Abstract
Temporal lobe epilepsy (TLE) is the most prevalent type of human epilepsy, yet the causes for its development, and the processes involved, are not known. Most individuals with TLE do not have a family history, suggesting that this limbic epilepsy is a consequence of acquired rather than genetic causes. Among suspected etiologies, febrile seizures have frequently been cited. This is due to the fact that retrospective analyses of adults with TLE have demonstrated a high prevalence (20->60%) of a hisrory of prolonged febrile seizures during early childhood, suggesting an etiological role for these seizures in the development of TLE. Specifically, neuronal damage induced by febrile seizures has been suggested as a mechanism for the development of mesial temporal sclerosis, the pathological hallmark of TLE. However, the statisrical correlation between febrile seizures and TLE does not necessarily indicate a causal relationship. For example, preexisting (genetic or acquired) ‘causes’ that result independently in febrile seizures and in TLE would also result in tight statistical correlation. For obvious reasons, complex febrile seizures cannot be induced in the human, and studies of their mechanisms and of their consequences on brain molecules and circuits are severely limited. Therefore, an animal model was designed to study these seizures. The model reproduces the fundamental key elements of the human condition: the age specificity, the physiological temperatures seen in fevers of children, the length of the seizures and their lack of immediate morbidity. Neuroanatomical, molecular and functional methods have been used in this model to determine the consequences of prolonged febrile seizures on the survival and integrity of neurons, and on hyperexcitability in the hippocampal-limbic network. Experimental prolonged febrile seizures did not lead to death of any of the seizure-vulnerable populations in hippocampus, and the rate of neurogenesis was also unchanged. Neuronal function was altered sufficiently to promote synaptic reorganization of granule cells, and transient and long-term alterations in the expression of specific genes were observed. The contribution of these consequences of febrile seizures to the epileptogenic process is discussed.
Introduction: The Human Problem
Among the epilepsies, temporal lobe epilepsy is often intractable and is associated with significant morbidity in terms of cognitive and psychosocial dysfunction. The most common pathology identified in resected temporal lobe tissue from patients with intractable TLE is the constellation of mesial temporal lobe sclerosis.6 This entity is characterized by selective neuronal loss, gliosis and synaptic reorganization in discrete regions of the hippocampal formation and related structures.6,16,30,56 The neuroanatomical alterations of the mesial temporal lobe, and particularly the hippocampus, can be observed using sophisticated neuroimaging studies, including magnetic resonance imaging15,17,21,33 permitting their recognition in vivo, in individual patients. The convergence of temporal lobe seizures, MRI changes and the pathological findings of mesial temporal sclerosis have been increasingly recognized as a distinct entity, mesial temporal lobe epilepsy, which is quite likely the most common of all epileptic syndromes in humans.25
Whereas the neuroparhological features of mesial TLE, i.e., mesial temporal sclerosis, have been defined and extensively studied for decades, the relationship of the anatomical abnormalities to the seizures has remained controversial.27,52,55,57,81 A significant body of evidence, including the presence of early features of mesial temporal sclerosis in young children, suggests that in some patients mesial temporal sclerosis precedes the TLE, and is thus not a consequence of the seizures.18,31,55 This has been interpreted to suggest that the hippocampal injury is also the cause of the TLE. In contrast, progression of the hippocampal lesion on magnetic resonance imaging in individuals with TLE who were imaged repetitively, and a correlation of the hippocampal atrophy with the number of partial and generalized seizures have also been reported.13,49,61,78 These observations are in support of the notion of induction of mesial temporal sclerosis by the seizures themselves. These conflicting views illustrate that an understanding of the causal relationship of neuronal loss in the hippocampal formation to temporal lobe seizures remains incomplete, and is further hampered by the difficulty inherent in human studies, i.e., their correlational nature.
A second striking correlation found in patienrs with TLE is the frequent history of childhood febrile seizures, and particularly prolonged ones.72 Thus, whereas the overall frequency of febrile seizures of the general population in western countries is 2-5%,42,74 retrospective analyses of populations with intractable TLE indicate a frequency of a febrile seizure history of 20->60%.1,17,35,40,65 This remarkable statistical relationship has raised the hypothesis that febrile seizures—particularly complex ones (i.e., focal, prolonged or repetitive)—may produce hippocampal injury that evolves into mesial temporal sclerosis.41,48,76,79 However, the high concordance of childhood febrile seizures in patients with mesial TLE is also consistent with a functional or structural ‘predisposing factor’ that leads independently to both conditions. Put differently, a genetically determined malformation or molecular dysfunction, or an early ‘acquired’ lesion or insult (e.g., pre- or perinatal injury or infection) may predate and actually cause both the hippocampal injury/mesial temporal sclerosis, as well as the complex febrile seizures.8,17,18,31,52,73
Given the high frequency of both febrile seizures and TLE, understanding the true impact of the former, and specifically their causal relationship to TLE, is of enormous clinical significance. However, studying the key questions relating to the acute and chronic effects of febrile seizures on neuronal integrity and function cannot be achieved in the human, for obvious reasons: Ethical considerations prevent the induction of febrile seizures in humans, and naturally occurring febrile seizures are typically sudden and unexpected, and rarely occur in circumstances where electrographic monitoring is possible. Funhermore, the evolution of molecular and fine structural alterations cannot be studied in the live human with currently available technology. Thus, studying febrile seizures and their consequences on the immature brain requires controlled and reproducible experiments which can only be achieved in an appropriate animal model. Here we describe such an immature rat model for prolonged febrile seizures, and discuss the contribution of data obtained using this model to the understanding of the consequences of complex febrile seizures on the developing hippocampal circuit.
The ‘Optimal’ Animal Model
Animal models used for the study of human conditions are by their nature only an approximation of the ‘real’, actual disorder. Therefore, care should be taken to define the key characteristics which are essential for any meaningful modeling of the human condition. In addition, the features and nature of a given model dictate the scope of questions that can be addressed using it. For example, substitution of hyperthermia for fever (it should be noted that hyperthermia, typically drug-induced, is a not uncommon cause of seizures in children22,47,54) does not permit determination of the mechanisms involved in fever generation. Thus, it is conceivable that separate models might be needed ro address different questions related to the same human problem. In addition to reproducing certain key elements of the human condition, it is advantageous for a model to be relatively simple and inexpensive, and much should be known about the relevant brain structures. These considerations have led to the choice of rodents over primates. In the following paragraphs we discuss several other characteristics of a meaningful animal model for prolonged febrile seizures.
Age Specificity
Febrile seizures are seen almost exclusively in infants and young children, specifically between 6 months and ~ 5 years of age, with peak incidence at ~ 18 months.42 An appropriate rat model for febrile seizures should therefore employ rats which are in a developmental age equivalent to the seizure-sensitive period in humans. However, data comparing rodent and human brain development are rare. In addition, different brain regions develop at variable rates and chronological ages, in terms of neurogenesis, migration, connectivity and function, and these processes are not necessarily parallel in human and rat. These facts are important considerations in defining the ‘appropriate age’ of an animal model of febrile seizures.
Early, detailed studies correlated rat and human brain development based on neuronal birth dates, myelination and saltatory growth stages, and suggested that the 5-7 day old rat may be “equivalent” to the human full-term newborn.24,39 More selective comparative neuroanatomical scudies have focused on maturational milestones in discrete limbic regions, specifically the hippocampus and the prefrontal cortex.7,43 Overall, these converging and complementary studies suggest that in the rat the first postnatal week may be comparable to the third trimester gestational period of the human fetus, and the second postnatal week to the first year of human life. For the hippocampal formation, specifically, we have recently summarized the information on comparative developmental milestones in human and rodent (see Table 1).7 For example, comparison of the maturation of synaptic communication indicates that the maturational state of the hippocampus of a 8 day old rat4,64 is roughly equivalent to the maturational state of the human hippocampus at 7 months of age (see refs. 7, 71 for analysis of hippocampal neurogenesis, connectivity and maturation of select synapses). Thus, based on anatomical data, rat hippocampal development during the second postnatal week seems to correspond best to me developmental stage at which human infants and young children are most susceptible to febrile seizures.
Table 1.
Selected milestones in hippocampal development: Human and rat
| Category | Event | Human | Rat |
|---|---|---|---|
| General | Maximal growth velocity | 2-3 postnatal months | 8-12 postnatal days |
| Hippocampal volume approximates that of adult | 10 postnatal months | 11-30 postnatal days | |
| Hippocampal-dependent learning/ memory function | 4-5 postnatal years | 15-16 postnatal days | |
| Neuronal | Birth of pyramidal cells | 1st half of gestation | 2nd half of gestation |
| Formation | Onset: | before 15th week | ~15th day |
| End: | 24th week | ~19th day | |
| Birth of dentate gyrus (DG) granule cells | ~70% prenatal (majority by 34th week) | ~85% postnatal (majority by 1st postnatal month) | |
| Onset: | 13-14th weeks | ~18th prenatal day | |
| End: | throughout life | throughout life | |
| Differentiation, Synaptogenesis | ‘Thorny excrescences’ on proximal CA3 pyramidal cell dendrites. | postnatal years | 1st postnatal month |
| Onset: | 3-7 months | ~9th day | |
| Maturation: | 3-5 years | 21st day | |
| Pedunculate spines: distal CA3 pyramidal cell dendrites | postnatal years | 1st postnatal month | |
| Onset: | birth | 7th day | |
| Maturation: | 3-5 years | 21st day | |
| Peak synapse overshoot (DG) | 1-2 postnatal years | 9-14 postnatal days | |
| Synapse density reaches adult levels: DG molecular layer | 7-10 postnatal months | 21st postnatal day | |
| Period of maximal mossy cell differentiation (DG) | 7-30 postnatal months (adult-like by 5 years) | 7-14 postnatal days (adult-like by 14th day) | |
| Afferent input | Entorhinal cortex toDG | Prenatal: 19-20th weeks | 17th prenatal day |
| Supramammillary afferents reach juxtagranular and CA2 pyramidal cell layers | Prenatal: ~20th week | Presumed 1st postnatal week |
Note: Gestation lasts 270-280 and 21 days in human and rat, respectively.
Modified from ref. 7, with permission.
The ontogenetic profile of physiological responses of the developing rat brain to hyperthermia supports the end of the second postnatal week as the ‘rat equivalent’ of the human susceptibility period for febrile seizures: The threshold temperatures required to generate experimental febrile seizures are lowest during postnatal days 10-13, and rise rapidly thereafter44 (Eghbal-Ahmadi & Baram, unpublished observations). Thus, because febrile seizures are a developmental phenomenon confined to infants and young children, and because limbic neuronal circuits (and particularly the hippocampal formation) are those suspected of involvement in and vulnerability to febrile seizures, models of febrile seizures should employ developing animals in which the stage of development of these limbic structures corresponds to the state of maturation of the human infant and young child, i.e., the middle of the second postnatal week in rat, and the end of the second postnatal week in mouse.
Temperature
Febrile seizures are defined as those occurring in children with fever (rectal temperarure of at least 38.4°C) but without evidence of intracranial infection.60 In addition, it has been suggested that in most normal children, threshold temperatures for febrile seizures exceed ~ 41°C.50 Hence, animal models for febrile seizures should involve temperatures which are relevant to the human condition, and are observed in children with fever and should not rely on extreme temperatures (e.g., ref 59). The ability to tightly regulate the temperature is an advancage of a hyperthermia-based model compared to fever-inducing agents. In addition, the majority of established pyrogens do not induce substantive fever in the immature rat32,51 (Hatalski & Baram, unpublished observations).
The relationship of brain and core temperatures should be carefully considered. In animal models, core temperature is routinely monitored, rather than direct measurements of brain temperatures. It should be noted that the relationship between the two may not be consistent throughout the range of temperatures induced to provoke a hyperthermic seizure.75 This may result in core temperatures that do not reflect actual brain temperatures. Therefore, calibration and standardization of parameters that influence the relationship of core and brain temperatures: the rate of heating, and volume, direction and diffusion of the heat, should carefully be standardized. Ideally, chronic measurement of brain temperature should be employed, but this is not feasible in the immature rat.
Ascertainment and Localization the Seizures
The behaviors induced by hyperthermia may resemble those seen during seizures, but automatisms, stiffening and other motor phenomena may result from nonconvulsive discharges in brainstem, basal ganglia or other subcortical regions. Therefore, electrophysiological correlation of such observed behaviors should be obtained from behaving animals. In infant rats, hyperthermia induces stereotyped seizure behaviors27,44 consisting of tonic body flexion accompanied by biting and chewing (‘facial myoclonus’). These are typical for seizures of limbic origin.9,46 Electrographic correlates should therefore be sought also in limbic regions, particularly in amygdala and hippocampus. In addition to pinpointing the likely source of the seizures, electrophysiological recording also from limbic rather than only from cortical regions is justified by the normal sequence of maturation in these regions: cortical maturation is incomplete during the second postnatal week in the rat, resulting typically in poorly organized and low-voltage cortical EEG activity.9,69 It should be noted that because EEG correlations of genuine febrile seizures in humans are exceedingly rare,59 they are not helpful in guiding placement of electrodes in experimental animals.
Absence of Immediate Morbidity and Mortality
Febrile seizures, whether single, short and nonfocal (simple) or recurrent, more prolonged or focal (complex) are typically not associated with immediate morbidity or mortality. Therefore, an appropriate animal model of febrile seizures should demonstrate similar low morbidity and mortality. In addition, a fundamental question related to hyperthermic seizures in the young human is whether they result in long-term effects, i.e., loss of hippocampal neurons and/or alteration of hippocampal circuitry leading to epilepsy. Therefore, an optimal model should be suitable for long-term survival without the confounding effects of major stress, burns, infection or general moribund states. An optimal model should use a defined, benign mechanism for increasing brain and core temperatures, which is suitable for repeated exposures. In summary, benign outcome not only reproduces the human situation, but permits meaningful prospective long-term studies of the long-term consequences of these seizures on epileprogenesis and neuronal function in general.
Hyperthermic Controls
The goals of setting up models of febrile seizures are to study the mechanisms or the outcomes of these seizures. However, by definition, each model involves subjecting me brain to hyperthermia, to simulate fever. Therefore, the effects of the hyperthermia per se must be distinguished from those of the associated seizures. The potential effects of hyperthermia may not be inconsequential Hyperthermia induces a significant number of genes (e.g., heat shock proteins), and both deleterious and protective effects on neuronal function and integrity. For example, hyperthermia enhances seizure severity and the neuronal loss induced by kainic acid,53 and when extreme, results in neuronal injury by itself.38 Therefore, experiments using febrile seizure models should compare three sets of animals: normothermic controls, hyperthermic controls, in which hyperthermia was induced, but seizures were prevented14,19,27,76 and the experimental group which has experienced both hyperthermia and seizures.
The Immature Rat Model
Based on the criteria outlined above, a rat model for prolonged febrile seizures was developed:27,28,78 In this model, 10-11 day-old Sprague-Dawley rats are subjected to hyperthermia which raises body and brain temperatures gradually via a regulated stream of moderately heated air (using a hair-dryer, low-intermediate settings, air temperature ~43°C). The air stream is directed ~ 30 cm above the rats, which are placed (1-2 at a time) on a towel in a 3 liter glass jar. Core temperatures are measured prior to initiating the hyperthermia, then every two minutes as well as at the onset of hyperthermia-induced seizures. These core temperatures have been extensively correlated with brain temperatures (Eghbal-Ahmadi and Baram, unpublished observations). In over 400 animals, we have found that raising core and brain temperatures to an average 40.88°C resulted in behavioral seizures in over 98%.
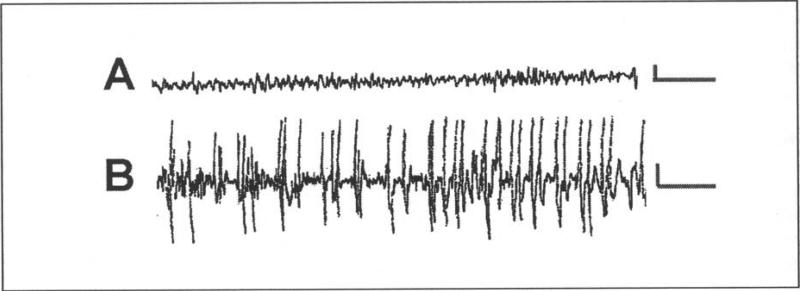
As mentioned above, the behavioral seizures are stereoryped, consisting of arrest of the heat-induced hyperkinesis, body flexion and biting of an extremity, occasionally followed by clonus. The epileptic nature of these seizures was confirmed by electrophysiological recording from the hippocampi of behaving pups, using bipolar electrodes. As shown in (Fig. 1), hyperthermia induces a change in hippocampal activity from a nonrhythmic pattern in the theta range (Fig. 1A) to the onset of rhythmic epileptiform spikes (Fig. 1B), which correlate wim the onset of behavioral seizures. In hyperthermic controls, given the rapid- and short-acting barbiturate pentobarbital prior to the procedure, the behavioral as well as the electrophysiological seizures are blocked.
Figure 1.
Electrophysiological characteristics of hyperthermia-induced seizures in immature rats. Records were performed via bipolar hippocampal electrodes in freely-moving 11-day-old rats. A) Baseline tracing of hippocampal activity during normothermia, showing a nonrhythmic pattern in the theta range. B) The hyperthermia procedure provoked hippocampal electrographic seizures, manifest as trains of spike-waves. Calibration: vertical, 50 mV; horizontal 1 sec.
Animals are maintained hyperthermic (39-41.5°C) for 30 minutes, which is designed to generate seizures lasting about 20 minutes. This reproduces the human condition of prolonged, or complex febrile seizures (defined as longer than 15 minutes, and comprising only ~ 10% of all febrile seizures.12 It is these longer seizures which have been statistically implicated in the development of TLE.5,12,76 The 20-minute duration also avoids the onset of status epilepticus (defined as continuous seizures for 30 minutes), which may carry distinct implications for outcome (see refs. 2, 23). Following the hyperthermia, animals are moved to a cool surface to regain normal body and brain temperatures, then returned to their mothers for rehydration. It should be noted that weighing the animals before and after the procedure indicates little evidence of dehydration < 3% change in body weight). Furthermore, animals regain normal activity rapidly after the procedure, and mortality has been <1%.
In summary, the immature rat model described above reproduces key features of prolonged febrile seizures, those that have been statistically correlated with the development of epilepsy and/or the presence of mesial temporal sclerosis. Using this model, we have started addressing the question of whether the relationship of these prolonged febrile seizures to neuronal loss and hyperexcitability is causal. In other words, the experiments described below query whether prolonged experimental febrile seizures cause epilepsy. Furthermore, if these seizures do induce hippocampal hyperexcitability, what are the underlying mechanisms?
Do Prolonged Experimental Febrile Seizures Increase Seizure Susceptibility?
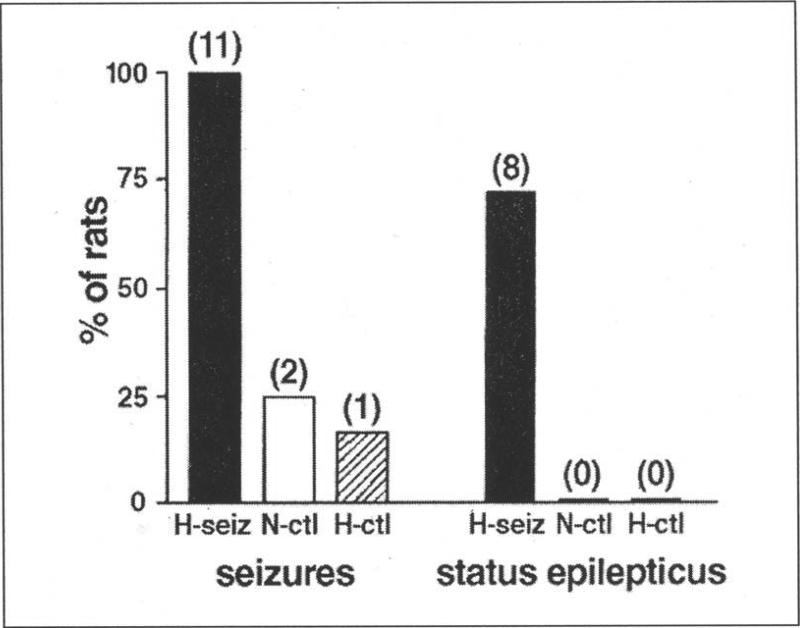
To determine whether the susceptibility to seizures is altered after prolonged febrile seizures, rats were allowed to mature (three months), and then underwent extensive hippocampal-electrophysiology and behavioral seizure monitoring.27 Both the hippocampal tracings and the behavioral measures to date have failed to demonstrate the occurrence of spontaneous seizures. However, when challenged with a sub-convulsant dose of the AMPA/kainate-type glutamate receptor agonist kainic acid, adult animals which had sustained developmental febrile seizures were far more sensitive than age-matched controls to the development of further seizures. In essence, a dose that failed to provoke seizures in normothermic and hyperthermic littermate controls led to severe seizures in all adult animals which had sustained prolonged experimental febrile seizures early in life (Fig. 2), demonstrating a ~four-fold increased sensitivity to kainic acid. This increased susceptibility to limbic convulsants was confirmed in vitro:27 Spontaneous epileptiform discharges were not observed in hippocampal-entorhinal cortex slices derived from either control or experimental groups. However, Schaffer collateral stimulation induced prolonged, self-sustaining, status-epilepticus-like discharges exclusively in slices from experimental rats. These data indicate that experimental prolonged febrile seizures do not cause spontaneous limbic seizures during adulthood. However, they induce persistent enhancement of hippocampal excitability that may facilitate the emergence of subsequent seizures in response to even a mild (and perhaps not clearly demonstrable in the human situation) trigger later in life.
Figure 2.
Differential induction of seizures and of status epilepticus in adult rats by low-dose kainic acid as a function of prolonged hyperthermic seizures early in life. Kainic acid led to seizures in all adult rats that had experienced prolonged hyperthermic seizures on postnatal days 10-11 (H-seiz; n=11). The majority of these (n=8) developed status epilepticus. In contrast, only 2 out of 8 normothermic (N-ctl) and 1 out of 6 hyperthermic control rats developed brief seizures, none of them status epilepticus (reproduced from ref. 27, with permission).
Do Prolonged Experimental Febrile Seizures Cause Neuronal Death and/or Synaptic Reorganization?
Neuronal loss and resulting changes in hippocampal circuitry (e.g., mossy fiber sprouting) in specific hippocampal subfields are characteristic of mesial temporal sclerosis in patients with TLE (reviewed in refs. 6, 45). The loss of seizure-sensitive neuronal populations can critically alter the balance of excitation and inhibition in the hippocampus, which may lead co long-term hyperexcitability and a reduced seizure threshold later in life, as indeed found in this model of prolonged febrile seizures. Therefore, we studied the short- and long-term effects of experimental febrile seizures on neuronal survival and synaptic connectivicy.
Acute neuronal death was studied using the in situ end labeling (ISEL) technique for visualizing apoptotic cell death, as well as using the Gallyas silver stain method (“dark” neuron36) for visualizing neuronal injury. ISEL demonstrated no evidence for acute neuronal death in the hippocampus when studied 1, 4, 8.5, 24 or 48 hours after the seizures. However, the seizures did impact neuronal structure: the Gallyas method demonstrated dark, argyrophilic neurons starting within 24 hours and lasting as long as two weeks after the seizures. Whereas the precise mechanisms which render neurons argyrophilic are not known, selective uptake of the silver stain is considered to arise from alterations in proteins constituting the cytoskeleton. These changes have often been suggested to signify cell death. However, such ‘dark’ neurons can also be generated by subjecting the brain to postmortem trauma, indicating that this process is independent from the process of cell death.37 Indeed, neuronal counts carried out in the central nucleus of the amygdala, where ~30% of neurons became silver-stained after experimenral febrile seizures, demonstrated no loss of cells.78 These findings suggest that the onset of the avidity to silver may not necessarily mean neuronal death: the changes or injury which render a cell argyrophilic may be reversible and not lead to cell loss.
That experimental prolonged febrile seizures do not cause neuronal cell death was further confirmed in a long-term study,11 in which neuronal densities in the hippocampal formations of seizure-experiencing animals and age-matched controls were analyzed three months after the seizures. No difference was evident in the neuronal numbers of specific, seizure-sensitive hippocampal cell populations of these experimental groups. However, the density of the mossy fibers, the axons of granule cells, in granule cell and molecular layers was significantly increased in seizure-experiencing compared to control rats 3 months after the seizures. These findings indicate that despite the absence of seizure-induced neuronal loss, reorganization of the hippocampal circuit, evident by mossy fiber sprouting, did occur.
Do Prolonged Experimental Febrile Seizures Alter the Rate of Granule Cell Neurogenesis?
Altered neurogenesis of dentate gyrus granule cells, promoting aberrant, excitatory connectivity in the hippocampus, has recently been proposed as an additional mechanism by which seizures can modulate the hippocampal network.63 Seizure-induced neurogenesis may be particularly disruptive during hippocampal development, since neurogenesis in the dentate gyrus peaks during the first and second postnatal weeks.3,70 Therefore, we examined the influence of experimental prolonged febrile seizures on granule cell proliferation: Rats experiencing experimental febrile seizures and age-matched controls were injected with BrdU 3, 7 or 28 days after the seizures, and numbers of BrdU-labeled cells were determined 48 hrs later. No differences were found between seizure-experiencing and control animals at any of the time-points studied.11 Thus, although granule cell neurogenesis in the immature hippocampus may be influenced by seizures during development.11,58,66 prolonged febrile seizures had no significant effect on this process. This might be due to the relatively short duration of these seizures, or to other, as yet unresolved model-specific factors.
Molecular Plasticity after Experimental Prolonged Febrile Seizures
Electrophysiological analyses in acute hippocampal slices from seizure-experiencing rats revealed a surprising result: Despite the increased network hyperexcitability in the hippocampus, the inhibitory perisomatic drive onto CA1 pyramidal cells was increased, rather than decreased.19 Conversion of enhanced, GABA-mediated hyperpolarization into neuronal depolarization may be mediated by activation of the hyperpolarization-activated, cyclic nucleotide-gated (Ih) current.26,62 This led to the hypothesis that the Ih-current was altered after prolonged febrile seizures.20,77 Indeed, whole-cell patch clamp recordings from CA1 pyramidal cells demonstrated that the biophysical properties of the Ih-current were altered by the experimental febrile seizures.20 In slices from seizure-experiencing rats, the Ih-current was activated and deactivated much more slowly, and its half-maximal activation (V50) was shifted towards a more depolarized membrane potential. Both changes opposed the increased presynaptic hyperpolarizing input, and could convert it to a depolarizing overshoot and action potential burst firing.20,77 These changes persisted for at least 3 months.
In teasing out the mechanisms which might mediate these changes of the Ih-current, it was found that, unlike typical short-term modulation of the properties of the current, they did not depend on alteration of cellular cyclic nucleotides. This led to the notion that this long-lasting alteration of the Ih-current might derive from transcriptional regulation of the molecules which constitute the h-channels: The Ih-current is generated by a specific type of channels, the hyperpolarization-activated cyclic nucleotide-gated cation channels (HCNs). Recently, four different genes encoding HCN isoforms (HCN1-4) have been discovered, each isoform forming channels with significantly differing physiological properties (reviewed in ref. 67). Three of these isoforms (HCN1, HCN2, HCN4) are expressed in CA1 pyramidal cells of the immature rat during the age when febrile seizures can be provoked.10 The relative abundance of each of these isoforms in a given cell has been shown to be critical for the physiological properties of the channels, which, in turn, govern the overall HCN properties of the cell34,68 In CA1 pyramidal cells, the HCN1 isoform, forming fast activating and deactivating channels with limited conductance, seems to be dominant under normal conditions.34,68 However, recent results indicate that prolonged experimental febrile seizures—occurring during a period of rapid evolution of the HCN isoform expression pattern10—influence the mRNA and protein expression of these channel molecules. The expression of HCN1 mRNA was significantly decreased and the expression of HCN2 mRNA significantly increased in seizure-sustaining animals by one week later.14 Both of these changes increase the relative abundance of HCN2 compared to HCN1 in a given neuron, favoring the formation of slower kinetics (and potentially larger-conductance) HCN2-homomeric channels, with altered biophysical properties. This alteration in the molecular make-up of the HCNs would promote neuronal activity-dependent depolarization and enhance the excitability of the hippocampal circuit.
Summary
What Has the Immature Rat Model of Prolonged Febrile Seizures Taught Us so Far?
The original working hypotheses driving these studies of experimental febrile seizures suggested that these seizures would either kill vulnerable neurons, or lead only to transient injury, without long-term effects on the hippocampal circuit. As evident from the data above, both hypotheses were refuted. The scenario emerging from the experimental data indicates that the process of epileptogenesis in the immature rat—the transformarion of a ‘normal’ limbic network to a pro-epileptic one—is far more intricate and subtle than a simple composite of direct or compensatory changes in response to cell death.
Indeed, the data cearly indicate that experimental prolonged febrile seizures do not result in death of neurons in amygdala and hippocampus. Vulnerable populations, such as the mossy cells or specific interneuronal subtypes, were specifically labeled and counted, and no loss or reduction in their numbers were found.11 The preservation of neuronal numbers was not due to the birth of new neurons, since BrdU analyses demonstrated that the rate of neurogenesis was not altered.
However, prolonged experimental febrile seizures were not “benign”. In the aftermath of the seizures, the hippocampus was far more susceptible to minor excitatory input (electrical current in the slice, kainic acid in vivo) compared with a hippocampus not previously involved in febrile seizures. These striking and long-lasting changes rendered the animal more likely to generate seizures. Thus, pro-epileptogenic changes may occur without the requirement for neuronal death.
Insight into the mechanisms contributing to the hyperexcitability resulting from prolonged experimental febrile seizures was derived from electrophysiological and molecular analyses, which, to date, have provided specific clues. Early (within hours of the seizures) regulation of calcium entry is modified, due to transient reduction of GluR2 expression and creation of calcium-permeable AMPA channels.29 By several days after the seizures, striking changes in ion channels, specifically in the molecular make-up—and hence the kinetics and voltage-dependence—of the HCNs, emerge and persist long-term. These contribute significantly to conversion of augmented GABA-induced hyperpolarization to activity-dependent hyperexcitation. Many questions remain: How do the seizures lead to down-regulation of GluR2? What other critical alterations result from altered calcium entry? What are the mechanisms governing HCN expression in a spatially and temporally constrained pattern?
These and related questions are the focus of ongoing studies. These studies are carried out in the hope that they will lead to further clues and to the discovery of the specific molecular targets, the key determinants, of this seizure-induced long-term hyperexcitability. It is the discovery of such specific molecular targets which could lead to the design of compounds which will specifically prevent the consequences of the seizures, and thus perhaps prevent these pro-epileptogenic effects of prolonged febrile seizures.
Acknowledgment
Authors work supported by NIH NINDS 35439 (TZB) and Epilepsy Foundation and Milken family awards (RAB, CD).
References
- 1.Abou-Khalil B, Andermann E, Andermann F, et al. Temporal lobe epilepsy after prolonged febrile convulsions: excellent outcome after surgical treatment. Epilepsia. 1993;34:878–883. doi: 10.1111/j.1528-1157.1993.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 2.Alldredge BK, Lowenstein DL. Status epilepticus: new concepts. Curr Opin Neurol. 1999;12:183–190. doi: 10.1097/00019052-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 4.Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 5.Annegers JF, Hauser WA, Shins SB, et al. Factors prognoStic of unprovoked seiwrcs after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neu ropath Exp Neurol. 1993;52:433–443. doi: 10.1097/00005072-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Avishai-Elincr S, Brunson KL, Sandman CA, et al. Stressed out or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baram TZ. Mechanisms and outcome of febrile seiwrcs: What have we learned from basic science approaches, and what needs studying? In: Baram TZ, Shinnar S, editors. Febrile seiwres. Academic Press; San Diego, CA: 2002. pp. 325–328. [Google Scholar]
- 9.Baram TZ, Hirsch E, Snead OC, III, et al. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender RA, Brewster A, Santoro B, et al. Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus. Neuroscience. 2001;106:689–698. doi: 10.1016/s0306-4522(01)00314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender RA, Dubé C, Gonzalez-Vega R, et al. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2002;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126–133. doi: 10.1111/j.1528-1157.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 13.Bower SP, Kilpatrick CJ, Vogrin SJ, et al. Degree of hippocampal atrophy is not related to a histOry of febrile seizures in patients with proved hippocampal sclerosis. J Neurol Neurosurg Psychiatry. 2000;69:733–8. doi: 10.1136/jnnp.69.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewster A, Bender RA, Chen Y, et al. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform and cell-specific manner. J Neurosci. 2002:22A591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briellmann RS, Kalnins RM, Berkovic SF, et al. Hippocampal pathology in refractory temporal lobe epilepsy. T2-weighted signal change reflects dentate gliosis. Neurology. 2002;58:265–271. doi: 10.1212/wnl.58.2.265. [DOI] [PubMed] [Google Scholar]
- 16.Bruton CJ. The neuropathology of temporal lobe epilepsy (Maudsley Monographs. No 31) Oxford University Press; New York, NY: 1988. [Google Scholar]
- 17.Cendes F, Andermann F, Dubeau F, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures and temporal lobe epilepsy. An MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- 18.Cendes F, Cook MJ, Watson C, et al. Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology. 1995;45:2058–2064. doi: 10.1212/wnl.45.11.2058. [DOI] [PubMed] [Google Scholar]
- 19.Chen K, Baram TZ, Soltesz I, et al. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K, Aradi I, Thon N, et al. Persistently modified h-channels after complex febri le seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook MJ, Fish DR, Shorvon SD, et al. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain. 1992;115:1001–1015. doi: 10.1093/brain/115.4.1001. [DOI] [PubMed] [Google Scholar]
- 22.Cooper AJ, Egleston C. Accidental ingestion of Ecstasy by a toddler: unusual cause for convulsion in a febrile child. J Accid Emerg Med. 1997;14:183–184. doi: 10.1136/emj.14.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulter DA, Delorenzo RJ. Basic mechanisms of status epilepticus. Adv Neurol. 1999;79:725–733. [PubMed] [Google Scholar]
- 24.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy. In: Engel J Jr, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincon-Raven Publishers; Philadelphia, PA: 1997. pp. 2417–2426. [Google Scholar]
- 26.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 27.Dubé C, Chen K, Eghbal-Ahmadi M, et al. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- 28.Dubé C. Do prolonged febrile seizures in an immature rat model cause epilepsy? In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 215–229. [Google Scholar]
- 29.Eghbal-Ahmadi M, Yin H, Stafstrom CE, et al. Altered expression of specific AMPA type glutamate receptor subunits after prolonged experimental febrile seizures in CA3 of immature rat hippocampus. Soc Neurosci Abstr. 2001;31:684.6. [Google Scholar]
- 30.Falconer MA, Serafetinides EA, Corsellis JAN. Etiology and pathogenesis of temporal lobe epilepsy. Arch Neurol. 1964;10:233–248. doi: 10.1001/archneur.1964.00460150003001. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez G, Effenbcrger O, Vinz B, et al. Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis. Neurology. 1998;50:909–917. doi: 10.1212/wnl.50.4.909. [DOI] [PubMed] [Google Scholar]
- 32.Fewell JE, Wong VH. Interleukin-1beta-induced fever does not alter the ability of 5- to 6-day-old rat pups to autoresuscitate from hypoxia-induced apnoea. Exp Physiol. 2002;87:17–24. doi: 10.1113/eph8702271. [DOI] [PubMed] [Google Scholar]
- 33.Fish DR, Spencer SS. Clinical correlations: MRI and EEG. Magn Reson Imaging. 1995;13:1113–1117. doi: 10.1016/0730-725x(95)02020-t. [DOI] [PubMed] [Google Scholar]
- 34.Franz O, Liss B, Neu A, et al. Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (lh) in central neurons. Eur J Neurosci. 2000;12:2685–2693. doi: 10.1046/j.1460-9568.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- 35.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 36.Gallyas F, Guldner FH, Zoltay G, et al. Golgi-like demonstration of “dark” neurons with an argyrophil III method for experimental neuropathology. Acta Neuropathol (Berlin) 1990;79:620–628. doi: 10.1007/BF00294239. [DOI] [PubMed] [Google Scholar]
- 37.Gallyas F, Zohay G, Horvath Z, et al. Light microscopic respo nse of neuronal somata, dendrites, axons to postmortem concussive head injury. Ana Neuroparhoi (Berlin) 1992;83:499–503. doi: 10.1007/BF00310026. [DOI] [PubMed] [Google Scholar]
- 38.Germano IM, Zhang YF, Sperber EF, et al. Neuronal migration disorders increase seizure susceptibility to febrile seizures. Epilepsia. 1996;37:902–910. doi: 10.1111/j.1528-1157.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 39.Gonlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- 40.Hamati-Haddad A, Abou-Khalil B. Epilepsy: diagnosis and localization in patients with antecedent childhood febrile convulsions. Neurology. 1998;50:917–922. doi: 10.1212/wnl.50.4.917. [DOI] [PubMed] [Google Scholar]
- 41.Harvey AS, Cranan-Smith JD, Desmond PM, et al. Febrile seizures and hippocampal sclerosis: frequent and rdated findings in intracrable temporal lobe epilepsy of childhood. Pediatr Neurol. 1995;12:201–206. doi: 10.1016/0887-8994(95)00022-8. [DOI] [PubMed] [Google Scholar]
- 42.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(Suppl 2):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 43.Herschkowin N, Kagan J, Zilles K. Neurobiological bases of behavioral development in the first year. Ncuropcdiatrics. 1997;28:296–306. doi: 10.1055/s-2007-973720. [DOI] [PubMed] [Google Scholar]
- 44.Hjeresen DL, Diaz J. Ontogeny of susceptibility to experimental febrile seizures in rats. Dev Psychobiol. 1988;21:261–275. doi: 10.1002/dev.420210307. [DOI] [PubMed] [Google Scholar]
- 45.Houser CR. Neuronal loss and synaptic rcorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–761. [PubMed] [Google Scholar]
- 46.Ikonomidou-Turski C, Cavalheiro EA, Turski WA, et al. Convulsant action of morphine. [D-A1a2, D-Leu5] -enkephalin and naloxone in the rat amygdala: electroencephalographic. morphological and behavioral sequelae. Neuroscience. 1987;20:671–686. doi: 10.1016/0306-4522(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 47.Ioos C, Fohlen M, Villeneuve N, et al. Hot water epilepsy: A benign and unrecognized form. J Child Neurol. 2000;15:125–128. doi: 10.1177/088307380001500211. [DOI] [PubMed] [Google Scholar]
- 48.Jackson GD, Mcintosh AM, Briellmann RS, et al. Hippocampal sclerosis studied in identical twins. Neurology. 1998;51:78–84. doi: 10.1212/wnl.51.1.78. [DOI] [PubMed] [Google Scholar]
- 49.Kälviäinen R, Salmonperä T, Pananen K, et al. Recurrent seizures may cause hippocampal damage in temporal lobe epilepsy. Neurology. 1998;50:1377–1382. doi: 10.1212/wnl.50.5.1377. [DOI] [PubMed] [Google Scholar]
- 50.Knudsen FU. Febrile seizures—treatment and outcome. Brain Dev. 1996;18:438–449. doi: 10.1016/s0387-7604(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 51.Lagerspetz KY, Vaatainen T. Bacterial endotoxin and infecrion cause behavioural hypothermia in infant mice. Comp Biochem Physiol A. 1987;88:519–521. doi: 10.1016/0300-9629(87)90074-0. [DOI] [PubMed] [Google Scholar]
- 52.Lewis DV. Febrile convulsions and mesial temporal lobe sclerosis. Curr Opin Neurol. 1999;12:197–201. doi: 10.1097/00019052-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z, Can A, Mikati M, et al. Effect of temperature on kainic acid-induced seizures. Brain Res. 1993;631:51–58. doi: 10.1016/0006-8993(93)91185-u. [DOI] [PubMed] [Google Scholar]
- 54.Mani KS, Mani AJ, Ramesh CK, et al. Hot-water epilepsy—a peculiar type of reflex epilepsy: clinical and EEG features in 108 cases. Trans Am Neurol Assoc. 1974;99:224–226. [PubMed] [Google Scholar]
- 55.Mathern GW, Babb TL, Vickrey BG, et al. The clinical-pathogenic mcchanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilcpsy. Brain. 1995;118:105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- 56.Mathern GW, Babb TL, Armstrong DL. Hippocampal sclerosis. In: Engel J Jr, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincott-Raven Publishers; Philadelphia, PA: 1997. pp. 133–155. [Google Scholar]
- 57.Mathern CW, Pretorius JK, Leite JP, et al. Hippocampal neuropathology in children with severe epilepsy. In: Nehlig A, Mone J, Moshe SL, Plouin P, editors. Childhood epilepsies and brain development. John Libbey & Co.; London. England: 1999. pp. 171–185. [Google Scholar]
- 58.McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morimoto T, Nagao H, Sano N, et al. Elecrroencephalographic study of rat hyperthermic seizures. Epilepsy. 1991;32:289–293. doi: 10.1111/j.1528-1157.1991.tb04653.x. [DOI] [PubMed] [Google Scholar]
- 60.Nationall nsitu tes of Heahh Febrile seizures: Consensus development conference summary. 2. Vol. 3. National Institutes of Health; Bethesda, MD: 1980. [Google Scholar]
- 61.O'Brien TJ, So EL, Meyer FB, et al. Progressive hippocampal atrophy in chronic intractable temporal lobe epilepsy. Ann Neurol. 1999;45:526–529. [PubMed] [Google Scholar]
- 62.Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 63.Parent JM, Yu TW, Leibowin RT, et al. Dentate granule cell neurogenesis is increased by seizures and contrib utes to aberrant network reorganilzation in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribak CE, Seress L, Amaral DG. The development, ultraslructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 65.Rocca WA, Sharbrough FW, Hauser WA, et al. Risk factors for complex partial seizures: a population-based case-control study. Ann Neural. 1987;21:22–3l. doi: 10.1002/ana.410210106. [DOI] [PubMed] [Google Scholar]
- 66.Sankar R, Shin D, Liu H, et al. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia Suppl. 2001;7:53–56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 67.Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann NY Acad Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- 68.Santoro B, Chen S, Lüthi A, et al. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schickerova R, Mares P, Trojan S. Correlation between electrocorticographic and motor phenomena induced by pentamerhylenetetrazol during ontogenesis in rats. Exp Neurol. 1984;84:153–164. doi: 10.1016/0014-4886(84)90012-8. [DOI] [PubMed] [Google Scholar]
- 70.Schlessinger AR, Cowan WM, Gottlieb ID. An auroradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–176. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- 71.Seress L, Mrzljak L. Postnatal development of mossy cells in the human dentate gyrus: a light microscopic Goigi study. Hippocampus. 1992;2:127–142. doi: 10.1002/hipo.450020205. [DOI] [PubMed] [Google Scholar]
- 72.Shinnar S. Prolonged febrile seizures and mesial temporal lobe sclerosis. Ann Neurol. 1998;43:411–412. doi: 10.1002/ana.410430402. [DOI] [PubMed] [Google Scholar]
- 73.Shinnar S. Human Data: What do we know about febrile seizures and what further information is needed? In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 317–324. [Google Scholar]
- 74.Stafsuom CE. The incidence and prevalence of febrile seizures. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 1–25. [Google Scholar]
- 75.Sundgren-Andersson AK, Ostlund P, Bartfai T. Simultaneous measurement of brain and core temperature in the rat during fever, hyperthermia, hypothermia and sleep. Neuroimmunomodulation. 1998;5:241–247. doi: 10.1159/000026344. [DOI] [PubMed] [Google Scholar]
- 76.Theodore WH, Bhatia S, Hatta J, et al. Hippocampal atrophy, epilepsy duration and febrile seizures in patients with partial seizures. Neurology. 1999;52:132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 77.Thon N, Chen K, Aradi I, et al. Physiology of limbic hyperexcitability after experimental complex febrile seizures: interactions of seizure-induced alterations at multiple levels of neuronal organization. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 203–213. [Google Scholar]
- 78.Toth Z, Yan XX, Hafroglou S, et al. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanlandingham KE, Heinz ER, Cavazos JE, et al. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 80.Van Paesschen W, Duncan JS, Stevens JM, et al. Longitudinal quantitative hippocampal magnetic resonance imaging study of adults with newly diagnosed partial seizures: one-year follow-up results. Epilepsia. 1998;39:633–639. doi: 10.1111/j.1528-1157.1998.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 81.Zimmerman HM. The histopathology of convulsive disorders in children. J Pediatr. 1940;13:359–390. [Google Scholar]