fig. 4.
Decreased incidence of NEC in IL-18−/− mice. Scored on an ileal damage scale of 0 (normal) to 4 (necrosis), animals with histological damage of 2 or greater were considered to have developed NEC.
Ileal inflammatory cytokines
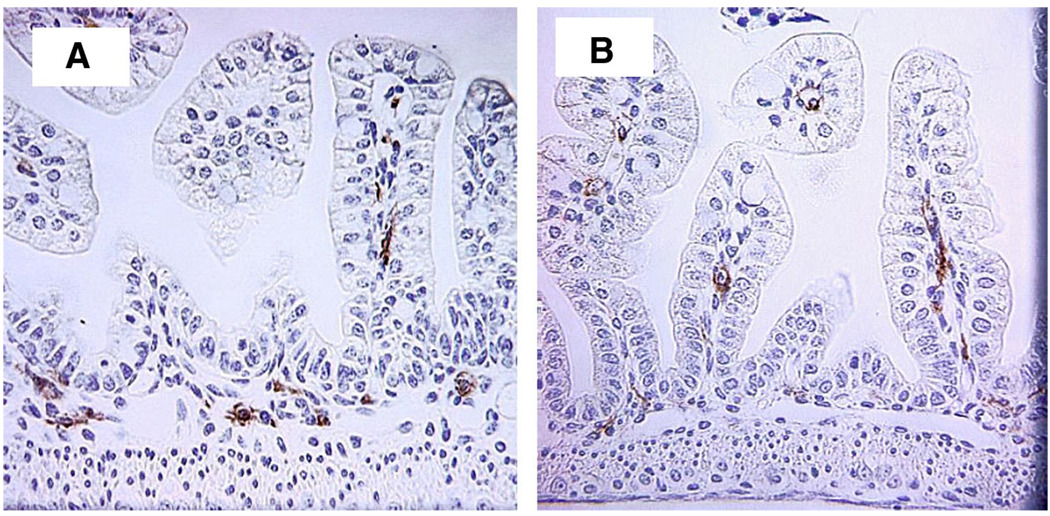
To determine how the absence of IL-18 affects ileal inflammation during NEC, we stained ileal samples from each animal with antibodies directed against proinflammatory cytokines IL-1β and IL-12. There were no statistically significant differences in the number of ileal IL-1β- or IL-12-positive cells between NEC IL-18−/− and NEC WT or DF WT and DF IL-18−/− groups (data not shown). In addition, ileal macrophages were counted from ten ×40 microscopic fields from each animal after immunohistological staining for the mouse macrophage marker BM8. Mean ileal macrophages were significantly decreased in NEC IL-18−/− mice (Table 1, Fig. 5) to numbers comparable to those in both DF WT and DF IL-18−/− mice.
Table 1.
Ileal macrophages and hepatic IL-1β
| Group | n | Mean Ileal Macrophages | Mean Hepatic IL-1β |
|---|---|---|---|
| NEC WT | 30 | 28.9±3.7* | 32.3±3.3* |
| NEC IL-18−/− | 34 | 12.2±2.3 | 10.5±3.0 |
| DF WT | 10 | 15.9±3.5 | 9.8±4.1 |
| DF IL-18−/− | 10 | 13.2±4.6 | 8.1±2.5 |
NEC WT, wild-type mice subjected to necrotizing enterocolitis (NEC) by formula feeding and cold stress; NEC IL-18−/−, IL-18 knockout mice subjected to NEC protocol; DF WT, dam-fed wild-type mice; DF IL-18−/−, dam-fed IL-18 knockout mice.
P ≤ 0.01 vs. NEC IL-18−/−, DF WT, and DF IL-18−/− groups.
Fig. 5.
Ileal macrophages in NEC WT (A) and NEC IL-18−/− mice (B). Magnification, ×400.
Hepatic inflammatory markers
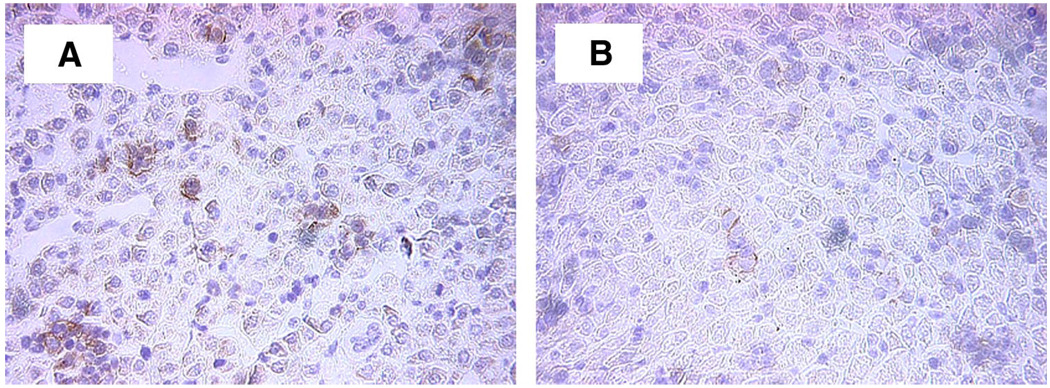
We have previously shown that hepatic inflammation is increased during experimental NEC in rats. To determine whether hepatic inflammatory mediators are altered in NEC IL-18−/− mice, we counted the number of Kupffer cells and IL-1β- and TNF-α-positive cells in immunohistologically stained liver tissue from each animal. There was no change in Kupffer cells or TNF-α-positive cells between groups, however, hepatic IL-1β-positive cells were significantly decreased in NEC IL-18−/− mice compared with NEC WT, with numbers similar to those in both the DF WT and DF IL-18−/− groups (Table 1, Fig. 6).
Fig. 6.
Hepatic IL-1β-positive cells in NEC WT (A) and NEC IL-18−/− mice (B). Magnification, ×200.
NF-κB in IL-18−/− mice subjected to the NEC protocol
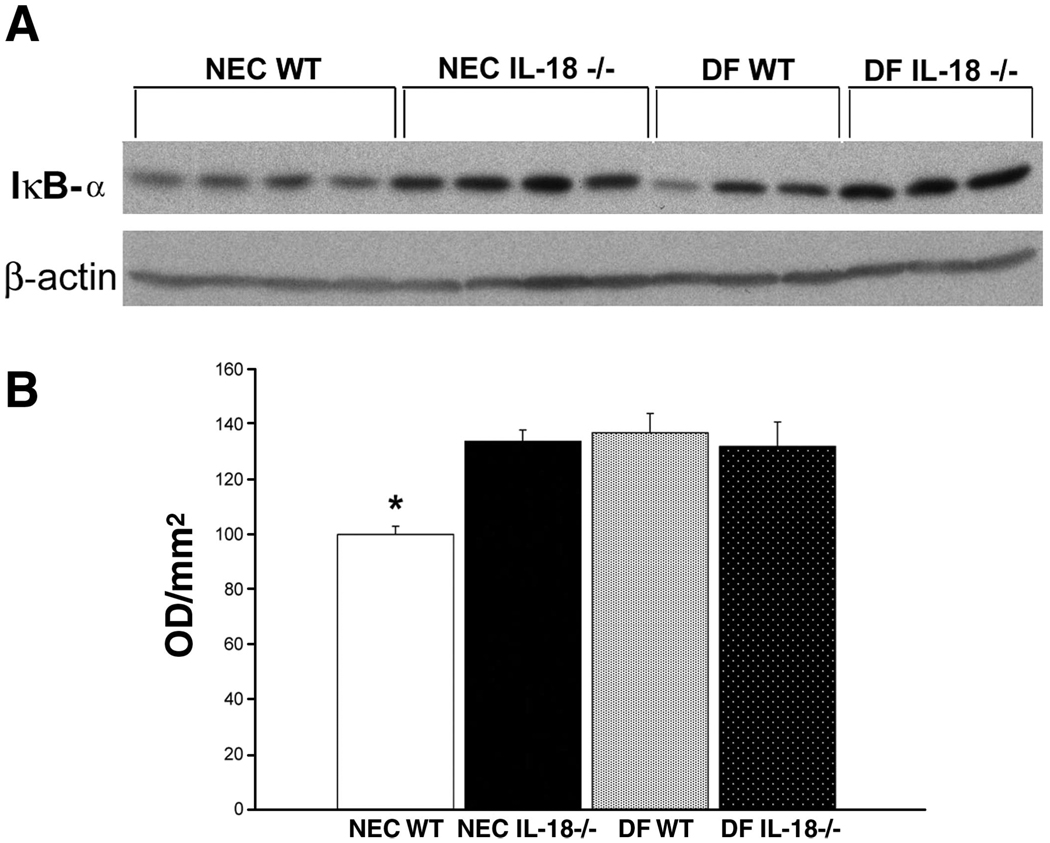
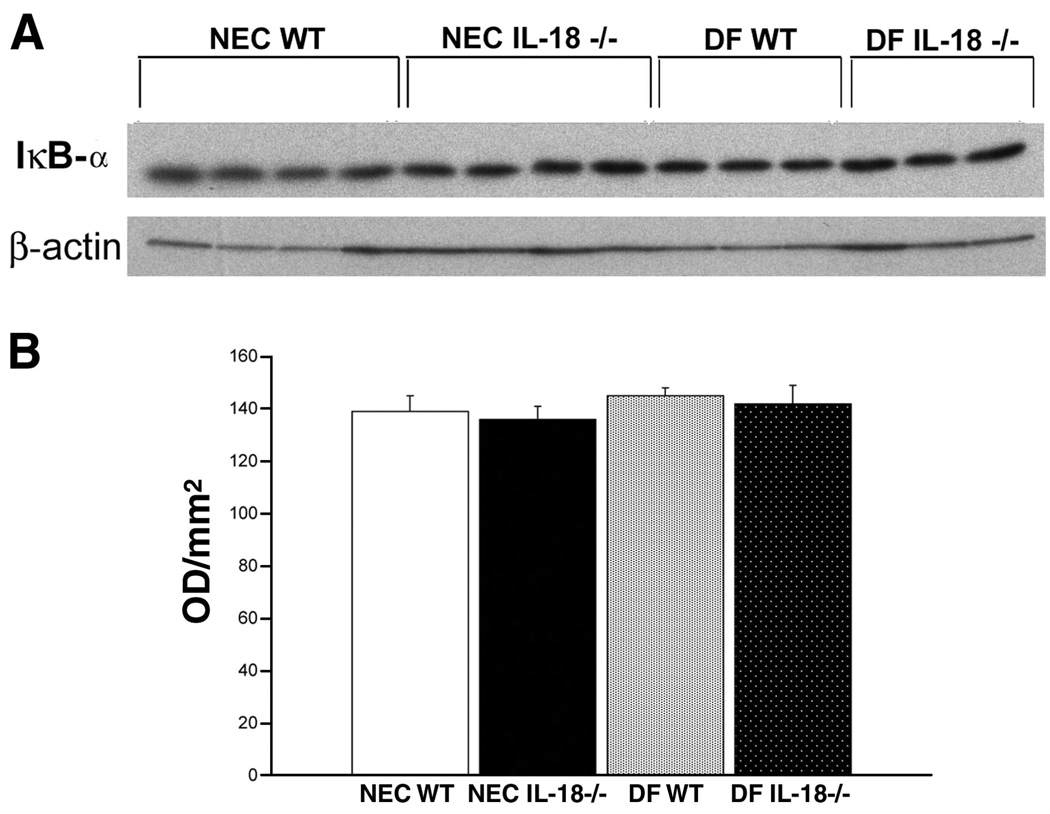
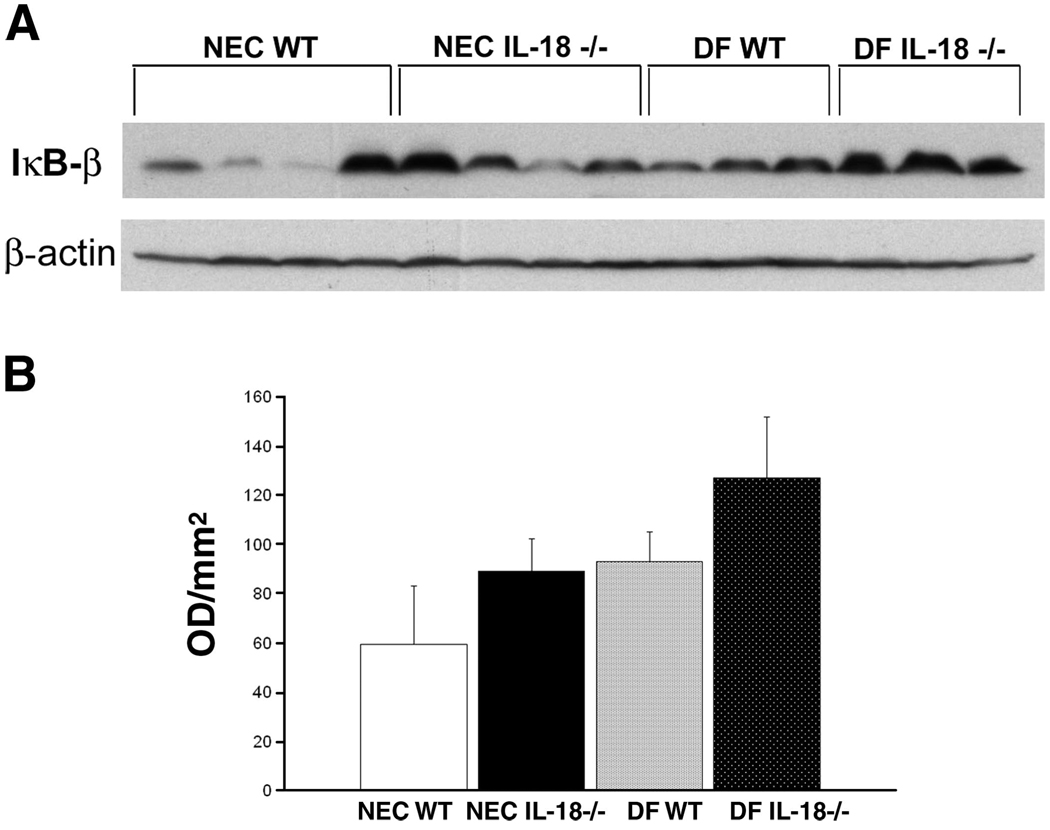
Activation of NF-κB is a hallmark of the inflammatory cascade and has been implicated in NEC pathogenesis. Because of the limited amount of ileal tissue obtainable from 3-day-old mouse pups, we did not have enough tissue to determine NF-κB activation using nuclear extracts. NF-κB is held inactive in the cell cytoplasm by the inhibitor protein IκB. Stimuli such as TNF-α or IL-18 induce selective IκB phosphorylation, which is then ubiquitinylated and degraded. Activated NF-κB is then free to migrate to the nucleus (7, 26, 38). As activated NF-κB levels increase in the nucleus, cytoplasmic IκB-α and IκB-β levels should decrease (11, 33, 40). Therefore, we evaluated ileal and hepatic IκB-α and IκB-β levels using Western blot analysis. Ileal IκB-α levels were significantly lower in NEC WT mice compared with NEC IL-18−/−, DF WT, and DF IL-18−/− mice (Fig. 7), indicating greater activation of NF-κB in the NEC WT group. Liver IκB-α levels were similar in all groups (Fig. 8). Ileal IκB-β levels were significantly lower in NEC WT mice compared with NEC IL-18−/−, DF WT, and DF IL-18−/− mice. However, IκB-β levels were extremely variable in the NEC WT group; thus statistically significant differences were not achieved (Fig. 9). Hepatic IκB-β levels were significantly lower in NEC IL-18−/− mice compared with the other groups (Fig. 10).
Fig. 7.
A: representative Western blot for ileal IκB-α from NEC WT, NEC IL-18−/−, DF WT, and DF IL-18−/− mice. Ileal tissues from 3-day-old mice were probed using anti-IκB-α. B: mean optical density (OD) for ileal IκB-α taken from NEC WT (n = 10), NEC IL-18−/− (n = 10), DF WT (n = 10), and DF IL-18−/− (n = 10) mice. *P ≤ 0.05 vs. NEC IL-18−/−, DF WT, and DF IL-18−/−.
Fig. 8.
A: representative Western blot for hepatic IκB-α levels from NEC WT, NEC IL-18−/−, DF WT, and DF IL-18−/− mice. Livers from 3-day-old mice were probed using anti-IκB-α. B: mean OD for hepatic IκB-α taken from NEC WT (n = 10), NEC IL-18−/− (n = 10), DF WT (n = 10), and DF IL-18−/− (n = 10) mice.
Fig. 9.
A: representative Western blot for ileal IκB-β from NEC WT, NEC IL-18−/−, DF WT, and DF IL-18−/− mice. Ileal tissues from 3-day-old mice were probed using anti-IκB-β. B: mean OD for ileal IκB-β taken from NEC WT (n = 10), NEC IL-18−/− (n = 10), DF WT (n = 10), and DF IL-18−/− (n = 10) mice.
Fig. 10.
A: representative Western blot for hepatic IκB-β levels from NEC WT, NEC IL-18−/−, DF WT, and DF IL-18−/− mice. Livers from 3-day-old mice were probed using anti-IκB-β. B: mean OD for hepatic IκB-β taken from NEC WT (n = 10), NEC IL-18−/− (n = 10), DF WT (n = 10), and DF IL-18−/− (n = 10) mice. *P ≤ 0.05 vs. NEC IL-18−/−, DF WT, and DF IL-18−/−.
DISCUSSION
Our previous studies suggested that IL-18 plays an important role in the pathogenesis of experimental NEC (13, 16). In the present studies, we have shown that IL-18 is a crucial component in the development of NEC, since IL-18 knockout mice have significantly lower incidence and severity of NEC than do WT mice, which are able to produce IL-18.
Our previous studies were conducted using the well-characterized neonatal rat model of NEC. In this model, NEC is developed in newborn rats by feeding milk-based formula coupled with exposure to hypoxia and cold stress. The major advantage of the neonatal rat NEC model is that many clinical and pathological changes are similar to those found in humans; the abdomen is distended, blood is detected in stool, and the ileum and proximal colon are the most affected parts of the intestine. In addition, the major risk factors for human NEC (intestinal immaturity, enteral formula feeding, and hypoxia/ ischemia) are essential factors to develop disease in the rat model (4–6).
The main disadvantage of this model is the lack of knockout or genetically modified rat strains with which to investigate mechanisms of disease development and pathology. Currently, mechanistic evaluations in the rat model require inhibition of potential disease contributors with specific antibodies or specific chemical inhibitors. The paucity of congenic, knockout, or spontaneous mutant rat strains has severely hampered research into this disease. Thus the ability to utilize a neonatal mouse model of NEC increases the ability to investigate disease pathogenesis and the molecular mechanisms underlying these processes. By developing a mouse model of NEC that encompasses the major risk factors for human disease and applying this model to investigate the role of IL-18 in disease pathology, we have been able to show that IL-18 is critical for disease development.
Inflammation requires a cascade of events and the upregulation and activation of a number of proinflammatory mediators. Thus some disease pathology would be expected even in the absence of a specific proinflammatory mediator such as IL-18. Although disease development was not completely halted in NEC IL-18−/− mice, our finding that the incidence of NEC was decreased from 61 to 12% in the NEC IL-18−/− group offers proof that IL-18 is critical for disease pathology.
The complexities of inflammation are also illustrated in this model, given that some proinflammatory cytokines were decreased in NEC IL-18−/− mice while others were unchanged. The neonatal rodent intestine is immunologically immature. In fact, the majority of IL-18 in rat (16) and mouse (unpublished data) models of NEC is produced by intestinal epithelial cells rather than immune cells. We found essentially no TNF-α, IFN-γ, or IL-1β produced in the ileum during experimental NEC treatment but high levels of hepatic IL-1β and TNF-α (15). Thus the lack of decreased IL-1β and TNF-α in the ileum of NEC IL-18−/− mice is likely a reflection of the small quantities of these cytokines found in the ileum during disease development rather than a direct consequence of the lack of IL-18. The parameters that are decreased in NEC IL-18−/− mice (hepatic IL-1β and ileal macrophages) are parameters that are increased during experimental NEC. The only exception is hepatic TNF-α, which is increased in NEC but unchanged in IL-18−/− mice subjected to the NEC protocol. Although the reasons for this observation are unclear, it is not without prior precedent, since Sakao et al. (37) found elevated TNF-α levels in IL-18−/− mice after LPS challenge. They hypothesized that during sepsis, IL-18 can actually downregulate TNF-α. It is possible, therefore, that in the NEC IL-18−/− group, the lack of IL-18 prevents downregulation of TNF-α, resulting in similar levels of hepatic TNF-α in all groups.
NF-κB has been shown to play a role in intestinal inflammation in a number of models of colitis and intestinal epithelial cell lines (21, 33, 35, 41, 42). The importance of NF-κB is underscored by the NF-κB-induced expression of many cytokines, enzymes, and adhesion molecules potentially involved in the pathogenesis of NEC. NF-κB regulates transcription of and can be activated by a number of inflammatory mediators including TNF-α, IL-18, and IL-12 (8, 18, 29), which we have shown to increased during NEC (13, 15, 16). In addition, NOS2, a proinflammatory target gene of NF-κB, is increased in intestinal inflammation (28, 36) and NEC (10, 31) and has been implicated in the pathogenesis of gut-barrier failure in inflammatory bowel disease (1–3). Therefore, a crucial regulatory role of NF-κB in the pathogenesis of NEC is likely. With the use of IκB-α and IκB-β as indirect identifiers, our data suggest that there is a significant decrease in NF-κB activation in IL-18−/− mice subjected to the NEC protocol. We speculate that hepatic TNF-α, which we have previously shown is the source of elevated TNF-α in the ileal luminal contents during NEC (12, 15), may be responsible for both the activated ileal and hepatic NF-κB.
In conclusion, we have shown that IL-18 is critical for the ileal pathology observed in experimental NEC. The results of these studies provide proof that IL-18 is crucial for disease development and suggest that neutralization of IL-18 could be utilized as a potential therapeutic approach to human NEC.
ACKNOWLEDGMENTS
Present address for M. Yajima: Hokkaido Univ., Nishi 11, Kita 21, Kita-ku, Sapporo, Hokkaido 011-0021, Japan.
GRANTS
This research was supported by The University of Arizona Foundation and the Office of the Vice President for Research, Graduate Studies and Economic Development and by National Institutes of Health Grants DK067953 and HD047237.
REFERENCES
- 1.Boughton-Smith NK. Pathological and therapeutic implications for nitric oxide in inflammatory bowel disease. J R Soc Med. 1994;87:312–314. doi: 10.1177/014107689408700602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet. 1993;342:338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- 3.Boughton-Smith NK, Evans SM, Whittle BJ, Moncada S. Induction of nitric oxide synthase in rat intestine and its association with tissue injury. Agents Actions. 1993 doi: 10.1007/BF01991159. 38 Spec No: C125-C126. [DOI] [PubMed] [Google Scholar]
- 4.Caplan MS, MacKendrick W. Necrotizing enterocolitis: a review of pathogenetic mechanisms and implications for prevention. Pediatr Pathol. 1993;13:357–369. doi: 10.3109/15513819309048223. [DOI] [PubMed] [Google Scholar]
- 5.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 6.Caplan MS, Russel T, Xiao Y, Amer M, Kaup S, Jilling T. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res. 2001;49:647–652. doi: 10.1203/00006450-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 7.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, Yoon DY, Reznikov LL, Kim SH, Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 9.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 10.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275–282. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 12.Halpern MD, Clark JA, Saunders TA, Doelle SM, Molla Husseini D, Stagner AM, Dvorak B. Reduction of experimental necrotizing enterocolitis with anti-TNF-α. Am J Physiol Gastrointest Liver Physiol. 2006;290:G757–G764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 13.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Halpern MD, Holubec H, Clark JA, Saunders TA, Williams CS, Dvorakova K, Dvorak B. Epidermal growth factor reduces hepatic sequelae in experimental necrotizing enterocolitis. Biol Neonate. 2005;89:227–235. doi: 10.1159/000090015. [DOI] [PubMed] [Google Scholar]
- 15.Halpern MD, Holubec H, Dominguez JA, Meza YG, Williams CS, Ruth MC, McCuskey RS, Dvorak B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G695–G702. doi: 10.1152/ajpgi.00353.2002. [DOI] [PubMed] [Google Scholar]
- 16.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res. 2002;51:733–739. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006;130:359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 19.Hoshiba J. Method for hand-feeding mouse pups with nursing bottles. Contemp Top Lab Anim Sci. 2004;43:50–53. [PubMed] [Google Scholar]
- 20.Hsueh W, Caplan MS, Tan X, MacKendrick W, Gonzalez-Crussi F. Necrotizing enterocolitis of the newborn: pathogenetic concepts in perspective. Pediatr Dev Pathol. 1998;1:2–16. doi: 10.1007/s100249900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobin C, Sartor RB. The IκB/NF-κB system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 22.Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis. 2003;16:349–355. doi: 10.1097/00001432-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kanai T, Watanabe M, Okazawa A, Sato T, Hibi T. Interleukin-18 and Crohn’s disease. Digestion. 2001;63:37–42. doi: 10.1159/000051909. [DOI] [PubMed] [Google Scholar]
- 24.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. Am J Dis Child. 1981;135:603–607. doi: 10.1001/archpedi.1981.02130310009005. [DOI] [PubMed] [Google Scholar]
- 25.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a cnine-year experience. II. Outcome assessment. Am J Dis Child. 1981;135:608–611. doi: 10.1001/archpedi.1981.02130310014006. [DOI] [PubMed] [Google Scholar]
- 26.Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 27.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg JO, Hellstrom PM, Lundberg JM, Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994;344:1673–1674. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto S, Tsuji-Takayama K, Aizawa Y, Koide K, Takeuchi M, Ohta T, Kurimoto M. Interleukin-18 activates NF-kappaB in murine T helper type 1 cells. Biochem Biophys Res Commun. 1997;234:454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 30.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 31.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 32.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996;43:409–432. doi: 10.1016/S0031-3955(05)70413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43:856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- 35.Potoka DA, Upperman JS, Nadler EP, Wong CT, Zhou X, Zhang XR, Ford HR. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. J Surg Res. 2002;106:7–14. doi: 10.1006/jsre.2002.6423. [DOI] [PubMed] [Google Scholar]
- 36.Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–255. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakao Y, Takeda K, Tsutsui H, Kaisho T, Nomura F, Okamura H, Nakanishi K, Akira S. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int Immunol. 1999;11:471–480. doi: 10.1093/intimm/11.3.471. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson IR. The physicochemical environment of the neonatal intestine. Am J Clin Nutr. 1999;69:1028S–1034S. doi: 10.1093/ajcn/69.5.1028s. [DOI] [PubMed] [Google Scholar]
- 39.Schanler RJ, Hurst NM, Lau C. The use of human milk and breastfeeding in premature infants. Clin Perinatol. 1999;26:379–398. vii. [PubMed] [Google Scholar]
- 40.Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schottelius AJ, Baldwin AS., Jr A role for transcription factor NF-kappa B in intestinal inflammation. Int J Colorectal Dis. 1999;14:18–28. doi: 10.1007/s003840050178. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yajima M, Hoshiba J, Terahara M, Yajima T. Reduced thymic size and numbers of splenic CD4+ and CD8+ cells in artificially reared mouse pups. Biosci Biotechnol Biochem. 2007;71:2420–2427. doi: 10.1271/bbb.70121. [DOI] [PubMed] [Google Scholar]