Abstract
The maintenance of genome stability requires efficient DNA double-stranded break (DSB) repair mediated by the phosphorylation of multiple histone H2AX molecules near the break sites. The phosphorylated H2AX (γH2AX) molecules form foci covering many megabases of chromatin. the formation of γ-H2AX foci is critical for efficient DNA damage response (DDR) and for the maintenance of genome stability, however, the mechanisms of protein organization in foci is largely unknown. To investigate the nature of γH2AX foci formation, we analyzed the distribution of γH2AX and other DDR proteins at DSB sites using a variety of techniques to visualize, expand and partially disrupt chromatin. We report here that γH2AX foci change composition during the cell cycle, with proteins 53BP1, NBS1 and MRE11 dissociating from foci in G2 and mitosis to return at the beginning of the following G1. In contrast, MDC1 remained colocalized with γ-H2AX during mitosis. In addition, while γH2AX was found to span large domains flanking DSB sites, 53BP1 and NBS1 were more localized and MDC1 colocalized in doublets in foci. H2AX and MDC1 were found to be involved in chromatin relaxation after DSB formation. Our data demonstrates that the DSB repair focus is a heterogeneous and dynamic structure containing internal complexity.
Keywords: DNA double strand breaks, phosphorylated H2AX, MDC1, 53BP1, NBS1
Introduction
Upon DNA double-strand break (DSB) induction by ionizing radiation (IR), hundreds of molecules of multiple DNA damage response (DDR) protein species accumulate at DNA DSB sites forming foci known as ionizing radiation induced foci (IRIF).1-4 Phosphorylated H2AX is a key component of numerous signaling pathways responsive to DNA DSBs.1,5 PI3 kinases, such as ATM, DNA-PKcs and ATR, phosphorylate H2AX at Ser 139 rapidly after DNA DSB induction.6-8 This initial γH2AX is required for further signal amplification by these kinases, indicating that γH2AX is necessary for rapid DDR signal amplification.9 In addition, γH2AX directly binds MDC1 and NBS1 which then allows the recruitment of other DDR proteins, including the MRE11/RAD50/NBS1 (MRN) complex, RNF8, BRCA1 and 53BP1 at DNA DSB sites.3,10,11 Focal accumulation of chromatin remodeling factors including TIP60 and INO80 also depend on γH2AX.12-15 Thus γH2AX is necessary for proper IRIF formation and DSB repair. There is considerable evidence suggesting that efficient IRIF formation is essential for the preservation of genome integrity. MEFs from H2AX deficient mice exhibit genomic instability and deficient accumulation of DNA repair proteins such as NBS1 and BRCA1.11 In addition, naturally occurring mutations in several DDR proteins result in human genetic diseases, such as Nijmegen breakage syndrome (mutation of NBS1), Ataxia Telangiectasia (ATM) and Bloom’s syndrome (BLM).16 Although there are numerous studies indicating that γH2AX is necessary for efficient DNA repair and genome integrity, the organization of DDR proteins in IRIF is still largely unknown. First, while γH2AX foci have been shown to form in all phases of cell cycle including mitosis,4,17 studies of the focal accumulation of other DDR proteins have been primarily limited to interphase cells.3,18,19 To further investigate the structure of IRIF, we examined the localization of γH2AX and other DDR proteins at DSB sites in cells at various stage of the cell cycle. Second, evidence that DDR proteins in IRIF might not be uniformly distributed throughout the focus comes from chromatin immunoprecipitation (ChIP) studies in yeast and in human cells containing a single inducible DNA DSB site.20,21 These studies revealed that H2AX is phosphorylated in a large region flanking the DNA DSB site, but the other DDR proteins such as Mre11 and Rad51 exhibited a different distribution, accumulating in smaller regions at the DSB site. These data suggest that different DDR proteins may have their own territory in a DSB focus. However, these analyses are limited to within a few kilobase pairs of DNA flanking the DNA DSB site, while H2AX phosphorylation spans several megabase pairs DNA flanking the DNA DSB site.4 Thus to investigate the wide-distribution of DDR proteins in IRIF, we analyzed the localization of γ-H2AX and other DDR proteins at DSB sites by utilizing metaphase chromosomes spreads and chromatin swelling techniques. We also analyzed the involvement of several DDR proteins in global chromatin relaxation after irradiation exposure. Our data taken together demonstrate that IRIF is a heterogeneous and dynamic structure containing internal complexity that is regulated by cell cycle progression.
Results
γH2AX foci change composition during the cell cycle
Many DDR proteins have been found to colocalize with γ-H2AX in foci in interphase cells.22-24 While γH2AX foci are known to appear throughout the cell cycle after DNA DSB induction, the behavior of other proteins at these foci is unknown. We examined γH2AX foci for the presence of several proteins, 53BP1, MDC1, NBS1 and MRE11 at various phases of the cell cycle (Fig. 1, Suppl. Fig. S1, and data not shown). These protein species exhibited two temporal patterns.
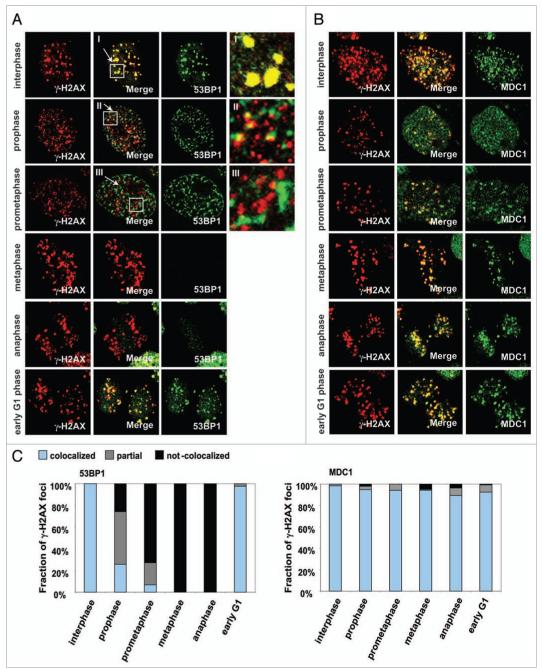
Figure 1.
The components of IRIF are reorganized during the cell cycle. HeLa cells were synchronized by colcemid, exposed to 1 Gy IR and fixed after 30 min incubation. Mitotic stages were classified by DAPI staining. (A) Immunostaining for γH2AX and 53BP1. I-III refer to enlargements of the noted areas. (B) Immunostaining for γH2AX and MDC1. (C) Fraction of γH2AX foci colocalized with 53BP1 (left) or MDC1 (right).
One pattern included 53BP1, NBS1 and MRE11. These species appeared to colocalize with γ-H2AX foci in interphase cells (Fig. 1A for 53BP1; Suppl. Fig. S1 for NBS1; MRE11, not shown). However, these proteins separate from the γ-H2AX foci and were incompletely colocalized during mitotic prophase (before nuclear membrane disintegration) (Fig. 1A, II and Suppl. Fig. S1A, II). Notably, there appears to be disperse and almost completely separated from γ-H2AX foci during prometaphase (after nuclear membrane disintegration) (Fig. 1A, III and Suppl. Fig. S1A, III). During mitotic prophase, approximately 26% of γH2AX foci colocalized with 53BP1 (Fig. 1C, left) and 55% with NBS1 (Fig. S1B); during prometaphase, 6.6% of γ-H2AX foci colocalized with 53BP1 and 30% with NBS1. While these foci appeared to disintegrate by metaphase, 53BP1 and NBS1 foci were again found colocalized with γ-H2AX in early G1. Recently, another group has reported that 53BP1 foci dispersed in mitotic cells.25 However, 53BP1 has also been reported to colocalize with kinetochores during mitosis,26 separate from γH2AX.
The second pattern is exemplified by MDC1, which appeared to colocalize with γH2AX foci throughout the cell cycle (Fig. 1B and C, right). However, as will be discussed below, other techniques reveal that γ-H2AX and MDC1 do not completely colocalize in foci.
To help confirm these findings, we analyzed metaphase chromosome spreads from cells exposed to 1 Gy for foci formation of γH2AX, 53BP1, NBS1, MRE11 and MDC1. As with metaphase cells, these protein species exhibited two patterns: γH2AX and MDC1 which formed IRIF on chromosomes in metaphase spreads and 53BP1, NBS1 and MRE11 which did not (Suppl. Fig. S2A). Some NBS1 and MRE11 foci were detected on metaphase chromosomes in both irradiated and un-irradiated cells (Suppl. Fig. S2B), though in neither case did these proteins colocalize with γH2AX, indicating that foci of these two proteins during mitotic stages were not related to DNA DSBs.
The loss of 53BP1, NBS1 and MRE11 from mitotic chromosomes may be attributed to cell cycle processes or just to the state of chromatin condensation. These two alternative processes can be dissected by the induction of premature chromosome condensation. Burkitt’s lymphoma CA46 cells, 30 min after exposure to 1 Gy, were treated with lasonolide A,27 a compound which induces premature chromosome condensation (PCC) without affecting phosphatase activity (personal communication with Dr. Zhang) (Suppl. Fig. S3A). Immunostaining of γH2AX and 53BP1 revealed a large decrease in the number of 53BP1 foci after lasonolide A treatment (23.3 per cell versus 4.5 per cell) whereas γH2AX focal numbers were not affected (Suppl. Fig. S3A and B). Thus, these results indicate that it appears to be the state of chromosome condensation rather than cell cycle position that determines whether or not 53BP1 colocalizes to IRIF.
γH2AX form foci large domains flanking DSB sites, 53BP1 and NBS1 are more localized, and MDC1 is colocalized in doublets in γH2AX foci
The first evidence of a defined substructure in γ-H2AX foci came from studies in yeast containing a single inducible DNA DSB site.21 Chromatin immunoprecipitation (ChIP) techniques showed that γ-H2AX was found in a region greater than 20 Kbp surrounding the break site but was depleted at the break site, while Mre11 was found only within 5 Kbp of the break site. Similar results showing differential binding of NBS1 and ATM along 10 Kbp of the chromatin flanking a DSB site were obtained in human cells.20 Another technique to dissect the foci structure in mammalian cells containing megabase γ-H2AX domains is swelling of DNA by incubation in lower Mg2+ concentrations as reported by Cole.28 A previous study reported that mild hypotonic treatment (100 mM NaCl at RT for 1 hr) induced phosphorylation of ATM without DNA damage induction,29 however, neither phosphorylation of ATM nor H2AX were detected under the condition we used (Suppl. Fig. S4 and data not shown).
When these swelling techniques were applied to interphase cells, γ-H2AX foci were found to expand (Fig. 2A, left images). As before, two patterns of behavior were found among the other proteins examined. The foci of 53BP1, NBS1 and MRE11 foci remained tight after the swelling process, while MDC1 foci expanded with the γ-H2AX foci (Fig. 2A). Intensity quantitation through these foci confirmed that γH2AX and MDC1 foci expanded in swollen nuclei in contrast to other repair proteins, suggesting that γH2AX and MDC1 are distributed over larger domains in the foci while 53BP1, NBS1 and MRE11 are concentrated in tighter domains.
Figure 2.
γH2AX foci enlarge upon chromatin swelling as do MDC1 foci, while 53BP1, NBS1 and MRE11 foci do not. (A, left images) At 30 min after exposure to 1 Gy, HeLa cells were subjected to swelling, then double-immunostained for γH2AX and 53BP1, MRE11, NBS1 or MDC1. The top row shows a no-swelling control with 53BP1. The bottom row shows a reversed-staining control. (A, right graphs) Density scanning along the white line shown in the merged images. (B) Immunostaining of γH2AX and either 53BP1 or NBS1 on chromatin fibers from Indian Muntjac cells. Chromatin fibers were prepared at 30 min after 1 Gy-exposure and immunostained for γH2AX and either 53BP1 or NBS1.
When extended chromatin fibers were immunostained for 53BP1 and NBS1, both were found to localize in relatively short regions at the end of longer regions containing γ-H2AX (Fig. 2B). MRE11 exhibited similar behavior (data not shown). These results provide visual substantiation of the findings from ChIP experiments that MRE11 and NBS1 are bound in short regions near the break site while H2AX is phosphorylated over larger regions.20,21
γH2AX foci were found to expand also when metaphase chromosomes were subjected to the same swelling techniques as those used with interphase cells (Fig. 3A). However, instead of swelling evenly in all directions as in chromatin, the γ-H2AX foci appeared to extend linearly. In cells of the Indian Muntjac which possess three pairs of chromosomes, swelling also expanded and extended the γH2AX foci to form extended filamentous structures on the swollen chromosomes (Fig. 3B), findings also supporting mechanisms in which H2AX is phosphorylated over large chromatin regions along the same DNA strand.4
Figure 3.

γH2AX foci enlarge on swollen metaphase chromosomes. (A) HeLa metaphase chromosomes immunostained for γH2AX with (right) or without (left) swelling. The metaphase spreads from HeLa cells were incubated with sterile water at room temperature for 15 min. After the solution was carefully removed from the cells, they were fixed by 80% ethanol at −20°C for 30 min. (B) Indian Muntjac cell metaphase chromosomes immunostained for γH2AX after (right) or without (left) swelling. The right panel shows a greater magnification of the noted area in the middle panel.
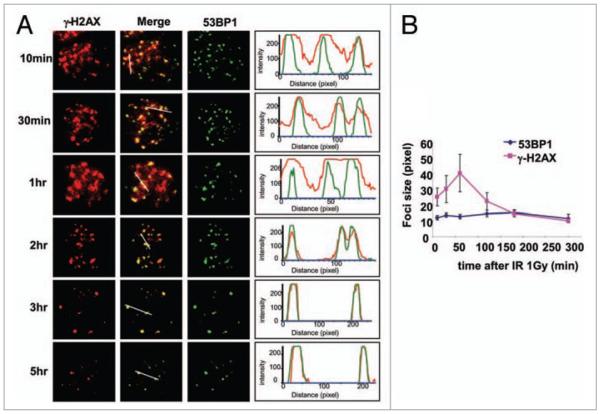
These results were obtained 30 min after DSB induction when γH2AX foci formation is maximal. While the number of γ-H2AX foci decrease after that time, a substantial fraction remains for several hours or even days post exposure.30 γH2AX foci disappear at a relatively rapid rate for the first few hr after IR-exposure, with a half-life about 2 hr, but after that the rate of foci disappearance slows greatly, with a half-life ten-fold slower.30 Recently, Goodarzi et al. showed that the γH2AX foci remaining after a few hr post exposure are enriched in heterochromatic foci.31 To examine whether the capabilities of persistent γ-H2AX foci to expand was similar to those of initial γ-H2AX foci, we subjected chromatin containing γ-H2AX foci to the swelling procedure at various time after IR exposure. Strikingly, the γ-H2AX foci remaining after 2 hr post-exposure did not expand, but remain tight similar to the 53BP1 and NBS1 foci (Fig. 4A and B, and data not shown). These findings might suggest that heterochromatic DNA DSBs, which marked by persistent γ-H2AX foci, require factors to make the heterochromatic regions more accessible to DSB repair factors, factors which are not released by the swelling procedures.
Figure 4.
Until 2 hr post IR, γH2AX localizes to a large region around the DNA DSB site. (A, left images) Swollen interphase nuclei were prepared from HeLa cells at the noted times after 1 Gy-exposure and immunostained for γH2AX and 53BP1. (A, right graphs) Density scanning along the white line shown in the merged images. Note that at 2 hr and beyond, γH2AX foci do not expand on swollen chromatin. (B) Graphical presentation of the data in (A). Average from three independent experiments. At least 25 foci were counted in each experiment. Error bars signify standard deviation.
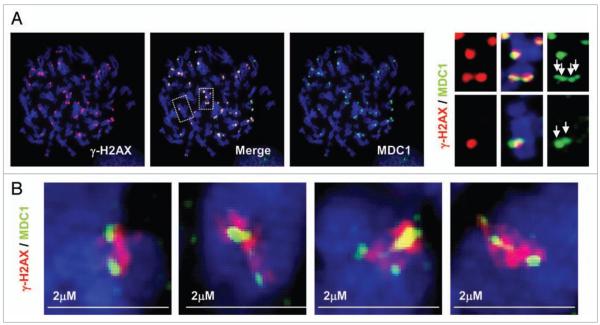
Careful observation of metaphase spreads double-stained for γ-H2AX and MDC1 revealed that some MDC1 foci appeared as doublets bracketing γ-H2AX foci (Fig. 5A and enlarged images). To confirm these observations, we utilized a DeltaVision OMX™ 3D-SIM™ Super Resolution Imaging System. As shown in Figure 5B, this technique revealed that most of the γ-H2AX foci present in the images exhibit a fiber-like structure. In addition, the γ-H2AX foci were usually flanked by two, sometimes more, MDC1 sub-foci. These observations further confirm the notion that γ-H2AX foci are complex entities with proteins non-homogeneously distributed in the focus (Fig. 7).
Figure 5.
MDC1 form double foci appearing to bracket γH2AX foci. (A) HeLa metaphase chromosomes stained for γH2AX and MDC1. The right panels show magnified images of the boxed areas in the merged image. (B) HeLa metaphase chromosomes stained for γH2AX and MDC1 and analyzed by the DeltaVision OMX™ 3D-SIM™ Super Resolution Imaging System.
Figure 7.
Distribution model of DNA repair proteins in foci surrounding a DSB. γH2AX form IRIF in a large area around DNA DSB site. MDC1 accumulate primarily at the outside edges of γ-H2AX focus. In contrast, 53BP1, NBS1 and MRE11 accumulate primarily in a smaller region near the DNA DSB site.
H2AX and MDC1 are involved in global chromatin relaxation after IR exposure
One function of γ-H2AX foci may be to alter the conformation of chromatin in the region near the DSB site to facilitate DNA repair. Recently, it has been demonstrated that global chromatin relaxation is induced by DNA DSBs and this process is mediated by the DNA repair machinery.32-34 Utilizing micrococcal nuclease (MNase) to probe for chromatin accessibility, we asked whether γH2AX and MDC1 may play roles in the process of global chromatin relaxation (Fig. 6). Increased MNase sensitivity was apparent in the inter-nucleosomal region 1 hr after IR exposure in the chromatin from wild type MEF cells (Fig. 6B, top). In contrast, the chromatin from the H2AX knock-out MEFs or phosphorylation site mutant S139A MEFs did not exhibit increased sensitivity (Fig. 6B). These data suggested that phosphorylation of H2AX may aid chromatin relaxation after DNA damage induction. HeLa cells also exhibited greater MNase sensitivity after exposure to IR, while MDC1-depleted HeLa cells exhibited considerably smaller extents of global chromatin relaxation in response to IR exposure (Fig. 6A and B, Suppl. Fig. S5). Thus one function of γ-H2AX foci appears to be the facilitation of IR-induced chromatin relaxation, and MDC1 appears to be involved in this process. Although our data did not address whether this relaxation is DNA DSB site specific, H2AX deficiency is known to impair the accumulation of DNA repair proteins at DSB sites as well as chromatin remodeling proteins such as TIP60 complex.12,13
Figure 6.
γH2AX and MDC1 are involved in chromatin relaxation after IR exposure. (A) Increased MNase accessibility of chromatin after IR exposure. Cells from H2AX wild type MEFs (WT), H2AX-null MEFs (KO), H2AX-S139A mutated H2AX MEFs (S139A), HeLa cells (CTR) and MDC1-depleted HeLa cells (MDC1 siRNA) were treated by MNase for 5 min, and the fragments analyzed by gel electrophoresis. (B) Profiles of the various lanes of the gel presented in (A). N1-N7 denote the numbers of nucleosomes per oligonucleosome. Note the shift in oligonucleosome size in wild type H2AX or MDC1 cells after IR exposure.
Discussion
In this study, we have shown that the DNA repair foci are complex and dynamic structures. We have identified two protein groups on the basis of their temporal and spatial assembly with γ-H2AX foci. These two groups, one containing 53BP1, NBS1 and MRE11, and the other containing γH2AX and MDC1 exhibit different behavior during the cell cycle due to the state of chromatin condensation rather than the cell cycle position. Mitosis is known to be the most radiosensitive phase of the cell cycle35 and agents that arrest cells in G2/M are effective radiosensitization agents (reviewed in ref. 36). Our findings demonstrate that DNA repair proteins are re-organized during mitosis and at least one group critical for DSB repair, the one including 53BP1, NBS1 and MRE11, is absent from γ-H2AX foci during mitosis. These findings are similar to those of Nelson et al. regarding 53BP1,25 and indicate that DSB repair may be suspended during mitosis, and that the DSB sites remain marked for rapid access by repair factors during G1. These observations also suggest a mechanistic explanation for the radiosensitivity of mitotic cells.
In addition to these different temporal patterns, these two groups exhibit different spatial patterns relative to γ-H2AX after low salt/Mg swelling procedures (Fig. 7). ChIP assays have shown that NBS1 and MRE11 accumulate near DSB sites, and the resistance of these proteins to swelling procedures also suggests that this group resides in a small region of the chromatin. 53BP1 behavior in these studies paralleled that of NBS1 and MRE11, suggesting it may also be located near the break site. The other group with MDC1 appears to reside near the outer ends of the γ-H2AX region. This is supported by its appearance as double sub-foci bracketing the γ-H2AX focus and by its sensitivity to swelling procedures.
Interestingly, the function of these DNA repair proteins in DDR is reflected in their grouping. NBS1 and MRE11 are known to be directly involved in DNA rejoining and this logically places them near break sites (reviewed in ref. 37),21 and our data are consistent with NBS1 and MRE11 localization near break sites. Foci formation of 53BP1 is reported to be regulated by MDC1,38,39 however, the function of the two proteins do not completely overlap.40 It has been reported that 53BP1 plays a role primarily in XRCC4-dependent non-homologous end-joining while MDC1 plays a role primarily in homologous recombination.41,42 Our data indicate that 53BP1 and MDC1 exhibit different distribution patterns in DNA damage foci that could reflect their non-overlapping function in DDR. Surprisingly, although MDC1 binds directly to γ-H2AX by its BRCT domains,43 our data indicated that MDC1 localizes heterogeneously along the γ-H2AX foci. These results suggest that MDC1 distribution is not simply reflected to its affinity to γH2AX. Because immunocytochemical techniques require multiple protein molecules for detection, we cannot eliminate the possibility that a few molecules of these proteins may bind at locations in the γ-H2AX foci other than those visualized. Thus the study of the architecture of γH2AX foci and their relationship to DSB repair may help elucidate not only the process of DSB repair but also how cell architecture plays an important role in facilitating such process.
DNA DSBs are often intentionally induced during cancer chemotherapies to introduce sufficient DSBs into cancer cells to activate cell death pathways. Understanding the spatial and temporal complexities of γ-H2AX focal structure may reveal novel therapeutic targets that involve disrupting these DNA repair foci structure and allow increasing the efficiency of chemotherapeutic drugs.
Materials and Methods
Cell culture and irradiation procedure
HeLa and Indian Muntjac cell lines were obtained from ATCC (Manassas, VA). HeLa cell lines were cultured in D-MEM containing 10% FBS. Muntjac cells were cultured in F-10 medium containing 20% FBS. Cells were maintained in humid incubator at 37°C, 5% CO2 and 20% O2. For cell cycle synchronization, cells were incubated with different concentration of colcemid depend on cell type (SIGMA, St. Louis, MO). Cells were exposed γ-IR in a Mark-1 γ-irradiator (JL Shepherd & Associates, San Fernando, CA); control samples were sham-irradiated.
Immunofluorescence
For analysis of IRIF formation throughout cell cycle, cells were fixed by 2% paraformaldehyde at room temperature for 20 min and then the slides were immunostained for a variety of DDR proteins with mouse monoclonal anti-γH2AX (Upstate Biotechnology, Inc., Lake Placid, NY or Abcam, Inc., Cambridge, MA), rabbit polyclonal anti-53BP1 (Novus Biologicals, Inc., Littleton, CO), rabbit polyclonal anti-NBS1 (Novus Biologicals, Inc., Littleton, CO), rabbit polyclonal anti-MDC1 (Abcam, Inc., Cambridge, MA) antibodies. Secondary antibodies were either anti-rabbit or anti-mouse Alexa-488 or 555-conjugated IgG (Invitrogen, Eugene OR). The metaphase spreads were prepared as described previously.44 DNA was counterstained by 4′,5-diamidino-2-phenylinodole, dihydrochloride (DAPI).
Swelling of interphase cells
HeLa cells were exposed to 1 Gy IR. At every time point after IR, cells were trypsinized and incubated with swelling solution (0.05% Trypsin, 0.05 M EDTA) at room temperature for 15 min. Swollen cells were cytospun to the slide and fixed by 80% ethanol at −20°C for 30 min. The slides were immunostained for a variety of DDR proteins as described above. Both immunoblotting and immunostaining showed that phosphorylation of neither ATM not H2AX levels were altered by the DNA swelling procedure (Suppl. Fig. S4 and data not shown).
Chromatin fiber analysis
Chromatin fiber analysis was performed as described previously.45 Briefly, Muntjac cells were exposed to 1 Gy IR and after 30 min, 10,000 cells were incubated with hypotonic buffer (0.075 M KCl) at room temperature for 10 min. Then the cells were cytospun to the slide. Cells were incubated in Lysis buffer [25 mM Tris-HCl (pH 7.5), 0.5 M NaCl, 1% Triton X-100, 0.5 M Urea] at room temperature for 7 min. The slides were lifted up slowly and fixed by 2% paraformaldehyde at room temperature for 20 min. The slides were immunostained for a variety of DNA repair proteins as described above.
Swelling of metaphase chromosomes
The metaphase spreads were prepared as described previously.44 After the cytospinning, cells were incubated with MgCl2 solution or sterile water at room temperature for 15 min. The solution was removed carefully and then cells were fixed by 80% ethanol at −20°C for 30 min. The slides were immunostained for a variety of DDR proteins as described above.
Deltavision OMX™ 3D-SIM™ super resolution imaging system
The metaphase spreads were prepared as described previously.44 Immunostaining were performed using the primary mouse monoclonal anti-γH2AX antibody (Abcam Inc., Cambridge, MA), rabbit polyclonal anti-MDC1 (Abcam, Inc., Cambridge, MA), antibodies and the secondary anti-mouse Alexa-555 or anti-rabbit Alexa-488 conjugated IgG (Invitrogen, Eugene, OR). The slides were analyzed by Applied Precision, Inc., (Issaquah, WA). Excitation used: 405 nm laser for DAPI, 488 nm laser for Alexia 488 and 592.2 nm laser for Alexia 555.
For more detail, www.api.com/lifescience/DeltaVisionOMX.html.
Micrococcus nuclease (MNase) assay
The assay was performed as previously described with some modification.33 Briefly, cells were exposed to 1 Gy IR and incubated for 30 min. Nuclear fraction was extracted by nuclear extraction buffer [10 mM HEPES-KOH (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA] following by 0.6% NP-40 treatment. Nuclear extractions were incubated with 500 μL MNase reaction buffer [30 U/mL MNase (Fermentas Inc., Glen Bernie, MD)] at 37°C for 5 min. Reaction was stopped by stop solution (200 mM EDTA, 20 mM EGTA). Genomic DNA was purified by phenol/chloroform method and separated by electrophoresis in 1.25% agarose gel in 1X Tris/Borate/EDTA (TBE). The gel was stained by ethidium bromide and analyzed by BioRad Gel Doc System (BioRad Life Science, Hercules, CA).
Supplementary Material
Acknowledgements
We thank Jennifer S. Dickey, Christophe E. Redon and Olga A. Sedelnikova, for critical reading of this manuscript, and other members of Laboratory of Molecular Pharmacology for help in this work. We thank Brittany Flood for technical assistance. We are grateful to Peter Franklin with the technical support for DeltaVision OMX™ 3D-SIM™ Super Resolution Imaging System. This work was supported by the Intramural Research Program of the National Cancer Institute, CCR, NIH.
Footnotes
Note Supplementary materials can be found at: www.landesbioscience.com/supplement/NakamuraCC9-2-Sup.pdf
References
- 1.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev. 2008;8:957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Capetillo O, Celeste A, Nussenzweig A. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle. 2003;2:426–7. [PubMed] [Google Scholar]
- 3.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 4.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–16. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickey JS, Redon CE, Nakamura AJ, Baird BJ, Sedelnikova OA, Bonner WM. H2AX: functional roles and potential applications. Chromosoma. 2009 doi: 10.1007/s00412-009-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–67. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–6. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 8.Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25:5775–82. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stucki M, Jackson SP. MDC1/NFBD1: a key regulator of the DNA damage response in higher eukaryotes. DNA Repair. 2004;3:953–7. doi: 10.1016/j.dnarep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 11.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–7. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, et al. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27:7028–40. doi: 10.1128/MCB.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, et al. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell. 2004;119:767–75. doi: 10.1016/j.cell.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR, 3rd, et al. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 2004;306:2084–7. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- 15.van Attikum H, Fritsch O, Hohn B, Gasser SM. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell. 2004;119:777–88. doi: 10.1016/j.cell.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 17.Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50 and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–12. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 18.Carbone R, Pearson M, Minucci S, Pelicci PG. PML NBs associate with the hMre11 complex and p53 at sites of irradiation induced DNA damage. Oncogene. 2002;21:1633–40. doi: 10.1038/sj.onc.1205227. [DOI] [PubMed] [Google Scholar]
- 19.Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–40. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkovich E, Monnat RJ, Jr, Kastan MB. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nature Cell Biol. 2007;9:683–90. doi: 10.1038/ncb1599. [DOI] [PubMed] [Google Scholar]
- 21.Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, et al. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol. 2004;14:1703–11. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao VA, Fan AM, Meng L, Doe CF, North PS, Hickson ID. Phosphorylation of BLM, dissociation from topoisomerase IIIalpha, and colocalization with gamma-H2AX after topoisomerase I-induced replication damage. Mol Cell Biol. 2005;25:8925–37. doi: 10.1128/MCB.25.20.8925-8937.2005. et ak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–14. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.di Masi A, Viganotti M, Polticelli F, Ascenzi P, Tanzarella C, Antoccia A. The R215W mutation in NBS1 impairs gamma-H2AX binding and affects DNA repair: molecular bases for the severe phenotype of 657del5/R215W Nijmegen breakage syndrome patients. Biochem Biophys Res Comm. 2008;369:835–40. doi: 10.1016/j.bbrc.2008.02.129. [DOI] [PubMed] [Google Scholar]
- 25.Nelson G, Buhmann M, von Zglinicki T. DNA damage foci in mitosis are devoid of 53BP1. Cell Cycle. 2009;8:3379–83. doi: 10.4161/cc.8.20.9857. [DOI] [PubMed] [Google Scholar]
- 26.Jullien D, Vagnarelli P, Earnshaw WC, Adachi Y. Kinetochore localisation of the DNA damage response component 53BP1 during mitosis. J Cell Sci. 2002;115:71–9. doi: 10.1242/jcs.115.1.71. [DOI] [PubMed] [Google Scholar]
- 27.Song HY, Joo JM, Kang JW, Kim DS, Jung CK, Kwak HS, et al. Lasonolide A: structural revision and total synthesis. J Org Chem. 2003;68:8080–7. doi: 10.1021/jo034930n. [DOI] [PubMed] [Google Scholar]
- 28.Cole A. Chromosome structure. Theoretical and experimental biophysics. 1967;1:305–75. [Google Scholar]
- 29.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 30.Redon C, Dickey JS, Bonner WM, Sedelnikova O. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res. 2009;43 doi: 10.1016/j.asr.2008.10.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–77. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Kaneko I. Changes in nuclease sensitivity of mammalian cells after irradiation with 60Co., gamma-rays. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;48:389–95. doi: 10.1080/09553008514551391. [DOI] [PubMed] [Google Scholar]
- 33.Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nature Cell Biol. 2006;8:870–6. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
- 34.Murga M, Jaco I, Fan Y, Soria R, Martinez-Pastor B, Cuadrado M, et al. Global chromatin compaction limits the strength of the DNA damage response. J Cell Biol. 2007;178:1101–8. doi: 10.1083/jcb.200704140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terasima T, Tolmach LJ. Variations in several responses of HeLa cells to x-irradiation during the division cycle. Biophys J. 1963;3:11–33. doi: 10.1016/s0006-3495(63)86801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson GD. Radiation and the cell cycle, revisited. Cancer Metastasis Rev. 2004;23:209–25. doi: 10.1023/B:CANC.0000031762.91306.b4. [DOI] [PubMed] [Google Scholar]
- 37.Zha S, Boboila C, Alt FW. Mre11: roles in DNA repair beyond homologous recombination. Nat Struct Mol Biol. 2009;16:798–800. doi: 10.1038/nsmb0809-798. [DOI] [PubMed] [Google Scholar]
- 38.Eliezer Y, Argaman L, Rhie A, Doherty AJ, Goldberg M. The direct interaction between 53BP1 and MDC1 is required for the recruitment of 53BP1 to sites of damage. J Biol Chem. 2009;284:426–35. doi: 10.1074/jbc.M807375200. [DOI] [PubMed] [Google Scholar]
- 39.Bekker-Jensen S, Lukas C, Melander F, Bartek J, Lukas J. Dynamic assembly and sustained retention of 53BP1 at the sites of DNA damage are controlled by Mdc1/NFBD1. J Cell Biol. 2005;170:201–11. doi: 10.1083/jcb.200503043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minter-Dykhouse K, Ward I, Huen MS, Chen J, Lou Z. Distinct versus overlapping functions of MDC1 and 53BP1 in DNA damage response and tumorigenesis. J Cell Biol. 2008;181:727–35. doi: 10.1083/jcb.200801083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Ma Z, Treszezamsky A, Powell SN. MDC1 interacts with Rad51 and facilitates homologous recombination. Nat Struct Mol Biol. 2005;12:902–9. doi: 10.1038/nsmb991. [DOI] [PubMed] [Google Scholar]
- 42.Xie A, Hartlerode A, Stucki M, Odate S, Puget N, Kwok A, et al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. Mol Cell. 2007;28:1045–57. doi: 10.1016/j.molcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–26. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura A, Sedelnikova OA, Redon C, Pilch DR, Sinogeeva NI, Shroff R, et al. Techniques for gamma-H2AX detection. Methods Enzymol. 2006;409:236–50. doi: 10.1016/S0076-6879(05)09014-2. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan BA, Karpen GH. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol. 2004;11:1076–83. doi: 10.1038/nsmb845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.