Abstract
Background
Human papillomavirus (HPV) infection of the genital mucosa is thought to require trauma to the cervicovaginal epithelium. Therefore, we determined whether a cytology specimen collection procedure (Pap smear), which disrupts the epithelium by design, renders the cervix more susceptible to HPV infection in a primate model.
Methods
In a series of female rhesus macaques, a speculum examination was performed with (n = 8) or without (n = 4) a cytology specimen collection procedure as it is commonly practiced in a gynecology clinic. An internal digital examination was performed after specimen collection using Surgilube (n = 4) or 1% iota-carrageenan, a previously indentified HPV inhibitor (n = 4) as the lubricant. The cervix was then inoculated with HPV16 pseudovirions expressing red fluorescent protein. After 3 days, the reproductive tracts were excised and the cervix was cryosectioned. Sections were analyzed by fluorescent confocal microscopy for the number of red fluorescent protein–positive keratinocytes.
Results
Substantial infection of the ectocervix, the transformation zone, and the endocervix was detected, but only in conjunction with the cytology specimen collection procedure (cytology using Surgilube vs without cytology using Surgilube, mean = 84 infectious events per section vs mean = 0.05 infectious events per section, difference = 84 infectious events per section, 95% confidence interval = 19 to 384 infectious events per section). When the carrageenan gel was substituted for Surgilube for an internal digital examination, the mean number of infectious events decreased (carrageenan gel vs Surgilube, mean = 3.5 events per section vs mean = 84 infectious events per section difference = 81 events per section, 95% confidence interval = 33 to 213 events per section).
Conclusions
These findings indicate that cytology screening in women might lead to a transient enhancement of susceptibility to HPV infection and that use of a carrageenan-based gel during the examination might mitigate this enhancement.
CONTEXT AND CAVEATS
Prior knowledge
Although the incidence of cervical cancer has decreased, the rate of human papillomavirus (HPV) infection, the main cause of cervical cancer, has increased. Damage to the cervical epithelium such as that produced during a routine cytology screening procedure (Pap smear) may increase susceptibility to HPV infection.
Study design
Female rhesus macaques were subjected to cervical examination with or without Pap smear in the presence of Surgilube or 1% iota-carrageenan (a lubricant that can inhibit HPV infection) followed by exposure to a pseudovirus composed of the HPV16 virion proteins. Three days later, the cervix was examined to determine the number of infected cells.
Contribution
Pap smear was associated with an increase in the number of infectious events. When iota-carrageenan was applied to the cervical tract before exposure to the pseudovirus, the mean number of infectious events decreased compared with that when Surgilube was used.
Implications
In this model, Pap smear was associated with increased susceptibility to HPV infection, but the risk was reduced by use of a carrageenan-based gel during the procedure.
Limitations
This study used relatively few monkeys, and the anatomy of the vagina and cervix of the rhesus macaque differs from that of humans. Although a variety of devices are used in the clinic to perform Pap smears, this study used only a standard cytobrush and spatula. The duration of increased susceptibility to HPV infection after cytology specimen collection is unknown.
From the Editors
Human papillomavirus (HPV) is one of the most common sexually transmitted diseases worldwide (1). A subset of HPV genotypes has been identified as oncogenic; cervical infection with these types produce cellular dysplastic changes that can lead to invasive cancer (2). Worldwide, it is estimated that approximately 500 000 cervical cancers occur per year, and greater than 95% of cases are HPV-associated (3).
The rates of cervical cancer diagnoses and deaths in developed countries have fallen dramatically, despite evidence for increased rates of genital HPV infections (4). This decrease coincides with the advent of cervical cytology screening, perhaps the most successful cancer screening strategy ever instituted. The vast majority of HPV-induced dysplasia occurs in a small ring of tissue at the opening of the cervix (the os) known as the transformation zone, in which the stratified squamous epithelium of the ectocervix transitions to the simple columnar epithelium of the endocervix. A simple speculum examination allows for direct visual assessment and sampling of the cells in this tissue, which exhibits characteristic and progressive changes in the slow progression to malignancy.
The procedure for collecting cervical cytology specimens, commonly known as a Pap smear, inherently disrupts the cervical epithelium. To effectively collect specimens for testing, the instrument used must dislodge cells from the deepest (basal) layer of the stratified squamous epithelium, the only layer in which low-grade dysplastic lesions are detectable. In addition, in the delicate tissue of the endocervix, which is a single layer epithelium, removing cells creates erosion that exposes the basement membrane to material in the endocervical canal. In a mouse model of HPV genital infection, we previously showed that this physical disruption of the endocervix leads to binding of pseudovirus (PsV) to the basement membrane and to a dramatic increase in susceptibility to infection (5,6).
We hypothesized that if infectious HPV capsids are present in the human genital tract during cytology screening, increased infection of the very tissue that is most vulnerable to HPV-induced oncogenesis could result. To test this hypothesis, the rhesus macaque was chosen because of the similarity to the human female genital tract in physiology (28 day menstrual cycle), anatomy (simplex uterus), and histology (transformation zone between stratified squamous epithelium of ectocervix to columnar epithelium of endocervix occurs at the cervical os). In addition, rhesus macaques are susceptible to squamous cell cancer of the cervix caused by rhesus papillomavirus (RhPV1), which is closely related phylogenetically to HPV16, the PsV type commonly used in studies to model infection (6–8). Last, the cytology screening procedure (cytobrush for endocervix; spatula for ectocervix) could be reproduced in the macaque with a high degree of fidelity.
Carrageenans are sulfated polysaccharides used in a wide variety of foods, cosmetics, and hygiene products, including sexual lubricants. The potent inhibitory action of iota-carrageenan against HPV infection has been demonstrated both in vitro and in vivo in the mouse cervicovaginal challenge model (6,9). The second aim of the study was to determine if this inhibitory effect could be recapitulated in the macaque model. Substituting iota-carrageenan as the lubricant during the internal digital examination component of a full gynecological examination represents a potentially simple and inexpensive intervention. To test the hypothesis that this intervention could mitigate Pap smear–induced susceptibility to infection in the nonhuman primate model, we performed an internal digital examination after the cytology collection procedure and PsV instillation. In one group of monkeys, a 1% iota-carrageenan solution was used as the lubricant, and in another group of monkeys, we used Surgilube, a lubricant that was previously determined to have no inhibitory activity against HPV infection in vitro and in vivo (unpublished observations) and is commonly used in gynecological examinations.
Methods
Monkeys
Adult female rhesus monkeys (Macaca mulatta; N = 13) aged 6–12 years and weighing 4–9 kg were culled from a recycled pool that had previously completed a research protocol. The monkeys were previously involved either in behavioral studies or in a trial of a vaccinia-vectored SHIV vaccine. None were SHIV positive. The serum of each monkey tested negative for neutralizing activity against HPV16 and RhPV1 (10). The experimental protocol was approved by the National Cancer Institute’s Animal Care and Use Committee.
PsV Production
HPV16 red fluorescent protein (RFP) PsV was produced according to the standard production protocol published on our laboratory Web site (http://home.ccr.cancer.gov/lco/pseudovirusproduction.htm). Nucleotide maps of the HPV16 packaging plasmid (p16sheLL) and the RFP reporter plasmid (pRwB) can be found at http://home.ccr.cancer.gov/lco/target.htm. PsV titer, which represents a quantum measure of the number of capsids capable of infectious entry, was determined by reporter gene expression in 293TT cells as detailed in Note 10 of the standard production protocol, and titer is referred to in terms of infectious units per milliliter (IU/mL).
PsV Challenge and Cytology Collection Procedure (Pap smear)
Fourteen days before PsV challenge, each monkey was given 30 mg of Depo-Provera (Pfizer, New York, NY) by intramuscular injection to decrease variability of reproductive tract physiological parameters associated with the menstrual cycle. A standard 100 μL PsV inoculum consisting of 75 μL of opti-prep purified HPV16-RFP (approximately 3.75 × 108 infectious units on the basis of a titer of approximately 5 × 109 IU/mL) mixed with 25 μL of 4% carboxymethylcellulose (an inert gel; Sigma, St. Louis, MO) was prepared in advance for each monkey. Under sedation with ketamine (10 mg/kg delivered by intramuscular injection; Fort Dodge, Overland Park, KS), the monkeys were placed in the dorsal lithotomy position and a modified Pederson vaginal speculum (Miltex, York, PA) was used to visualize the cervix. In the monkeys that underwent PsV challenge without cytology (n = 4), 25 μL of the inoculum was instilled with a positive displacement pipette atraumatically approximately 1 cm into the endocervix; the remaining 75 μL was applied to the ectocervix before the speculum was removed. In the monkeys that underwent PsV challenge in conjunction with cytology (n = 8), 25 μL of inoculum was applied to the ectocervix. The ectocervix and the transformation zone were then swiped several times with a standard plastic spatula (Cooper Surgical, Inc, Trumbull, CT) used to obtain cells from this region. Next, a Cytobrush cell collector (Cooper Surgical, Inc) was inserted approximately 1–2 cm into the endocervix and swirled several times, followed by the atraumatic instillation of 25 μL of inoculum into the cervix, and 50 μL onto the ectocervix. After removing the speculum, a bimanual examination was performed in which one digit was inserted into the vagina to palpate the cervix from below and the other hand was placed on the abdomen above the pubic symphysis to palpate the uterus from above. Immediately beforehand, 1 mL of Surgilube (Fougera, Melville, NY) was placed on the gloved digit and applied to the external genitalia as a lubricant for the bimanual examination (n = 4). In the carrageenan group (n = 4), each monkey underwent PsV challenge in conjunction with the cytology collection procedure as described above, except that 1 mL of 1% iota-carrageenan (Sigma) was substituted for Surgilube as the lubricant for the bimanual examination. One monkey served as a negative control and was challenged with a dummy inoculum of 4% carboxymethylcellulose mixed with phosphate-buffered saline in conjunction with the cytology specimen collection. The mean age in years of the monkeys in each group were as follows: PsV alone, 7.5 years (range, 6–9 years); PsV plus cytology, 9.5 years (range, 7–12 years), PsV plus cytology and carrageenan, 7.5 years (range, 7–10 years); negative control, 9 years. Supplementary Table 1(available online) lists the age and number of live births for each monkey.
Histology and Confocal Microscopy
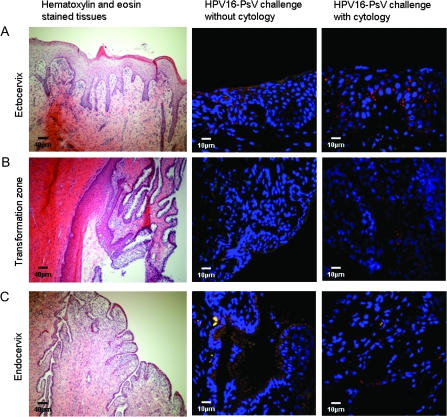
Three days post-challenge, each monkey was euthanized with Beuthanasia (80 mg/kg intravenous; Schering-Plough, Union, NJ) and the cervix was dissected out and stored in phosphate-buffered saline on ice for not more than 4 hours until it could be dissected for histology. Six wedge-shaped biopsy specimens were dissected from each individual cervix with the goal of obtaining a tissue mass lined on one side with an uninterrupted surface of cervical epithelium extending from approximately 1 cm on the ectocervix to 1 cm into the endocervix; by definition, this area includes the transformation zone. These specimens were frozen in tissue freezing media (Triangle Biomedical Services, Inc, Durham, NC). At cryosectioning, 6 μm sections were obtained at five levels through the tissue mass at approximately 250 μm increments. The quantity of evaluable epithelium on individual slides varied. This variation could not be accurately quantified because histological characteristics were not uniform across slides (Figure 1). The resulting 30 slides per monkey (six biopsy specimens, five sections each) were evaluated by confocal microscopy (×40 magnification) for the total number of infectious events in the cervical epithelium. Confocal images were acquired with a Zeiss LSM 510 Confocal Microscope (Carl Zeiss, Inc, Thornwood, NY). The unit is fitted with an Axiovert 100M inverted microscope and operates with a 1 mW HeNe laser tuned to 543 nm. RFP signal was collected in PMT 1 with a LP560 filter after excitation with the 543 nm laser line. Each cell displaying the characteristic punctate pattern of cytoplasmic RFP expression was counted as an infectious event. Before confocal examination, the slides were randomized and relabeled so that each of two observers (J. N. Roberts and R. C. Kines) could perform a separate blinded count. After infectious events per slide were totaled, the results were unblinded and the counts of the two observers were averaged and reported as the mean number of infectious events per biopsy specimen. Only one of the two observers (J. N. Roberts) tracked the distinction between ectocervical vs endocervical infection.
Figure 1.
Histological depiction of the rhesus macaque cervix and its infection by human papillomavirus (HPV) pseudovirus. Representative images from the three regions of the cervix are shown: A) ectocervix, B) transformation zone, and C) endocervix. Hematoxylin- and eosin-stained tissues are depicted in micrographs in the first column (scale bar = 40 μm). The images in the second and third columns are representative images of cervical specimens from monkeys challenged with HPV16-pseudovirus (PsV) transducing the red fluorescent protein without and with cytology sample collection, respectively (scale bar = 10 μm). Red stained cells indicate PsV-infected cells.
Hematoxylin–Eosin Staining
After cryosectioning, a set of slides from each animal were fixed for 10 minutes in 2% paraformaldehyde, rinsed for 1 hour in 1X phosphate-buffered saline and stored at 4°C. Hematoxylin and eosin staining was performed using the Frozen Section Staining Kit according to the manufacturer’s protocol (Richard-Allan Scientific, Kalamazoo, MI). Images were acquired using a Nikon Eclipse E600 (Nikon, Inc, Melville, NY) at ×10 magnification.
Statistical Methods
We estimated the average number of infectious events per slide within biopsy specimen by treatment. To estimate the confidence interval for each average, we modeled the average number of infectious events with a negative binomial regression model (11) and estimated separate variances for each observer and treatment. We accounted for the correlation in number of infectious events within and between biopsies by the use of an autoregressive correlation structure. We fit the model with the use of Generalized Estimating Equation methods and estimated confidence intervals that are robust to incorrect specification of the correlation. We fit separate models to counts of infectious events in the endocervix and in the ectocervix. To approximate the upper limit of the confidence interval for the zero endocervix counts for PsV + Surgilube, we added a single count to a single slide from the four monkeys and used the upper limit of the confidence interval assuming 0.0083 average counts (1 of 120) as the proxy for the upper limit for zero counts. All models were fit with the use of PROC GLIMMIX in SAS version 9.1 (SAS Institute, Cary, NC).
Results
Histological Analysis of HPV Infection of the Cervix After the Cytology Collection Procedure
The hematoxylin- and eosin-stained sections in Figure 1 demonstrate the typical morphology of the macaque cervix. HPV infection of the cervix was detected using replication incompetent HPV16 PsV that express RFP. Individual infectious events in the cervical epithelium were detected as red fluorescent cells in cryosections by confocal microscopic analysis. Figure 1 shows the characteristic punctate appearance of RFP expression in the cytoplasmic space in PsV infected cells. The ectocervix, the transformation zone, and the endocervix were analyzed and found to have a substantial number of infectious events in the monkeys that were challenged with PsV in conjunction with the cytology collection procedure. Virtually no infection was observed in any of the three regions after PsV challenge alone. This finding established that cytology specimen collection enhanced infection in each region of the cervix.
Quantitation of the Effect of Cell Collection Procedure and Carrageenan on Infection
A system was devised for quantifying the overall level of infection by counting the number of infectious events per biopsy specimen obtained from each monkey. Two independent and blinded observers counted events in each biopsied specimen, and these values were then combined and averaged for analysis. Infectious events were rare in the monkeys exposed to PsV in the absence of the cytology collection procedure despite challenge with approximately 3.75 × 108 infectious units (Table 1). In contrast, the cytology-exposed monkeys had 1843-fold (95% confidence interval [CI] = 404 to 8391) higher average number of infectious events per biopsy specimen than nonexposed monkeys (cytology using Surgilube vs without cytology using Surgilube, mean = 84 infectious events per section vs mean = 0.05 infectious events per section, difference = 84 infectious events per section, 95% CI = 19 to 384 infectious events per section) (Table 1). The number of infectious events varied substantially between the monkeys in each group (data not shown). Although observer 2 regularly recorded lower numbers of counts than observer 1, both observers counted more infectious events in the presence of cell collection (Table 1). The lower counts recorded by observer 2, who evaluated the slides after observer 1, may be because of the prolonged confocal assessment necessary to count the large number of events, which tended to extinguish signal. The use of carrageenan instead of Surgilube resulted in a 24-fold (95% CI = 9.5 to 60.8) decrease in detectable infectious events (carrageenan gel vs Surgilube, mean = 84 infectious events per section, difference = 81 events per section, 95% confidence interval = 33 to 213 events per section) (Table 1).
Table 1.
Individual observer and combined mean number of infectious events (95% confidence intervals) per biopsy specimen*,‡
| Observer | Cytology + Surgilube† | PsV + Surgilube | Cytology + PsV + Surgilube | Cytology + PsV + iota-carrageenan |
| Observer 1 | 0 | 0.17 (0.02 to 0.95) | 112 (44 to 285) | 5.4 (2.1 to 14.6) |
| Observer 2 | 0 | 0.03 (0.004 to 0.19) | 67 (29 to 155) | 2.9 (1.2 to 7.3) |
| Combined | 0 | 0.05 (0.01 to 0.18) | 84 (45 to 158) | 3.5 (1.8 to 6.9) |
PsV = pseudovirus.
One monkey served as the negative control (no PsV). Each of the other groups contained four monkeys per group. Six specimens were taken from each cervix.
A 95% confidence interval was not calculated for the negative control because zero events were observed.
Infection in the Ectocervix vs the Endocervix
In addition to comparing the total number of infectious events in the animals with and without cytology specimen collection, the number in the ecto- and endocervix was evaluated separately by observer 1 (observer 2 did not discriminate between these regions during data acquisition) (Table 2). Although PsV was inoculated directly into the endocervix, no infectious events were observed in the endocervix in the absence of the cytology collection procedure and only two infectious events were detected in the ectocervix (n = 4 in each group) (Table 2). In both the endo- and ectocervix, the number of infectious events was clearly higher in the sampled compared with non-sampled monkeys (a mean of 106 and 12.1 infectious events in the ectocervix and endocervix of sampled monkeys, respectively, vs 0.003 and 0.0 infectious events in the ectocervix and endocervix of non-sampled monkeys, respectively) (Table 2). Administration of carrageen was associated with a 56-fold reduction in the number of infectious events at the ectocervix (carrageen vs Surgilube, mean = 1.9 infectious events vs mean = 106 infectious events, difference = 104 infectious events, 95% CI =7.6 to 413.8 infectious events). Administration of carrageen was associated with a threefold reduction the number of infectious events at the endocervix (cytology with carrageen vs carrageen only, mean = 3.6 infectious events vs mean = 12.1 infectious events, difference = 9 infectious events 95% CI = 0.7 to 15.3 infectious events) (Table 2).
Table 2.
Mean number of infectious events (95% confidence intervals) observed in the endocervix and the ectocervix of monkeys after exposure to human papillomavirus-16 pseudovirus (PsV) with and without cytology and according to lubricant used*
| Site | PsV + Surgilube | Cytology + PsV + Surgilube | Cytology + PsV + iota-Carrageenan |
| Ectocervix | 0.003 (0.001 to 0.011) | 106 (41 to 271) | 1.9 (0.32 to 10.9) |
| Endocervix | 0 (0 to 0.008) | 12.1 (4.0 to 37) | 3.6 (1.3 to 10.2) |
The number of infectious events per specimen was determined by observer 1. Each group contained four monkeys.
Discussion
In our study, we confirmed the histological similarities of the human and macaque cervix and established that the transformation zone was not preferentially susceptible to infection in the absence of trauma. Infectious events did occur in the endocervical epithelium, but they were exceedingly rare and occurred after exposure to a very large HPV16 PsV inoculum. The trauma associated with a Pap smear collection substantially enhanced experimental infection. This potentiation was largely reversed by the simple intervention of replacing the lubricant commonly used for the internal digital examination with an iota-carrageenan gel.
Data from human studies are conflicting as to whether trauma may occur in the area of the transformation zone as a result of consensual intercourse. Recent consensual intercourse (<24 hours) was strongly associated with minor trauma of the genital mucosa, including the cervix, albeit on the forniceal surface thereof (12). However, magnetic resonance imaging of the anatomy of the coital act shows that the cervical os may not contact the male genitalia (13). Therefore, infection may occur more frequently in the vaginal epithelium, particularly in the lower portions which are more prone to intercourse-associated microtrauma, and the resulting foci continually shed infectious virus into the vaginal lumen. The transformation zone would then be regularly exposed so that even rare events or narrow windows of susceptibility could be expected to lead to infection. Consistent with this model, ascending infections from the lower genital tract to the cervix were frequently observed in a natural history study of HPV infection in women in which various portions of the genital tract were independently and frequently sampled (14). It is also possible that foci of inflammation may sufficiently make the epithelium permeable to cervical infection, although further studies are needed to test this hypothesis.
We recognized the importance of diligently mimicking the cytology collection procedure routinely done in gynecology clinics to test the hypothesis that a cytology collection procedure can induce susceptibility to HPV16 infection of the cervix. To a large degree, this replication was accomplished, although there are some limitations to our model. In particular, the relatively small size of the rhesus macaque vagina and cervix made the cytology collection procedure technically difficult in some monkeys. The rhesus macaque endocervix is also somewhat different from human, both ultrastructurally (S-shaped vs straight, respectively) and histologically (the rhesus macaque endocervical epithelium is more papillary and frond-like, probably resulting in a higher surface area in the endocervical canal) (7). In addition, several different devices are routinely used to sample the cervix for cytology collection in gynecology clinics and the extent to which these results would be replicated with devices other than the cytobrush and plastic spatula used in this study are unknown. However, any device that succeeds in the intended outcome of dislodging basal keratinocytes would be expected to have a similar potentiating effect compared to that of a cytobrush and spatula.
If Pap smears similarly potentiate HPV infection in women, then the cervical HPV infection rates measured in many natural history studies may need to be reconsidered because most studies involve a Pap smear at entry and multiple repeats at relatively closely spaced intervals thereafter. Our results indicate that it might be informative to compare rates of HPV16 acquisition (and clearance) using nondisruptive sampling devises, such as Dacron swaps, to determine if infection rates differ from the ones determined in association with frequent collection of cytology specimens.
Carrageenans are candidate HPV microbicides previously shown to be highly effective in vitro, with IC50s in the nanogram per milliliter range (9). In addition, iota-carrageenan eliminated detectable infection in the mouse challenge model, even when instilled in the vagina 6 hours before PsV inoculation (6). In this study, carrageenan, which is the primary gelling agent in several over-the-counter sexual lubricants (9), was not associated with complete elimination of susceptibility to HPV16 infection induced by the cytology collection. To some degree, this result was expected, given that the carrageenan gel was simply applied to the examiners gloved digit for lubrication with no conscious effort made to instill the material into the vagina or onto the cervical os. An intervention against cytology collection procedure–induced HPV16 susceptibility might be more effective if a carrageenan gel were administered directly into the cervical os immediately following the procedure. Further studies are needed to determine the validity of this hypothesis.
The implications of these findings for cervical cancer prevention are debatable. Certainly, the tremendous success of cervical cytology screening in the prevention of cervical cancer must be acknowledged. It would be a mistake to interpret these results as an implication that cervical cytology screening programs should be curtailed or abandoned. Indeed, even if the potentiation of HPV infection occurs in humans, the disease outcome is not apparent a priori. Increased HPV infection of the transformation zone could lead to more dysplastic lesions. However, the physical disruption of repeated cytology collection procedures could also stimulate the immune system to recognize HPV infection that may have previously escaped immune surveillance. Thus, potentiation of infection might be counterbalanced by an enhancement of immune cell-mediated viral clearance and may explain previous reports that found an inverse association of dysplastic lesions with the number of Pap smears in patients (15).
The increase in cervical adenocarcinoma rates in screened Western populations, in which squamous cell carcinomas have fallen dramatically, remains unexplained (16–19). Although the rate of detection of adenocarcinoma in situ has increased in the United States, the risk of invasive cervical adenocarcinomas and related mortality has increased in white women younger than 50 years (16,19). Our findings raise the possibility that the increased use and frequency of cytobrush collection procedures to specifically sample endocervical cells may contribute to this increase. Cytobrush cell collection might render cells within the endocervical canal that are not normally traumatized susceptible to HPV infection. Women screened at a young age have high rates of prevalent HPV16 and HPV18 infection (20–22), and so many are therefore likely to have productive lesions shedding infectious virus into the vaginal lumen. Younger women also tend to have more pronounced ectopy (reversion of endocervical columnar epithelium out onto the face of the cervix), which exposes a greater surface area of fragile single layer epithelium to physical disruption, from the spatula or other device meant to sample the ectocervix and transformation zone. As women age, ectropion recedes (23,24), so that lesions that were once near the cervical os may migrate higher into the cervical canal and become less detectable in cervical cytology examinations.
Several other factors likely contribute to the observed increasing rates of cervical adenocarcinoma, including improvements in histological classification and increased prevalence of oncogenic HPV infections (25,26). Also, removing a competing cause of mortality generally increases the observed rates of the diseases still present (27). Therefore, because current screening modalities preferentially detect and remove squamous lesions and not adenomas, observed rates of adenocarcinoma might increase without a change in the distribution of known or unknown risk factors.
In summary, the speculative nature of the possible association of cytology screening with adenocarcinoma must be stressed. The issue may be largely rendered moot for future generations of women by the fact that the vast majority of adenocarcinomas are caused by HPV16 and HPV18, and HPV vaccine uptake is likely to be highest among girls who will eventually be screened most regularly. However, adenocarcinoma is causing deaths in more women and at younger ages now, and adenocarcinoma in situ, for which the treatment is almost invariably hysterectomy, is destroying the reproductive capability of these patients. The field might benefit from an epidemiological examination of a potential temporal or geographical association between the frequency and aggressiveness of cervical cytology cell collection and the incidence of cervical adenocarcinoma and adenocarcinoma in situ. Our results also indicate that it may be important to determine if the high sensitivity of HPV DNA-based tests for cervical precancerous lesions is maintained in the absence of endocervical sampling.
Funding
This work was supported by the intramural program of the National Cancer Institute, National Institutes of Health.
Supplementary Material
Footnotes
The sponsor had no role in the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit it for publication.
References
- 1.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.Levi F, Lucchini F, Negri E, Franceschi S, la Vecchia C. Cervical cancer mortality in young women in Europe: patterns and trends. Eur J Cancer. 2000;36(17):2266–2271. doi: 10.1016/s0959-8049(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 5.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106(48):20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 7.Hafez ESE, Jaszcak S. Comparative anatomy and histology of the cervix uteri in non-human primates. Primates. 1972;13(3):297–316. [Google Scholar]
- 8.Wood CE, Chen Z, Cline JM, Miller BE, Burk RD. Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J Virol. 2007;81(12):6339–6345. doi: 10.1128/JVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2(7):e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastrana DV, Buck CB, Pang YY, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 11.McCullagh P, Nelder J. Generalized Linear Models. London: Chapman & Hall; 1989. [Google Scholar]
- 12.Fraser IS, Lahteenmaki P, Elomaa K, et al. Variations in vaginal epithelial surface appearance determined by colposcopic inspection in healthy, sexually active women. Hum Reprod. 1999;14(8):1974–1978. doi: 10.1093/humrep/14.8.1974. [DOI] [PubMed] [Google Scholar]
- 13.Faix A, Lapray JF, Callede O, Maubon A, Lanfrey K. Magnetic resonance imaging (MRI) of sexual intercourse: second experience in missionary position and initial experience in posterior position. J Sex Marital Ther. 2002;28(suppl. 1):63–76. doi: 10.1080/00926230252851203. [DOI] [PubMed] [Google Scholar]
- 14.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro S, Hoffman M, Constant D, et al. Papanicolaou smears induce partial immunity against sexually transmitted viral infections. Epidemiology. 2007;18(6):709–715. doi: 10.1097/EDE.0b013e31815774fc. [DOI] [PubMed] [Google Scholar]
- 16.Sherman ME, Wang SS, Carreon J, Devesa SS. Mortality trends for cervical squamous and adenocarcinoma in the United States. Relation to incidence and survival. Cancer. 2005;103(6):1258–1264. doi: 10.1002/cncr.20877. [DOI] [PubMed] [Google Scholar]
- 17.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States–a 24-year population-based study. Gynecol Oncol. 2000;78(2):97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 18.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75(4):536–545. doi: 10.1002/(sici)1097-0215(19980209)75:4<536::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100(5):1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 20.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 21.Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10):696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 22.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson DL, Peralta L, Graham NM, Zenilman J. Histologic development of cervical ectopy: relationship to reproductive hormones. Sex Transm Dis. 2000;27(5):252–258. doi: 10.1097/00007435-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Singer A. The uterine cervix from adolescence to the menopause. Br J Obstet Gynaecol. 1975;82(2):81–99. doi: 10.1111/j.1471-0528.1975.tb02204.x. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4 suppl):S20–S26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Laukkanen P, Koskela P, Pukkala E, et al. Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J Gen Virol. 2003;84(pt 8):2105–2109. doi: 10.1099/vir.0.18995-0. [DOI] [PubMed] [Google Scholar]
- 27.Kalbfleish JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. Hoboken, NJ: Wiley; 1998. Chap 8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.