Abstract
The purpose of this study was to delineate the mechanisms by which stromal components of cancer may induce tumour thermotolerance and exploit alterations in stromal and tumour physiology to enhance radiation therapy. The vascular thermoresponse was monitored by daily one-hour 41.5°C heatings in two murine solid tumour models, SCK murine mammary carcinoma and B16F10 melanoma. A transient increase was seen in overall tumour oxygenation for 2–3 days, followed by a progressive decline in tumour pO2 upon continued daily heatings. Vascular thermotolerance was further studied by treating tumours with different heating strategies, i.e. (1) a single 60 min 41.5°C treatment; (2) two consecutive daily treatments of 41.5°C for 60 min; (3) a single 60 min 43°C treatment or (4) two days of 41.5°C for 60 min followed by treatment with 43°C for 60 min on the third day. Pre-heating tumours with mild temperature hyperthermia induced vascular thermotolerance, which was accompanied by evidence of vessel normalisation, i.e. a decrease in microvessel density and an increase in pericyte coverage. Rational scheduling of fractionated radiation during heat-induced increases in tumour oxygen levels rendered a significantly greater, synergistic, tumour growth inhibition. In vitro clonogenic survival responses of the individual cell types associated (endothelial cells, fibroblasts, pericytes and tumour cells) indicated only a direct cellular thermotolerance in endothelial cells. Overall, this suggests that tumour thermotolerance is a physiological phenomenon mediated through improvement of functional vasculature.
Keywords: Hyperthermia, radiation, thermotolerance, tumour microenvironment, vessel normalisation
Introduction
Over the last several decades, thermal therapy has shown great promise as cancer treatment due to its proven ability to reduce cancer morbidity and mortality, minimally or in a non-invasive way, and its progressive advances in devices and techniques [1–3]. However, tumours are known to be able to develop thermotolerance, a transient resistance to heat, thought to possibly limit the direct anti-tumour effects of thermal therapy [4]. Conversely, the phenomenon known as vascular thermotolerance has been described [5]. The tentative definition of vascular thermotolerance is that the blood flow response of the tumour to a second hyperthermia exposure is significantly greater, at least two-fold on average, than in response to a single thermal dose [6–8]. This vascular tolerance would have the premise of an improved vasculature condition or function after a priming heat dose. Regardless, the canonical notion has been that individual cellular resistance must be at the root of the increased physiological reaction to a second heating. Namely, that the survival rate of a cell type pre-exposed to heat is greater, as compared to those cells which were not pre-exposed. Several heat resistance or tolerance mechanisms have been proposed; yet thus far most studies have focused on molecular (heat shock) pathways of resistance within a cell type. Delineating the mechanism of action(s) and dynamics of cellular and physiological thermotolerance will contribute to the improvement of heat as an anti-cancer strategy alone and in conjunction with radiation and possibly with drug delivery.
The tumoural vascular bed and blood circulation play key roles in heat-induced tissue damage. Delineating vascular thermotolerance is warranted, since heat dissipation through blood perfusion hinders the thermal dose delivered to the tumour [9, 10]. Moreover, in order to intelligently design combinatorial clinical trials involving hyperthermia it is essential to comprehend the tumour vascular consequences of single or multiple heatings. Whereas disregarding the tumour physiological responses to hyperthermia might be detrimental to the clinical outcome, utilising the changes might even be able to amplify treatment success. It has been postulated that the tumour microenvironment plays a role in the development of thermotolerance, as the kinetics of this process in cells of the same origin varied significantly in vitro as compared to in vivo. Namely, hyperthermia induced tumour cell apoptosis in vivo was about three times greater than that for tumour cells in vitro [4]. Therefore, the overall thermotolerance of the tumour may be attributed by thermotolerance development in either the circulatory system, stroma or parenchyma individually, or by thermotolerance of the tissue en mass. To date, the molecular and cellular events during tumour thermotolerance and their dynamics have not been completely elucidated.
The functional involvement of the cellular component of the stroma, e.g. endothelial cells, pericytes and perivascular fibroblasts, within tumour progression is increasingly being acknowledged. A substantial portion of tumour vessels are abnormal: tortuous, leaky, misshaped, and lack pericyte coverage [11]. Since it was recently shown that anti-angiogenic treatment can transiently improve tumour physiology by the destruction of these immature and dysfunctional tumour vessels [12–14] (similar to post-natal vascular maturation and remodelling) [15], we hypothesised that tumour vascular thermotolerance, a transient resistance to heat, may also be mediated by vessel normalisation. In murine tumour models, B16F10 melanoma and SCK murine mammary carcinoma, we monitored the oxygen and vessel maturation levels during a regimen designed to induce vascular thermotolerance and observed that repeated 1 h 41.5°C heatings induced a transient increase in overall tumour oxygenation for 2–3 days, followed by a progressive decline in tumour pO2 upon continued daily heatings.
Even though thermotolerance can hamper the direct effects of hyperthermia, we capitalised on this temporal improvement of tumour physiology to improve radiation therapy via increased oxygenation. Here we present the results of various combinations of hyperthermia exposures and radiation therapy. Mechanistically, since direct cellular thermotolerance was only seen in endothelial cells and to a low level at these thermal doses, it appears that tumour thermotolerance is mainly a physiological phenomenon mediated through improved functional vasculature, rather than a direct cellular effect in tumour cells or those of the stroma.
Materials and methods
Mouse studies
Mice were purchased from the National Cancer Institute, Bethesda, MD, USA and allowed to acclimate to local conditions for at least 1 week. Animals were given water and standard chow ad libitum and were kept on a 12-h light/dark cycle. Experiments were approved by the University of Minnesota Research Animal Resources ethical committee. B16F10 murine melanoma cells (kindly provided by J. Fidler, Houston, TX), and SCK murine mammary carcinoma (kindly provided by C.W. Song, Minneapolis, MN) were cultured in RPMI 1640 with 10% bovine calf serum. A solution of 100 µL with 2 × 105 of syngeneic B16F10 and SCK were injected subcutaneously in the right rear leg of male C57BL/6 or A/J mice, respectively.
When tumours reached a size of approximately 100 mm3 (7 days for B16F10 and 5 days for SCK), treatment or tumour oxygenation studies were initiated after randomisation [16].
Hyperthermia
Each anaesthetised mouse was placed on a specially designed Plexiglas jig and the tumour-bearing leg was vertically extended and loosely anchored with masking tape to a support on the jig. The jigs were then placed on a Plexiglas shelf, which was positioned over a thermostatically regulated water bath preheated to the temperature indicated, and the anchored legs were immersed into the water for 60 min. The temperature in representative tumours, measured with a 29-gauge needle-type thermocouple (Physitemp Instruments, Clifton, NJ), averaged 0.1°–0.3°C lower than the water temperature near the centre of the tumour, while the periphery nearly reached the water temperature (typically within 0.1°–0.2°C of the water temperature) [17]. Therefore, for all in vitro work, we used the same temperatures as used in vivo due to the fact that a substantial portion of the tumour cells in vivo also were heated at the set-temperature in our water bath immersion set-up.
Tumour oxygenation studies
Tumour partial pressure of oxygen (pO2) was measured using an Eppendorf pO2 histograph (Hamburg, Germany), as described previously [14, 18]. A pO2 electrode (300 µm diameter, Eppendorf) was inserted by hand into tumours through small incisions made in the skin on the distal side of the tumour. The electrode, which was advanced into the tumour via computer-control, measured pO2 along the track. For this procedure, the electrode was advanced by 0.4 mm forward steps, and then withdrawn by 0.3 mm to reduce compression pressure, prior to recording the pO2 value. The pO2 values reported are the average of approximately 100 individual readings derived from 3 to 5 mice per group per day, with 3 tracks per mouse and 10 values per track.
Immunofluorescence for tumour vessel density and pericyte involvement
At the end of the experiment similar size tumours without apparent widespread necrosis were excised and embedded in tissue freezing medium (Miles, Elkart, IN) and snap frozen in liquid nitrogen. The frozen tumours were cut in 5 µm sections and after rehydration and antigen retrieval the slides were stained. In brief, sections were incubated in a 1 : 50 dilution with phycoerythrin (PE)-conjugated monoclonal antibody to mouse CD-31 (PECAM-1, Pharmingen, San Diego, CA) or a fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-α-smooth muscle actin antibody (α-SMA, Sigma, St Louis, MO) to stain for microvessel density (MVD) or pericytes, respectively [14, 19]. A minimum of three tumours and ten randomly chosen images per treatment group were acquired using an Olympus BX-60 microscope at 200× magnification and digitally analysed and differentially quantified by morphometric analysis [14]. After binarisation of the images, microvessel density or pericytes were estimated by scoring the total number of representative coloured pixels per field.
Immunofluorescent visualisation of tumour hypoxia
An i.p. injection of 60 mg/kg pimonidazole was given to each mouse. Pimonidazole, a substituted 2-nitroimidazole with a molecular weight of 290.7, is preferentially reduced in hypoxic viable cells and forms irreversible protein adducts which have been optimised for detection with immunohistochemistry. At 3 h post-injection, the mice were sacrificed, and similar size tumours without apparent widespread necrosis were excised, and embedded in tissue freezing medium (Miles) and snap frozen in liquid nitrogen. Following sectioning of the tumour tissue, a FITC-conjugated monoclonal antibody against protein adducts of pimonidazole was added (hypoxyprobe-1 Mab1, Chemicon, Temecula, CA), as described earlier [14]. A minimum of 3 tumours and 10 randomly chosen images per treatment group was acquired using an Olympus BX-60 microscope at 200× magnification [20].
Radiation-induced tumour growth delay assays
The mice were locally irradiated with 5 Gy using a Philips 250 Kv X-ray machine at a dose rate of 1.4 Gy/min. The body was shielded with lead, and only the tumour and the tumour bearing limb were exposed to the X-ray beam, as described earlier [16]. Tumour volumes were measured using a caliper (Scienceware, Pequannock, NJ) and were calculated according to the equation: (a2 × b)/2, where ‘a’ is the width and ‘b’ the length of the tumour.
Efficacy determination of combination therapy
To determine whether and to what degree the combination of heat and radiation had supra-additive effects on tumour growth inhibition, the following formula was applied [21]:
A ratio >1 indicates a synergistic (greater than additive) effect.
In vitro cell clonogenic assay
Primary cultures of human umbilical vein endothelial cells (HUVEC) and fibroblasts were obtained from the Ob/Gyn department at the University of Minnesota. Perivascular cells, pericyte-like cell line 10T1/2, were obtained from American Type Culture Collection (Manassas, VA) [22]. Cells in exponential growth phase were trypsinised, washed, counted and seeded into 25 cm2 tissue culture flasks in duplicate for all conditions. The flasks were incubated overnight at 37°C, 5% CO2. Approximately 16 hours after seeding, the flasks were treated with the following schedules: (1) mild temperature hyperthermia (MTH), where the flasks were heated at 41.5°C for 60 min two consecutive days in a row, day 1 and 2; (2) a single treatment of 43°C for 60 min on the third day of heat treatments; (3) pretreatment of MTH followed by treatment with 43°C for 60 min on the third day. Care was taken to gently move the flasks back and forth from the incubator to avoid satellite colony formation or the detachment of the cells from the flask. When resulting colonies were formed by approximately 20–30 cells each, they were stained with either crystal violet in methanol/acetic acid (10 : 1) or Simply Blue Safe Stain (Invitrogen, Carlsbad, CA) and counted by hand, as described earlier [16].
Thermal tolerance or sensitivity was determined by the following formula [21]:
(i.e. fraction of colonies after MTH × fraction of colonies after 43°C for 60 min).
A ratio >1 indicates induced thermal tolerance by MTH pretreatment, whereas a ratio of <1 indicates an induction of thermal sensitisation by MTH pretreatment.
Statistical analysis
Tumour volume was analysed using general linear mixed models [23, 24]. During the process of model selection, the likelihood ratio test was used to determine random effects and variance-covariance structures. The final general linear mixed model of tumour volume in a natural log scale, i.e. ln (tumour volume +1), contained the interaction of treatment with time only. The variance-covariance structure of random effects was unstructured, whereas that of random errors was first-order auto-regression.
Using derived parameters, model-based tumour growth curves for raw data were drawn, and the integral from day 7 (start of treatment) to day 20 for each curve was estimated from the area under the curve. This integral provided estimates of percentage inhibition in tumour growth for treatment versus control groups over the time course of the study, rather than just on a single day.
The Student’s t-test was used where indicated to determine the validity of the differences between control and treatment data sets. A p value of 0.05 or less was considered significant.
Results
Transient increased overall tumour oxygenation by mild hyperthermia
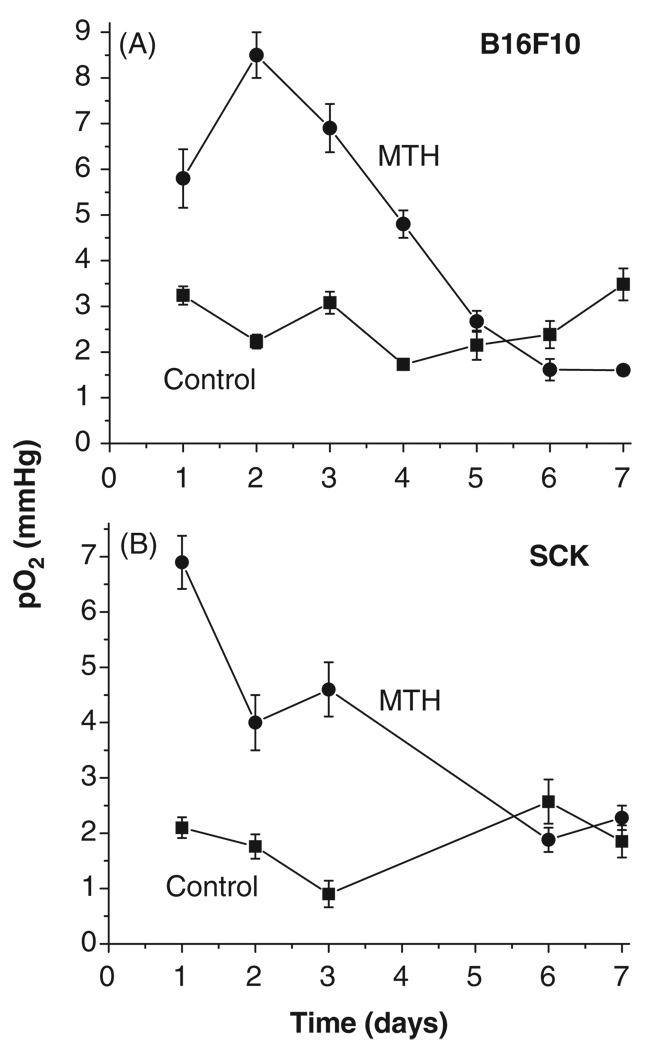
The overall daily tumour oxygenation levels in mice were measured directly and in real time using an Eppendorf pO2 histograph at 24 h after each heating was applied to the tumour. This technique varies in that most oxygenation measurement methods use indirect indicators, such as immunohistochemistry or electron paramagnetic resonance oximetry [13, 25]. Here we show that daily treatment with mild hyperthermia (41.5°C) resulted in an approximately 3-fold increase in overall tumour oxygenation up to day four of treatment (compared to vehicle treated mice) in both murine tumour models examined: melanoma B16F10 and murine mammary carcinoma SCK (Figure 1). In B16F10 model the tumours reached a peak pO2 increase after two daily heatings and began a decline afterwards. In SCK tumours the oxygenation enhancement peaked after a single heating and began to fall off afterwards towards control levels, but maintained a 2-fold elevation after 2 and 3 heatings. After day four, tumour oxygenation fell to levels around those in vehicle-treated mice (Figure 1).
Figure 1.
Tumour oxygenation as a function of daily fractionated mild temperature hyperthermia (41.5°C, 60 min). Tumour oxygenation is transiently increased by fractionated mild temperature hyperthermia (MTH) treatment in B16F10 and SCK tumors as measured in real time by Eppendorf histograph. -■- control and -●- 41.5°C (60 min) heatings.
Hyperthermia-induced vascular thermotolerance
Subsequently, the induction of tumour vascular thermotolerance was investigated. The oxygen and vessel maturation levels were examined during different heating strategies, i.e. (1) a single 60 min 41.5°C treatment, (2) two consecutive daily treatments of 41.5°C for 60 min, (3) a single 60 min 43°C treatment, or (4) two days of 41.5°C for 60 min followed by treatment with 43°C for 60 min on the third day.
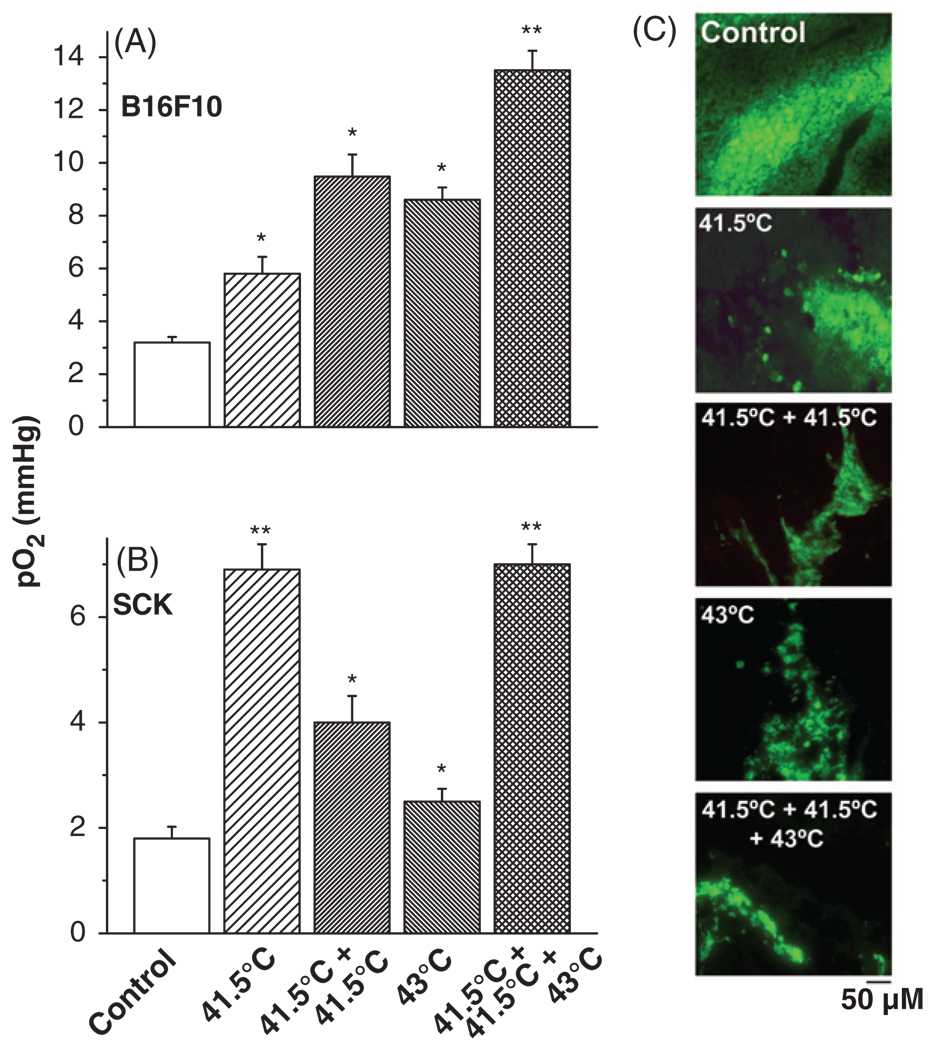
The tumour oxygenation was significantly increased (p < 0.05) in response to heating at 43°C when the tumour was pre-exposed to mild hyperthermia, as compared to a single treatment of 43°C alone (Figure 2). This expression of thermotolerance via improved oxygenation occurred in both tumour models, the B16F10 melanoma and the SCK murine mammary carcinoma as measured by the Eppendorf pO2 histograph. Pimonidazole distribution analysis subsequently confirmed these differences in tumour oxygenation levels. Pimonidazole, a substituted 2-nitroimidazole, is preferentially reduced in hypoxic viable cells and forms irreversible protein adducts. This can be monitored and has been optimised for immunofluorescence (IF) detection. Pimonidazole staining validated that the tumours pre-exposed to hyperthermia for two days at 41.5°C before a third treatment at 43°C were better oxygenated compared to either the 41.5°C or 43°C single treatment or the two consecutive daily 41.5°C hyperthermia treatments (Figure 2C).
Figure 2.
Vascular thermal tolerance. Pre-treatment of tumours with mild temperature hyperthermia (41.5°C, 60 min) increases tumour oxygenation by conventional hyperthermia (43°C, 60 min) treatment in B16F10 (A) and SCK (B) tumours. Representative pimonidazole stainings of control, and different hyperthermia schedules in B16F10 tumours (C). A minimum of 3 tumours and 10 randomly chosen images per treatment group were acquired. Original magnification, ×200; Scale bar 50 µM. *p < 0.05 versus control; **p < 0.05 versus 43°C treatment group.
Tumour vascular thermotolerance parallels evidence of vessel normalisation
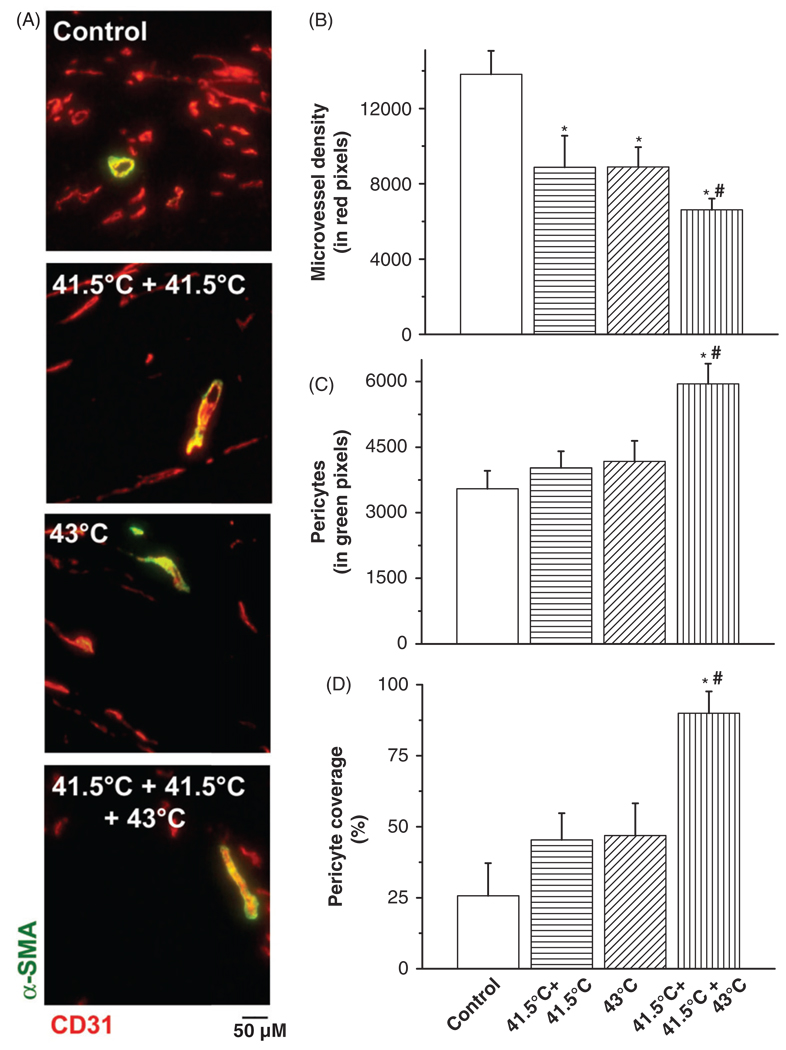
Furthermore, we desired to clarify the cellular changes in the tumour vasculature. Additional IF analysis revealed differences in the vascular composition after the various hyperthermia strategies. Namely, repeated mild hyperthermia at 41.5°C reduced tumour microvessel density and increased the pericyte coverage of those vessels that remained (Figure 3). Interestingly, mice pre-exposed to mild hyperthermia followed by 43°C thermal treatment showed the greatest reduction in microvessel density (p < 0.05) and greatest increase in pericyte coverage (p < 0.05). This indicated that the development of vascular or tumour thermotolerance in vivo is reflected in compositional changes in the tumour vasculature, matching what has been termed vessel normalisation or maturation, namely the increase in pericyte covered blood vessels.
Figure 3.
Histological analysis of pericytes and microvessel density (MVD) after hyperthermia treatment schedules. Co-localisation staining of pericytes (α-SMA, green) and microvessel density (CD31, red) in B16F10 tumours (A). Quantification of IF staining for microvessel density (CD31) (B), pericytes (α-SMA) (C) and pericyte covered vessels (D) by morphometric analysis. Original magnification, ×200; scale bar 50 µM. *p < 0.05 versus control; *#p < 0.05 versus 43°C treatment group. After binarisation of the images, microvessel density or pericytes were estimated by scoring the total number of representative coloured pixels per field. A pericyte-covered tumour blood vessel is considered a mature, normalised vessel.
Hyperthermia increases fractionated radiation response
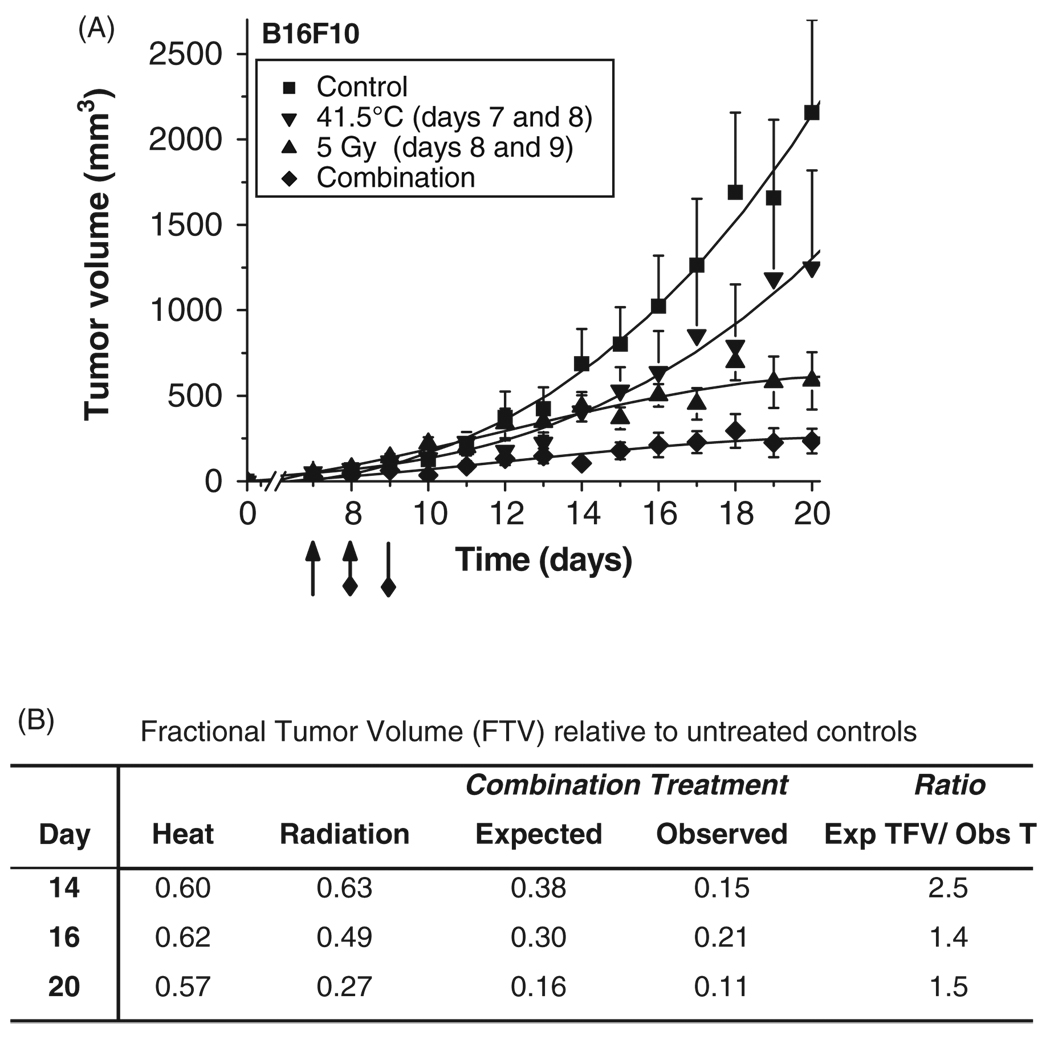
Since oxygen is the seminal radiosensitiser [26, 27], we subsequently investigated the therapeutic relevance of hyperthermia-induced increases in tumour oxygenation to enhance radiation treatment (Figure 4). B16F10 tumour bearing mice were treated daily with mild hyperthermia (41.5°C) for two days (days 7 and 8 after inoculation) followed by fractionated radiation treatment (5 Gy; days 8 and 9).
Figure 4.
Fractionated radiation treatment enhancement by rational scheduling of mild temperature hyperthermia. B16F10 tumour volumes after hyperthermia, radiation or combination treatments (A). Tumour-bearing animals were treated with control (-■-), heat (-▼-, 41.5°C 60 min on days 7 and 8; indicated by arrowhead), radiation (-▲-, 5 Gy, day 8 and 9, indicated by diamond arrow tail), or combined (-◇-). Data are plotted as means ± SEM over time with third order polynomial line fitting. Efficacy determination of combination therapy of heat and fractionated radiation (B). The following formula was applied to determine efficacy [21]: the expected tumour growth inhibition from combination treatment (tumour growth inhibition by hyperthermia × tumour growth inhibition by radiation) / observed tumour growth inhibition. A ratio >1 indicates a synergistic (greater than additive) effect.
Initially, we compared the inhibition of tumour growth on day 20 (the last day when all four groups can be compared), as well as by using the area under the model-based tumour growth curve (see Methods section) for each group from start of treatment, day 7, to the end of the measurement period, day 20. On day 20, hyperthermia and radiation as single-agent therapies, inhibited tumour growth by approximately 42% and 73%, respectively, whereas combination of the two resulted in tumour growth inhibition of 90% compared to controls, which was a supra-additive effect [16, 21, 23] and caused tumour stasis (Figure 4). The area under the curve approach rendered a comparable percentage inhibition: hyperthermia (37%), radiation (49%), and combination (81%).
The general linear mixed model for the ln (tumour volume + 1) also demonstrated that tumours in the control and hyperthermia groups grew faster and became larger than those in the radiation and combination groups. Compared to the combination group, the control group showed significantly different linear time trends, i.e. tumour growth rates (t = 4.29, p = 0.0002), hyperthermia marginally significant (t = 1.95, p = 0.0595), whereas the radiation group (t = 1.58, p = 0.1238) did not. On day 20, the least squares means of ln (tumour volume + 1) of the four groups were: control (7.93) > hyperthermia (6.9) >radiation (6.73) >combination (5.12). The combination group had a significantly lower least squares mean ln (tumour volume +1) than control (t = 5.17, p < 0.0001), hyperthermia (t = 3.27, p = 0.0101) and radiation (t = 2.96, p = 0.0233) groups.
Body weights of mice were monitored as an indirect measurement of general toxicity. The treatment schedule of hyperthermia (alone or in combination with radiation) showed no signs of toxicity as assessed by unaltered behaviour, weight gain during experiments, and macro- and microscopic morphology of internal organs on autopsy (data not shown).
Vascular thermotolerance is a physiological phenomenon
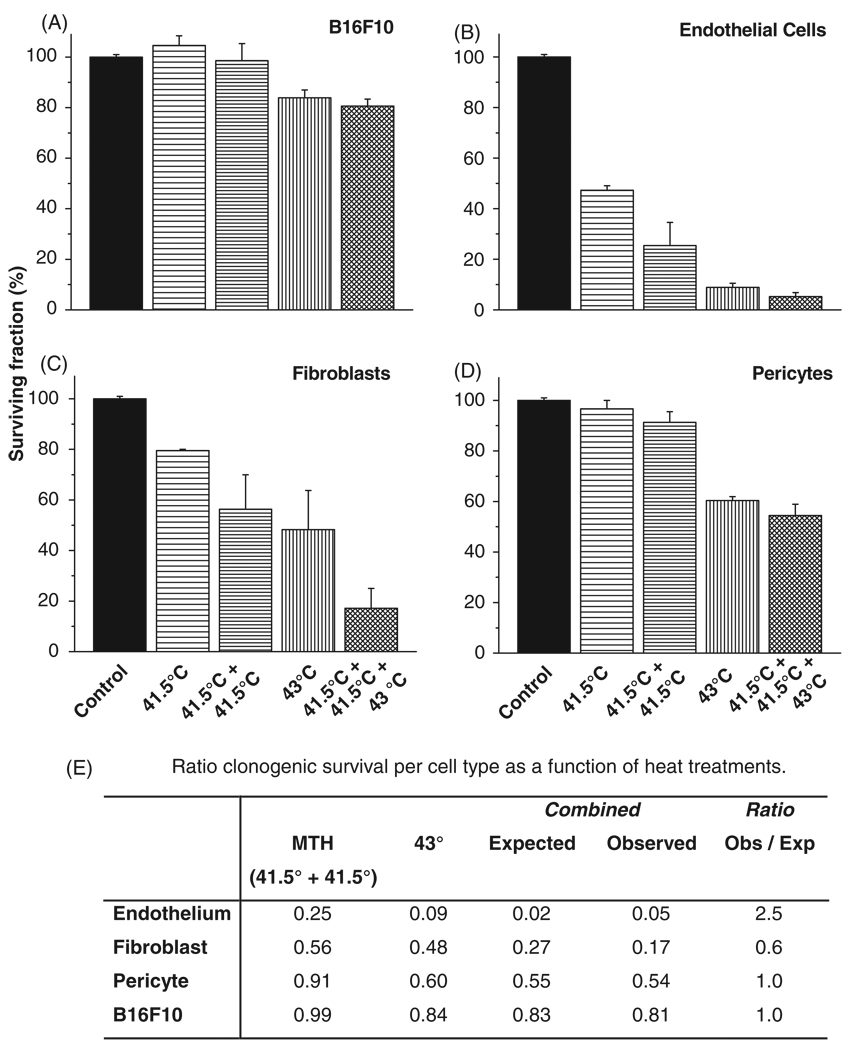
To further elucidate the process and mechanisms by which hyperthermia may induce features previously ascribed as evidence of vascular thermotolerance, we investigated the heat response of parenchymal and stromal cell types independently in vitro (Figure 5). We analysed the colony forming ability of the B16F10 melanoma cells, endothelial cells, fibroblasts, perivascular 10T1/2 cells after heating sequences as applied in vivo (Figures 2 and 3), namely (1) MTH, two consecutive daily treatments of 41.5°C for 60 min, (2) a single 60 min 43°C treatment, or (3) two days of 41.5°C for 60 min followed by treatment with 43°C for 60 min on the third day. After the hyperthermia regimens the cells were returned to the incubator and allowed to form colonies (approximately one week for B16F10 and pericytes; 2 weeks for the fibroblasts and HUVEC). Two days of mild temperature hyperthermia showed little to no effect on the colony formation of B16F10 cells as compared to control cell plating efficiency (99% ± 7% survival), whereas 60 min of 43°C treatment caused the surviving fraction to decrease to 84% (±3%) (Figure 5A). MTH pretreatment of B16F10 did not significantly influence the overall colony formation as compared to one-day treatment of 43°C, a survival of 81% ± 3%. On the contrary, MTH alone reduced the surviving HUVEC colony formation to approximately 25% (±9%) and one day of 43°C reduced the surviving colony formation to approximately 9% (±2%) (Figure 5B). MTH pretreatment of HUVEC induced thermal tolerance (Figure 5E), and reduced the overall colony formation to 5% (±2%) (Figure 5B). As for the fibroblasts, MTH before heating at 43°C reduced the surviving colony formation to 17% ± 8%, which was lower than but not statistically different compared to a single 43°C heating alone (48% ± 15% survival; Figures 5C and E). MTH itself reduced the colony formation of fibroblasts to 56% (±14%). As for the pericytes, MTH alone showed little to no effect on the colony formation (91% ± 4% survival), whereas 60 min at 43°C alone dropped the survival fraction to 60% (±2%). MTH pretreatment of pericytes prior to 60 min of 43°C heating did not significantly influence the overall colony formation as compared to 43°C alone with a resulting surviving fraction of 54% ± 4% (Figures 5D and E).
Figure 5.
Clonogenic potential of parenchymal and stromal cells after different hyperthermia schedules. Survival of B16F10 (A), HUVEC (B), fibroblasts (C), and 10T pericyte cells (D), after different hyperthermia treatments as indicated. Acquisition of thermotolerance (E). Treatment schedules were as follows: (1) mild temperature hyperthermia (MTH), where the flasks were heated at 41.5°C for 60 min two consecutive days in a row, day 1 and 2; (2) a single treatment of 43°C for 60 min on the third day of heat treatments; (3) pretreatment of MTH followed by treatment with 43°C for 60 min on day 3. Thermal tolerance was determined by the following formula [21]: the observed fraction of colony formation after MTH pretreatment/the expected fraction of colony formation (i.e. fraction of colonies after MTH × fraction of colonies after 43°C for 60 min). A ratio >1 indicates induced thermal tolerance by MTH pretreatment, whereas a ratio of <1 indicates an induction of thermal sensitisation by MTH pretreatment.
Discussion
Tumour thermotolerance or vascular thermotolerance, an induced resistance of the tumour to hyperthermia, is a potentially powerful and clinically pertinent by-product observed in patients receiving multiple hyperthermia treatments [28]. Thus far, the basic concept of vascular thermotolerance has been characterised as a markedly increased blood flow response of the tumour to a second hyperthermia exposure compared to the response to a single thermal dose, even at temperatures that would normally cause vascular damage [6, 7]. Since oxygen delivery is dependent upon the blood flow, we hypothesised that the tumour oxygenation may be improved upon two or more sequential mild heating sessions. Previously, Lin and Song observed that tumour oxygenation and blood flow were maintained at a higher level in tolerant tumours than naїve tumours in response to relatively high temperatures that normally cause vascular shutdown and hypoxia [6]. However, few studies have explored the possible increase in tumour oxygenation that may occur after multiple treatments with mild temperature hyperthermia [8]; where significant vascular damage and shutdown are not expected. Since the majority of clinical hyperthermia treatments are in the range of 40–42°C temperatures, we focused on 41.5°C multi-treatment studies. As postulated, daily treatment of mild hyperthermia resulted in an increase in overall tumour oxygenation up to day four of treatment in both murine tumour models, B16F10 and SCK, after which it dropped to untreated levels (Figure 1). Interestingly, in B16F10 tumours the pO2 increase reached its height after the second daily heating, whereas in SCK tumours the oxygenation improvement peaked at 24 h after a single heating and began to fall off afterwards towards control levels. This rapid response of SCK tumours to heat is in line with previous reports, where one heating at 41.5°C for 60 min resulted in tumour growth delay [29]. In both models the peak increase in overall tumour oxygenation was approximately 3-fold, similar to what was previously seen in the FSaII model [8, 29]. We have previously observed that SCK tumours are quite sensitive to moderate thermal doses and that the oxygenation was improved for a longer duration when a single heating at 41.5°C was applied for 30 min versus 60 min [30]. In addition, our recent work has indicated that upon heating the FSaII murine fibrosarcoma daily, the overall heat-induced reoxygenation begins to decrease upon second and third heat applications [8]. Therefore, a decrease in oxygenation improvement upon two heat applications of 41.5°C for 60 min would be in line with these previous observations. In Figure 2B, we observed that although two heat applications at 41.5°C or a single 43°C exposure did not improve oxygenation to the same extent as a single 41.5°C exposure in SCK tumours (thus indicating an increase in vascular damage), the induction of some degree of vascular thermotolerance (i.e. improved vascular response to subsequent thermal stress) occurred when tumours were heated at 41.5°C for two days in a row and then heated with 43°C on the third day. Evidence of this is the increase in oxygenation observed after three heat exposures to a level similar to that induced with a single 41.5°C heating; a result not expected if the only effect of additional heat exposures was to increase the amount of vascular damage accumulated in the tumour. The results between different tumour types are certainly a function of distinct physiology and intrinsic heat sensitivity for each tumour cell type, as well as the degree of vascular damage or vascular thermotolerance that is caused by each successive heat exposure. Furthermore, additional studies are needed to measure the immediate or long term effects of hyperthermia on tumour physiology in general and tumour oxygenation in particular, since the described results in this manuscript are only focused on tumour pO2 measurements 24 h after the heating(s). Regardless, we observed that multiple mild temperature hyperthermia exposures are able to induce a dynamic tumour oxygenation profile. To date, this type of ‘tumour oxygenation window’ has only been described for anti-angiogenic and possibly chemotherapeutic agents [12–14].
In principle, the overall tumour oxygenation level depends on the balance of oxygen supply via blood flow on one side, and oxygen consumption by cells on the other. Studies have shown that heat-induced changes in tumour oxygenation parallel the changes in tumour blood flow [17, 31–34]. Under physiological conditions, blood flow is tightly regulated by vasodilating factors that stimulate endothelial cells to release nitric oxide (NO), which then causes relaxation of the vascular smooth muscle and subsequent increased blood flow. NO is a pluripotent molecule which has cytotoxic as well as radiosensitising properties in hypoxic cells [17]. Moreover, it has an essential function in vascular tone, and its production is influenced by thermal treatment, since nitric oxide synthase expression and overall tumoural nitric oxide production is increased after hyperthermia in tumours [17, 35]. However, it has also been shown that blocking NO production can increase heat-induced tumour growth delay, likely the result of reduced dissipation of the thermal stress in the tumours [17]. The ability of tumours to increase blood flow after heating is limited compared to normal tissue blood flow [32]. As a result, the heat dissipation by blood flow in tumours is slower as compared to normal tissue, and as a consequence the temperature in tumours rises higher than normal tissue during hyperthermia [32]. Additional factors which influence tumour blood flow and subsequent tumour oxygenation, are vessel obstructions by circulating (blood, tumour or bone marrow derived) cells, the collapse of vessels in regions of high tumour interstitial pressure or the spontaneous vasomotor activity in peripheral normal tissue vessels incorporated into the tumour, which all subsequently affects the blood flow in downstream tumour microvessels and causes intermittent blood flow in tumours, which results in acute hypoxia [36, 37]. In the case of heat-induced tumour blood flow, these same peripheral normal tissues likely drive the increased perfusion in the actual tumour due to the heating of, and direct connection between, vessels originating in the host organ that supply the tumour tissue.
Conversely, tumour cell oxygen consumption rate has also been shown to be an important influence on tumour oxygenation levels [38]. Thermal exposures as applied similarly in this study (at a range of 41–42°C) were shown to transiently increase oxygen consumption. At temperatures above this threshold of 42°C the oxygen consumption rate declines rapidly [39, 40]. An additional concern is that the heating of tumours by submerging them in preheated water can create osmotic effects resulting in oedema formation [41]. Although this normal tissue response and potential damage is mostly seen at higher thermal exposures (>43°C) [41], it is conceivable that this osmotic oedema causes some degree of vascular congestion and could be an unintended source of vascular inefficiency and may be a factor in our results. Nonetheless, even if the postulated increased oxygen consumption and vasocongestion occurs after repeated mild hyperthermia treatments, we found that the overall tumour oxygenation was still increased by mild hyperthermia.
Subsequently we observed that in tumours heated more than once, in an attempt to induce a thermotolerant state, the oxygenation was significantly increased as compared to control groups during 3–4 days of daily heatings. On a tissue level this increase in oxygenation was correlated with vessel normalisation, i.e. a decrease in microvessel density and an increase in pericyte coverage. Hence, in addition to creating a ‘tumour oxygenation window’, our results indicate that mild hyperthermia is a novel vessel normalisation inducing modality.
The clonogenic assays show differential sensitivity of the cell types to heat. This in vitro assay is used to investigate potential diverse responses to heat by the cell types involved. Interestingly, the thermal treatment strategies only slightly reduced B16F10 melanoma cells or the pericyte clonogenicity, while the fibroblasts and the endothelial cells were significantly affected by 41.5°C and 43°C treatments. Nonetheless, in this in vitro assay a degree of cellular thermotolerance was established in the endothelial cells that remained viable after two heatings at 41.5°C. In vivo however, analysis of the tumour tissue revealed that the ‘immature’ vessels were clearly more affected by the repeated heat exposures than ‘mature’, i.e. pericyte covered, vessels after thermal treatments. As seen in vitro, this might be explained by either the relative thermal resistance of pericytes individually, or as seen in vivo by a thermal tolerance displayed by mature stabilised peri-vasculature pericyte covered vessels. These data implicate that a fully established collective stroma survives to a greater extent and demonstrates thermal tolerance as compared to immature separate stromal components.
Overall, this suggests that, mechanistically, tumour thermotolerance is a physiological phenomenon mediated through retention or recruitment of improved functional vasculature in the tumour while more sensitive and immature vasculature fall away as repeated exposures to thermal energy are given. Moreover, that tumour thermotolerance in vivo is in fact a consequence of the presence and development of a transient state of functional vasculature that is able to deal with the effects of applied heating in a more efficient manner than would occur in a typical solid tumour microenvironment.
To interrogate the possible beneficial effects of the improved oxygenation observed via sensitisation of previously hypoxic tumour tissue [26, 27], we subsequently combined multiple heat treatments of the tumour with two fractions of radiation therapy. We indeed found that rational scheduling of fractionated radiation during a course of repeated hyperthermia treatments caused a synergistic tumour growth inhibition compared to either treatment modalities alone.
Ultimately, the goal of this work is to catalogue the potential effects of hyperthermia treatments, in order to be able to rationally design the most optimal clinical treatment strategy. The finding that mild hyperthermia is a novel vessel normalisation-inducing modality leading to better radiation response, will aid toward this. Moreover, these results are of immediate translational importance calling for more precise and proper scheduling of hyperthermia frequency and radiation doses to capitalise on periods of tumour peak radiosensitivity, i.e. heat-induced increased tumour oxygen levels. Moreover, at least in theory, since tumour perfusion is a crucial parameter in drug pharmacokinetics [42], this transient improvement of tumour physiology and oxygenation can also be employed to enhance the clinical success of other conventional cancer treatments, such as improvement of chemotherapeutic delivery.
Acknowledgements
This work was supported by grants CA44114 and CA107160 from the NIH.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Stauffer PR, Goldberg SN. Introduction: Thermal ablation therapy. Int J Hyperthermia. 2004;20:671–677. doi: 10.1080/02656730400007220. [DOI] [PubMed] [Google Scholar]
- 2.Shinohara K. Thermal ablation of prostate diseases: Advantages and limitations. Int J Hyperthermia. 2004;20:679–697. doi: 10.1080/02656730412331286876. [DOI] [PubMed] [Google Scholar]
- 3.Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischof JC. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther. 2006;5:1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 4.Rhee JG, Schuman VL, Song CW, Levitt SH. Difference in the thermotolerance of mouse mammary carcinoma cells in vivo and in vitro. Cancer Res. 1987;47:2571–2575. [PubMed] [Google Scholar]
- 5.Song CW, Lin JC, Chelstrom LM, Levitt SH. The kinetics of vascular thermotolerance in SCK tumors of A/J mice. Int J Radiat Oncol Biol Phys. 1989;17:799–802. doi: 10.1016/0360-3016(89)90069-2. [DOI] [PubMed] [Google Scholar]
- 6.Lin J-C, Song CW. Influence of vascular thermotolerance on the heat-induced changes in blood flow, pO2 and cell survival in tumors. Cancer Res. 1993;53:2076–2080. [PubMed] [Google Scholar]
- 7.Nah BS, Choi IB, Oh WY, Osborn JL, Song CW. Vascular thermal adaptation in tumors and normal tissue in rats. Int J Radiat Oncol Biol Phys. 1996;35:95–101. doi: 10.1016/s0360-3016(96)85016-4. [DOI] [PubMed] [Google Scholar]
- 8.Griffin RJ, Dings RP, Jamshidi-Parsian A, Song CW. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int J Hyperthermia. 2010;26:256–263. doi: 10.3109/02656730903453546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewhirst M, Gross JF, Sim D, Arnold P, Boyer D. The effect of rate of heating or cooling prior to heating on tumor and normal tissue microcirculatory blood flow. Biorheology. 1984;21:539–558. doi: 10.3233/bir-1984-21413. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Determinants of tumor blood flow: A review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 11.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 13.Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Gregoire V, Feron O, Gallez B. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res. 2005;11:743–750. [PubMed] [Google Scholar]
- 14.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, Griffin RJ. Scheduling of radiation with angiogenesis inhibitors Anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 16.Dings RP, Williams BW, Song CW, Griffioen AW, Mayo KH, Griffin RJ. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int J Cancer. 2005;115:312–319. doi: 10.1002/ijc.20850. [DOI] [PubMed] [Google Scholar]
- 17.Griffin RJ, Ogawa A, Williams BW, Song CW. Hyperthermic enhancement of tumor radiosensitization strategies. Immunol Invest. 2005;34:343–359. doi: 10.1081/imm-200066270. [DOI] [PubMed] [Google Scholar]
- 18.Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: Evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 19.Dings RP, Chen X, Hellebrekers DM, van Eijk LI, Zhang Y, Hoye TR, Griffioen AW, Mayo KH. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J Natl Cancer Inst. 2006;98:932–936. doi: 10.1093/jnci/djj247. [DOI] [PubMed] [Google Scholar]
- 20.Dings RP, van der Schaft DW, Hargittai B, Haseman J, Griffioen AW, Mayo KH. Anti-tumor activity of the novel angiogenesis inhibitor anginex. Cancer Lett. 2003;194:55–66. doi: 10.1016/s0304-3835(03)00015-6. [DOI] [PubMed] [Google Scholar]
- 21.Dings RP, Yokoyama Y, Ramakrishnan S, Griffioen AW, Mayo KH. The designed angiostatic peptide anginex synergistically improves chemotherapy and antiangiogenesis therapy with angiostatin. Cancer Res. 2003;63:382–385. [PubMed] [Google Scholar]
- 22.Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. Faseb J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 23.Dings RP, Van Laar ES, Webber J, Zhang Y, Griffin RJ, Waters SJ, MacDonald JR, Mayo KH. Ovarian tumor growth regression using a combination of vascular targeting agents anginex or topomimetic 0118 and the chemotherapeutic irofulven. Cancer Lett. 2008;265:270–280. doi: 10.1016/j.canlet.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dings RP, Van Laar ES, Loren M, Webber J, Zhang Y, Waters SJ, Macdonald JR, Mayo KH. Inhibiting tumor growth by targeting tumor vasculature with galectin-1 antagonist anginex conjugated to the cytotoxic acylfulvene, 6-hydroxylpropylacylfulvene. Bioconjug Chem. 2010;21:20–27. doi: 10.1021/bc900287y. [DOI] [PubMed] [Google Scholar]
- 25.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 28.Olch AJ, Kaiser LR, Silberman AW, Storm FK, Graham LS, Morton DL. Blood flow in human tumors during hyperthermia therapy: Demonstration of vasoregulation and an applicable physiological model. J Surg Oncol. 1983;23:125–132. doi: 10.1002/jso.2930230217. [DOI] [PubMed] [Google Scholar]
- 29.Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, Park H, Song CW. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia. 2000;2:555–560. doi: 10.1038/sj.neo.7900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okajima K, Griffin RJ, Iwata K, Shakil A, Song CW. Tumor oxygenation after mild-temperature hyperthermia in combination with carbogen breathing: Dependence on heat dose and tumor type. Radiat Res. 1998;149:294–299. [PubMed] [Google Scholar]
- 31.Bicher HI, Hetzel FW, Sandhu TS, Frinak S, Vaupel P, O’Hara MD, O’Brien T. Effects of hyperthermia on normal and tumor microenvironment. Radiology. 1980;137:523–530. doi: 10.1148/radiology.137.2.7433686. [DOI] [PubMed] [Google Scholar]
- 32.Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984;44:4721s–4730s. [PubMed] [Google Scholar]
- 33.Vaupel PW, Otte J, Manz R. Oxygenation of malignant tumors after localized microwave hyperthermia. Radiat Environ Biophys. 1982;20:289–300. doi: 10.1007/BF01323754. [DOI] [PubMed] [Google Scholar]
- 34.Griffin RJ, Okajima K, Ogawa A, Song CW. Radiosensitization of two murine tumours with mild temperature hyperthermia and carbogen breathing. Int J Radiat Biol. 1999;75:1299–1306. doi: 10.1080/095530099139467. [DOI] [PubMed] [Google Scholar]
- 35.Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res. 2001;155:515–528. doi: 10.1667/0033-7587(2001)155[0515:iotobm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 37.Masunaga S, Nagasawa H, Uto Y, Hori H, Suzuki M, Nagata K, Kinashi Y, Ono K. The usefulness of continuous administration of hypoxic cytotoxin combined with mild temperature hyperthermia, with reference to effects on quiescent tumour cell populations. Int J Hyperthermia. 2005;21:305–318. doi: 10.1080/02656730500060574. [DOI] [PubMed] [Google Scholar]
- 38.Kirkpatrick JP, Brizel DM, Dewhirst MW. A mathematical model of tumor oxygen and glucose mass transport and metabolism with complex reaction kinetics. Radiat Res. 2003;159:336–344. doi: 10.1667/0033-7587(2003)159[0336:ammoto]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Thews O, Li Y, Kelleher DK, Chance B, Vaupel P. Microcirculatory function, tissue oxygenation, microregional redox status and ATP distribution in tumors upon localized infrared-A-hyperthermia at 42°C. Adv Exp Med Biol. 2003;530:237–247. doi: 10.1007/978-1-4615-0075-9_23. [DOI] [PubMed] [Google Scholar]
- 40.Vaupel P, Ostheimer K, Muller-Klieser W. Circulatory and metabolic responses of malignant tumors during localized hyperthermia. J Cancer Res Clin Oncol. 1980;98:15–29. doi: 10.1007/BF00413173. [DOI] [PubMed] [Google Scholar]
- 41.Lindegaard JC, Nielsen OS. Thermotolerance in the mouse foot estimated at various levels of normal tissue damage. Int J Radiat Oncol Biol Phys. 1989;16:1543–1549. doi: 10.1016/0360-3016(89)90960-7. [DOI] [PubMed] [Google Scholar]
- 42.Saleem A, Price PM. Early tumor drug pharmacokinetics is influenced by tumor perfusion but not plasma drug exposure. Clin Cancer Res. 2008;14:8184–8190. doi: 10.1158/1078-0432.CCR-08-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]