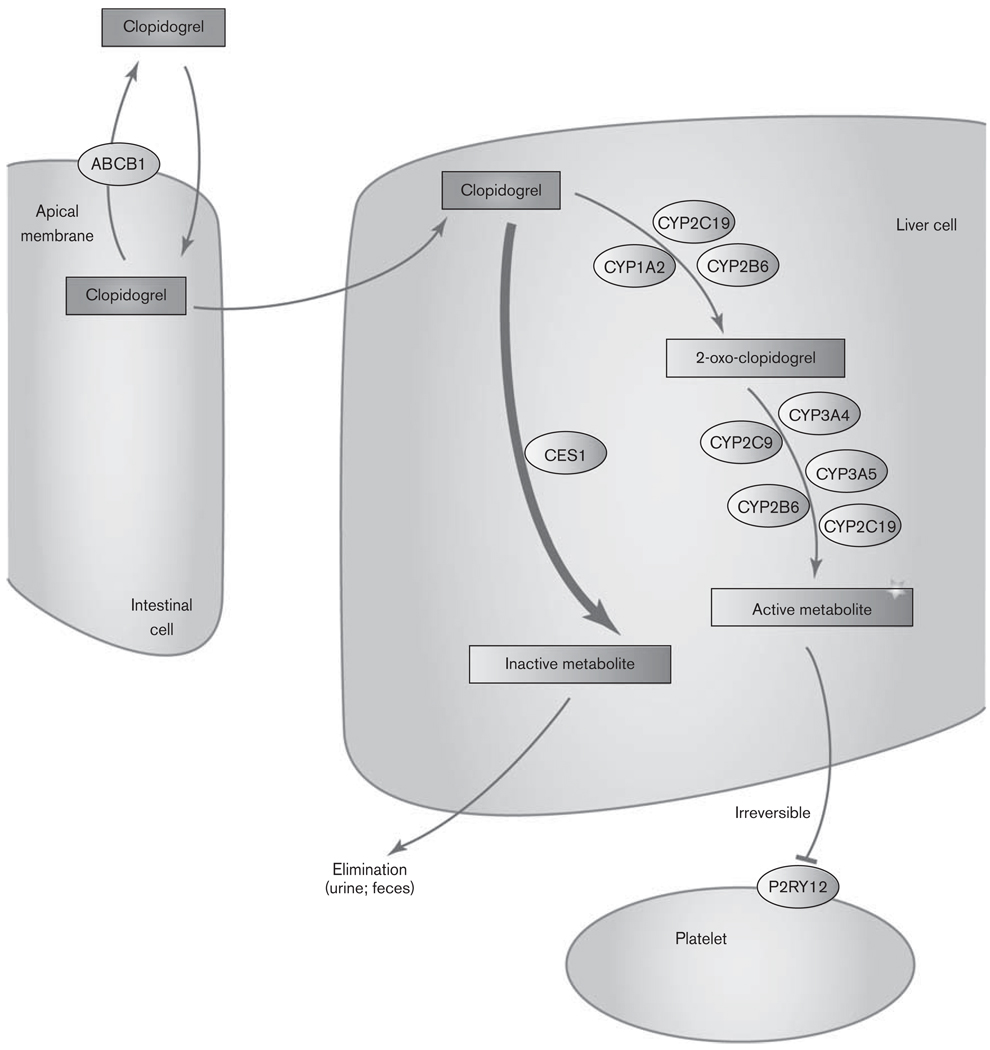
Clopidogrel, a thienopyridine derivative, binds specifically and irreversibly to the platelet P2RY12 purinergic receptor, inhibiting ADP-mediated platelet activation and aggregation [1,2].
After oral administration, clopidogrel is rapidly absorbed. Owing to its extensive metabolism, clopidogrel is not detected in human plasma. Clopidogrel is a prodrug that is absorbed in the intestine [3,4] and activated in the liver [5]. The conversion of clopidogrel to its active metabolite requires two sequential oxidative steps. As shown in Fig. 1, the first step leads to formation of 2-oxo-clopidogrel, followed by the conversion of 2-oxoclopidogrel to the active metabolite. CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4/5 are implicated as cytochrome P450 enzymes involved in the metabolism of clopidogrel. However, the relative importance of the individual enzymes and which part of the reaction they are involved in are controversial, as discussed in the literature. Savi et al. [1] showed that clopidogrel was converted into 2-oxo-clopidogrel by cytochrome P450 monooxygenase-dependent metabolism in vitro and that hydrolysis of 2-oxo-clopidogrel generates the active metabolite. Several publications indicate a major role for CYP3A4 [6,7]. Other in-vitro studies showed that CYP1A2, CYP2B6, and CYP2C19 were capable of forming the 2-oxo-clopidogrel form from clopidogrel in liver microsomes [8,9]. When 2-oxo-clopidogrel was used as a substrate, the enzymes CYP3A4, CYP2C9, CYP2C19, and CYP2B6 produced the active metabolite [8,9]. The study by Kazui et al. [9] concluded that CYP2C19 contributes substantially to both oxidative steps and that CYP3A4 contributes substantially to the second oxidative step.
Fig. 1.
Representation of the candidate genes involved in the metabolism of clopidogrel and its primary mechanism of action.
In a competing metabolic reaction, about 85% of the drug is hydrolyzed to an inactive carboxylic acid derivative by esterases [3,10]. The active metabolite of clopidogrel contains a thiol group which binds to a free cysteine on the P2RY12 receptor and irreversibly blocks ADP binding and receptor activation (Fig. 1) [1]. Once this blockage has occurred, platelets are affected for their entire lifespan of approximately 7–10 days.
Drug–drug interactions of clopidogrel were reported with atorvastatin [11], the calcium-channel antagonist verapamil [12], and the proton-pump inhibitor omeprazole [13–15]. The clinical implications of these findings are still under investigation [16,17]. Several clinical studies did not support the finding that atorvastatin can interfere with the effect of clopidogrel [18–20].
Recent studies indicate that the pharmacodynamic response to clopidogrel is variable, with 20–40% of patients being classified as nonresponders, poor responders or resistant to clopidogrel because of low inhibition of ADP-induced platelet aggregation or activation [21]. Nongenetic factors influencing the clopidogrel response include age, diabetes, renal failure, and cardiac failure [22]. As described above, the prodrug clopidogrel requires activation in the liver. A growing number of studies investigated the effect of pharmacokinetic variables (intestinal absorption and metabolic activation) on response to clopidogrel. ABCB1 is involved in the intestinal absorption of clopidogrel. Two recent studies found an influence of the C3435T variant (rs1045642) in ABCB1 on clopidogrel absorption in patients with cardiovascular diseases [23,24]. A genome-wide association study of ADP-stimulated platelet aggregation in response to clopidogrel found no association between this single-nucleotide polymorphism (SNP) and clopidogrel response [25].
CYP2C19 is one of the hepatic cytochrome P450 enzymes involved in the formation of clopidogrel’s active metabolite. Genetic polymorphisms of CYP2C19 are associated with impaired clopidogrel metabolism in healthy volunteers and in patients [21,24,26–30]. This poor metabolizer phenotype has also been associated with an increased risk of cardiovascular events. The CYP2C19*2 genetic variant, 681 G > A (rs4244285), was identified as a major determinant of prognosis in young patients who received clopidogrel treatment after myocardial infarction [29]. Furthermore, patients carrying any two CYP2C19 loss-of-function alleles [*2, *3 (rs4986893), *4 (rs28399504), or *5 (rs56337013)] had a higher rate of cardiovascular events than patients who did not have these alleles [24]. Similarly, another study showed that carriers of a reduced-function CYP2C19 allele had significantly lower levels of clopidogrel’s active metabolite, diminished platelet inhibition, and a higher rate of major adverse cardiovascular events [30]. A genome-wide association analysis identified 13 SNPs on chromosome 10q24 within the CYP2C18-CYP2C19-CYP2C9-CYP2C8 cluster showing strong evidence for association with clopidogrel response in an Amish population. The SNP rs12777823 within this cluster was the most significantly associated variant. All 13 SNPs were in strong linkage disequilibrium with each other and also with the loss-of-function variant CYP2C19*2, which further findings showed accounted for most of all the association with diminished platelet response to clopidogrel. CYP2C19*3 and *5 were not polymorphic in the Amish population. The extension and replication of these results in a population with high risk of cardiovascular disease showed an association of CYP2C19*2 with poorer cardiovascular outcomes [25].
Acknowledgement
PharmGKB is supported by the NIH/NIGMS Pharmacogenetics Research Network (PGRN; UO1GM61374).
Footnotes
Supplemental digital content for the drug clopidogrel (PA449053) and the clopidogrel pathway (PA154424674) is available at http://www.pharmgkb.org/do/serve?objId=PA449053&objCls=Drug and http://www.pharmgkb.org/do/serve?objId=PA154424674&objCls=Pathway.
References
- 1.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 2.Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003;3:113–122. doi: 10.1055/s-2003-40669. [DOI] [PubMed] [Google Scholar]
- 3.Lins R, Broekhuysen J, Necciari J, Deroubaix X. Pharmacokinetic profile of 14C-labeled clopidogrel. Semin Thromb Hemost. 1999;25(Suppl 2):29–33. [PubMed] [Google Scholar]
- 4.Caplain H, Donat F, Gaud C, Necciari J. Pharmacokinetics of clopidogrel. Semin Thromb Hemost. 1999;25(Suppl 2):25–28. [PubMed] [Google Scholar]
- 5.Savi P, Herbert JM, Pflieger AM, Dol F, Delebassee D, Combalbert J, et al. Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol. 1992;44:527–532. doi: 10.1016/0006-2952(92)90445-o. [DOI] [PubMed] [Google Scholar]
- 6.Farid NA, Payne CD, Small DS, Winters KJ, Ernest CS, II, Brandt JT, et al. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007;81:735–741. doi: 10.1038/sj.clpt.6100139. [DOI] [PubMed] [Google Scholar]
- 7.Clarke TA, Waskell LA. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos. 2003;31:53–59. doi: 10.1124/dmd.31.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Kurihara AHK, Kazui M, Ishizuka T, Farid NA, Ikeda T. In vitro metabolism of antiplatelet agent clopidogrel: cytochrome P450 isoforms responsible for two oxidation steps involved in the active metabolite formation. Drug Metab Rev. 2005;37(Suppl 2):99. [Google Scholar]
- 9.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 10.Levin LS, Cloyd WH, Beaudry RJ, Jr, Yingling TH. Hemangioma of the incisive papilla: a case report. J Md State Dent Assoc. 1981;24:21–22. [PubMed] [Google Scholar]
- 11.Lau WC, Waskell LA, Watkins PB, Neer CJ, Horowitz K, Hopp AS, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation. 2003;107:32–37. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 12.Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008;52:1557–1563. doi: 10.1016/j.jacc.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 13.Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) Study. J Am Coll Cardiol. 2008;51:256–260. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 14.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 15.Cuisset T, Frere C, Quilici J, Poyet R, Gaborit B, Bali L, et al. Comparison of omeprazole and pantoprazole influence on a high 150-mg clopidogrel maintenance dose the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol. 2009;54:1149–1153. doi: 10.1016/j.jacc.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 16.Kleiman NS. Clopidogrel and calcium-channel antagonists: another drug-drug interaction for the ever-wary clinician? J Am Coll Cardiol. 2008;52:1564–1566. doi: 10.1016/j.jacc.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Rude MK, Chey WD. Proton-pump inhibitors, clopidogrel, and cardiovascular adverse events: fact, fiction, or something in between? Gastroenterology. 2009;137:1168–1171. doi: 10.1053/j.gastro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Saw J, Steinhubl SR, Berger PB, Kereiakes DJ, Serebruany VL, Brennan D, Topol EJ. Lack of adverse clopidogrel-atorvastatin clinical interaction from secondary analysis of a randomized, placebo-controlled clopidogrel trial. Circulation. 2003;108:921–924. doi: 10.1161/01.CIR.0000088780.57432.43. [DOI] [PubMed] [Google Scholar]
- 19.Saw J, Brennan DM, Steinhubl SR, Bhatt DL, Mak KH, Fox K, Topol EJ. Lack of evidence of a clopidogrel-statin interaction in the CHARISMA trial. J Am Coll Cardiol. 2007;50:291–295. doi: 10.1016/j.jacc.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 20.Lotfi A, Schweiger MJ, Giugliano GR, Murphy SA, Cannon CP. High-dose atorvastatin does not negatively influence clinical outcomes among clopidogrel treated acute coronary syndrome patients – a Pravastatin or Atorvastatin Evaluation and Infection Therapy – Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) analysis. Am Heart J. 2008;155:954–958. doi: 10.1016/j.ahj.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, II, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 22.Geisler T, Schaeffeler E, Dippon J, Winter S, Buse V, Bischofs C, et al. CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics. 2008;9:1251–1259. doi: 10.2217/14622416.9.9.1251. [DOI] [PubMed] [Google Scholar]
- 23.Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 25.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the anti-platelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 27.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10+ 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007;17:1057–1064. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- 28.Fontana P, Senouf D, Mach F. Biological effect of increased maintenance dose of clopidogrel in cardiovascular outpatients and influence of the cytochrome P450 2C19*2 allele on clopidogrel responsiveness. Thromb Res. 2008;121:463–468. doi: 10.1016/j.thromres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 30.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]