Abstract
Whereas periodontal disease is ultimately of bacterial etiology, from multispecies biofilms of gram-negative anaerobic microorganisms, much of the deleterious effects are caused by the resultant epithelial inflammatory response. Hence, development of a treatment that combines anti-biofilm antibiotic activity with anti-inflammatory activity would be of great utility. Antimicrobial peptides (AMPs) such as defensins are naturally occurring peptides that exhibit broad-spectrum activity as well as a variety of immunomodulatory activities. Furthermore, bacteria do not readily develop resistance to these agents. However, clinical studies have suggested that they do not represent optimal candidates for exogenous therapeutic agents. Small-molecule mimetics of these AMPs exhibit similar activities to the parent peptides, in addition to having low toxicity, high stability and low cost. To determine whether AMP mimetics have the potential for treatment of periodontal disease, we examined the activity of one mimetic, mPE, against biofilm cultures of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. Metabolic assays as well as culture and biomass measurement assays demonstrated that mPE exhibits potent activity against biofilm cultures of both species. Furthermore, as little as 2 µg ml−1 mPE was sufficient to inhibit interleukin-1β-induced secretion of interleukin-8 in both gingival epithelial cells and THP-1 cells. This anti-inflammatory activity is associated with a reduction in activation of nuclear factor-κB, suggesting that mPE can act both as an anti-biofilm agent in an anaerobic environment and as an anti-inflammatory agent in infected tissues.
Keywords: Aggregatibacter actinomycetemcomitans, antibiotic, biofilm, cytokine
INTRODUCTION
Periodontitis is the most common cause of tooth loss in adults in the USA (Borrell et al., 2005), occurring in 15–25% of the US population. Its etiology can be considered to be the result of bacterial colonization by a variety of pathogenic microorganisms, including Porphyromonas gingivalis, which is associated with chronic periodontitis, and Aggregatibacter actinomycetemcomitans, which is associated with aggressive periodontitis. This colonization and subsequent invasion into the gingival epithelium leads to an innate immune response, including the production of such mediators as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) (Graves & Cochran, 2003). This leads to inflammation, which ultimately results in the bone loss seen in this disease (reviewed in Cochran, 2008). Standard treatment involves the mechanical removal of the biofilm but the use of systemic antibiotics has also been examined (reviewed in Herrera et al., 2008), as has the identification of therapeutic targets in the inflammatory response (reviewed in Kirkwood et al., 2007). Although the development of new antibiotics can temporarily address the bacterial colonization, the increase in antibiotic-resistant organisms makes this approach less effective.
Naturally occurring antimicrobial peptides have been proposed as a novel alternative to standard antibiotics because they exhibit broad-spectrum activity with little development of antibiotic resistance. However, their development as exogenous antibiotics has been hampered by a variety of factors, including the difficulty of their large-scale production, poor tissue distribution and systemic toxicity. The development of small molecule antimicrobial peptide mimetics has provided a novel direction for the development of new antibiotics (reviewed in Som et al., 2008). We recently demonstrated the potent activity of one such compound, mPE, a mimetic whose design was based on the amphiphilic structure of the peptide magainin (Beckloff et al., 2007). This compound was active against numerous oral pathogens, both gram-positive and gram-negative, including biofilm cultures of Streptococcus mutans. It also inhibited the lipopolysaccharide (LPS) -mediated induction of TNF-α from a macrophage cell line, presumably because of its predicted binding of LPS.
Based on these results, we hypothesized that mPE could be developed as a treatment for periodontal disease. To examine this, we tested the ability of mPE to kill the biofilm cultures of two periodontal pathogens, A. actinomycetemcomitans and P. gingivalis. We also examined further its ability to suppress the inflammatory response of epithelial and myeloid-derived cells.
METHODS
Bacterial strains and culture
Aggregatibacter actinomycetemcomitans 1005 (rough phenotype, serotype f, obtained from Dr Helen Schreiner, New Jersey Dental School) were cultured on TSB agar (4% trypticase soy broth, 0.6% yeast extract, 0.8% dextrose, 0.4% NaHCO3, 75 µg ml−1 bactracin, 5 µg ml−1 vancomycin) at 37°C in 10% CO2. Single colonies were inoculated to TSB broth in 75-cm2 tissue culture flasks, and maintained in culture at 37°C, 10% CO2. Bacteria were harvested by scraping, and resuspended into 1 ml phosphate-buffered saline. This suspension was vortexed vigorously for 1 min and allowed to settle for 10 min. The supernatant was then diluted to 2.5 × 107 before seeding into 96-well plates. These were cultured at 37°C in 10% CO2 until biofilms were formed.
Porphyromonas gingivalis strain 381 (obtained from Dr Christopher Cutler, Stony Brook University Dental School, NY) were cultured on TSB–blood agar (3% trypticase soy broth, 5% defibrinated sheep blood, 5 µg ml−1 hemin, 0.5 µg ml−1 menadione, 0.2 mg ml−1 KNO3) in an anaerobic chamber (80% N2, 10% H2, 10% CO2) at 37°C. For biofilm formation, single colonies were dispersed in 1 ml brain–heart infusion (BHI) broth. The suspension was vortexed vigorously for 30 s and allowed to settle for 10 min. The supernatant was diluted to 2.5 × 107 in BHI medium. The assay plate (96-well or 12-well tissue culture treated plates; BD Falcon, Bedford, MA) was pre-coated with pooled (n = 3), clarified, filtered (0.2 µm) fresh human saliva for 3 h at 37°C under anaerobic conditions, before seeding with bacteria.
Antimicrobial assays
Biofilms of A. actinomycetemcomitans were cultured into 96-well or 12-well plates (tissue culture treated, Falcon) for 18 h; P. gingivalis biofilms were allowed to grow for 72 h before the assay. For metabolic assays, biofilims were grown in 96-well plates, and serial dilutions of the mimetic compounds were made in 100 µl RPMI-1640 without Phenol red and added directly to the wells. Plates were cultured at 37°C in 10% CO2 for 24 h (or for the times indicated). Medium was removed, and cell viability was evaluated by XTT assay using the In Vitro Toxicology Assay kit (Sigma, St Louis, MO) according to the manufacturer’s protocol. Metabolic activity was measured by reading in a plate-reader at 450 nm. To determine cell viability by plating, biofilms were grown in 12-well plates, and treated with mPE as described above. The wells were scraped using a sterile rubber scraper until the wells were visually clear, and the scrapings were resuspended in growth medium, and plated onto agar. Colonies were counted after 72 h. All assays were performed in duplicate.
Cell culture and stimulation
The oral keratinocyte cell line OKF6/TERT (obtained from Dr James Rhinewald, Harvard University, Cambridge, MA) was cultured in keratinocyte growth medium (Lonza, Basel, Switzerland) with human epidermal growth factor and bovine pituitary extract. Cells were subcultured in six-well dishes 18 h before stimulation. Cells were treated with 2 µg ml−1, 5 µg ml−1 mPE with and without IL-1β stimulation (100 ng ml−1, 24 h) for 2, 4 and 18 h. THP-1 cells were grown in suspension in RPMI-1640 with 10% fetal bovine serum, and stimulated similarly.
Cytokine and inflammation assays
Growth medium from stimulated cultures was collected either by aspiration (from keratinocytes) or after centrifugation at 600 g for 15 min (for THP-1 cells). Cell debris was removed by centrifugation at 8000 g for 10 min at 4°C. To quantify IL-8 levels, the human IL-8 Single Analyte ELISArray kit (SA Biosciences, Frederick, MD) was used according to the manufacturer’s protocol. The Cellular Activation of Signaling ELISA kit IκBα (SA Biosciences) was used to quantify both phosphorylated and whole IκBα levels in OKF6/TERT cells grown in a 96-well plate. All assays were performed in duplicate.
Polymerase chain reaction
Total cellular RNA was isolated from cultures using QIA-shredder and RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA was reverse transcribed using a Superscript II reverse transcriptase kit as described by the manufacturer (Invitrogen, CA). Quantitative polymerase chain reaction (qPCR) was carried out using SYBR Green in a MyiQ iCycler (Bio-Rad, Hercules, CA). A total of 1 µl cDNA (described above) was analysed using a final concentration of 100 nm of primers, 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) in a volume of 20 µl. Primer sequences were as follows: hBD-2: forward 5′-GATGCCTCTTCCAGGTGTTTTTGG-3′, reverse 5′-TTG TTCCAGGACCACAGGTG-3′; IL-8: forward 5′-GCAGCTCTGTGTGAAGGTGCAGTTTTGC-3′, reverse 5′-TTTCTGTGTTGGCGCAGTGTGGTCC-3′; β2-microgloublin (control): forward 5′-CTCCGTGGCCTTAGCTGTG-3′, reverse 5′-TTGGAGTACGCTGGATAGCCT-3′.
Amplification was carried out for 50 cycles (95°C, 15 s; 60°C, 60 s). Relative mRNA levels in each sample were calculated based on its Ct value in comparison to the Ct of the housekeeping gene. The data were presented as 2−DDCt, an arbitrary unit. All amplified products showed a single peak in the dissociation curve test. Real-time qPCR was performed in triplicates for each sample. This procedure was conducted in at least three independent experiments.
RESULTS
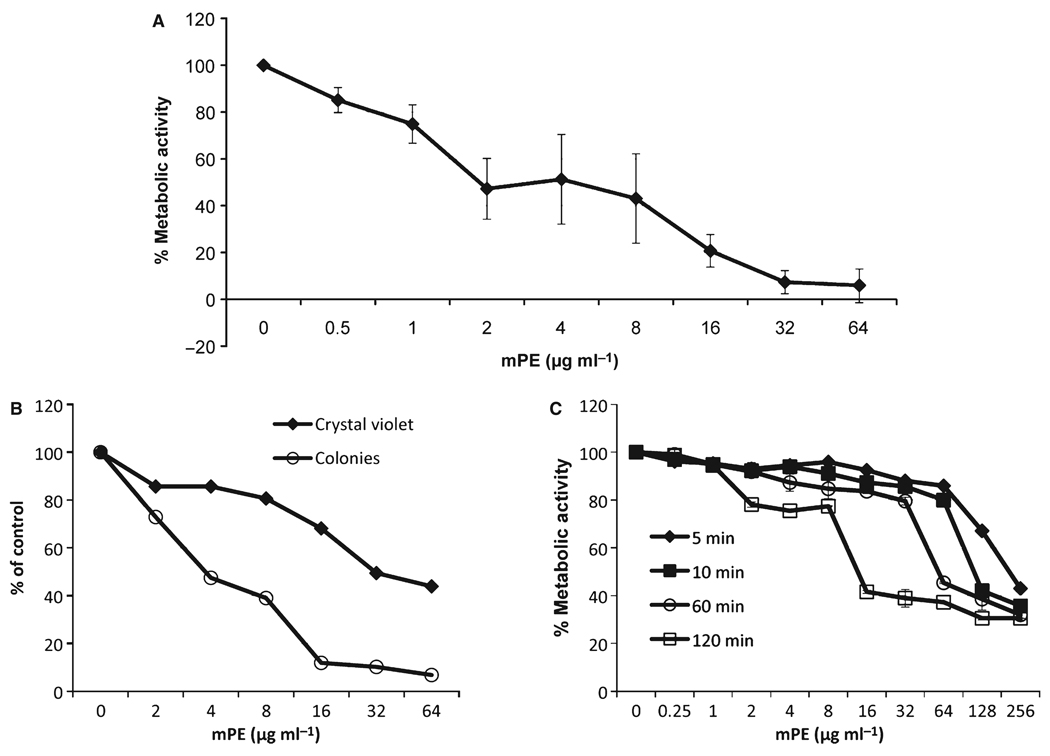
To quantify the activity of antimicrobial peptide mimetics on biofilms, we assayed for activity against two bacterial species associated with periodontitis, A. actinomycetemcomitans and P. gingivalis under conditions that lead to biofilm formation (Kaplan et al., 2003; Davey, 2006). We have previously published the minimum inhibitory concentration of this compound against these species in planktonic form (0.4 µg ml−1 for A. actinomycetemcomitans, and 2.5 µg ml−1 for P. gingivalis) (Beckloff et al., 2007). To assess the activity against A. actinomycetemcomitans biofilms, mPE was added in two-fold dilutions beginning at 64 µg ml−1, as in a standard minimum inhibitory concentration assay. After 24 h, the growth medium was replaced with RPMI (without Phenol Red) and an XTT assay was carried out to quantify the metabolic activity. The results in Fig. 1A demonstrate that mPE exhibits potent antimicrobial activity against A. actinomycetemcomitans grown in biofilms. To confirm that a reduction in metabolic activity led to a reduction in bacterial biofilm and viable bacteria, we stained the wells from a separate experiment with Crystal violet and plated the bacteria from a parallel experiment to that shown in Fig. 1A, and showed that the reduction in viable colonies paralleled the results from the XTT assay (Fig. 1B). Similarly, there was a dose-dependent reduction in Crystal violet staining, indicating desorption of the bacteria from the biofilm. Killing of A. actinomycetemcomitans occurs rapidly, as demonstrated in Fig. 1C, where even a 2-h exposure was sufficient to reduce metabolic activity by 60% at 16 µg ml−1. Exposures up to 6 h produced a similar result to the 2-h exposure (not shown).
Figure 1.
Activity of mPE against Aggregatibacter actinomycetemcomitans biofilms. Strain 1005 of A. actinomycetemcomitans was grown in AAGM in 96-well plates until complete confluence. Two-fold dilutions of mPE were added for 24 h (A) or 5 min to 2 h (C). Medium was removed and replaced with RPMI-1640 with XTT and grown for a further 3 h. Metabolic activity was quantified by measuring the optical density (OD) at 450 nm and 600 nm. Results are shown as % reduction in the OD450–OD600 from untreated cultures. The experiment in (A) was carried out in triplicate, and error bars are ± SD. In (B) parallel wells were stained with Crystal violet, destained and quantified by reading OD600; bacteria were removed and plated on AAGM agar plates to quantify viable colonies. Results are shown as % reduction from untreated control.
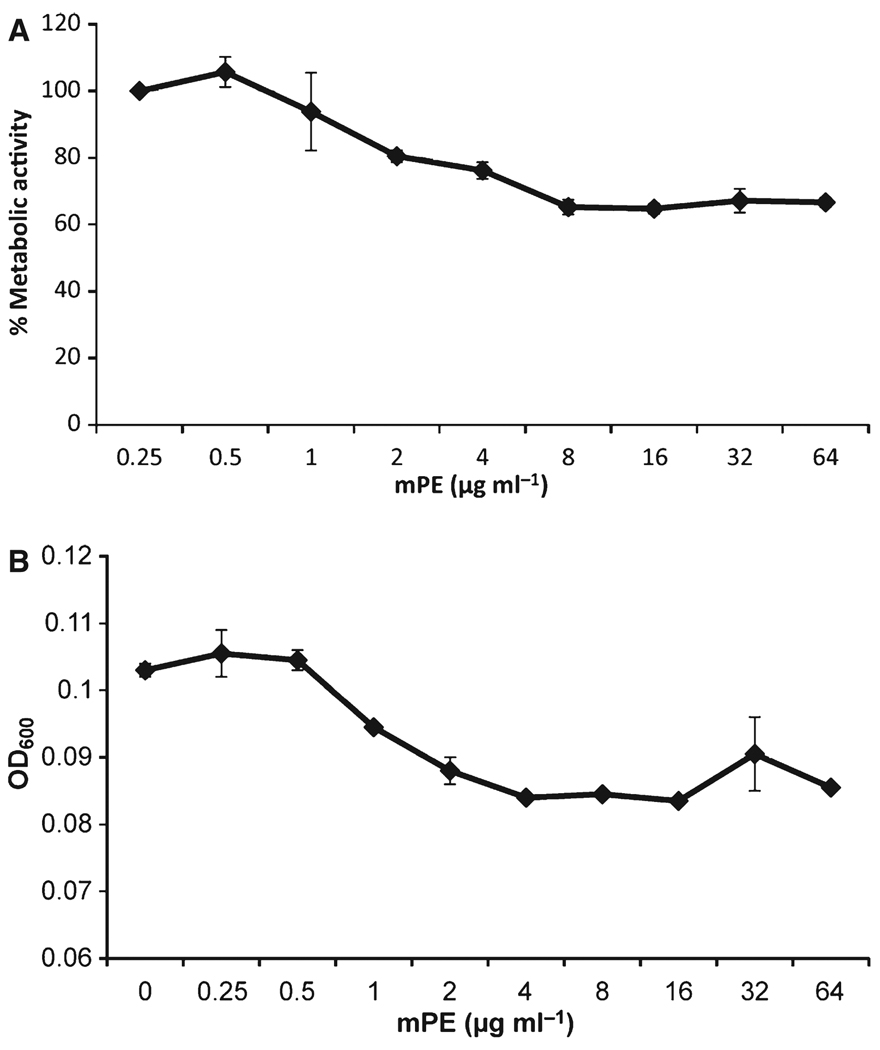
To test the activity against P. gingivalis biofilms, we used strain 381, grown in 96-well plates under conditions that favor biofilm formation (Davey, 2006). The mPE was added in serial dilutions, incubated anaerobically for 24 h, and the medium was replaced with XTT in RPMI-1640. Metabolic activity was quantified as above. To confirm the ability of XTT to measure activity in the biofilm, the growth medium was removed, and biomass was quantified by Crystal violet staining, followed by destaining and quantification of the optical density. The results, shown in Fig. 2, indicate that there is a decrease in both metabolic activity (Fig. 2A) and biofilm bacteria (Fig. 2B) to a baseline at 4–8 µg ml−1 mPE.
Figure 2.
Activity of mPE against biofilms of Porphyromonas gingivalis. Biofilms were grown in a 96-well plate for 21 days in an anaerobic chamber in brain–heart infusion (BHI) medium. (A) The mPE was added for 24 h, and replaced with XTT in RPMI-1640 as described in Fig. 1. (B) After XTT measurement, wells were stained with Crystal violet, and staining was quantified by reading optical density at 600 nm (OD600). Values are presented as mean ± standard deviation. The difference in both metabolic activity and OD between the lowest concentration and 8 µg ml−1 was significant at P < 0.002 as determined by Student’s t-test.
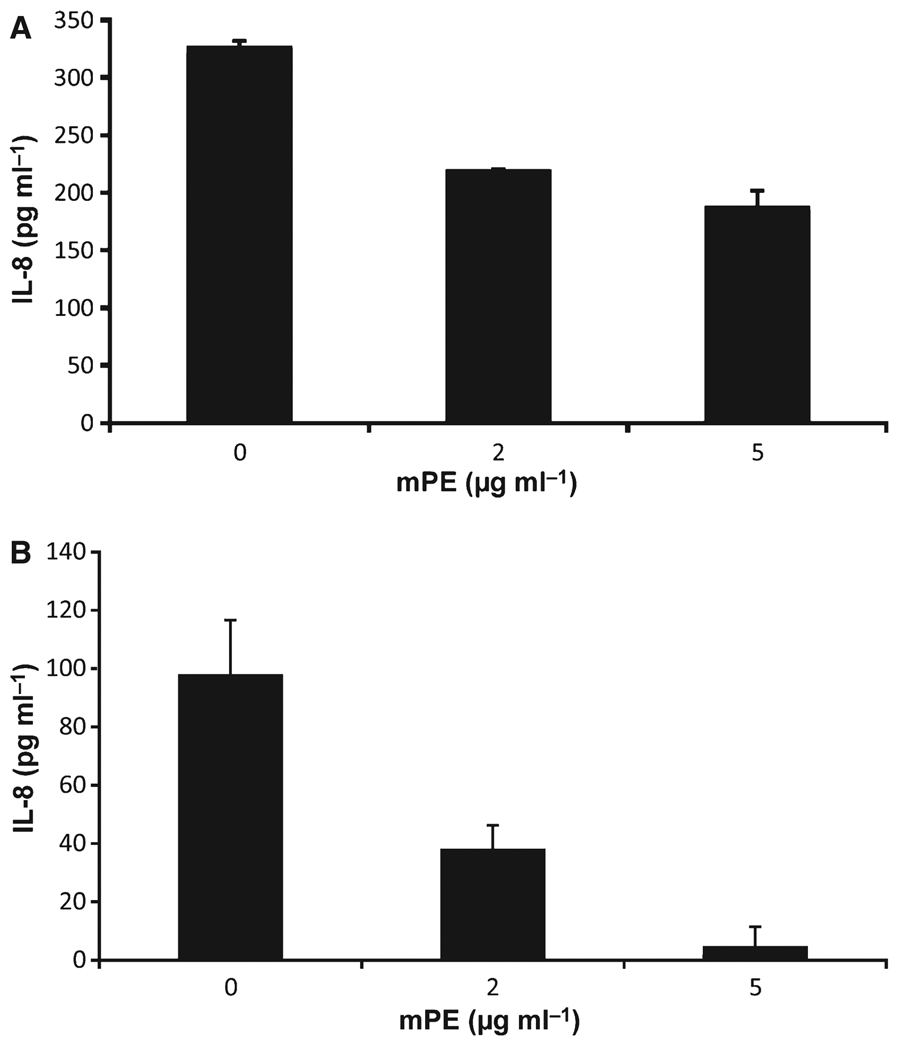
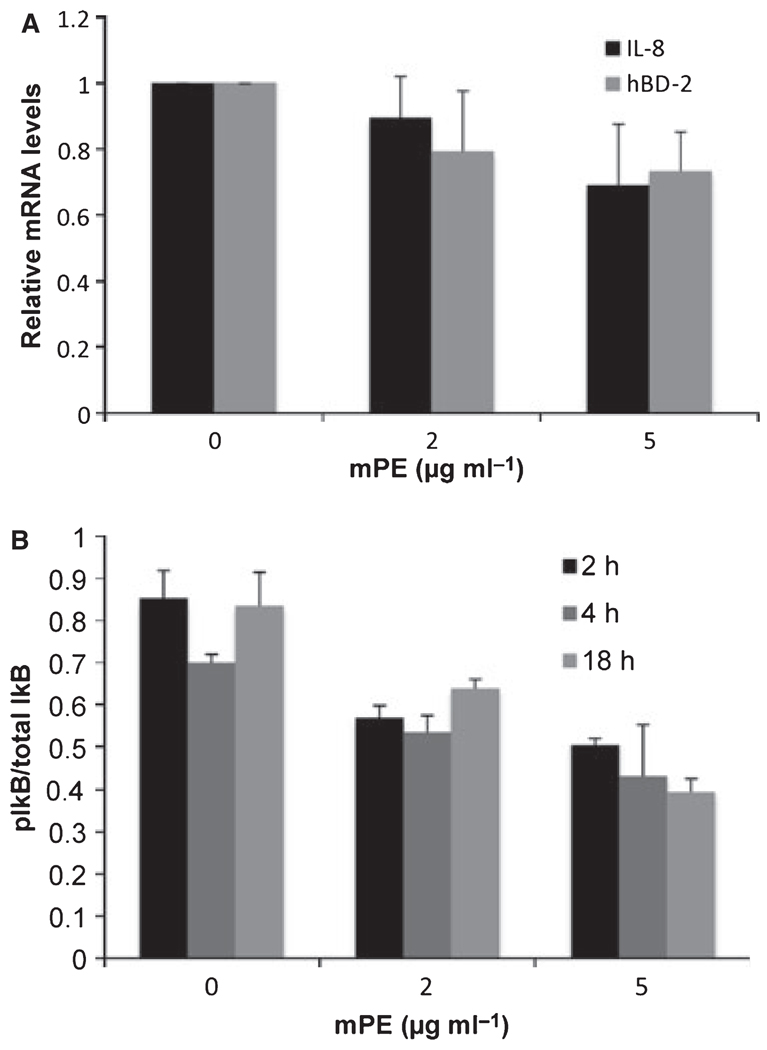
To examine the effect of mPE on the inflammatory response, we treated oral keratinocyte cells (the OKF6/TERT cell line) and the monocytic cell line, THP-1 with IL-1β (100 ng ml−1) in the presence of increasing concentrations of mPE. Secreted levels of IL-8 were measured by enzyme-linked immunosorbent assay (ELISA). The results shown in Fig. 3 demonstrate a dose-dependent inhibition of IL-8 secretion by mPE. This was not a result of cytotoxicity, as cell viability (as measured by XTT assay and trypan blue exclusion) was no lower than 93–96% at the highest concentration of mPE. To determine whether this was because of an effect on IL-8 secretion or on gene regulation, we isolated mRNA from treated cells and quantified IL-8 mRNA levels by qPCR. The results in Fig. 4A mirror the reduction of IL-8 protein and show that the inhibitory effect is at the level of gene expression. There was a similar inhibitory response in steady-state mRNA levels of another IL-1β-stimulated host defense gene, hBD-2 (Fig. 4A).
Figure 3.
Anti-inflammatory activity of mPE. The oral keratinocyte cell line OKF6/TERT (A) or THP-1 cells (B) were treated with 100 ng ml−1 recombinant human interleukin-1β (IL-1β) in the presence of 0, 2 or 5 µg ml−1 mPE for 6 h (A) or 2 h (B). The concentration of IL-8 secreted into the medium was quantified by enzyme-linked immunosorbent assay (SA Biosciences). No IL-8 was observed in either case in the absence of IL-1β (not shown). The experiment was carried out in quadruplicate; error bars represent + SD. The inhibition by mPE was significant at both concentrations with P < 0.01, as determined by Student’s t-test.
Figure 4.
Mechanism of anti-inflammatory activity of mPE. (A) Effect on nuclear factor-κB-regulated genes. OKF6/TERT cells were treated with mPE as above in the presence of 100 ng ml−1 interleukin-1β (IL-1β). Total mRNA was isolated and IL-8 and hBD-2 mRNA levels were quantified by quantitative polymerase chain reaction normalized to β2-microglobulin. Levels are shown relative to the no-mPE sample for each group. The experiment was carried out in triplicate; error bars represent + SD. (B) Effect on IκB phosphorylation. OKF6/TERT cells were grown in 96-well plates, treated with 100 ng ml-1β IL-1β for 2 or 4 h in the presence of 0, 2 or 5 µg ml−1 mPE. Phosphorylation of IκB was measured using the CASE assay (SA Biosciences) and quantified relative to total IκB levels. Phosphorylated IκB levels/total IκB of IL-1β-treated cultures are shown compared with untreated cultures. The experiment was carried out twice in quadruplicate. Reductions in pIκB/total IκB are significant at P < 0.002.
To determine whether this was the result of an inhibition of nuclear factor-κB activation, gingival epithelial cells were treated with mPE in the presence or absence of 100 ng ml−1 IL-1β, and IκB phosphorylation levels were quantified using the CASE assay. The results shown in Fig. 4B demonstrate a rapid, dose-dependent reduction in IL-1β-stimulated phosphorylated IkB levels by mPE.
DISCUSSION
Periodontal disease is associated with the adherence and colonization of pathogenic bacteria at the gingival epithelium, followed by inflammation that occurs in response to this colonization and invasion. Although development of new antibiotics can temporarily address this, the increase in antibiotic-resistant organisms makes this approach less effective. As bacteria do not readily develop resistance to antimicrobial peptides, their potential as therapies is great. However, their development as exogenous antibiotics has been hampered by a variety of factors, including difficulty in their large-scale production. The development of antimicrobial peptide mimetics that exhibit broad-spectrum antimicrobial activity with low toxicity (reviewed in Som et al., 2008) has provided a novel direction for the development of new antibiotics. Our earlier results demonstrated that an antimicrobial peptide mimetic exhibited potent activity against oral pathogens (Beckloff et al., 2007). Our results shown here demonstrate that this mimetic exhibits potent activity against both A. actinomycetemcomitans and P. gingivalis biofilms. The rapidity with which the compound acts supports its further examination as antibiotic therapy for periodontal disease as well as other biofilm-related infections.
Most antimicrobial peptides act on bacterial membranes, and many of them bind LPS as well (Beckloff et al., 2007). Indeed, a computational model of mPE demonstrates predicted binding to LPS, and incubation of mPE with macrophage cells inhibited LPS-mediated TNF-α production, similar to that seen with the LPS-binding antibiotic Polymyxin B (Beckloff et al., 2007). It was therefore surprising that mPE demonstrated similar anti-inflammatory properties inhibiting the IL-1β-induced inflammatory response in both epithelial and myeloid cells. This suggested that the anti-inflammatory property may be the result of an intracellular mechanism. Our data that mPE inhibited both IL-8 protein secretion and mRNA levels, as well as hBD-2 expression and IκB-phosphorylation, suggest that it acts on the NF-κB signal transduction pathway induced by IL-1β. This suggests that mPE could also be used as an anti-inflammatory agent in other disorders.
Anti-inflammatory properties of both synthetic and naturally occurring antimicrobial peptides have been observed (Capparelli et al., 2009; Mookherjee et al., 2009; Nan et al., 2009). Furthermore, whereas some antibiotic agents used to treat periodontal disease can induce the production of inflammatory mediators, at least one (Chlorhexidine) appears to reduce their levels (Houri-Haddad et al., 2008). However, antimicrobial peptide mimetics have numerous advantages over both peptides and agents such as chlorhexidine. Specifically, the mimetics are more stable than peptides, and are protease resistant. They are inexpensive to produce, and exhibit low cytotoxicity. Hence, our demonstration of the activity of this peptide mimetic against biofilms of periodontal pathogens and its ability to suppress IL-1β-mediated cytokine production, support its further examination as a potential therapy for periodontal disease.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by US Public Health Service grant R43 DE18371 to R.W.S. and G.D.
REFERENCES
- Beckloff N, Laube D, Castro T, et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 2007;51:4125–4132. doi: 10.1128/AAC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Burt BA, Taylor GW. Prevalence and trends in periodontitis in the USA: the [corrected] NHANES, 1988 to 2000. J Dent Res. 2005;84:924–930. doi: 10.1177/154405910508401010. [DOI] [PubMed] [Google Scholar]
- Capparelli R, Romanelli A, Iannaccone M, et al. Synergistic antibacterial and anti-inflammatory activity of temporin A and modified temporin B in vivo. PLoS ONE. 2009;4:e7191. doi: 10.1371/journal.pone.0007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- Davey ME. Techniques for the growth of Porphyromonas gingivalis biofilms. Periodontol 2000. 2006;42:27–35. doi: 10.1111/j.1600-0757.2006.00183.x. [DOI] [PubMed] [Google Scholar]
- Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- Herrera D, Alonso B, Leon R, Roldan S, Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J Clin Periodontol. 2008;35:45–66. doi: 10.1111/j.1600-051X.2008.01260.x. [DOI] [PubMed] [Google Scholar]
- Houri-Haddad Y, Halabi A, Soskolne WA. Inflammatory response to chlorhexidine, minocycline HCl and doxycycline HCl in an in vivo mouse model. J Clin Periodontol. 2008;35:783–788. doi: 10.1111/j.1600-051X.2008.01290.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Meyenhofer MF, Fine DH. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J Bacteriol. 2003;185:1399–1404. doi: 10.1128/JB.185.4.1399-1404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee N, Lippert DN, Hamill P, et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol. 2009;183:2688–2696. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- Nan YH, Bang JK, Shin SY. Design of novel indolicidin-derived antimicrobial peptides with enhanced cell specificity and potent anti-inflammatory activity. Peptides. 2009;30:832–838. doi: 10.1016/j.peptides.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Som A, Vemparala S, Ivanov I, Tew GN. Synthetic mimics of antimicrobial peptides. Biopolymers. 2008;90:83–93. doi: 10.1002/bip.20970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.