Abstract
Background
Many complex systems can be represented and analysed as networks. The recent availability of large-scale datasets, has made it possible to elucidate some of the organisational principles and rules that govern their function, robustness and evolution. However, one of the main limitations in using protein-protein interactions for function prediction is the availability of interaction data, especially for Mollicutes. If we could harness predicted interactions, such as those from a Protein-Protein Association Networks (PPAN), combining several protein-protein network function-inference methods with semantic similarity calculations, the use of protein-protein interactions for functional inference in this species would become more potentially useful.
Results
In this work we show that using PPAN data combined with other approximations, such as functional module detection, orthology exploitation methods and Gene Ontology (GO)-based information measures helps to predict protein function in Mycoplasma genitalium.
Conclusions
To our knowledge, the proposed method is the first that combines functional module detection among species, exploiting an orthology procedure and using information theory-based GO semantic similarity in PPAN of the Mycoplasma species. The results of an evaluation show a higher recall than previously reported methods that focused on only one organism network.
Background
Sequence similarity has proven to be useful for many years in attempting to annotate genomes [1,2]. A simple way to infer the possible function of a protein is to use an alignment procedure such as PSI-BLAST [3], to find possible homologues in sequence databases, such as UniProt [4]. However, sequence homology has its limitations. Only a fraction of newly discovered sequences have identifiable homologous genes in current databases, and its viability is limited to cases where substantial sequence similarity to annotated proteins can be found [5]. Moreover, the growing number of annotations extrapolated from sequence similarity is prone to errors [6-8], hence, new bioinformatics methods are developed to complement traditional sequence homology-based methods.
The development of high throughput technologies has resulted in large amounts of predicted Protein-Protein Interaction networks (PPI) for different genomes and, subsequently, methods using PPI data for functional inference [6,9-12] have been developed. It has been demonstrated that we may be able to use the semantics of gene annotations [13,14] and that we can obtain greater precision to predict new annotations using Gene Ontology (GO) information inside PPI [9,10,15]. Several semantic similarity measures using the GO database have been applied to gene products annotated with high ratios of prediction accuracy [13,15-19].
Recently, other methods using PPI to predict functions for individual genes or proteins have been developed by considering modularisation in biological networks [20] These methods attempt to first identify coherent groups of proteins and then assign functions to all of the proteins in each group. In terms of topology, a functional module can be typically understood as a group of proteins that are densely interconnected and contribute to perform for a specific biological function [21]. Once a module is obtained, the function prediction within the module [22] is usually conducted in a straightforward way by simple methods like orthology exploitation [10].
However, most of these approaches use PPI and, while very useful, they have limitations. The obtained PPI data result in a rich, but quite noisy and still incomplete, source of information. Also, PPIs are only available for a reduced group of organisms [15] due to the problems of using high throughput technologies in important study organisms such as Mycoplasma.
This last case implies a significant restriction since Mycoplasma genitalium is one of the most studied species, as it is the smallest organism having a small genome size [23,24] and has limited metabolic capabilities [25]. Due to all of the above, it has become a close approximation to the minimal set of genes required for bacterial growth [2,24], so, in order to study the Proteomes of these species, other type of protein-protein networks is necessary.
In many ways, Protein-Protein Association Networks (PPAN) are a more informative way of describing proteins and their mutual interactions [26]. In contrast to PPI, PPAN make no assertion as to how exactly two proteins interact: Proteins can show a productive functional interaction without physically interacting with each other (i.e. performing subsequent metabolic reactions in the same metabolic pathway). Therefore, whenever two proteins form a specific functional partnership, they can be thought of as being associated, independently of what the actual mechanism of their association is [26]. PPAN have been widely used recently in order to predict protein function [27-31].
In this work, a new protocol is described for predicting new gene annotations involving different approaches using PPAN for Mycoplasmas: Functional module identification, orthology exploitation and Gene Ontology (GO) Semantic similarity-based measures [32]. We have developed a simple, but effective, procedure in order to assign function transferring GO terms to unannotated protein nodes inside functionally conserved modules between two Mycoplasma species.
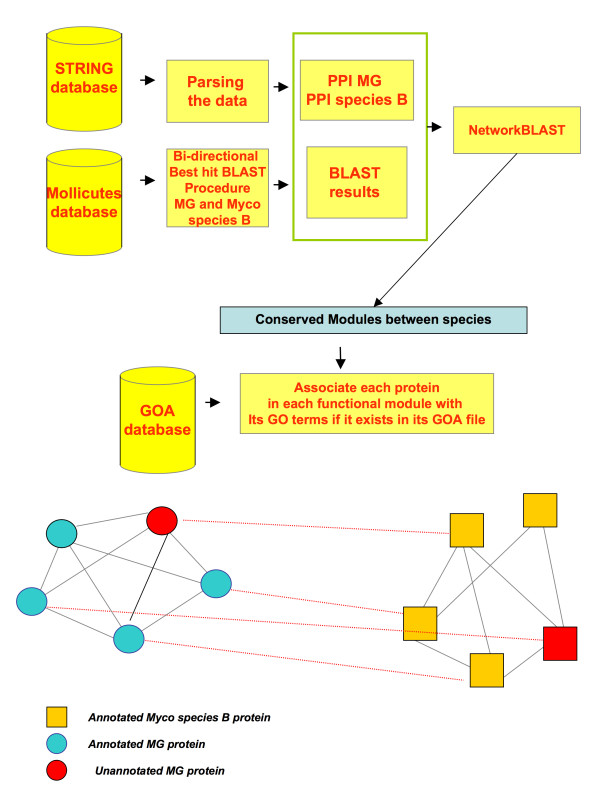
The procedure is as follows: First, identify functional modules between two Mycoplasma PPAN (species A and B); second, assign Gene Ontology (GO) terms to each protein inside the species B modules (proteins without GO terms remain unannotated); third, calculate the orthology value between each potential pair of orthologs (A and B), and if the value exceeds a threshold, transfer GO terms specifically from Species B ortholog proteins to the Species A unannotated protein; Finally, the transferred GO terms list of unannotated Species A protein was analysed, comparing them with the annotated proteins GO terms inside the Species A module that they belong to, using GO semantic similarity measures. If the similarity value is above a threshold, then the Species A unannotated protein is associated with these assigned GO terms. A schematic view of the algorithm is depicted in Figure 1
Figure 1.
Information theory-based semantic similarity (ITTS) for predicting function through interacting proteins. A. If Orthology value exceed a threshold, we considered the protein pair between 2 functional modules as real orthologs and transfer GO terms specifically between annotated protein one species (yellow) to un-annotated × M. genitalium protein (red). B. Calculate semantic similarity between this GO terms assigned and GO terms from annotated neighbouring proteins inside the M. genitalium functional module. C. Consider GO terms A with similarity above a threshold, then associate protein × with GO. Orange dotted lines indicate Semantic similarity calculations.
Results
1. Calculations and algorithm procedure
1.2. Protein-protein association networks for Mycoplasma species
We have performed this study on seven Mycoplasma species proteomes. One of the main limitations in using protein-protein interactions for function prediction is the availability of interaction data, especially if one wishes to work with Mollicutes. The entries in STRING [33] correspond to protein-protein functional associations for more than 600 organisms, including Mycoplasmas. A protein-protein functional association can mean either a direct physical binding or an indirect interaction, such as participation in the same metabolic pathway or cellular process. The associations are derived from high-throughput experimental data, from the mining of databases and literature and from predictions based on genome context analysis. STRING carefully assesses and integrates all of these data in order to obtain a single confidence score for all protein interactions, taking a more generalised perspective on proteins and their associations than other databases whose main purpose is to collect and curate direct experimental evidence about protein-protein physical interactions.
Moreover, the improvement of the use of STRING in Protein Function network procedures has been indicated [15], and in a recent work, STRING has been used to study the proteome organisation of Mycoplasma pneumoniae.
The PPAN for each species were obtained from the STRING database (2008 release), the information for Mycoplasmas being extracted and then a Protein-Protein network for each of the species being constructed, resulting in undirected PPAN. The networks were built using high-associations (weighted score> 0.7). See Additional File 1 for all the Mycoplasma PPANs used in this study. These PPAN have been used for the following calculations. The following procedure is depicted on Figure 2.
Figure 2.
Method procedure. First, we obtained the PPANs for each of the two species from STRING database. Secondly, a BLAST bi-directional procedure is executed for both species. Then, a NetworkBLAST analysis using the PPANs and BLAST best hits results is executed and conserved functional modules between the two species are obtained. The GO terms for each protein node in the conserved modules are assigned using GOA files for each species. Proteins with no entry in GOA file remain unannotated.
1.2. Functional Module identification
We first identified the functional modules shared between M. genitalium and every other Mycoplasma species using NetworkBLAST [22,34,35] as follows:
First, the proteomes in FASTA format were obtained for each Mycoplasma species from the GenBank repository dated October 2010 ftp://ftp.ncbi.nih.gov/genbank/, and for every two species analyzed, we performed a bi-directional BLAST [3] search of M. genitalium was performed over the other Mycoplasma's species 2 and vice versa. The algorithm was fed with the BLAST results and PPAN s of each of the species analyzed. NetworkBLAST outputs a set of modules that are evolutionarily conserved across the two PPAN. (See Table 1 for the number of interactions and functional modules detected between each genome and M. genitalium.)
Table 1.
Number of interactions and functional modules detected for each genome and M. genitalium
| Genome | Number of interactions | Modules detected |
|---|---|---|
| M. penetrans | 37106 | 485 |
| M. pneumoniae | 22270 | 136 |
| M. hyopneumoniae | 18038 | 105 |
| M. capricolum | 28610 | 84 |
| M. mycoides | 19158 | 99 |
| M. pulmonis | 27365 | 81 |
1.3. Gene Ontology (GO) terms assignation to proteins inside the modules
The standardized Gene Ontology vocabulary (GO) [36] was used as a standard to annotate the proteins inside the PPAN. The annotation for each gene in each proteome was obtained from Gene Ontology Annotation files (GOA) [37] available for each Mycoplasma proteome in the GO database http://www.geneontology.org. (See Table 2 for the total number of GO annotated genes in each genome). The filtered GO terms were associated for each node in each conserved module detected by NetworkBLAST. Each M. genitalium protein with no GO terms assigned in the GOA file is susceptible to be annotated by using its orthologs in the other species.
Table 2.
Genomes, their number of genes and genes with GO annotations
| Genomes | Number of genes | Number of GO annotations | GO annotations |
|---|---|---|---|
| M. genitalium | 483 | 435 | 5124 |
| M. pneumoniae | 687 | 563 | 5809 |
| M. hyopneumoniae | 671 | 424 | 4670 |
| M. capricolum | 812 | 499 | 5697 |
| M. penetrans | 1028 | 629 | 5889 |
| M. mycoides | 978 | 711 | 6136 |
| M. pulmonis | 778 | 484 | 5355 |
1.4. Transferring GO terms to unannotated protein
The orthology value calculated between each potential pair of orthologs between conserved modules using ORTHOMCL [38]. If the value exceed a threshold, the pair was considered as real orthologs and the procedure of Jaeger and Laeser [10] was followed for transferring GO terms specifically between those ortholog protein pairs and generating a list for the unannotated M. genitalium protein.
1.5. Semantic similarity analysis of transferred GO terms
The transferred GO annotations list of unannotated M. genitalium proteins was analyzed, comparing them with the GO annotations from annotated proteins inside the module that they belong to. The Information theory-based semantic similarity (ITTS) for predicting function through interacting proteins was followed [10,14]:
• To calculate Semantic Similarity between the transferred GO terms from unannotated protein and GO terms from interacting neighbours inside the same functional module.
• To consider GO terms with a similarity value above a threshold, then associate unannotated protein with these GO terms
However, we can accept the predictions if they are similar to the annotations in the module, i.e., have similar GO ontology annotations, then a "manual curation" procedure is needed. A schematic view of the ITTS procedure is depicted in Figure 1.
2. Performance measures
2.1. Effectiveness of the method
The performance of the method was measured as an average value in a five-fold cross-validation analysis, where the GOA dataset for M. genitalium was randomly divided into five parts. Four parts for model learning (training), and the remaining part for validation (testing). Known GO annotations were removed from the test set and it was tried to predict the terms of the proteins in the test set using the rest of the sets (training sets).
Finally, the predicted terms were compared with original annotations to determine the amount of correctly predicted annotations. Effectiveness is validated using standard information retrieval measures: recall and precision. Several terms have defined:
A: set of annotated GO functions (in test set)
P: set of predicted GO functions
F: GO functions in train set
So, we can establish:
| (1) |
| (2) |
| (3) |
| (4) |
then, define:
| (5) |
| (6) |
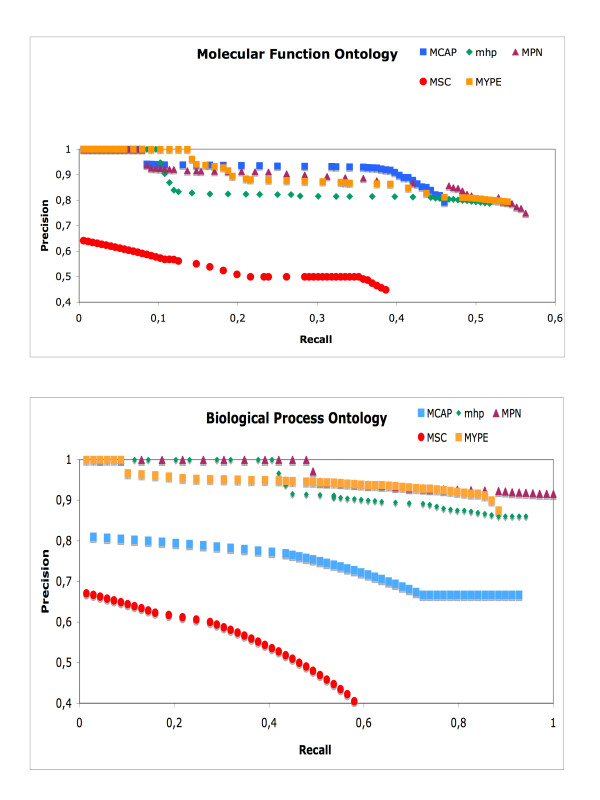
The variance of the reconstructed annotation was computed in order to see if it is affected by the random split choice. The performance of the method was measured as an average value in a five-fold cross-validation analysis using each of the Mycoplasma genomes (M. penetrans, M. pneumoniae, M. capricolum, M. hyopneumoniae, M. pulmonis and M. mycoides) to predict the M. genitalium annotations for two ontologies: Biological Process and Molecular Function. These genomes vary greatly in the availability of annotations and interaction data, which provides a good setup to study the strengths and limitations of our procedure. As shown in Table 3 for Molecular Function ontology, the average of recall is nearly 90% in all species (except M. pneumoniae which reaches 98% and, on the other hand, M. pulmonis only reaches 81.5%) and the average of precision is 65%. On the other hand, the average of precision is 30% and the average of recall is 80% in the Biological Process ontology. As expected, recall is higher in the Molecular Function ontology due to, in previous studies, researchers having found that sequence similarity is strongly correlated with semantic similarity based on the Molecular Function aspect of GO [39]. This aspect fits with the biological expectations. The sequence of a protein determines its molecular function but does not necessarily relate to the biological process that it is involved in.
Table 3.
The performance of the method measured as an average value in a 5-fold cross-validation analysis for two ontologies: Biological Process and Molecular Function.
| PROTEOME | AVERAGE PRECISION | AVERAGE RECALL | ||
|---|---|---|---|---|
| BP | MF | BP | MF | |
| M. hyopneumoniae | 0,305 | 0,661 | 0,815 | 0,879 |
| M. pneumoniae | 0,331 | 0,653 | 0,987 | 0,987 |
| M. penetrans | 0,317 | 0,635 | 0,899 | 0,895 |
| M. mycoides | 0,309 | 0,655 | 0,793 | 0,837 |
| M. capricolum | 0,322 | 0,643 | 0,928 | 0,921 |
| M. pulmonis | 0,306 | 0,664 | 0,754 | 0,815 |
Average Precision and Average Recall values for the 5 folds in each genome and for each ontology is presented. Left side Biological Process, right side Molecular Function.
The high recall in both ontology cases indicates that a large number of GO terms is recovered by our method from all of the GO terms that are relevant to the search. The high precision in the Molecular Function ontology case indicates that a large proportion of the GO terms are relevant to the search among all of the GO terms recovered by our method.
2.2. Predictions in the M. genitalium dataset using different GOA files
Two GOA files for M. genitalium were used in this study. The GOA2005 in this article is dated March 2005, and was obtained directly from NCBI. GOA2005 contains 443 distinct gene-GO entries. The second GO annotation file, referred to as GOA2010, is dated October 2010 and was obtained from NCBI also. Table 4 summarises the content of both files. It is seen that the two files are consistently similar in terms of GO annotations for each gene (i.e., a similar number of genes with more than 10 annotations...) despite the number of annotation gaps between them.
Table 4.
Content of GO terms and genes for each Gene Ontology Annotation (GOA) file used in our experiments.
| Database | GO terms | |||
|---|---|---|---|---|
| Total | >10 annotations (%) | 3-9 annotations (%) | <2 annotations (%) | |
| GOA2005 | 443 | 36(9,7%) | 56 (12,6%) | 263(59,36%) |
| GOA2010 | 483 | 46(9,3%) | 60 (12,15%) | 291(58,9%) |
See text for details
In order to determine the accuracy of the predictions in real-world conditions where most genes are poorly annotated or not at all (less than 10 gene annotations per GO term), the same procedure as above was followed (using each of the Mycoplasma genomes to predict the M. genitalium annotations), but now using the older GO2005 file and then validating the newly predicted annotations using the newer GOA2010 association file as a second evaluation. Using a similar procedure as Tao's [14], those GO terms marked as 'obsolete' and the ambiguous terms 'Biological Process unknown', 'Molecular Function unknown' were excluded from GOA2005. We limited our testing dataset to include only those GO terms that had at least three associated genes [14]. Two separate experiments were conducted: one to predict annotations for the Molecular Function ontology and another for the Biological Process ontology. The semantic similarity was calculated using different measures according to Couto [32] (See Methods for details). The cut-off values of semantic similarities were varied to generate the different data points on the curves. The results are shown in Figure 3. Due to space constrictions, only M. genitalium predictions using M. pneumoniae results are presented here. However, the rest of the results can be obtained from the authors upon request (See Additional File 2).
Figure 3.
Precision and Recall values for Resnik semantic similarity measure using GOA2005 file. The performance of the method using each of the Mycoplasma genomes (M. penetrans, M. pneumoniae, M. capricolum, M. hyopneumoniae, M. pulmonis and M. mycoides) to predict the M. genitalium annotations for two ontologies: Biological Process and Molecular Function, see Methods for details. The cut-off values of semantic similarities were varied to generate the different data points on the curves.
We found that our procedure performed well, for the Molecular Function Ontology, and high precision scores (more than 80%) and recall values of nearly 60% were obtained. In the Biological Process Ontology, precision scores obtained were also high (more than 90%) and recall values of nearly 85% were obtained for those GO terms that were associated with two or more genes. However, it can be seen that in both experiments (Molecular Function and Biological Process Ontology) poor values were obtained compared with the other genome predictions in the case of M. mycoides. We believe that the cause of these results could be that the higher semantic similarity values obtained for all of the GO terms predicted and those that are part of the modules are barely 0.6 (data not shown), while the higher semantic similarity values for the other genomes oscillate between 0.7 and 0.85.
2.3. Predictions of informative GO terms in the M. genitalium dataset using different approaches
We also, wish to determine if our method can be compared with other similar method in terms of precision and recall when trying to predict informative GO terms. Informative GO terms were defined as those terms that are annotated to at least n proteins (n = 10) and has at least a level-4 or higher. Defining:
t: Relevant/Informative GO terms
n: Retrieved GO terms
| (7) |
and define now Precision and Recall as:
| (8) |
| (9) |
Here, how well our procedure detects informative GO terms, as compared to other similar approximations is studied. FS-Weight is an algorithm which predicts the function of a protein based on the idea that the interaction between indirect neighbours inside a PPI is likely to share common functions [9]. These indirect neighbours may interact with the same protein due to some common physical or biochemical characteristics, especially if they share many common interaction neighbours. The FS-weight algorithm was chosen, from others for several reasons: First, because the method is one of the latest PPI-based algorithms for predicting protein function using GO annotations. Secondly, because it was tested to predict protein function using the STRING database, showing significant improvement and, thirdly, because FS-Weight can predict protein function effectively for all of the three categories of GO across different genomes, indicating that it is a robust approach [15]. We also compared our procedure with two other approximations: The first one [6] attempts to learn a linear model of how likely a protein is to have a function given the frequency with which a proteins neighbours have that term. Parameters of the model are estimated using quasi-likelihood estimation techniques. In order to provide a fair comparison, the proposed method has been compared to a sequence based annotation method which is based on the transfer of annotations in a closely related species, which is BYPASS [5]. This method predicts the putative function for the protein from its sequence integrating the results from the PSI-BLAST programme and a fuzzy logic algorithm using several protein sequence characteristics which have been checked, with regards to their ability to rearrange a PSI-BLAST profile according more to their biological functions.
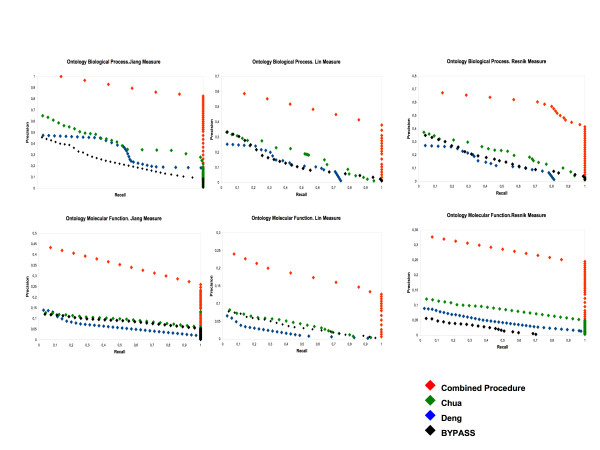
We wish to study the prediction performance of our procedure for detecting conserved modules among Mycoplasma species. Due to space constrictions, only M. genitalium and M. pneumoniae results are presented here, however, the rest of the results can be obtained from the authors upon request. The precision versus recall graphs for the prediction of informative GO terms are depicted in Figure 4 for both Molecular function and Biological Process Ontology.
Figure 4.
Predictions of informative GO terms in the M. genitalium dataset using different approaches. The Precision versus Recall graphs for the prediction of informative GO terms Biological Process (upper) and Molecular function (lower side) Ontology using different GO semantic similarity measures for our combined procedure and FS-Weight.
Different semantic similarity measures were used to obtain scores between the GO terms included inside of each predicted module and the GO terms obtained using our procedure. For PPI predictions, the modules obtained were assigned using NetworkBLAST and then calculated the semantic similarity between the GO terms included and the GO terms predicted. For BYPASS predictions, the GO terms were assigned to the predictions using the Gene Ontology database directly. The semantic similarity values were then used as variable thresholds. (See Material and Methods for details).
It can be seen in Figure 4 that, for the three semantic similarity calculations our algorithm makes predictions with better precision and recall, as compared to PPI (Chua and Deng) and sequence-analysis methods. The main advantage of our procedure is that, despite obtaining a low number of predicted GO terms, they are related to the terms inside of each module, while the PPI predictions use only one PPAN (in this case the M. genitalium) and, then, the predicted GO terms have lower similarity values than do our predictions. It can be seen, however, that our results have the same problem as do the PPI aproximations [15]. Due to the lack of annotation information and our previous parsing in trying to avoid inconsistency in GO terms, the informative terms chosen are a low number (nearly 50) and may not provide statistically strong comparisons.
Finally, the number of M. genitalium unnanotated genes with function predictions, with respect to GO annotations, can be found in Table 5. Using our method, not only general functional categories are assigned to unannotated proteins, but they are also assigned very specific functions to unannotated proteins (complete results are available upon request to the authors). Predictions are consistent with effectiveness results. These genomes vary greatly in the availability of annotations and interaction data. As shown in Table 5 the coverage of annotations vary from 17% to nearly 70%, for the species that have more functionally conserved modules with M. genitalium, M. pneumoniae, M. hyopneumoniae and M. penetrans.
Table 5.
M. genitalium using different genomes and number of genes unannotated. Coverage of annotations using our method (Predictions over number of unnanotated genes)
| Genomes | Coverage of annotations | Number of GO terms assigned |
|---|---|---|
| M. pulmonis | 0.23 | 120 |
| M. pneumoniae | 0.69 | 535 |
| M. hyopneumoniae | 0.66 | 470 |
| M. capricolum | 0.17 | 163 |
| M. penetrans | 0.57 | 460 |
| M. mycoides | 0.27 | 155 |
Discussion
It has been shown herein, that our procedure out-performs other similar algorithms to predict GO-based annotations using Protein-Protein networks, with equal or higher overall precision from a significantly broader range of GO terms. The incorporation of other approximations such as functional module detection, conserved between species and orthology exploitation, predict function with higher precision and recall in two ontologies of the GO database. As compared to other GO search engines, our algorithm is capable of finding GO terms with high semantic similarity values due to using orthology information between proteins predicted inside functional modules conserved between species, and it has been also shown to recapitulate "known" future GO annotations artificially removed from the dataset using five-fold cross-over validation, with high precision and recall.
Conclusion
We believe that our combined approach could be applied in future as a high-precision Mollicute genome annotation procedure: Moderately well GO-annotated Mycoplasma genomes could help to improve protein function in other Mycoplasma less-annotated genomes in a more effective way than ab initio classic annotation methods. However, caution must be exercised when using this technique. We have shown how critical neighbour genomes with good GO annotation are: The performance of this procedure is limited because it needs Mycoplasma genomes with a high GO annotation degree and a high number of predicted conserved modules. If genomes were used with low predicted modules and a low number of GO terms annotated within those modules, a reliable number of predictions could not be achieved (data not shown). Future work will cover this by using the latest development of the GO database, and which evolutionary distances between Mollicute genomes are still allowable in order to predict a reliable number of conserved functional modules between them.
Methods
Gene Ontology annotation file
The standardised Gene Ontology vocabulary (GO) was used [36] as a standard to annotate the proteins inside the PPAN. The annotation for each gene in each proteome was obtained from the Gene Ontology Annotation file (GOA) [37] available for each Mycoplasma proteome in the GO database http://www.geneontology.org. The version of GO used in this study dated from October, 2008, and was parsed for each of the species, thus avoiding several problems:
• Excluding terms annotated as obsolete and terms that no have relation with Mycoplasma such as: GO:0000004. (Biological process unknown).
• Nearly all Mycoplasma proteomes are annotated in GOA files mainly with GO terms with evidence code IEA (Inferred from Electronic Annotation). To be retained, IEA annotations must be manually reviewed in order to be assigned an upgraded Evidence Code such as ISS (Inferred from Sequence or Structural Similarity). All the of IEA GO terms that do not have an associated ISS code were removed.
• IEA annotations are generalised to apply to a diverse range of species and usually only represent very broad functions such as 'Protein binding' and 'Enzyme binding'. In effect, this means that as functional genomics data are modelled using GO annotation, a large proportion of the remaining data describes only very broad biological concepts [40]. GO terms are arranged in a hierarchical manner with more general terms at the lower level and more specific terms at the higher level. The GO term "biological process" is defined as level 0, its children terms as level 1, and so on. Wong's work was followed and only GO terms were considered to contribute to "annotated protein" if they had at least one level-4 GO term or higher.
GO semantic similarity calculations
The semantic similarity between two concepts has been used for investigating the relationships between GO annotations and gene sequences [13,39] as well as in clustering genes functionally [19].
The information content of each GO term for each protein inside the detected modules was calculated and then three measures were applied to estimate the semantic similarity between GO terms assigned using orthology exploitation to un-annotated proteins and the GO terms assigned to the annotated proteins inside the same functional module. Information content is defined as the frequency of each term which occurs in the GO corpus.
The semantic similarity of one GO term go1 and a GO terms set GO = {go1,go2...gok} is dfined as:
| (10) |
As GO allows for multiple parents for each concept, two GO terms can share parents by multiple paths. We take the minimum p(go), where there is more than one shared parent. pms is defined as;
| (11) |
where S(go1, go2 ) is the set of parent terms shared by go1 and go2.
Different similarity calculations were then followed depending upon the GO ontology. For the Molecular Function ontology, Lord's procedure [39] was followed and the Resnik Similarity measure was applied [41]:
| (12) |
For the Biological Process ontology, we followed Couto [32] and Lin was applied [42]
| (13) |
and also, Jiang [43] measure
| (14) |
| (15) |
Therefore, given two GO terms sets, one for the annotated protein GOann = {goann1, goann2...goannk} and another for the Functional module GO = mod{gomod1, gomod 2 ...gomod k}, the semantic similarity between them is defined as:
| (16) |
Depending on the similarity between the GO annotations for the protein and the GO annotations for the module which it belongs to, this score ranges from between 0 and 1, where 1 indicates functional equality and 0 indicates maximal functional distance. Low values can be discriminated among, indicating low precision, and higher values, indicating high precision.
Abbreviations
PPI: Protein-protein interaction; PPAN: Protein-Protein Association Networks; GO: Gene Ontology; IEA: Inferred from Electronic Annotation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EQ and AG conceived the study. AP participated in conceptualization and discussion. AG and JC designed the main algorithm and developed the PERL STRING processing scripts and R Similarity calculations scripts. IA performed the BLAST and functional modules detection. JP made the performance measurements. AG drafted the manuscript. All authors read and approved the final manuscript.
Supplementary Material
PPANs for each Mycoplasma genome extracted from STRING. Each sheet corresponds to one PPAN Mycoplasma genome (M. Genitalium, Mycoplasma genitalium, M. Gallisepticum: Mycoplasma gallisepticum, MPN: Mycoplasma pneumoniae, MYPE: Mycoplasma penetrans, MYPU: Mycoplasma pulmonis, Synoviae: Mycoplasma synoviae S53, MMOB: Mycoplasma mobile MCAP: Mycoplasma capricolum, mhp: Mycoplasma hyopneumoniae) The sheets are tabulated in two columns (the two interacting proteins that have a functional association as described in STRING). The STRING scores are not described in this file but can be available from authors upon request.
M. genitalium predictions using M. pneumoniae PPAN. The file is tabulated in four columns: M. genitalium ORF, Number of functional module conserved between M. genitalium and M. pneumoniae, Resnik GO Similarity Measure between GO terms list assigned from M. pneumoniae ortholog and GO terms from neighbor M. genitalium protein inside its functional module, List of GO terms assigned to the protein.
Contributor Information
Antonio Gómez, Email: antonio.gomez@bioinf.uab.es.
Juan Cedano, Email: juan.cedano@uab.es.
Isaac Amela, Email: isaac.amela@bioinf.uab.es.
Antoni Planas, Email: antoni.planas@iqs.url.edu.
Jaume Piñol, Email: jaume.pinol@uab.es.
Enrique Querol, Email: enric.querol@uab.es.
Acknowledgements
This research was supported by Grants BIO2007-67904-C02 and BFU2010-22209-C02 from the MCYT (Ministerio de Ciencia y Tecnología, Spain), and from the Centre de Referència de R+D de Biotecnologia de la Generalitat de Catalunya. The English of this manuscript has been corrected by Mr. Chuck Simmons, a native English-speaking Instructor of English of Autonomous University of Barcelona (UAB). EQ is holder of a Chair of Knowledge and Technology Transfer Parc de Recerca UAB-Santander. AG acknowledges his postdoctoral fellowship from Parc de Recerca UAB-Santander.
References
- Galperin MY, Koonin EV. Who's your neighbor? New computational approaches for functional genomics. Nat Biotechnol. 2000;18:609–613. doi: 10.1038/76443. [DOI] [PubMed] [Google Scholar]
- Koonin EV. Bridging the gap between sequence and function. Trends Genet. 2000;16:16. doi: 10.1016/S0168-9525(99)01927-7. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Universal Protein Resource (UniProt) 2009. Nucleic Acids Res. 2009;37:D169–174. doi: 10.1093/nar/gkn664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Cedano J, Espadaler J, Hermoso A, Pinol J. et al. Prediction of protein function improving sequence remote alignment search by a fuzzy logic algorithm. Protein J. 2008;27:130–139. doi: 10.1007/s10930-007-9116-x. [DOI] [PubMed] [Google Scholar]
- Deng M, Zhang K, Mehta S, Chen T, Sun F. Prediction of protein function using protein-protein interaction data. J Comput Biol. 2003;10:947–960. doi: 10.1089/106652703322756168. [DOI] [PubMed] [Google Scholar]
- Devos D, Valencia A. Practical limits of function prediction. Proteins. 2000;41:98–107. doi: 10.1002/1097-0134(20001001)41:1<98::AID-PROT120>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Devos D, Valencia A. Intrinsic errors in genome annotation. Trends Genet. 2001;17:429–431. doi: 10.1016/S0168-9525(01)02348-4. [DOI] [PubMed] [Google Scholar]
- Chua HN, Sung WK, Wong L. Exploiting indirect neighbours and topological weight to predict protein function from protein-protein interactions. Bioinformatics. 2006;22:1623–1630. doi: 10.1093/bioinformatics/btl145. [DOI] [PubMed] [Google Scholar]
- Jaeger S, Leser U. High-Precision Function Prediction using Conserved Interaction. Proc of the German Conference on Bioinformatics. 2007. pp. 146–163.
- Samanta MP, Liang S. Predicting protein functions from redundancies in large-scale protein interaction networks. Proc Natl Acad Sci USA. 2003;100:12579–12583. doi: 10.1073/pnas.2132527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Su Z, Mao F, Olman V, Xu Y. Prediction of functional modules based on comparative genome analysis and Gene Ontology application. Nucleic Acids Res. 2005;33:2822–2837. doi: 10.1093/nar/gki573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto FM, Silva MJ, Lee V, Dimmer E, Camon E. et al. GOAnnotator: linking protein GO annotations to evidence text. J Biomed Discov Collab. 2006;1:19. doi: 10.1186/1747-5333-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Sam L, Li J, Friedman C, Lussier YA. Information theory applied to the sparse gene ontology annotation network to predict novel gene function. Bioinformatics. 2007;23:i529–538. doi: 10.1093/bioinformatics/btm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HN, Sung WK, Wong L. Using indirect protein interactions for the prediction of Gene Ontology functions. BMC Bioinformatics. 2007;8 Suppl 4:S8. doi: 10.1186/1471-2105-8-S4-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud MB, Costanzo MC, Shah P, Skrzypek MS, Sherlock G. Gene Ontology and the annotation of pathogen genomes: the case of Candida albicans. Trends Microbiol. 2009;17:295–303. doi: 10.1016/j.tim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio MG, Collmer CW, Lomax J, Ireland A. Applying the Gene Ontology in microbial annotation. Trends Microbiol. 2009;17:262–268. doi: 10.1016/j.tim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Pesquita C, Faria D, Bastos H, Ferreira AE, Falcao AO. et al. Metrics for GO based protein semantic similarity: a systematic evaluation. BMC Bioinformatics. 2008;9 Suppl 5:S4. doi: 10.1186/1471-2105-9-S5-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Du Z, Payattakool R, Yu PS, Chen CF. A new method to measure the semantic similarity of GO terms. Bioinformatics. 2007;23:1274–1281. doi: 10.1093/bioinformatics/btm087. [DOI] [PubMed] [Google Scholar]
- Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- Sharan R, Ulitsky I, Shamir R. Network-based prediction of protein function. Mol Syst Biol. 2007;3:88. doi: 10.1038/msb4100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA. et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR. et al. Essential genes of a minimal bacterium. Proc Natl Acad Sci USA. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, Voelker LL. Molecular biology of mycoplasmas. Annu Rev Microbiol. 1996;50:25–57. doi: 10.1146/annurev.micro.50.1.25. [DOI] [PubMed] [Google Scholar]
- von Mering C. In: Protein-Protein Interaction Networks: Assembly and Analysis. Ron Appel, Ernest Feytmans, Lausanne, editor. SIoB; 2008. [Google Scholar]
- Alexeyenko A, Sonnhammer EL. Global networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res. 2009;19:1107–1116. doi: 10.1101/gr.087528.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabow AP, Leach SM, Baumgartner WA, Hunter LE, Goldberg DS. Improving protein function prediction methods with integrated literature data. BMC Bioinformatics. 2008;9:198. doi: 10.1186/1471-2105-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Janga SC, Babu M, Diaz-Mejia JJ, Butland G. et al. Global functional atlas of Escherichia coli encompassing previously uncharacterized proteins. PLoS Biol. 2009;7:e96. doi: 10.1371/journal.pbio.1000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Chan CH, Tsai CH, Lai JM, Wang FS. et al. Ortholog-based protein-protein interaction prediction and its application to inter-species interactions. BMC Bioinformatics. 2008;9 Suppl 12:S11. doi: 10.1186/1471-2105-9-S12-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromatis K, Chu K, Ivanova N, Hooper SD, Markowitz VM. et al. Gene context analysis in the Integrated Microbial Genomes (IMG) data management system. PLoS One. 2009;4:e7979. doi: 10.1371/journal.pone.0007979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto FM Coutinho PM Silva MJ Measuring semantic similarity between Gene Ontology terms Data & Knowledge Engineering 200761137–152. 10.1016/j.datak.2006.05.00321516604 [DOI] [Google Scholar]
- Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C. et al. STRING 8--a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan R, Ideker T. Modeling cellular machinery through biological network comparison. Nat Biotechnol. 2006;24:427–433. doi: 10.1038/nbt1196. [DOI] [PubMed] [Google Scholar]
- Sharan R, Suthram S, Kelley RM, Kuhn T, McCuine S. et al. Conserved patterns of protein interaction in multiple species. Proc Natl Acad Sci USA. 2005;102:1974–1979. doi: 10.1073/pnas.0409522102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell D, Dimmer E, Huntley RP, Binns D, O'Donovan C. et al. The GOA database in 2009--an integrated Gene Ontology Annotation resource. Nucleic Acids Res. 2009;37:D396–403. doi: 10.1093/nar/gkn803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camon E, Magrane M, Barrell D, Lee V, Dimmer E. et al. The Gene Ontology Annotation (GOA) Database: sharing knowledge in Uniprot with Gene Ontology. Nucleic Acids Res. 2004;32:D262–266. doi: 10.1093/nar/gkh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Mackey AJ, Stoeckert CJ Jr, Roos DS. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 2006;34:D363–368. doi: 10.1093/nar/gkj123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord PW, Stevens RD, Brass A, Goble CA. Investigating semantic similarity measures across the Gene Ontology: the relationship between sequence and annotation. Bioinformatics. 2003;19:1275–1283. doi: 10.1093/bioinformatics/btg153. [DOI] [PubMed] [Google Scholar]
- McCarthy FM, Bridges SM, Burgess SC. GOing from functional genomics to biological significance. Cytogenet. Genome Res. 2007;117:278–287. doi: 10.1159/000103189. [DOI] [PubMed] [Google Scholar]
- Resnik P. Using information content to evaluate semantic similarity in a taxonomy. Proceedings of the 14th International Joint conference on Artificial Intelligence. 1995.
- Lin D. An information-theoretic definition of similarity. Proceedings of the 15th International Conference on Machine Learning. 1998.
- Jiang J, Conrath D. Semantic similarity based on corpus statistics and lexical taxonomy. Proceedings of the 10th International Conference on Research on Computational linguistics. 1997.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PPANs for each Mycoplasma genome extracted from STRING. Each sheet corresponds to one PPAN Mycoplasma genome (M. Genitalium, Mycoplasma genitalium, M. Gallisepticum: Mycoplasma gallisepticum, MPN: Mycoplasma pneumoniae, MYPE: Mycoplasma penetrans, MYPU: Mycoplasma pulmonis, Synoviae: Mycoplasma synoviae S53, MMOB: Mycoplasma mobile MCAP: Mycoplasma capricolum, mhp: Mycoplasma hyopneumoniae) The sheets are tabulated in two columns (the two interacting proteins that have a functional association as described in STRING). The STRING scores are not described in this file but can be available from authors upon request.
M. genitalium predictions using M. pneumoniae PPAN. The file is tabulated in four columns: M. genitalium ORF, Number of functional module conserved between M. genitalium and M. pneumoniae, Resnik GO Similarity Measure between GO terms list assigned from M. pneumoniae ortholog and GO terms from neighbor M. genitalium protein inside its functional module, List of GO terms assigned to the protein.