Abstract
Objective
We tested the hypothesis of a role for the calcium-dependent protease calpain in the endothelial dysfunction induced by hyperglycemic activation of protein kinase C (PKC).
Methods and Results
Chronic hyperglycemia with insulin deficiency (Type-1 diabetes) was induced in rats by streptozotocin. Total PKC and calpain activities, along with activity and expression level of the two endothelial-expressed calpains isoforms, µ- and m-calpain, were measured in vascular tissue homogenates by enzymatic assays and western blot analysis, respectively. Intravital microscopy was used to measure and correlate leukocyte-endothelium interactions with calpain activity in the microcirculation. Expression levels and endothelial localization of the inflammatory adhesion molecule ICAM-1 were studied by western blot analysis and immunofluorescence, respectively. The mechanistic role of hyperglycemia alone in the process of PKC-induced calpain activation and actions was also investigated. We found that in the Type-1 diabetic vasculature PKC selectively upregulates the activity of the µ-calpain isoform. Mechanistic studies confirmed a role for hyperglycemia and PKCβ in this process. The functional implications of PKC-induced calpain activation were upregulation of endothelial expressed ICAM-1 and leukocyte-endothelium interactions.
Conclusions
Our results uncover the role of µ-calpain in the endothelial dysfunction of PKC. Calpain may represent a novel molecular target for the treatment of PKC-associated diabetic vascular disease.
Keywords: calpain, PKC, diabetes, vascular complications, leukocytes, endothelium
Introduction
Diabetic patients have an increased risk of cardiovascular disease and inflammatory-type microvascular complications of target organs. Hyperglycemia, the hallmark of diabetes, initiates macro- and micro-vascular complications in part by inducing an endothelial inflammatory phenotype1. The ensuing endothelial dysfunction is considered causative of vascular and organ damage and it is currently used as a prognostic indicator of diabetic complications2. Indeed, the dysfunctional endothelium becomes adhesive to circulating leukocytes 3, a process implicated not only in vascular wall damage but also in organ tissue injury 4. Hyperglycemia upregulates cell adhesion molecules expressed on the vascular endothelium (eCAMs) 5, 6, a process known to induce pathologic leukocyte-endothelium interactions 7. Accordingly, hyperglycemic expression of eCAMs has been extensively studied and mechanistically linked to macro- and microvascular complications in animal models of diabetes as well as in diabetic humans. Among the several adhesion molecules expressed by the inflamed endothelium, we focused on ICAM-1 based on recent evidence implicating ICAM-1 not only in atherogenesis 8 but also in diabetic organ complications 9, 10.
Hyperglycemic activation of protein kinase C (PKC) causes endothelial dysfunction with increased leukocyte-endothelium interactions 11, 12. Inhibition of PKC attenuates leukocyte-endothelium interactions in experimental animal models of diabetes as well as in human tissue exposed to elevated ambient glucose 7, 13. PKC regulates ICAM-1 expression 14, 15, but the pathways regulating ICAM-1 transcription by PKC have not been described 16. PKC was originally discovered as a kinase cleaved by a calcium-dependent protease, now known as calpain 17. Conversely, more recent in vitro studies have suggested that calpain can serve as a downstream target of PKC signaling. Thus, in platelets active calpain colocalizes with PKC at integrin signaling complexes18, 19. Studies in human lung cancer cells have also demonstrated that PKC increases calpain activity by phosphorylation 20.
Calpain is a cytosolic calcium-dependent cysteine protease. Two calpain isoforms, µ and m, are ubiquitously expressed and, thus, found in the vascular endothelium 21. Calpain has been recently implicated in the endothelial dysfunction of acute and chronic hyperglycemia 22, 23, as well as in the neurovascular dysfunction 24, platelet hyperaggregability 25, and cardiomyocyte apoptosis 26 of diabetes. Accordingly, we tested the novel hypothesis of a role for calpain in the endothelial dysfunction of hyperglycemic activation of PKC.
Research Design and Methods
The Temple University IACUC guidelines and the NIH Policy on Humane Care and Use of Laboratory Animals were observed in this study. Male Sprague-Dawley rats of 8 weeks of age were used (Ace Animals, Inc.; Boyertown, PA, USA).
Insulin-deficient diabetes
Insulin-deficiency with hyperglycemia, a condition similar to human Type-1 diabetes, was induced by a single i.p. injection of 60 mg/kg streptozotocin (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.9% saline 27. Streptozotocin-injected rats (STZ-diabetes) developed and average blood glucose levels of 23.8±1.1 mM (p<0.001 versus average values of 5.7±0.1 mM found in nondiabetic control rats). Nondiabetic control rats and diabetic rats were randomly assigned to one of the following experimental groups: 1) control + vehicle, 2) STZ diabetes + vehicle, 3) STZ-diabetes + 27 µg/kg ZLLal, 4) STZ-diabetes + 1 mg/kg PD150606, 5) STZ-diabetes + 30 nM BIM-I, 6) STZ-diabetes + 30 nM BIM-V.
Acute hyperglycemia of the peritoneal cavity
To dissect the role of hyperglycemia alone in these studies, we used a well-established animal model of acute hyperglycemia of the peritoneal cavity, which is characterized by PKC-dependent endothelial dysfunction with increased leukocyte adhesion in the mesenteric microcirculation12. Rats were randomly divided into three experimental groups: 1) control rats injected with 5 mM D-glucose, 2) rats injected with 25 mM L-glucose; 3) rats injected with 25 mM D-glucose, and 3) rats injected with 25 mM D-glucose + 30 nM BIM-I. Saline and glucose were administered intraperitoneally 14–18 hours prior to study, as previously described 28. L-glucose was used to rule out non-specific actions caused by osmolarity changes. For acute studies, BIM-I was administered as a single i.p. injection, 1 hour before intravital microscopy.
Inhibition of calpain and total PKC in vivo
Total calpain inhibition was achieved with either of two different calpain inhibitors, ZLLal or PD150606. Total PKC inhibition was achieved with Bisindolylmaleimide-I (BIM-I). Bisindolylmaleimide -V (BIM-V) was used as the inactive control for BIM-1.
Inhibition of PKCβ in rat heart microvascular endothelial cells (RHME)
A twofold approach was used to dissect the role of PKCβ: a) genetic inhibition using an adenovirus encoding a kinase-inactive rabbit PKCβ 29; and b) pharmacological inhibition using either the pan-PKC inhibitor BIM-I or the selective PKCβ blocker LY379196, which blocks both PKCβ-I and PKCβ-II 30.
Analyses of Calpain and PKC activities
were performed using a fluorescent-based assay and a standard radioactive assay, respectively.
Quantification of leukocyte-endothelium interactions
Leukocyte rolling and adhesion were studied by intravital microscopy of the mesenteric microcirculation. ICAM-1 expression levels were studied by fluorescent microscopy and western blot analysis.
Data Analysis
All data are expressed as mean values ± standard error mean (SEM). Data were analyzed by ANOVA with a Newman-Keuls Multiple Comparison post-test or with Student’s t test using GraphPad Prism version 5.0C for Macintosh (GraphPad Software, San Diego California USA, www.graphpad.com"). P values less than 0.05 were considered statistically significant.
For detailed methods please see http://atvb.ahajournals.org.
Results
Insulin-deficient diabetes increases PKC and calpain activities in the vascular mesentery
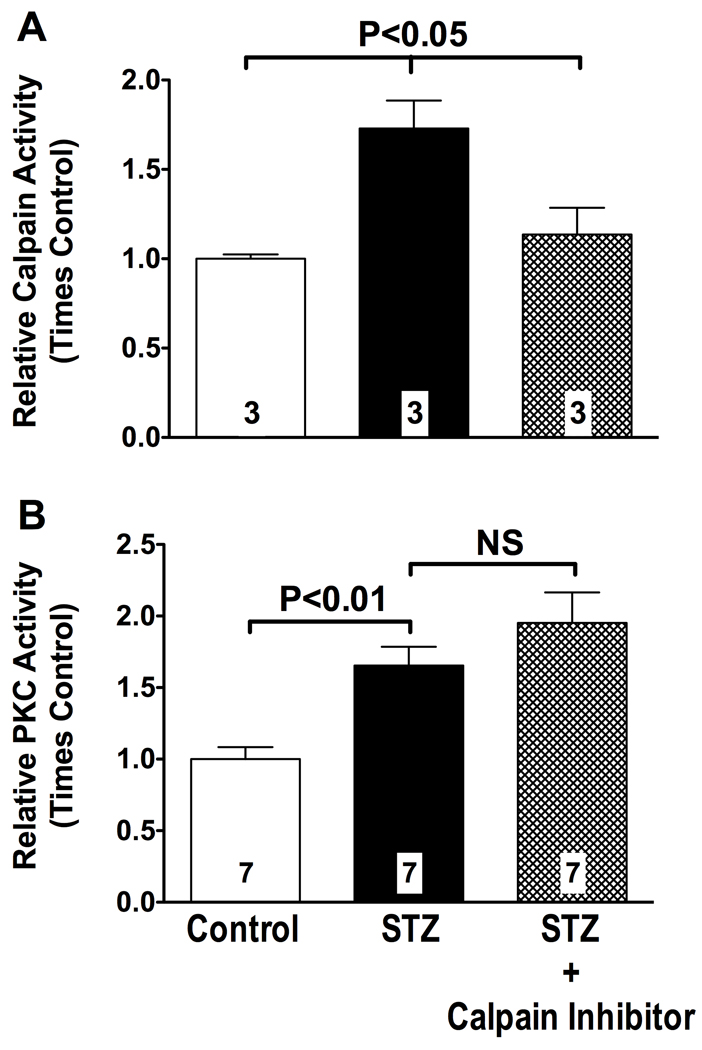
To study the relationship between PKC and calpain in STZ-diabetic rats, we first measured PKC and calpain activities in tissue extracts of vascularized sections of the mesentery, before and after pharmacological inhibition of calpain. STZ-diabetic rats exhibited an almost 2-fold increase in calpain activity (P<0.05 vs. control, Figure 1, Panel A) and a greater than 1.5-fold increase in PKC activity in the vascular mesentery (P<0.05 vs. control, Figure 1, Panel B). Treatment of STZ-diabetic rats with 27 µg/kg of the calpain inhibitor ZLLal returned calpain activity to the control values found in nondiabetic rats (P<0.05, Figure 1, Panel A), but failed to attenuate PKC activity (Figure 1, Panel B). Biochemical studies into the expression levels of the calpain isoform(s) found in the vascular wall revealed selective upregulation of µ-calpain but not m-calpain under our experimental conditions (Figure 1S).
Figure 1. Calpain and PKC activities are increased in the hyperglycemic, insulin-deficient mesenteric microcirculation.
Calpain (panel A) and PKC (Panel B) activities in all experimental groups of rats were measured in protein extracts of the vascularized mesentery using the fluorogenic substrate Succ-LLVY-AMC and by the incorporation of γ-phosphate from [γ-32P]ATP into the substrate QKRPSQRSKYL, respectively. The calpain inhibitor ZLLal effectively blocked calpain activity (Panel A) but failed to attenuate PKC activity (Panel B) in the STZ-diabetic microcirculation. Activity levels are expressed as fold change from nondiabetic control values. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
We next studied whether inhibition of PKC could prevent calpain activation in the intact microcirculation of STZ-diabetic rats. For these studies we coupled a fluorescent assay of calpain activity with in vivo microscopy of mesenteric post-capillary venules, which are microvessels comprised only by a layer of endothelial cells, a basement membrane, and a few scattered pericytes 31. The advantage of this approach over the biochemical one described above is that it allows for simultaneous quantification and spatial localization of active calpains within the vascular endothelium of the observed microvasculature in vivo. In fact, the results obtained with the aortic and mesenteric tissue homogenates described in Figures 1 and 1S could in theory have been contaminated with vascular smooth muscle cells, which also upregulate PKC signaling in hyperglycemic conditions 32. Fluorescent staining clearly demonstrated that the diabetic endothelium of post-capillary venules experiences increased calpain activity compared to control (Figure 2, Panel B). Quantification of the fluorescent staining revealed a 2-fold increase from control in endothelial-expressed calpain activity in STZ-diabetic rats, which was attenuated to control levels following pharmacological inhibition of calpain in vivo (P<0.05, Figure 2, bar graph). These results are consistent with the biochemical analysis for calpain activity reported in Figure 1, Panel A. Two 30 nM doses per day of the PKC inhibitor BIM-I significantly reduced endothelial calpain activity in STZ-diabetic rats (P<0.05, Figure 2, bar graph). In contrast, treatment of rats with two 30 nM doses per day of inactive PKC inhibitor BIM-V failed to attenuate calpain activity in STZ-diabetic rats (NS), confirming the pharmacological specificity of PKC inhibitor used in these studies. Since calpain inhibition failed to prevent upregulation of PKC, these data indicate that calpain lies downstream of PKC in the vascular inflammation of diabetes. They also demonstrate that hyperglycemia with insulin-deficiency upregulates calpain activity in the vascular endothelium and that this process is in part PKC-dependent.
Figure 2. PKC inhibition attenuates calpain activity in the hyperglycemic, insulin-deficient microcirculation.
Following superfusion of the rat mesentery with the fluorogenic calpain substrate t-BOC-Leu-Met-CMAC, active calpains in the venular endothelium were visualized by fluorescent intravital microscopy and measured by densitometry. Control post-capillary venules (V) had a very low basal level of calpain activity indicated by a low level of fluorescent staining (Panel A). Calpain activity was markedly increased in the post-capillary venular endothelium of STZ-diabetic rats, as demonstrated by intense fluorescent staining (Panel B, white arrows). The PKC inhibitor BIM-I reduced fluorescent staining for calpain activity in the STZ-diabetic microcirculation (Panel C). The calpain inhibitor PD150606 also attenuated fluorescent staining for calpain activity. White line in Panel A represents 10 µm scale. Yellow circle in panel B depicts a representative region of interest (ROI) used for densitometry. The bar graph shows densitometry quantification of calpain activity in all experimental groups of rats. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
None of the above inhibitors changed blood glucose levels in diabetic rats (data not shown), ruling out the possibility that the vascular protective actions of calpain and PKC inhibition in vivo in our study were due to lowered glucose levels in the plasma.
The role of PKCβ in the process of calpain activation
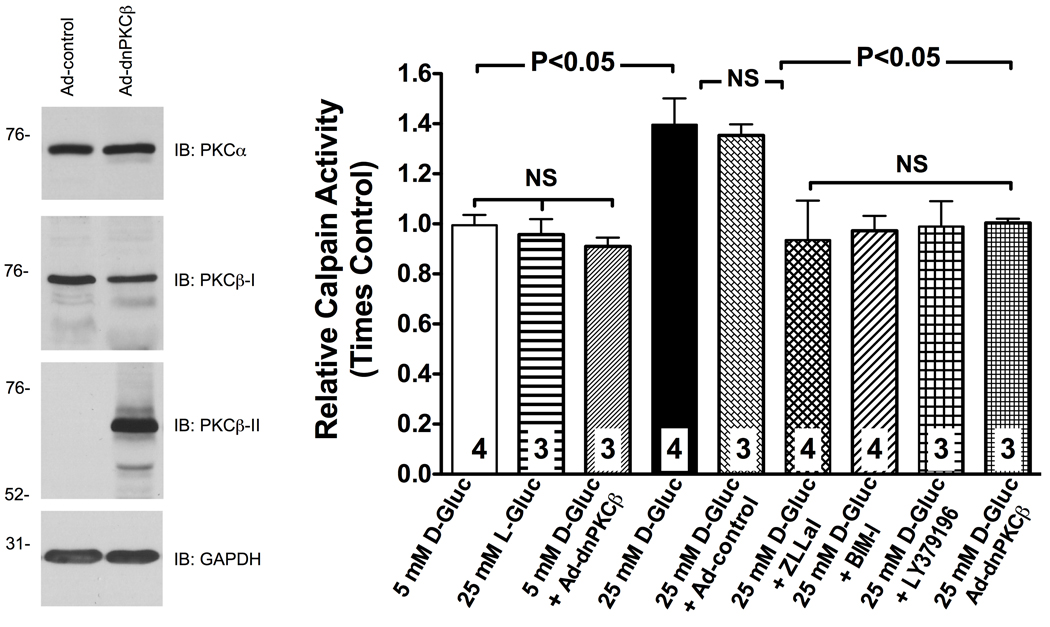
Among the several PKC isoforms, PKCβ plays a widely accepted role in the microvascular complication of hyperglycemia 33. Accordingly, we performed in vitro experiments to assess the role of PKCβ in the process of endothelial calpain activation by hyperglycemia. Consistent with our in vivo results (Figure 2, Panel A), rat heart microvascular endothelial cells (RHMEC) exposed to elevated ambient glucose showed an approximately 1.5-fold increase in calpain activity over control (Figure 3). Co-incubation of RHMEC with the calpain inhibitor ZLLal, the pan-PKC inhibitor BIM-I or the selective PKCβ blocker LY379196 prevented upregulation of calpain activity in the face of elevated glucose concentration (Figure 3). Moreover, adenovirus-mediate expression of a kinase-inactive dominant-negative (dn) PKCβ also blocked calpain activation in response to elevated ambient glucose, which further confirms the validity and specificity of the pharmacological results obtained in this study. Overall, these data agree with our in vivo observations reported in Figures 1 and 2 and they also demonstrate a role for the PKCβ isoform in the process of endothelial calpain activation by elevated ambient glucose.
Figure 3. RHMEC experience a PKCβ-dependent increase in calpain activity in response to high glucose.
Calpain activity was measured in attached RHMEC using the fluorogenic substrate Succ-LLVY-AMC and expressed as fold change from control. Genetic inhibition of PKCβ by expression of a kinase-inactive dominant-negative (dn) PKCβ, and pharmacological inhibition of either total PKC activity with BIM-I or selective PKCβ activity with LY379196 prevented upregulation of calpain in response to high glucose. The mutant protein constructed on rabbit PKCβ-II cDNA could be distinguished from the endogenously expressed PKCβ-I by antibodies raised against c-terminus of PKCβ-I and PKCβ-II. Expression of PKCα was not affected by the dnPKCβ over-expression (Immunoblot). The dnPPKCβ used in this study has been shown to inhibit select functions mediated by both PKCβ-I and PKCβ-II, but not by other PKCs such as PKCα44. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of experiments.
PKC/calpain upregulates leukocyte-endothelium interactions
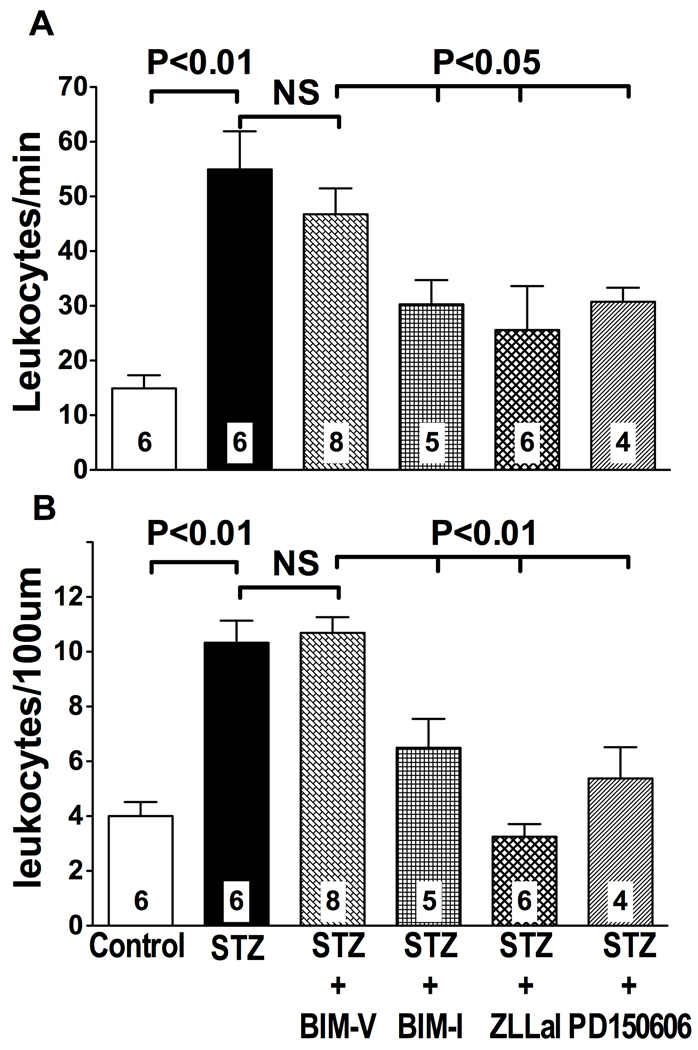
The post-capillary venules experiencing increased calpain activity (Figure 2, Panel B) are also the site of the microcirculation that regulates leukocyte-endothelium interactions, a process highly relevant to diabetic vascular complications. Accordingly, we measured leukocyte rolling (Figure 4, Panel A) and adhesion (Figure 4, Panel B) in mesenteric post-capillary venules by intravital microscopy. As shown in Figure 4, the microcirculation of STZ-diabetic rats experiences increased adhesiveness to circulating leukocytes in post-capillary venules, a site where increased calpain activity was also observed (Figure 2). Specifically, we found evidence of a 3-fold and 2.5-fold increase in leukocyte rolling and adhesion, respectively, in STZ-diabetic rats compared to nondiabetic rats (Figure 4). Both leukocyte rolling and adhesion were reduced following treatment of STZ-diabetic rats with the PKC inhibitor BIM-I (Figure 4). In contrast, leukocyte rolling and adhesion remained elevated after administration of the inactive PKC inhibitor BIM-V to diabetic rats (NS). Comparable results were obtained following inhibition of calpain activity with either one of the two calpain inhibitors used in this study (Figure 4). Administration of the calpain inhibitor ZLLal or PD150606 to diabetic rats each returned the values of leukocyte rolling and adhesion to near those found in nondiabetic control rats (Figure 4). Thus, hyperglycemia with insulin-deficiency induces abnormal leukocyte trafficking in the microcirculation through increased PKC/calpain signaling.
Figure 4. Leukocyte-endothelium interactions in mesenteric post-capillary venules.
Leukocyte rolling (A) and adhesion (B) were studied in all experimental groups of rats by brightfield intravital microscopy and expressed as the number of leukocytes per minute and the number of leukocytes per 100 µm vessel length, respectively. Calpain or PKC inhibition reduced leukocyte-endothelium interactions to a similar extent in the STZ-diabetic microcirculation. In contrast, the inactive PKC inhibitor, BIM-V, failed to attenuate leukocyte rolling and adhesion in STZ-diabetic rats. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
PKC/Calpain upregulate ICAM-1
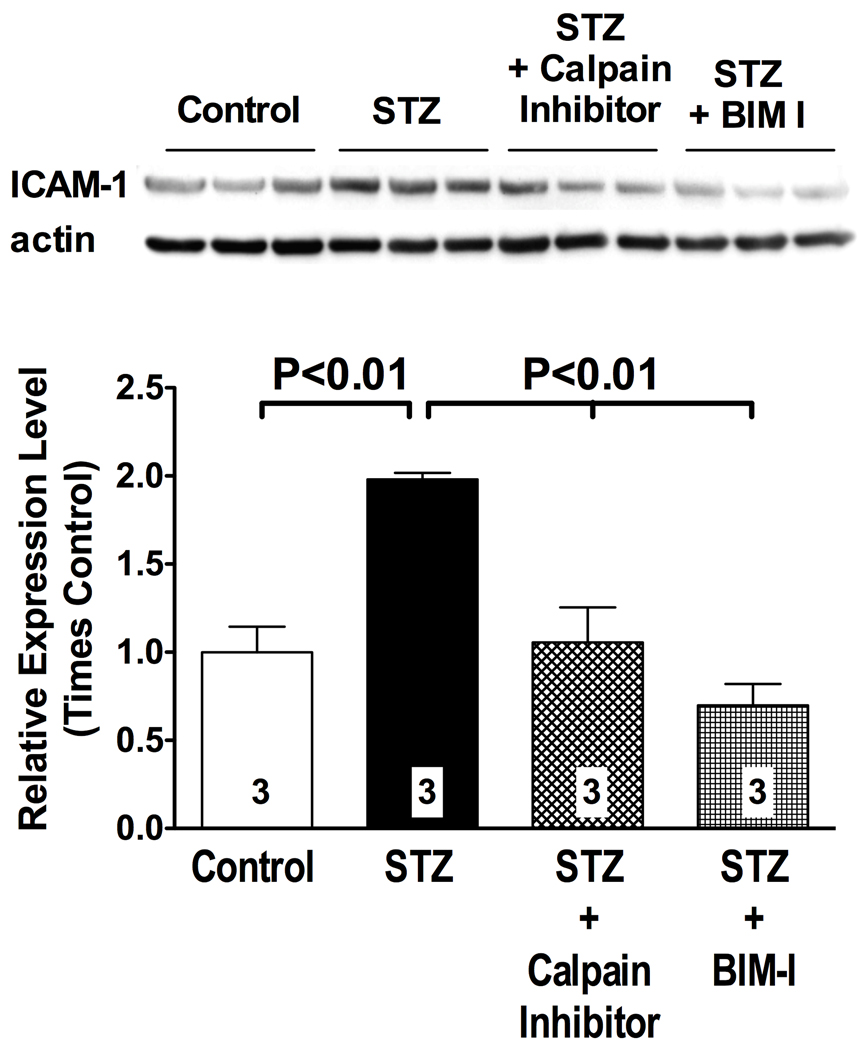
The dysfunctional endothelium expresses ICAM-1, a cell adhesion molecule upregulated by PKC and implicated in diabetic vascular complications9, 15. We used western blot analysis to study the impact of PKC/calpain signaling on ICAM-1 expression in the hyperglycemic, insulin-deficient vasculature of STZ diabetic rats (Figure 5). Consistent with data in the literature14, 15, we found evidence of increased ICAM-1 expression in the aorta of STZ diabetic rats (Figure 5), which was prevented by PKC inhibition in vivo (Figure 5). Interestingly, inhibition of calpain activity also prevented upregulation of ICAM-1 expression in diabetic rats (Figure 5), even in the face of increased PKC activity (Figure 1). These results were confirmed by immunofluorescence of mesenteric post-capillary venules, which specifically demonstrated a calpain-dependent increase in ICAM-1 expression in the vascular endothelium of the diabetic microcirculation (Figure S2). These data correlate with the anti-adhesive effects of calpain inhibition on leukocyte-endothelium interactions reported above, and they are consistent with published study implicating a role for PKC in the process of ICAM-1 transcription14, 15.
Figure 5. PKC-calpain signaling regulates ICAM-1 expression in the hyperglycemic, insulin-deficient vasculature.
ICAM-1 expression in the thoracic aorta was studied by western blot analysis. Expression of ICAM-1 was increased above control levels in STZ diabetic rats. Treatment of STZ diabetic rats with the calpain inhibitor ZLLal or with the PKC inhibitor BIM-I returned ICAM-1 expression levels to control values. The bar graph shows quantification by densitometry of the signal from western blot analysis. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
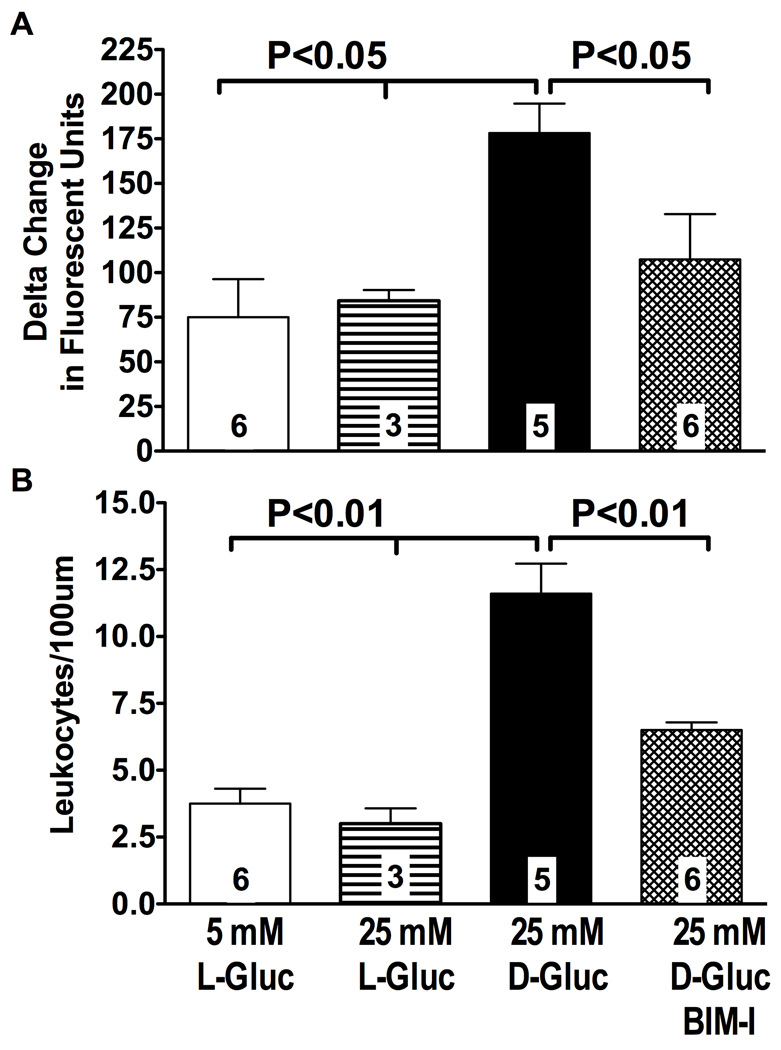
The role of hyperglycemia in the process of PKC-calpain signaling
Hyperglycemia is the hallmark of insulin-deficient diabetes, and, based on previous studies by our laboratory34, it is likely responsible for abnormal activation of endothelial-expressed calpains. Nonetheless the possibility exists that the obvious loss of insulin signaling occurring in STZ-diabetic rats played a role in the process of endothelial calpain activation in this study. Accordingly, we studied the role of hyperglycemia alone in the process of PKC-calpain activation and actions using a well-established animal model of experimental hyperglycemia of the peritoneal cavity. The mesenteric microcirculation of nondiabetic control rats with preserved insulin signaling was exposed to 25 mM D-glucose for 16 hours to acutely induce leukocyte-endothelium interactions28. Leukocyte adhesion and calpain activity were then measured simultaneously in mesenteric post-capillary vessels by intravital microscopy as reported above. The upper graph in Figure 6 shows that following a 16 hour exposure to elevated ambient glucose the post-capillary venules of the mesentery experience an approximately 2.5-fold increase in calpain activity (P<0.05 versus control), as detected with the calpain substrate t-BOC-Leu-Met-CMAC (see also Figure 2). A nearly 3-fold increase in leukocyte adhesion (P<0.001) was also observed in the same post-capillary venules (Figure 6). Administration of the PKC inhibitor BIM-I prevented glucose-induced calpain activation (P<0.05) and leukocyte adhesion (P<0.001). These results closely correlate with those found in STZ-diabetic rats (Figures 2 and 4), thus confirming a causative role for hyperglycemia in the process of PKC-induced calpain activation and actions in the vascular endothelium.
Figure 6. Acute hyperglycemia upregulates leukocyte-endothelium interactions and calpain activity in mesenteric post-capillary venules in a PKC-dependent manner.
Post-capillary venules from all experimental groups of rats were viewed under brightfield microscopy. Following superfusion of the rat mesentery with the fluorogenic calpain substrate t-BOC-Leu-Met-CMAC, active calpains in the venular endothelium were visualized by fluorescent microscopy and measured by densitometry, as shown in Figure 2. Elevated ambient glucose increased calpain activity (panel A) and leukocyte adhesion in post-capillary venules (Panel B). Treatment of rats with BIM-I prevented both calpain activity and leukocyte adhesion in post-capillary venules exposed to elevated ambient glucose. Panel A shows densitometry quantification of calpain activity in all experimental groups of rats. Panel B is leukocyte adhesion expressed as the number of cells per 100 µm in all experimental groups of rats. Bars represent mean ± SEM, and numbers at the base of the bars represent the number of rats studied in each group.
Discussion
The major findings of this study are that: a) under conditions of hyperglycemia with insulin-deficiency PKC activates µ-calpain, which leads to elevated expression levels of ICAM-1 and leukocyte-endothelium interactions; b) pharmacological inhibition of calpain attenuates leukocyte-endothelium interactions and ICAM-1 expression even in the face of increased PKC activity; c) hyperglycemia, more than loss of insulin signaling, is primarily responsible for increased PKC/calpain signaling. Overall these data demonstrate an emerging role for the calpain system in the inflammatory cascade of PKC and provide a new signaling pathway by which PKC regulates ICAM-1 expression in diabetic vascular disease.
PKC was originally recognized as a calpain substrate based on biochemical studies that demonstrated calpain proteolysis of PKC 17, 35. Accordingly, we had initially predicted that calpain would operate upstream of PKC. To the contrary, the results obtained in this study clearly show that in the hyperglycemic, insulin-deficient vasculature PKC regulates calpain activity, as demonstrated by the fact that inhibition of PKC attenuates calpain activation and not vice versa. This novel finding is supported by recent studies demonstrating a colocalization of PKC and calpain at the cell membrane upon platelet activation18. In vitro studies of cancer cells have also provided direct evidence that PKC is responsible for calpain activation through phosphorylation20, a process known to increases activity of the protease 36. With regard to endothelial function, one recent study in vitro has indirectly linked PKC and calpain in hyperglycemia. Thus, Wang et al 37 have suggested that the Na+/H+ exchanger, a known PKC substrate, is required for glucose-induced endothelial dysfunction via calpain 38. The data here presented support this working model in which primary activation of PKC results in elevated calpain activity and calpain-dependent endothelial dysfunction.
Based on cDNA cloning, the calpain family includes at least 15 isoforms, with the µ and m isoforms being ubiquitously expressed 21. The names of the latter two isoforms describe the calcium concentrations required for their activation in vitro: micromolar for µ-calpain and millimolar for m-calpain. However, such calcium concentrations are rarely, if ever, found under physiological conditions. Thus, substantial controversy over the in vivo activation of calpain still exists. Biochemical studies have now demonstrated that post-translational modifications of calpains by kinases result in their activation in the presence of reduced calcium levels 39. Thus, PKC may indirectly activate calpain by increasing its calcium sensitivity. Further studies need to be undertaken to identify the precise mechanism by which PKC activates calpain in diabetes, which will not only help understand the regulation of the PKC/calpain pathway but may also explain the molecular mechanisms responsible for preferential calpain isoforms(s) activation.
PKC is known to be an important signaling molecule in diabetic endothelial dysfunction, and several PKC isoforms have been involved in the pathophysiology of vascular-driven diabetic complications 11. There are at least 11 isoforms of PKC, and the activation of individual isoforms appears to be tissue specific 11. The β isoform has received much attention since it was first shown to be preferentially upregulated in diabetic vascular tissue 40. The development of a specific inhibitor of this isoform 41 has allowed the assessment of diabetic vascular disturbances as a function of PKCβ. Our results in cultured endothelial cells provide novel evidence that calpain may function as an important downstream mediator of PKCβ in diabetic endothelial dysfunction. They also may stimulate future research into the individual PKC isoforms implicated in the process of calpain activation and action in other relevant vascular tissues (e.g., vascular smooth muscle cells) with the overall goal of understanding how activation of PKC/calpain signaling impacts the homeostasis of the entire vascular wall under physiologic and pathophysiologic conditions.
A large amount of data in the literature has confirmed the association between deregulated PKC activity and vascular dysfunction, including but not limited to increased leukocyte-endothelium interactions 11, 16. Our laboratory was the first to report that calpain is uniquely activated in the endothelial dysfunction associated with acute hyperglycemia 22 and obesity 23. In line with our observations, others have now documented a role for calpain in the neurovascular dysfunction and platelet hyperaggregability of type 2 diabetes24,25, in the apoptosis of hyperglycemic cardiomyocytes 26, and in hyperlipidemic endothelial dysfunction 42. The present study provides novel evidence that calpain also serves as an important downstream signaling mediator of PKC in hyperglycemia with insulin deficiency. At the functional level, pharmacological inhibition of calpain activity abrogated leukocyte-endothelium interactions and ICAM-1 expression in the hyperglycemic vasculature, even in the face of increased PKC activity. These findings are in line with previous evidence demonstrating hyperglycemic expression of ICAM-1 through either PKC 7 or calpain 22. Relevant to vascular disease, upregulated ICAM-1 expression from exposure to the hyperglycemic environment is associated with enhanced atheroma formation 8 and organ tissue damage 10. Thus, targeting calpain and PKC together in diabetes may afford enhanced vascular protection by reducing the pathogenic expression of ICAM-1. Albeit both conventional and atypical PKC have been involved in regulation of ICAM-1 expression 14, 15, the pathways responsible for regulating ICAM-1 transcription during high-glucose concentration remains largely unknown. Our data demonstrate the mechanistic role of calpain in the process of endothelial ICAM-1 upregulation in the hyperglycemic vasculature experiencing also increased PKC activity, thus providing a novel signaling pathways by which PKC upregulates inflammatory signals in hyperglycemia.
Hyperglycemia is only one of the many metabolic alterations of diabetes. Nonetheless, hyperglycemia remains the diabetic disturbance most largely associated with vascular complications, as demonstrated by the results of the Diabetes Control and Complications Trial 43. Activation of PKC via the de novo production of DAG is one of the most accepted mechanisms by which hyperglycemia induce endothelial dysfunction and vascular inflammation. The experimental results obtained in our rat model of acute hyperglycemia of the peritoneal cavity further confirm the role of hyperglycemia in the process of PKC activation in the insulin deficient cardiovascular system and they also uncover a role for calpain in the endothelial dysfunction resulting by acute hyperglycemic activation of PKC. Finally, our study did not explore how sex differences impact on the PKC/calpain signaling cascade, since all results were obtained in male mice. Given the well-established role that sex hormones play both in the regulation of metabolic parameters and in the progression of cardiovascular disease, further studies are clearly needed to understand how sex-linked difference regulate endothelial PKC/calpain signaling under pathophysiological conditions.
In summary, we have shown that activation of PKC in hyperglycemia with insulin-deficiency increases endothelial calpain activity, with a preferential activation of the µ-calpain isoform. The pathophysiologic implications of this enhanced calpain activity is ICAM-1 upregulation with increased leukocyte trafficking in the microcirculation.
Supplementary Material
Acknowledgements
Sources of Funding. This work was supported by Grants NIH DK064344 and JDRF #1-2007-71 to Rosario G. Scalia, and Grants #HL076770 and AHA, Established Investigator Award #0740042N to Satoru Eguchi. Amanda R. Smolock is a student in the MD/PhD program of Thomas Jefferson University in Philadelphia, PA and she is supported in part by a Dubbs Scholar Fellowship Award and in part by a Percival E. and Ethel Brown Foerderer Foundation Fellowship from Thomas Jefferson University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. None
References
- 1.Rask-Madsen C, King GL. Mechanisms of disease: Endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 2.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 3.Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, Paolisso G, Giugliano D. Circulating adhesion molecules in humans: Role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–2251. doi: 10.1161/01.cir.101.19.2247. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner-Parzer SM, Wagner L, Pettermann M, Gessl A, Waldhausl W. Modulation by high glucose of adhesion molecule expression in cultured endothelial cells. Diabetologia. 1995;38:1367–1370. doi: 10.1007/BF00401771. [DOI] [PubMed] [Google Scholar]
- 7.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a nf-kb-dependent fashion. The Journal of clinical investigation. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlassara H, Fuh H, Donnelly T, Cybulsky M. Advanced glycation endproducts promote adhesion molecule (vcam-1, icam-1) expression and atheroma formation in normal rabbits. Mol Med. 1995;1:447–456. [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul K, Hodgkinson A, Tarr J, Kohner EM, Chibber R. Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Curr Diabetes Rev. 2010 doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Zhang J, Zhang Y, Wang Y, Wang B. Improvement of inflammatory responses associated with nf-kappa b pathway in kidneys from diabetic rats. Inflamm Res. 2008;57:199–204. doi: 10.1007/s00011-006-6190-z. [DOI] [PubMed] [Google Scholar]
- 11.Das Evcimen N, King GL. The role of protein kinase c activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Booth G, Stalker TJ, Lefer AM, Scalia R. Mechanisms of amelioration of glucose-induced endothelial dysfunction following inhibition of protein kinase c in vivo. Diabetes. 2002;51:1556–1564. doi: 10.2337/diabetes.51.5.1556. [DOI] [PubMed] [Google Scholar]
- 13.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappab by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 14.Rahman A, Anwar KN, Uddin S, Xu N, Ye RD, Platanias LC, Malik AB. Protein kinase c-delta regulates thrombin-induced icam-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase. Mol Cell Biol. 2001;21:5554–5565. doi: 10.1128/MCB.21.16.5554-5565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman A, Anwar KN, Malik AB. Protein kinase c-zeta mediates tnf-alpha-induced icam-1 gene transcription in endothelial cells. Am J Physiol Cell Physiol. 2000;279:C906–C914. doi: 10.1152/ajpcell.2000.279.4.C906. [DOI] [PubMed] [Google Scholar]
- 16.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase c in diabetes and insulin resistance. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. Ii. Proenzyme and its activation by calcium-dependent protease from rat brain. The Journal of biological chemistry. 1977;252:7610–7616. [PubMed] [Google Scholar]
- 18.Fox JE. On the role of calpain and rho proteins in regulating integrin-induced signaling. Thromb Haemost. 1999;82:385–391. [PubMed] [Google Scholar]
- 19.Kulkarni S, Saido TC, Suzuki K, Fox JE. Calpain mediates integrin-induced signaling at a point upstream of rho family members. The Journal of biological chemistry. 1999;274:21265–21275. doi: 10.1074/jbc.274.30.21265. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Deng X. Protein kinase ciota promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. The Journal of biological chemistry. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- 21.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 22.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. Faseb J. 2003;17:1511–1513. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 23.Stalker TJ, Gong Y, Scalia R. The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes. 2005;54:1132–1140. doi: 10.2337/diabetes.54.4.1132. [DOI] [PubMed] [Google Scholar]
- 24.Nangle MR, Cotter MA, Cameron NE. The calpain inhibitor, a-705253, corrects penile nitrergic nerve dysfunction in diabetic mice. European journal of pharmacology. 2006;538:148–153. doi: 10.1016/j.ejphar.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 25.Randriamboavonjy V, Pistrosch F, Bolck B, Schwinger RH, Dixit M, Badenhoop K, Cohen RA, Busse R, Fleming I. Platelet sarcoplasmic endoplasmic reticulum ca2+-atpase and mu-calpain activity are altered in type 2 diabetes mellitus and restored by rosiglitazone. Circulation. 2008;117:52–60. doi: 10.1161/CIRCULATIONAHA.107.719807. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Feng Q, Arnold JM, Peng T. Calpain activation contributes to hyperglycemia-induced apoptosis in cardiomyocytes. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp189. [DOI] [PubMed] [Google Scholar]
- 27.Szkudelski T. The mechanism of alloxan and streptozotocin action in b cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 28.Booth G, Stalker TJ, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: Effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–E856. doi: 10.1152/ajpendo.2001.280.6.E848. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe J, Ohba M, Ohno F, Kikuyama S, Nakamura M, Nakaya K, Arimura A, Shioda S, Nakajo S. Pituitary adenylate cyclase-activating polypeptide-induced differentiation of embryonic neural stem cells into astrocytes is mediated via the β isoform of protein kinase c. Journal of Neuroscience Research. 2006;84:1645–1655. doi: 10.1002/jnr.21065. [DOI] [PubMed] [Google Scholar]
- 30.Osto E, Kouroedov A, Mocharla P, Akhmedov A, Besler C, Rohrer L, von Eckardstein A, Iliceto S, Volpe M, Luscher TF, Cosentino F. Inhibition of protein kinase cbeta prevents foam cell formation by reducing scavenger receptor a expression in human macrophages. Circulation. 2008;118:2174–2182. doi: 10.1161/CIRCULATIONAHA.108.789537. [DOI] [PubMed] [Google Scholar]
- 31.Kierszenbaum AL. Histology and cell biology : An introduction to pathology. Philadelphia, PA: Mosby Elsevier; 2007. [Google Scholar]
- 32.Srivastava AK. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: A potential role in the pathogenesis of vascular dysfunction in diabetes (review) Int J Mol Med. 2002;9:85–89. [PubMed] [Google Scholar]
- 33.Das Evcimen N, King GL. The role of protein kinase c activation and the vascular complications of diabetes. Pharmacological Research. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Stalker TJ, Skvarka CB, Scalia R. A novel role for calpains in the endothelial dysfunction of hyperglycemia. FASEB J. 2003:1202fje–1213fje. doi: 10.1096/fj.02-1213fje. [DOI] [PubMed] [Google Scholar]
- 35.Takai Y, Yamamoto M, Inoue M, Kishimoto A, Nishizuka Y. A proenzyme of cyclic nucleotide-independent protein kinase and its activation by calcium-dependent neutral protease from rat liver. Biochemical and biophysical research communications. 1977;77:542–550. doi: 10.1016/s0006-291x(77)80013-2. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induces phosphorylation of mu- and m-calpain in association with increased secretion, cell migration, and invasion. The Journal of biological chemistry. 2004;279:53683–53690. doi: 10.1074/jbc.M409889200. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Peng Q, Zhang J, Liu L. Na+/h+ exchanger is required for hyperglycaemia-induced endothelial dysfunction via calcium-dependent calpain. Cardiovascular research. 2008 doi: 10.1093/cvr/cvn179. [DOI] [PubMed] [Google Scholar]
- 38.Williams B, Howard RL. Glucose-induced changes in na+/h+ antiport activity and gene expression in cultured vascular smooth muscle cells. Role of protein kinase c. The Journal of clinical investigation. 1994;93:2623–2631. doi: 10.1172/JCI117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain ii), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase c isoform beta ii and diacylglycerol levels in the aorta and heart of diabetic rats: Differential reversibility to glycemic control by islet cell transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral pkc beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 42.Dong Y, Wu Y, Wu M, Wang S, Zhang J, Xie Z, Xu J, Song P, Wilson K, Zhao Z, Lyons T, Zou MH. Activation of protease calpain by oxidized and glycated ldl increases the degradation of endothelial nitric oxide synthase. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The diabetes control and complications trial research group. The New England journal of medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg HJ, Whiteside CI, Fantus IG. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and sp1 transcriptional activation through protein kinase c-œ≤i and -œ¥. Journal of Biological Chemistry. 2002;277:33833–33841. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.