Abstract
Charge is an important characteristic of drug molecules, since ionization sites determine the pKa at a particular pH. The pKa in turn can affect many parameters, including solubility, dissolution rate, reaction kinetics, formulation, cell permeability, tissue distribution, renal elimination, metabolism, protein binding and receptor interactions. The impact of charge dynamics is amplified in human solid tumors that exhibit the glycolytic phenotype and associated acidic extracellular microenvironment. This phenotype is driven by hypoxia and creates a pH gradient in tumors that favors uptake of weak acids and exclusion of weak bases. Established anticancer drugs exhibit a range of pKa’s and thus variable ability to exploit the tumor pH gradient. The camptothecins are a prime example as they represent a diverse class of approved anticancer drugs and drug candidates whose charge distribution varies with pH. An in silico method was used to predict charge distribution of camptothecins at physiological versus acidic pH in both the lactone and carboxylate forms. A significant amount of uncharged carboxylate was predicted at acidic pH that could enter tumor cells and accumulate in mitochondria to inhibit mitochondrial topoisomerase I. A model is presented to describe the charge dynamics of a new camptothecin analog and the impact on nuclear and mitochondrial mechanism(s) of action. This example illustrates the importance of integrating tumor physiology and charge dynamics into anticancer drug development.
Keywords: Camptothecin, charge dynamics, glycolytic phenotype, hypoxia, pKa, topoisomerase I, tumor pH gradient
INTRODUCTION
The discovery and design of anticancer therapeutics is a complex process involving many factors that must be optimized. The current emphasis on advances in target identification and mechanism-based drug design has overshadowed the impact of fundamental pharmacological properties. The overall charge on a drug molecule in solution, determined by its intrinsic pKa value(s) and the solution pH is a case in point. As succinctly stated by Prankerd [1], “the extent of ionization for a drug can control is solubility, dissolution rate, reaction kinetics, complexation with drug carriers (e.g., cyclodextrins), absorption across biological membranes, distribution to the site of action, renal elimination, metabolism, protein binding, or receptor interactions”. Any one of these parameters could affect the apparent selectivity of a mechanistically-designed agent no matter how valid the molecular target. Drug charge is even more critical for cancer, because solid tumors often exhibit the glycolytic phenotype or Warburg Effect that is driven by tumor hypoxia and produces an acidic extracellular microenvironment. Hence, anticancer drugs with ionizable groups with pKa’s in the pH 6-8 range will exhibit charge dynamics that could significantly impact cellular uptake and retention. This point is illustrated by camptothecin and its many analogs, including the established anticancer drugs topotecan and irinotecan. Camptothecin contains an unstable E ring lactone that will spontaneously open to form the corresponding carboxylate at physiological pH. The lactone has two ionizable sites; the carboxylate has four. To explore charge dynamics of camptothecin and its analogs, an in silico method was employed (ACD/pKa DB) that relies on linear free-energy relationships (LFER) to predict charge distribution as a function of pH. This approach predicted presence of uncharged species of camptothecin carboxylates that could lead to novel charge dynamics. A model is proposed to describe how the charge dynamics of a new camptothecin analog in pre-clinical development could exploit the tumor pH gradient to enhance selectivity and to inhibit two forms of the molecular target, topoisomerase I.
HYPOXIA DRIVES THE TUMOR GLYCOLYTIC PHENOTYPE AND ASSOCIATED PH GRADIENT THAT REGULATES DRUG UPTAKE AND RETENTION
Oxygen concentration is a critical determinant of human physiology. Oxygen concentration in blood ranges from 10-12.5%, in healthy normal tissue from 3-6% (true “normoxia”), while solid tumors are typically hypoxic at 1-2%. In marked contrast, much of our understanding of cancer biology is based on standard cell culture conditions that utilize atmospheric oxygen levels (20-21%) [2, 3]. These hyperoxic conditions down-regulate hypoxia-inducing factor-1 (HIF-1), a transcription factor that controls over sixty genes with hypoxia response elements (HRE) [4]. Not surprisingly, drug mechanisms that involve HIF-1 are not manifest under standard culture conditions (e.g., irinotecan inhibition of HIF-1 in colon cancer cells [5]). HIF-1 is up-regulated in the majority of primary malignant tumors and in two thirds of metastases, but absent in most normal tissues [3, 6]. HIF-1 activates the glycolytic or tumor metabolic phenotype [7], a well known phenomenon [7-11], dating to the pioneering work of Otto Warburg [12, 13]. The glycolytic phenotype leads in turn to extracellular tumor acidification, even though tumor cells maintain a physiological intracellular pH. This produces a pH gradient unique to tumors [14] that has important consequences for anticancer drugs with ionizable groups. Negatively charged, weak acid drugs will exploit the gradient to accumulate in tumor cells where they will be trapped [14-16]. Conversely, positively charged, weak base drugs will tend to be excluded. A case in point is doxorubicin, one of the most widely used drugs in the armamentarium [17]. Consequently, charge dynamics at acidic versus physiological pH becomes a key parameter for anticancer drug development.
pKA’s OF ANTICANCER DRUGS
Many anticancer drugs display pH-dependent cytotoxicity both in vitro [15, 18] and in vivo [19]. In addition, most anticancer drugs have multiple ionization sites and therefore multiple pKa’s that contribute to the overall charge dynamic. Despite the importance of ionization state to drug action as a function of tissue pH, drug pKa’s are not readily accessible in the literature. For example, the monograph series Profiles of Drug Substances, Excipients, and Related Methodology (Brittain, HG, ed.; previously entitled Analytical Profiles of Drug Substances) that contains the most recent and rigorous compilation of these data [1] is not indexed by either PubMed or Web of Science databases. Moreover, drug pKa’s are often listed without citation in the indexed literature, which is critical because measurement of pKa is not standardized. Data quality varies considerably depending on the method used, instrument calibration, the reference standards and presence of co-solvents. The latter is of particular importance for drugs like the camptothecins that have limited water solubility and thus require inclusion of organic solvents. The pKa’s for selected anticancer drugs are presented in Fig. (1). Clearly, there is a range of behavior from weak acid to weak base. What is less clear for drugs with multiple ionization sites is the distribution of neutral or charged species that comprise the overall dynamic as pH varies in the physiological range.
Fig. (1).

Measured pKa values for selected anticancer drugs. Data from Prankerd [1], Mahoney [15] and Raghunand [35] were combined. Multiple listings for a drug indicate separate sources. Bar: one pH unit range of titration curve.
This distribution is important because according to pH partition theory [17, 20], neutral species are more likely to diffuse cross cell plasma membranes, while charged species will be excluded. Once inside cells, uncharged molecules will enter neutral compartments (nucleus), while weak bases will partition into acidic compartments (lysosomes) and weak acids into alkaline compartments (mitochondria) [15]. This subcellular compartmentalization therefore affects access to molecular targets that may also be localized.
PREDICTION OF pKa IN SILICO
Between 60-75% of all small-molecule pharmaceuticals have at least one ionizable group [21, 22]. Because pKa(s) can have such a dramatic impact on drug properties, particularly those related to adsorption, distribution, metabolism and excretion (ADME), and direct measurement is often not practical, much effort has focused on computational methods for high-throughput prediction of pKa. Some currently available programs include: ACD/pKa DB, ADME Boxes (Advanced Chemistry Development), ADMET predictor (Simulations Plus), Epik, Jaguar (Schrodinger), Marvin (ChemAxon), MoKa (Molecular Discovery), Pallas (CompuDrug International), Pipeline Pilot (SciTegic), and SPARC (University of Georgia/U.S. EPA)[21]. Several recent reviews have compared software using different datasets and statistical endpoints [21-23]. The reader is referred to these reviews for a detailed description of the underlying differences in prediction algorithms. While these tools are routinely used by medicinal chemists in drug design, consideration of the impact of tumor physiology, notably the tumor pH gradient, and the contribution of multiple ionization sites in the same molecule to overall charge as a function of pH have received much less attention.
PREDICTED CHARGE DYNAMICS OF CAMPTOTHECINS
The pH-dependent cytotoxicity of camptothecins is well established [24]. While generally considered weak acids [25], the numerous modifications of the camptothecin nucleus can alter charge characteristics. For example, Meloun and colleagues compared predicted versus measured pKa’s for the lactone forms of SN-38 and its precursors CPT, 10-hydroxy-CPT and 7-ethyl-CPT using Pallas and Marvin software [26]. Table 1 presents predicted pKa values for the lactone forms of various CPT analogs generated by ACD/pKa DB (v.12.01, ACD/Labs, Toronto, ON), the industry standard for pKa prediction [21, 23]. Tables 2 and 3 list related charge distribution results for the lactone and carboxylate forms, respectively, of camptothecin analogs at physiological versus acidic pH. These include approved drugs (topotecan and SN-38, the active metabolite of irinotecan), analogs in clinical trial (rubitecan, karenitecan, gimatecan), and a novel analog in pre-clinical development (7-butyl-10-amino-camptothecin, BACPT) specifically chosen for enhanced activity at acidic pH [27, 28]. The results confirm that with the exception of topotecan, the lactones of camptothecin and analogs are highly lipophilic and thus cell permeable at physiological pH, being 95-100% uncharged. This illustrates an early and central issue in camptothecin drug development, namely limited water solubility. The diethylaminoethyl substituent at the 9 position in topotecan is one solution. Another is to form water soluble pro-drugs via a dipiperodino group at the 10 position (irinotecan) or via a dipeptide at the 20 position (BACPTDP). Given the tendency for rapid E ring opening at pH 7.4, the charge distribution for the carboxylates is equally important. As expected, a majority of the carboxylate species are acidic (negatively charged). However, the model predicts a surprising percentage of topotecan carboxylate to remain uncharged (63%), followed by BACPT (33%). This raises the possibility that these carboxylates can enter cells and impact activity. The effect is more pronounced if the analog encounters an acidic extracellular environment common to solid tumors. At pH 6.8, 86% of topotecan and 66% of BACPT carboxylates are uncharged. Moreover, now SN-38 (46%) and karenitecin (39%) also generate significant amounts of uncharged carboxylate. Of course, the acidic extracellular environment will favor stability of the lactone form where most analogs will remain uncharged (83-100%). Topotecan, however, will primarily behave as a weak base, which could explain the drug’s limited potency.
Table 1.
Predicted pKa’s for Camptothecin Analogs as Lactones
| Analog | Ionizable Group | Apparent pKa | Measured pKa1 | |||||
|---|---|---|---|---|---|---|---|---|
| ACD/pKa DB | MARVIN2 | PALLAS2 | SPECFIT | SQUAD | ||||
| Camptothecin | 20-OH | 11.2 | +/− | 0.2 | 8.63 | 10.64 | 10.18 | 10.11 |
| 1-N | 4.76 | +/− | 0.4 | 3.07 | 4.17 | 2.90 | 2.83 | |
| Topotecan | 20-OH | 11.3 | +/− | 0.2 | ||||
| 10-OH | 8.92 | +/− | 0.4 | |||||
| 23-N | 7.65 | +/− | 0.3 | |||||
| 1-N | 4.04 | +/− | 0.4 | |||||
| SN-38 | 20-OH | 11.3 | +/− | 0.2 | 9.12 | 10.65 | 9.70 | 9.71 |
| 10-OH | 9.13 | +/− | 0.4 | 8.24 | 9.06 | 8.91 | 8.90 | |
| 1-N | 5.75 | +/− | 0.4 | 3.92 | 5.66 | 3.11 | 3.04 | |
| Rubitecan | 20-OH | 11.2 | +/− | 0.2 | ||||
| 1-N | 2.56 | +/− | 0.4 | |||||
| Karenitecan | 20-OH | 11.3 | +/− | 0.2 | ||||
| 1-N | 5.62 | +/− | 0.4 | |||||
| Gimatecan | 20-OH | 11.2 | +/− | 0.2 | ||||
| 1-N | 4.55 | +/− | 0.4 | |||||
| BACPT | 20-OH | 11.2 | +/− | 0.2 | ||||
| 1-N | 6.11 | +/− | 0.4 | |||||
| 10-N | 1.0 | +/− | 0.4 | |||||
data from Meloun et al. [26] obtained by multiwavelength spectrophotometric pH titration with analysis by the two algorithms listed.
predicted pKa’s from Meloun et al.
Table 2.
Predicted Charge Distribution for Camptothecin Analogs as Lactones
| pH | Charge | CPT | Topotecan | SN-38 | Rubitecan | Karenitecin | Gimatecan | ACPT | BCPT | BACPT |
|---|---|---|---|---|---|---|---|---|---|---|
| 6.8 | −1 | |||||||||
| 0 | 99% | 12% | 92% | 100% | 94% | 99% | 96% | 95% | 83% | |
| +1 | 1% | 88% | 1% | 6% | 1% | 4% | 5% | 17% | ||
| 7.4 | −1 | 1% | 2% | |||||||
| 0 | 100% | 35% | 96% | 100% | 98% | 100% | 99% | 99% | 95% | |
| +1 | 63% | 2% | 2% | 1% | 1% | 5% |
Table 3.
Predicted Charge Distribution for Camptothecin Analogs as Carboxylates
| pH | Charge | CPT | Topotecan | SN-38 | Rubitecan | Karenitecin | Gimatecan | ACPT | BCPT | BACPT |
|---|---|---|---|---|---|---|---|---|---|---|
| 6.8 | −1 | 92% | 12% | 54% | 100% | 61% | 95% | 73% | 68% | 34% |
| 0 | 8% | 86% | 46% | 39% | 5% | 27% | 32% | 66% | ||
| +1 | 1% | |||||||||
| 7.4 | −2 | 1% | 1% | |||||||
| −1 | 99% | 36% | 81% | 100% | 86% | 99% | 92% | 90% | 67% | |
| 0 | 2% | 63% | 17% | 14% | 1% | 8% | 10% | 33% | ||
| +1 |
Drugs that are weak acids can exploit the tumor pH gradient, because the acidic extracellular conditions will lower the overall charge and thereby facilitate diffusion across cell membranes. Fig. (2) indicates that the camptothecin carboxylates are most effective in this regard. At pH 6.8, net decrease in charge is most apparent for BACPT > SN-38 > karenitecin = topotecan. Meanwhile, camptothecin, gimatecan and rubitecan carboxylates do not significantly exploit the tumor pH gradient by this model. A tradeoff is that at pH 6.8, the lactone form of BACPT increases in charge by 12%; that for topotecan by 24%.
Fig. (2).
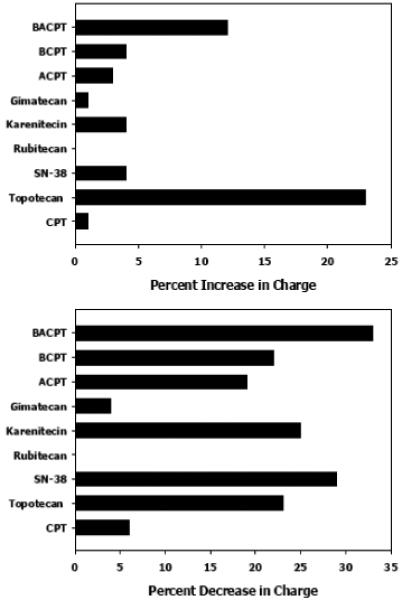
Predicted Charge dynamics for camptothecin analogs at physiological versus acidic pH. Charge distribution was predicted using ACD/Labs pKa DB software. The difference in charge for analogs at pH 7.4 compared to pH 6.8 is plotted for the respective lactones (top panel) and carboxylates (bottom panel).
The detailed ionization profile for BACPT lactone and carboxylate (Supplementary Figs. 1 and 2) indicates that BACPT lactone has four potential ionization sites, while the corresponding carboxylate has seven. The major determinant of the charge dynamic is the 1-nitrogen. In the lactone, the predicted pKa is 6.1, while in the carboxylate form, the pKa shifts to 7.1 due to the generation of the 21-OH with a pKa of 3.09.
CONSEQUENCES OF CHARGE DYNAMICS FOR CAMPTOTHECIN MECHANISM(S) OF ACTION
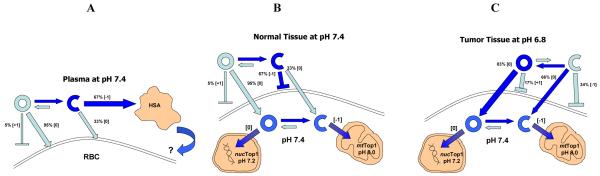
Charge dynamics are generally defined under aqueous or buffered conditions. For example, in buffer at pH 7.3 and 37°C, the rate of lactone hydrolysis of CPT, irinotecan, and SN-38 is rapid with a half life of approximately 30 min. The resulting percentage of lactone remaining at equilibrium is 14-21% [29]. Translation of charge dynamics to a physiological environment such as whole blood for intravenous drugs is complex and critical for camptothecins because the carboxylate form has a high affinity for human serum albumin [30], which is generally considered a sequestering and thus inactivating pathway. Opposing this process is partitioning of the uncharged lactone into lipophilic compartments such as red cell membranes [31]. Making the camptothecin nucleus more lipophilic or blocking E ring opening by addition of a 20(S) substituent are thus done to enhance blood stability of the “active” lactone form. However, this is an over-simplification, because early-on it was clear that the carboxylate form of camptothecin and certain analogs had activity. Hsiang et al. [32] demonstrated that 20(S) camptothecin sodium (carboxylate) was active in vivo against L1210 leukemia, in drug-treated cell lysates via deletion of top1 and in drug-induced top1-mediated cleavage of linear DNA. Moreover, de la Loza and Wellinger showed that both lactone and carboxylate forms of topotecan bound to human top1 by x-ray crystallography [33]. What mechanism could account for camptothecin carboxylate activity? One possibility is the recent discovery by Pommier and co-workers of a mitochondrial form of topoisomerase I [34]. Since the enzyme must function in the alkaline environment of mitochondria, the pH optimum of 8 was not surprising. However, inhibition of mtTop1 by 10 μM camptothecin after 1 h incubation at pH 8 was unexpected. Although one cannot rule out activity of the small amount of lactone present, the overwhelming majority of drug would be in the carboxylate form, suggesting that mtTop1 could be a significant molecular target. This is particularly important because negatively charged carboxylate species will accumulate in this basic organelle. Accordingly, topotecan was found to accumulate in yeast mitochondria [33], while camptothecin itself localized in cytoplasmic vacuoles. This difference could reflect cellular uptake of carboxylates at pH 7.4, where 99% of CPT carboxylate is charged and thus excluded, while 63% of topotecan carboxylate is uncharged (Table 2). Once the uncharged topotecan is intracellular at physiological pH, negatively charged forms can then regenerate and accumulate in mitochrondria. The process is summarized in Chart 1, which presents a model for the charge dynamics of the camptothecin analog BACPT in plasma, normal and tumor tissues. In plasma at pH 7.4, lactone hydrolysis and binding to human serum albumin will favor conversion to the carboxylate form, while affinity of the uncharged BACPT lactone for red cell membranes will protect the E ring from opening. In normal tissues, the equilibrium also favors conversion to the carboxylate form to inhibit cell uptake. In contrast, in the acidic extracellular environment of solid tumors, equilibrium will favor the uncharged lactone form and the carboxylate present will also be uncharged and cell permeable. Traversing the pH gradient, the drug encounters physiological pH to again yield neutral and weak acid species. The neutral lactone will diffuse into the nucleus to inhibit nucTop1, while the acidic carboxylate will concentrate in basic mitochondrion to inhibit mtTop1. This model accounts for observations that BACPT preferentially accumulates in MCF-7 cells at pH 6.8 vs pH 7.4, and correspondingly exhibits increased antiproliferative activity [27]. In addition, the model is consistent with the punctate cytoplasmic intracellular BACPT distribution pattern seen by fluorescence microscopy (D. Adams, unpublished observation). Confirmation of mitochondrial localization and the kinetics of these charge interactions for BACPT remain critical unknowns and must be determined to assess the relative contribution of each Top1 target to overall drug mechanism of action. Nevertheless, the model suggests the importance of carboxylate species and a mitochondrial site of action to drug activity, which is notable since mitochondria initiate the apoptotic response. In summary, this example demonstrates the importance of understanding anticancer drug charge dynamics in normal versus tumor tissues for drug uptake, retention and antitumor mechanism of action.
Chart 1. Predicted charge dynamics for BACPT lactone versus BACPT carboxylate.
Predominant species and dynamic pathways are denoted by dark blue. Hydrolysis of the pro-drug BACPTDP to BACPT is not shown and only the camptothecin E ring is represented. The predicted charged species for BACPT lactone (closed ring) are [+1] (basic), while those for BACPT carboxylate (open ring) are [−1] (acidic). Hence, the carboxylate is a weak acid, while the small amount of charged lactone is a weak base. Panel A: in plasma at pH 7.4, the equilibrium will favor conversion to the carboxylate form. The charged carboxylate species has a high affinity for human serum albumin, which further drives ring opening. Whether albumin-bound drug is terminally sequestered or still available (i.e., as in paclitaxel formulated in albumin nanoparticles; Abraxane ®), is not known. Counteracting the serum albumin pathway is the affinity of the uncharged BACPT lactone for red cell membranes where it is protected from ring opening. Panel B: in normal tissues, the equilibrium also favors conversion to the carboxylate form, which will yield a majority of charged species that cannot cross the plasma membrane. Panel C: in the acidic extracellular environment of solid tumors, equilibrium will favor the lactone form, most of which is not charged [0]. Furthermore, most of the carboxylate present will also be in an uncharged form that can enter cells. Once inside the tumor cell, the drug encounters physiological pH to again yield neutral and weak acid species. The neutral lactone will diffuse into the nucleus to inhibit nucTop1, while the acidic carboxylate will concentrate in basic mitochondrion to inhibit mtTop1. The kinetics of these charge interactions for BACPT remains a critical unknown and must be determined to assess the relative importance of each Top1 target to overall drug mechanism of action.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Darryl Reid and Arvin Moser of ACD/Labs for access to ACD/pKa DB software and instruction in its use and interpretation. This work was supported in part by Small Business Innovation Research grant 2R44CA125871-02 from the National Cancer Institute.
ABBREVIATIONS
- CPT
camptothecin
- ACPT
10-amino-camptothecin
- BCPT
7-butyl-camptothecin
- BACPT
7-butyl-10-amino-camptothecin
- BACPTDP
7-butyl-10-amino-camptothecin (20S) β-alaninelysine
- SN-38
7-ethyl-10-hydroxy-camptothecin
- nucTop1
nuclear topoisomerase I
- mtTop1
mitochondrial topoisomerase I
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.
REFERENCES
- [1].Prankerd R. Critical compilation of pKa values for pharmaceutical substances. Elsevier Academic Press; San Diego: 2007. [DOI] [PubMed] [Google Scholar]
- [2].Atkuri KR, Herzenberg LA. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc. Natl. Acad. Sci. U. S. A. 2005;102(10):3756–3759. doi: 10.1073/pnas.0409910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ivanovic Z. Hypoxia or In Situ Normoxia: The Stem Cell Paradigm. J. Cell. Physiol. 2009;219(2):271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- [4].Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- [5].Pencreach E, Guerin E, Nicolet C, Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP, Guenot D. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1 alpha axis. Clin. Cancer Res. 2009;15(4):1297–1307. doi: 10.1158/1078-0432.CCR-08-0889. [DOI] [PubMed] [Google Scholar]
- [6].Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1 alpha and HIF-2 alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 2000;157(2):411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stubbs M, Bashford CL, Griffiths JR. Understanding the tumor metabolic phenotype in the genomic era. Curr. Mol. Med. 2003;3(1):49–59. doi: 10.2174/1566524033361645. [DOI] [PubMed] [Google Scholar]
- [8].Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003;63(14):3847–3854. [PubMed] [Google Scholar]
- [9].Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J. Nucl. Med. 2008;49:24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- [10].Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66(18):8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- [11].Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ. Hypoxia-inducible factor-1 alpha and the glycolytic phenotype in tumors. Neoplasia. 2005;7(4):324–330. doi: 10.1593/neo.04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Warburg O. The Metabolism of Tumours. Biochem. Z. 1923;142:317. [Google Scholar]
- [13].Warburg O. On respiratory impaiment in cancer cells: reply. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- [14].Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996;56(6):1194–1198. [PubMed] [Google Scholar]
- [15].Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem. Pharmacol. 2003;66(7):1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- [16].Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics I. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem. Pharmacol. 2003;66(7):1219–1229. doi: 10.1016/s0006-2952(03)00468-4. [DOI] [PubMed] [Google Scholar]
- [17].Gerweck LE, Kozin SV, Stocks SJ. The pH partition theory predicts the accumulation and toxicity of doxorubicin in normal and low-pH-adapted cells. Br. J. Cancer. 1999;79(5-6):838–842. doi: 10.1038/sj.bjc.6690134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adams DJ, Dewhirst MW, Flowers JL, Gamcsik MP, Colvin OM, Manikumar G, Wani MC, Wall ME. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother. Pharmacol. 2000;46(4):263–271. doi: 10.1007/s002800000157. [DOI] [PubMed] [Google Scholar]
- [19].Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol. Cancer Ther. 2006;5(5):1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- [20].Jacobs M. Some aspects of cell permeability to weak electrolytes. Cold Spring Harbor Symp Quant Biol. 1940;8:30–39. [Google Scholar]
- [21].Liao CZ, Nicklaus MC. Comparison of nine programs predicting pK(a) values of pharmaceutical substances. J. Chem. Inform. Model. 2009;49(12):2801–2812. doi: 10.1021/ci900289x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Manchester J, Walkup G, Rivin O, You ZP. Evaluation of pK(a) estimation methods on 211 drug like compounds. J. Chem. Inform. Model. 2010;50(4):565–571. doi: 10.1021/ci100019p. [DOI] [PubMed] [Google Scholar]
- [23].Meloun M, Bordovska S. Benchmarking and validating algorithms that estimate pK(a) values of drugs based on their molecular structures. Anal. Bioanal. Chem. 2007;389(4):1267–1281. doi: 10.1007/s00216-007-1502-x. [DOI] [PubMed] [Google Scholar]
- [24].Adams DJ. The impact of tumor physiology on camptothecin-based drug development. Curr. Pharm. Med. Chem.-Anticancer Agents. 2005;5(1):1–13. doi: 10.2174/1568011053352596. [DOI] [PubMed] [Google Scholar]
- [25].Mellor HR, Callaghan R. Resistance to chemotherapy in cancer: A complex and integrated cellular response. Pharmacology. 2008;81(4):275–300. doi: 10.1159/000115967. [DOI] [PubMed] [Google Scholar]
- [26].Meloun M, Bordovska S, Vrana A. The thermodynamic dissociation constants of the anticancer drugs camptothecine, 7-ethyl-10-hydroxycamptothecine, 10-hydroxycamptothecine and 7-ethylcamptothecine by the least-squares nonlinear regression of multiwavelength spectrophotometric pH-titration data. Anal. Chim. Acta. 2007;584(2):419–432. doi: 10.1016/j.aca.2006.11.049. [DOI] [PubMed] [Google Scholar]
- [27].Adams DJ, Wahl ML, Flowers JL, Sen B, Colvin M, Dewhirst MW, Manikumar G, Wani MC. Camptothecin analogs with enhanced activity against human breast cancer cells. II. Impact of the tumor pH gradient. Cancer Chemother. Pharmacol. 2006;57(2):145–154. doi: 10.1007/s00280-005-0008-5. [DOI] [PubMed] [Google Scholar]
- [28].Adams D, Waud W, Wani M, Manikumar G, Flowers J, Driscoll T, Morgan L. BACPTDP: a water-soluble camptothecin pro-drug with enhanced activity in hypoxic/acidic tumors. Cancer Chemother. Pharmacol. 2010 doi: 10.1007/s00280-010-1388-8. Online First(22 June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chourpa I, Millot JM, Sockalingum GD, Riou JF, Manfait M. Kinetics of lactone hydrolysis in antitumor drugs of camptothecin series as studied by fluorescence spectroscopy. Biochimica Et Biophysica Acta-General Subjects. 1998;1379(3):353–366. doi: 10.1016/s0304-4165(97)00115-3. [DOI] [PubMed] [Google Scholar]
- [30].Burke TG, Mi ZH. Preferential binding of the carboxylate form of camptothecin by human serum-albumin. Anal. Biochem. 1993;212(1):285–287. doi: 10.1006/abio.1993.1325. [DOI] [PubMed] [Google Scholar]
- [31].Burke TG, Munshi CB, Mi ZH, Jiang Y. The important role of albumin in determining the relative human blood stabilities of the camptothecin anticancer drugs. J. Pharm. Sci. 1995;84(4):518–519. doi: 10.1002/jps.2600840426. [DOI] [PubMed] [Google Scholar]
- [32].Hsiang YH, Liu LF, Wall ME, Wani MC, Nicholas AW, Manikumar G, Kirschenbaum S, Silber R, Potmesil M. DNA topoisomerase I-mediated DNA cleavage and cyto-toxicity of camptothecin analogs. Cancer Res. 1989;49(16):4385–4389. [PubMed] [Google Scholar]
- [33].de la Loza MCD, Wellinger RE. A novel approach for organelle-specific DNA damage targeting reveals different susceptibility of mitochondrial DNA to the anticancer drugs camptothecin and topotecan. Nucleic Acids Res. 2009;37(4) doi: 10.1093/nar/gkn1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang HL, Barcelo JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, Pommier Y. Human mitochondrial topoisomerase I. Proc. Natl. Acad. Sci. U. S. A. 2001;98(19):10608–10613. doi: 10.1073/pnas.191321998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resistance Updates. 2000;3(1):39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.