Abstract
Ex vivo production of highly stimulator mature dendritic cells (DCs) for cellular therapy has been used to treat different pathological conditions with the aim of inducing a specific immune response. In the last decade, several protocols have been developed to mature monocyte-derived DCs: each one has led to the generation of DCs showing different phenotypes and stimulatory abilities, but it is not yet known which one is the best for inducing effective immune responses. We grouped several different maturation protocols according to the downstream pathways they activated and reviewed the shared features at a transcriptomic level to reveal the potential of DCs matured by each protocol to develop Th-polarized immune responses.
Keywords: Dendritic cells, Gene profiling, Maturation, Th polarization
Immunotherapy and dendritic cells
Dendritic cells (DCs) are considered essential for the initiation, programming, and regulation of antigen-specific immune responses [1]. They play a fundamental role in supporting the survival and the effector function of primed T cells while coordinating communication among cells of the immune system. The observation that patients with cancer have DCs that exhibit a reduced ability to activate Th1-polarized immune responses [2, 3] makes the understanding of DC maturation a prerequisite for the design of rational immunotherapies based on antigen-specific reactivation of adaptive immune responses. Since DCs were first generated ex vivo from monocytes for immunotherapy [4] and used in clinical trials [5, 6], they have been found to be effective in treating patients with lymphoma, melanoma, and renal cell carcinoma [7, 8], although their potential utilization is wider and goes beyond cancer therapy. However, the clinical response rates of DC therapies are only 10–15% [7, 9]. Various reasons for the limited efficacy of monocyte-derived DC-based vaccines have been hypothesized, with inefficient activation of Th1-polarized responses due to incomplete DC maturation being the most cited [7, 10, 11].
T-cell activation by DCs relies on three different signals [7, 8]: antigen presentation, co-stimulatory, and polarizing. The first two signals are intrinsic to mature DCs. During maturation, in fact, the antigen processing ability of DCs decreases while their antigen presentation and expression of co-stimulatory molecules increase. For example, the expression of the B7 family of co-stimulator molecules is increased in mature DCs [12]. Optimizing the expression of polarizing molecules is complicated: DCs can secrete different cytokines, growth factors, and chemokines that can, in turn, attract different immune cells and differentially influence their activation in situ [13–15]. Moreover, the methods that are currently used to produce DCs for adoptive immune therapy may not be optimized: immune responses against tumors, pathogens, and autoimmunities need different Th-specific polarization [16]. For these reasons, Th-specific immune responses should be obtained by stimulating DCs to produce cells with a specific Th polarizing ability; however, DC-induced Th polarization is still not well understood or characterized [16].
Over the last decade, a large body of information has accumulated concerning the functional properties of DCs generated ex vivo under different conditions [8]. However, the molecular determinants that regulate effective immune responses are still poorly defined [7]. Moreover, the heterogeneity of the clinical trials done so far makes it impossible to define the most efficient protocol according to clinical results [9]. DNA microarray technology has often been used to study the maturation of DCs, but only a small number of studies have systematically compared different conditions of maturation [14, 15]. This review summarizes the salient results from these studies following a pathway-associated principle and clustered the different maturation strategies according to conceptually convergent theorems with the aim of suggesting future studies and directions to improve the effectiveness of DC-based immunotherapy.
We first selected publications on PubMed using the following sequence of terms: “Monocyte” “Dendritic Cells” “Human” “Gene Profiling”. Then, we selected only those published between September 2000 and June 2009 that focused on DCs production for immunotherapy and that included a protocol for generating mature DCs. The compounds used for the maturation of DCs were clustered according to the overlapping downstream signaling pathways that they activated. By using this method, a less complex and sharper description of the maturation of DCs resulted. All the pathways used for this analysis were canonical pathways described by Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com, Mountain View, CA). To restrict the analyses to more reliable and consistent data, genes were selected as induced only when cited by at least two papers. The only exception was when only one paper described the transcription profiling of a specific maturation protocol [17]. Considering that the experiments used different microarray platforms and designs, we did not include any fold-change or p-value criteria for the selection of the genes, but trusted the methods used by the authors to define a gene as induced.
Different protocols for DC maturation
Eighteen reports fit the criteria for this study (Table 1). All reports were based on studies using monocyte-derived DCs. Generally, peripheral blood mononuclear cells (PBMCs) were collected by apheresis, and monocytes were obtained by elutriation, CD14 antibody selection, or selection of adherent cells after overnight culture on plates. Protocols to generate immature DCs from circulating monocytes most frequently used granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), although the concentrations of these factors and time in culture varied among studies. One study used interferon-α (IFN-α) instead of IL-4 during the differentiation step [18]. Then, because of the low T-cell activation potential of the resulting immature DCs, cells were matured with immune response activation stimuli. Several strategies have been used to produce mature DCs, which are characterized by high immune cell activation potential. Factors used to mature immature DCs included lipopolysaccharide (LPS), CD40 ligand (CD40L), tumor necrosis factor-α (TNF-α), IFN-α, and IFN-γ. Cocktails combining several factors to better recreate the inflammation environment have also been used. Factors used in maturation cocktails include prostaglandin E2 (PGE2), interleukin-1β (IL-1β), IL-6, and polyinosinic: polycytidylic acid (poly (I:C)).
Table 1.
Selected publications on DC maturation
| Protocol | Time for maturation | Methods | Reference |
|---|---|---|---|
| LPS (100 μg/ml) | 2 days | SAGEa | [83] |
| LPS (100 ng/ml) | 2 days | 2d electrophoresis + MSb | [22] |
| LPS (10 ng/ml) | 1 day | DD-PCRc | [23] |
| LPS (100 ng/ml) | n/a | microarray (Affymetrix) | [14] |
| LPS (1 μg/ml) | 16 h | microarray (in house) | [20] |
| LPS (100 ng/ml) | 2 days | microarray (Gearray) | [84] |
| CD40L (undefined) | 2 days | microarray (Affymetrix) | [46] |
| CD40L (undefined) | n/a | microarray (Affymetrix) | [14] |
| CD40L (1 μg/ml) | 1 day | subtractive hybridization | [47] |
| TNF-α (50 ng/ml) | 2 days | microarray (Clontech) | [48] |
| TNF-α (10 ng/ml) | 7 days | microarray (HuGeneFL) arrays (Affymetrix) | [49] |
| TNF-α (10 ng/ml) | 1 days | microarray (Affymetrix) | [85] |
| TNF-α (1,000 U/ml) | 2 days | microarray (Affymetrix) | [18] |
| TNF-α (5 ng/ml) | 1 day | DD-PCR | [23] |
| IFNα (1,000 U/ml) | 8 h | microarray (in house) | [86] |
| IFN-γ (1,000 U/ml) | 2 days | microarray (Clontech) | [87] |
| IL-1β (1,870 U/ml), | 3 days | microarray (Genome Systems) | [88] |
| IL-6 (1,000 U/ml), | |||
| TNF-α (1,100 U/ml), | |||
| PGE2 (1 μg/ml) | |||
| IL-1β (10 ng/ml), | 2 days | microarray (Affymetrix) | [15] |
| IL-6 (1,000 IU/ml), | |||
| TNFα (10 ng/ml), | |||
| PGE2 (1 μm/ml) | |||
| IL-1β (25 ng/ml), | 2 days | microarray (Affymetrix) | [15] |
| IFN-α (3,000 IU/ml), | |||
| IFN-γ (1,000 IU/ml), | |||
| TNF-α (50 ng/ml), | |||
| poly (I:C) (20 ng/ml) | |||
| IL-1β (10 ng/ml), | n/a | microarray (Affymetrix) | [14] |
| IL-6 (1,000 U/ml), | |||
| TNF-α (10 ng/ml), | |||
| PGE2 (1 μm/ml) | |||
| LPS (100 mg/ml), | 1 day | microarray (in house) | [17] |
| IFN-γ (1,000 IU/ml) |
aSerial analysis of gene expression
bMass spectrometry
cDifferential display-PCR
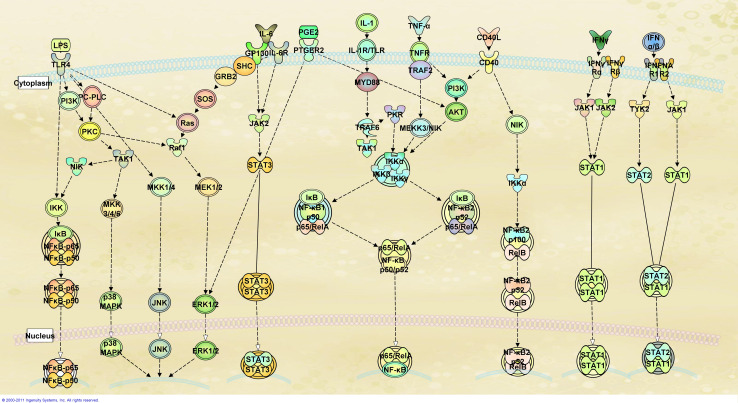
Based on the level of overlap of canonical pathways believed to be primarily activated by each compound (Fig. 1), we clustered various maturation protocols into three major categories: LPS, CD40L/TNF-α, and IFN-dependent maturation strategies. In addition, a fourth category was needed for protocols that included cocktails of factors that represented a combination of the other categories. For instance, LPS interacts with toll-like receptors (TLR)-4 leading to the activation of different transcription factors including nuclear factor-kB (NF-kB), p38 mitogen-activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK), and extra-cellular signal-regulated protein kinase (ERK1/2). On the other hand, although both CD40L and TNF-α activate NF-kB, this activation occurs through different and only partially overlapping signaling cascades. In contrast, IFN-α and IFN-γ signal through the Janus kinase (JNK) and tyrosine kinase (TYK) cascade leading to the activation of signal transducer and activator of transcription proteins (STATs), which leads to down-stream effects where the action of NF-kB is also complemented by the action of several interferon regulatory factors (IRFs). We summarized the effects of the different maturation protocols on the transcriptional profiles of DCs according to these broad categories. It should be kept in mind that this report is obviously limited by the protocols used for DC activation, which generally refer to a specific time point after the maturation process is started.
Fig. 1.
Pathways activated during the maturation of DCs. DCs can be matured using different compounds as stimuli. LPS interaction with toll-like receptors leads to a cascade of events leading to the activation of NF-κB, p38-MAPK, ERK1/2, and JNK. Although IL-1, CD40L, and TNF-α bind different receptors, their downstream signals include activation of the complex NF-κB RelA/B. In contrast, IFNs stimulate a different pathway that includes the activation of JAKs, TYK2, and STAT1/2. Adapted from Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com, Mountain View, CA)
Regarding the different monocyte purification methods, there are no specific studies aimed at identifying the effects of the purification method on the final product at the transcriptomic level. Dohnal showed that the greatest differences due to the purification method were on the purity and yield of the starting monocytes, but functionally no statistical differences were noticed [19]. In addition, the high degree of similarity of the phenotypic characteristics of the DCs derived from different monocyte selection techniques [14, 20–23], as well as the observation that the monocyte-derived DCs do not show differences at a transcriptomic level due to differences in processing [24], suggests that the purification method has only minor effects on the final product. Thus, it is possible that other information could have been gathered if analyses were performed at different time points as was clearly shown by Piqueras [25].
LPS
Because of its bacterial origin and its predominant role as a pathogen-associated pattern (PAP), LPS represents a prototypical model of DC maturation. Gene profiling studies (see Table 1) have shown that several chemokine genes are induced by LPS: CCL5 (RANTES), CCL4 (MIP-1β), CCL20, CCL18 (DC-CK1), CCL19 (MIP-3β), and CCL23 (MPIF-1) [20, 22, 23, 26, 27]. The expression of CCL5, CCL4, CCL20, and CCL18 has also been confirmed by RT–PCR, ELISA, or protein array [28–31]. CCL5 primarily mediates the trafficking and homing of classical lymphoid cells such as T cells and monocytes, but also acts on a variety of other chemokine receptor (CCR)-5-expressing cells, including basophils, eosinophils, natural killer (NK) cells, DCs, and mast cells [32]. Increased CCL5 expression has been associated with a wide range of inflammatory disorders and pathologies. It is thought to act by promoting leukocyte infiltration to sites of inflammation [33]. CCL4 has both chemotactic and pro-inflammatory effects, attracting, in particular, monocytes to the site of inflammation [34]. CCL20 interacts with CCR-6 and recruits immature DCs, their precursors, and NKT cells [35]. Both CCL18 and CCL23 can attract lymphocytes, immature DC, and monocytes. It remains unclear whether CCL18 predominantly favors pro-inflammatory (Th1 or Th2) or anti-inflammatory/tolerogenic responses depending upon the microenvironment in which it is secreted [36]. Another transcript that was often observed to be induced by LPS-matured DCs is the BCL-related protein A1 (BCL2A1), which, like BCL-2, has anti-apoptotic function and whose induction has been confirmed by ribonuclease protection assay [37]. It promotes the survival of immune cells and consequently leads to stronger immune responses [36]. Taken together, these data suggest that after DCs detect pathogens, they produce factors to enhance the recruitment of different immune system cells, and consequently, activate the primary immune response against pathogens in the area of infection.
Other genes whose expression has often been found to be enhanced in DCs matured with LPS are DUSP-5, DUSP-1, and TNFAIP3. The increased expression of these latter two has been confirmed at protein level [38, 39]. These three genes play an important role in inhibiting MAPK activity and in particular the activity of NF-kB, JNK, and ERK2 [40], indicating that the activation signal induced by LPS has a self-regulatory loop that dampens its effects over time. Other genes whose expression is increased are super-oxide dismutase 2 (SOD2) and ICAM1 (CD54) and whose induction has been confirmed at a protein level [41–43]. Both genes have a putative role in inflammation. The adhesive molecule ICAM1 is directly implicated in leukocyte adhesion and transmigration during inflammation [44]. SOD2, instead, is primarily involved in the catabolism of reactive oxygen species usually generated during inflammation [45].
TNF-α/CD40L
According to our selection criteria, TNF-α/CD40L induces the expression of a pattern of genes tightly associated with TNF family signaling: CCL17 (TARC), IL-10R, IL-13R, MYO1A, and GM-CSFR [14, 18, 23, 46–50]. CCL17, IL-10R, and Il-13R have been observed to be induced by TNF-α/CD40L also with other techniques [46, 51]. CCL17 is a chemokine that is able to recruit memory DCs, CD4+ cells, and Th2-T cells. High levels of CCL17 in serum are associated with progression-free survival in advanced melanoma patients in response to dendritic cell-based immunotherapy [52]. Both IL-10R and IL-13R are cytokines receptors involved in Th2 cell differentiation [53]; both receptors can also make DCs more responsive to the Th2-cytokine imbalance that has been observed in several tumor microenvironments [54], and thus in this way weakening the immunogenic properties of DCs. The transcription profile of DCs matured with TNF-α/CD40L suggests that these DCs polarize T cells toward a Th2 response. This observation perfectly correlates with the impaired ability of TNF-α-matured DCs to induce the Th1 immune response described by Decker [54]. In fact, TNF-α-matured DCs show a lack of secretion of IL-12 and IFN-γ and consequentially a reduced ability to activate cytotoxic T-cell responses in vitro [54].
IFNs
Due to the central role played by IFNs in the initiation of innate and adaptive immune responses, the effects on maturation of DCs by type I (IFN-α) and type II (IFN-γ) IFNs have been widely studied [55, 56]. Although the physiological role of IFNs in DC function in vivo is still not clear, it has been reported that in vitro IFN-α induces the differentiation and maturation of DCs and IFN-γ can be used alone or in combination with other compounds to mature DCs, leading to the secretion of large quantities of IL-12 [18, 57–59]. Moreover, type I IFNs enhance the maturation of DCs through a positive feedback loop, acting as autocrine mediators either when used alone or in combination with other factors [58, 60, 61]. Gene profiling studies indicate that the early response (6–8 h following stimulation) of DCs to IFN stimulation includes the expression of well-described IFN-stimulated genes (ISGs), such as the CXCR3 ligand chemokines CXCL-9/MIG, CXCL-10/IP-10, CXCL-11/I-TAC, MXa, and ISG-15 [62]. Furthermore, both type I and type II IFNs stimulate, though with different intensities, the STAT-1/IRF-1/IL-15 axis which has recently been shown to play a key role in immune-mediated tissue rejection [63, 64]. In general, it has been concluded that IFN-based maturation of DCs is strongly biased toward a Th1 immune response. This observation is also suggested by a study of Longhi et al., where the induction of Th1 immune response has been shown to require type I IFNs [65].
Since IFN-dependent signaling is time and cell type dependent [66], it is important to keep in mind that different methods and/or stimulation of different cell types will yield different results. For instance, type I IFNs induce phosphorylation of STAT1 or STAT4 in immature DCs and mature DCs, respectively [67]. These differences are, however, likely to be quantitative in most cases rather than qualitative and the large majority of ISGs induced by either type of IFNs will likely separate the transcriptional patterns of DCs induced by IFNs from those of all other methods of maturation. In fact, IFN-α induces a pattern of expression that is similar to the majority of the ISGs in both immature and mature DCs [67]. Obviously, since stimulation of several TLRs (i.e. TLR-7, -8 and -9) is tightly linked to the secretion of IFNs and activation of IRFs, it is likely that maturation protocols involving nucleic acid containing PAPs of damage-associated patterns (DAPs) may lead to the activation of similar pathways and produce similar Th1 polarization.
Maturation cocktails
In an attempt to recreate a physiological environment for DC maturation, some investigators advocate the utilization of balanced cocktails of maturation agents that may be the most representative of various inflammatory states [68]. At a cellular level, a cocktail of agents leads to the activation of several downstream pathways, and the consequent expression profile could differ from the simple sum of profiles of the genes induced by each single agent because of synergistic or antagonistic effects. The first and most frequently used cocktail contains TNF-α, IL-1β, IL-6, and PGE2. The rationale for the use of this cocktail is to enhance the pro-inflammatory effects of TNF-α by adding other pre-inflammatory cytokines in an attempt to mimic the inflammatory environment that physiologically leads to the maturation of DCs. It’s interesting to note that, although LPS can by itself induce the production and secretion of these same cytokines, gene expression profiles of DCs matured with LPS differ markedly from that of DCs matured with the cocktail. Like TNF-α alone, this cocktail leads to the induction of CCL17, CXCR4, and IL13R, but not IL10R and GM-CSFR [15]. Interestingly the expression of IL-6 is enhanced. IL-6 is a potent, pleiotropic, inflammatory cytokine that mediates a plethora of physiological functions, including the developmental differentiation of lymphocytes, cell proliferation, and cell survival [69]. Since IL-6 is biologically active only on cells that express constitutively IL-6R (B cells and hepatocytes), it is likely that it acts through transignaling in which IL-6 dimerizes with the soluble IL-6R shed by activated neutrophils and/or monocytes. However, this needs to be further explored in the context of DC activation. Other genes found to be increased by this cocktail are NF-kB1/2, CXCL-2/GRO-β, TNFAIP3/6, CXCL-8/IL-8, CXCL-16, CD58, IL-2R, IL-7R, CRIP1, ISG20, BCL2A1, IER3, CFLAR, NCK2, and RUNX3 [15]. The functions of these genes are associated with inflammatory response and apoptosis. In vitro assays have clearly shown that DCs matured with this cocktail strongly stimulate the proliferation of allogeneic lymphocytes [70, 71].
Although PGE2 is believed to play an important role in DC migration and lymph node homing, it is also considered responsible for some immune inhibitory properties of DCs [13]. In particular, the induction of indoleamine-2,3-deoxygenase (IDO), the increased secretion of IL-10 and the diminished secretion of IL-12 suggest that PGE2 may induce a tolerogenic immune response [15, 68, 72]. Additionally, several clinical studies have shown that plasma from patients with metastatic melanoma or renal cell carcinoma treated with DCs matured with this cocktail contain a mixture of Th1/Th2 cytokines, without resulting in a shifting toward a Th1-cytokines profile [73–75]. For these reasons, several groups use cocktails of cytokine that do not contain PGE2.
One of these alternative cocktails contains IL-1β, TNF-α, IFN-α, IFN-γ, and poly (I:C). This maturation protocol seems to bias the maturation of DCs toward a Th1 phenotype since IL-12p40, IL-1α/β, IL-2Rα/β/γ, IL-6, CXCL-8/IL-8, IL-15, CXCL-9/MIG, CXCL-10/IP10, and CXCL-11 are expressed at much higher levels compared with the PGE2-containing protocols [15]. Only the protocol containing poly (I:C) upregulates CCL4/MIP-1β, CCL5/RANTES, CCL20/MIP-3α, and CXCL-2/GROβ, but CCL17/TARC and CXCL16 are not induced by the poly (I:C) containing protocol [15]. However, this cocktail of cytokines also induces the expression of functionally active IDO [15], and it has been shown that the presence of poly(I:C) could affect the antigen expression ability of DCs [76]. In vitro stimulation of autologous T cells by poly (I:C) matured DCs, in fact, failed to show a clear improvement respect to the stimulation of PGE2-matured DCs [15].
Another of the PGE2-free maturation protocols contains LPS and IFN-γ [17]. Gene expression analysis of DCs matured under these conditions clearly demonstrates the expression of several chemokines, proinflammatory cytokines, and inflammatory-related genes. The expression of CCL2, CCL3, CCL4, CCL5, CCL8, CXCL-9, CXCL-10, CXCL-11, IL-1, IL-6, IL-8, IL-10, IL-12, TNF, DUSP5, and SOD2 were increased after 24 h, and these factors were found in the supernatants of DCs cultured with LPS and IFN-α, indicating a strong Th1 polarization potential [17]. In vitro assays have shown that DCs matured with LPS and IFN-γ induce the stimulation of antigen-specific CD4+ and CD8+ T cells much more than PGE2-matured DCs [77]. These data suggest that a cocktail of cytokines induces factors that predominantly could be anticipated from the effects of the individual cytokines with dominance by IFN-dependent activation when either type I or type II IFNs or TLR agonists are added to the mixture, resulting in the induction of Th1 cells and effector T-cell expansion [15].
Conclusion: understanding and developing Th-polarized immune responses
Although gene expression profiling does not provide conclusive information about cell function, it does indicate predominant signaling pathways induced by a defined state of stimuli. This review, which addressed the transcriptional activation of DCs upon various maturation stimuli, indicates that maturation protocols can be categorized according to target pathways and presumed effects on cell function (Table 2). Furthermore, because of the lack of comprehensive comparison studies on different DCs maturation protocols, this review addresses important concepts concerning the differences of maturation protocols. In fact, by looking at the downstream pathways, we have categorized the different protocols and have shown that some lead to similar expression patterns. Then, focusing on the most consistent data, we delineated the potential of DCs matured according to each protocol, showing the in vitro assay results that support the phenotype described by gene profiling. However, the ultimate comparison of protocols involves cellular assays and/or biomarkers associated with clinical outcomes. We, however, can speculate that gene expression profiling could identify biomarkers useful for quality control of DCs during manufacture and for potency evaluation of cellular products.
Table 2.
Genes consistently induced by each maturation agent and their immune-related functions
| Maturation compound | Induced genes | Effect |
|---|---|---|
| LPS | Chemokines: CCL5, CCL4, CCL20, CCL18, CCL19, CCL23 | Recruitment of a variety of immune cells; activation of a primary immune response against a pathogen |
| Inflammatory-related genes: ICAM1, SOD2 | ||
| MAPK inhibitors: DUSP1, DUSP5, TNFAIP3 | ||
| TNF-α/CD40L | Chemokines: CCL17 | Activation of the Th2-polarized immune response |
| Surface proteins: IL-10R, IL-13R, GM-CSFR | ||
| IFNs | IFN stimulated genes: CXCL-9, CXCL-10, CXCL-11, MXa, ISG-15 | Activation of the Th1-polarized immune response |
| Immune-mediated tissue rejection genes: STAT-1, IRF-1, IL-15 | ||
| IL-1β, IL6, TNF-α, PGE2 | Chemokines: CCL17, CXCL2, IL-8, CXCL16 | Activation of immune response with a mixture of Th1 and Th2 |
| Surface proteins: CXCR4, IL-13R, IL-2R, IL-7R, CD58 | ||
| IL-1b, IFN-α, IFN-γ, TNF-α, poly(I:C) | Cytokines: IL-12p40, IL-1, IL-6, IL-8, IL-15, | Activation of the Th1-polarized immune response |
| Chemokines: CXCL-9, CXCL-10, CXCL-11 CCL4, CCL5, CCL20 and CXCL-2 | ||
| LPS, IFN-γ | Cytokines: IL-1, IL-6, IL-8, IL-10, IL-12, TNF | Activation of the Th1-polarized immune response; recruitment of a variety of immune cells |
| Chemokines: CCL2, CCL3, CCL4, CCL5, CCL8, CXCL-9, CXCL-10, CXCL-11 | ||
| Inflammatory-related genes: DUSP5 and SOD2 |
Although antigen-specific immunotherapy holds promise as an approach to cancer therapy, from a practical standpoint, limited, if any, clinical benefits have been achieved for several possible reasons [9, 78]. None the less, it is likely that the function of DCs as antigen-presenting cells may play a central role, at least in the afferent phase, in the induction of adaptive immune responses. DCs, in particular, have attracted much attention because of their primary role in presenting antigens to lymphocytes and simultaneously contributing to the recruitment and activation of various immune cells through cell-to-cell contact or through secretion of soluble factors.
Fifteen years after the first studies of DC generation from monocytes, several different protocols have been described to differentiate DCs from circulating mononuclear cells and to mature them into efficient antigen-presenting units [8], but only a few have been widely tested in clinical trials [9]. Consequently, it still remains questionable which DC maturation protocol provides the best cellular products for in vitro activation of adaptive immune responses or the adoptive transfer of antigen-loaded DCs.
The first maturation protocols used clinically were based on a single compound and lead to a phenotypical complete maturation of DCs but not to a functional DC. For a long time, the cocktail containing TNF-α, PGE2, IL-6, and IL-1β was considered the gold standard maturation protocol because it increased the ability of DCs to home in lymph nodes and recruit T cells. However, recent studies have shown that DCs matured with this protocol enhance the recruitment and activation of CCR4-expressing T regulatory cells by secreting CCL22/MDC [13, 79] and display a reduced ability to secrete active IL-12, which is thought to be fundamental for the induction of a Th1 immune response. Thus, different groups are testing in clinical trials maturation protocols that contain IFNs since it appears that these cytokines are more directly linked and are likely essential for the induction of an effector immune response [63]. Indeed, gene expression profiling [15] as well as other experimental evidence suggests that the maturation of DCs in the presence of IFNs seems to lead to a more powerful Th1-polarized immune response [7].
Our analysis came to these same conclusions, but only by considering the most consistent genes induced by each maturation compound we did reveal several differences in DCs matured by different protocols. According to our analysis, TNF-α and CD40L-based maturation protocols shift the polarization of DCs toward a Th2 response; whereas IFNs-based protocols seem to be more able in activating Th1 cells. Moreover, our analysis suggested that the use of a cocktail of cytokines could lead to a more complete activation of DCs, better mimicking the one that occurs naturally in vivo where a combination of danger signals mature DCs. Among the cocktails of cytokines used, the potential of LPS in attracting different immune cells could be the best utilized when combined with the Th1 activating power of IFN-γ.
Further studies are necessary to define the best way to differentiate, mature, and use DCs in order to obtain an effective Th1 immunogenic response or a Th2 tolerogenic response. Moreover, although some antigen processing and presentation-related genes have been observed to be induced by maturation protocols [12], it is still not clear how specific maturation protocols affect antigen presentation. Antigen cross-presentation has been shown to be affected by maturation stimulus [80], but a comprehensive comparison of different protocols is lacking.
DC activation of T cells can be used as a marker of the co-presence of an activating environment and antigen presentation. In recent years, several lines of evidence have indicated that during in vivo maturation DCs secrete a well-defined cascade of cytokines [25]. These waves of cytokine production synergistically attract different immune cells in a time-dependent fashion triggering a logical and systematic activation of the immune response which evolves from the early induction of innate effector mechanisms to the subsequent expansion of effector adaptive immune responses and, in the end, the induction of regulatory mechanisms aimed at limiting and eventually concluding the host’s reaction to the pathogenic stimulus. While this physiological cascade is probably determined in vivo by a distinct group of maturing factors during the various phases of the immune response, the artificial over-exposure to higher than physiological doses of the same factors in the context of cellular therapy may effectively bias the natural behavior of DCs toward one or the other outcomes of the immune response.
The lack of comprehensive comparisons among protocols and the time-dependent effect of IFNs on DCs discussed before [25] shed light on the importance of defining and comparing protocols currently used for DC maturation in the clinical arena. It is likely that DCs are not only affected by the compounds/cocktail used, but also by the timing of their application in relation to the utilization of the DCs. In fact, several studies described a loss of DCs’ activating power after they gained full maturation that was associated with reduced IL-12 secretion [11], absence of DCs response to further stimulation [81, 82], and impaired ability in inducing T cells activation [11]. Perhaps, by using the optimal timing and maturation protocol combination, it will be possible to obtain mature DCs that mimic physiological maturation and activate an effective immune response.
Acknowledgments
This work is supported by the Intramural Programs of the National Institutes of Health Clinical Center and National Cancer Institute.
References
- 1.Steinman RM, Banchereau J. Taking Dendritic Cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Carbone DP. Tumor-host immune interactions and Dendritic Cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 3.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived Dendritic Cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 5.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed Dendritic Cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed Dendritic Cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 7.Kalinski P, Urban J, Narang R, Berk E, Wieckowski E, Muthuswamy R. Dendritic cell-based therapeutic cancer vaccines: what we have and what we need. Future Oncol. 2009;5:379–390. doi: 10.2217/fon.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuyaerts S, Aerts JL, Corthals J, et al. Current approaches in Dendritic Cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1513–1537. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engell-Noerregaard L, Hansen TH, Andersen MH, Straten P, Svane IM. Review of clinical studies on Dendritic Cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14. doi: 10.1007/s00262-008-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized Dendritic Cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008;84:319–325. doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giermasz AS, Urban JA, Nakamura Y, et al. Type-1 polarized Dendritic Cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z, Saltzman A. Understanding human Dendritic Cell biology through gene profiling. Inflamm Res. 2004;53:424–441. doi: 10.1007/s00011-004-1283-z. [DOI] [PubMed] [Google Scholar]
- 13.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature Dendritic Cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messmer D, Messmer B, Chiorazzi N. The global transcriptional maturation program and stimuli-specific gene expression profiles of human myeloid Dendritic Cells. Int Immunol. 2003;15:491–503. doi: 10.1093/intimm/dxg052. [DOI] [PubMed] [Google Scholar]
- 15.Moller I, Michel K, Frech N, et al. Dendritic cell maturation with poly(I:C)-based versus PGE2-based cytokine combinations results in differential functional characteristics relevant to clinical application. J Immunother. 2008;31:506–519. doi: 10.1097/CJI.0b013e318177d9e5. [DOI] [PubMed] [Google Scholar]
- 16.Kalinski P, Moser M. Opinion—consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 17.Jin P, Han TH, Ren J, et al. Molecular signatures of maturing Dendritic Cells: implications for testing the quality of Dendritic Cell therapies. J Transl Med. 2010;8:4. doi: 10.1186/1479-5876-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korthals M, Safaian N, Kronenwett R, et al. Monocyte derived Dendritic Cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007;5:46. doi: 10.1186/1479-5876-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohnal AM, Graffi S, Witt V, et al. Comparative evaluation of techniques for the manufacturing of Dendritic Cell-based cancer vaccines. J Cell Mol Med. 2009;13:125–135. doi: 10.1111/j.1582-4934.2008.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arico E, Wang E, Tornesello ML, et al. Immature monocyte derived Dendritic Cells gene expression profile in response to virus-like particles stimulation. J Transl Med. 2005;3:45. doi: 10.1186/1479-5876-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baltathakis I, Alcantara O, Boldt DH. Expression of different NF-kappaB pathway genes in Dendritic Cells (DCs) or macrophages assessed by gene expression profiling. J Cell Biochem. 2001;83:281–290. doi: 10.1002/jcb.1231. [DOI] [PubMed] [Google Scholar]
- 22.Pereira SR, Faca VM, Gomes GG, et al. Changes in the proteomic profile during differentiation and maturation of human monocyte-derived Dendritic Cells stimulated with granulocyte macrophage colony stimulating factor/interleukin-4 and lipopolysaccharide. Proteomics. 2005;5:1186–1198. doi: 10.1002/pmic.200400988. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga T, Ishida T, Takekawa M, Nishimura S, Adachi M, Imai K. Analysis of gene expression during maturation of immature Dendritic Cells derived from peripheral blood monocytes. Scand J Immunol. 2002;56:593–601. doi: 10.1046/j.1365-3083.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 24.Shin JW, Jin P, Fan Y, et al. Evaluation of gene expression profiles of immature Dendritic Cells prepared from peripheral blood mononuclear cells. Transfusion. 2008;48:647–657. doi: 10.1111/j.1537-2995.2007.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid Dendritic Cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto SI, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. Identification of genes specifically expressed in human activated and mature Dendritic Cells through serial analysis of gene expression. Blood. 2000;96:2206–2214. [PubMed] [Google Scholar]
- 27.Baltathakis I, Alcantara O, Boldt DH. Expression of different NF-kappaB pathway genes in Dendritic Cells (DCs) or macrophages assessed by gene expression profiling. J Cell Biochem. 2001;83:281–290. doi: 10.1002/jcb.1231. [DOI] [PubMed] [Google Scholar]
- 28.Duenas AI, Aceves M, Orduna A, Diaz R, Sanchez CM, Garcia-Rodriguez C. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int Immunol. 2006;18:785–795. doi: 10.1093/intimm/dxl015. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa H, Kurokawa M, Ozaki N, et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vulcano M, Dusi S, Lissandrini D, et al. Toll receptor-mediated regulation of NADPH oxidase in human Dendritic Cells. J Immunol. 2004;173:5749–5756. doi: 10.4049/jimmunol.173.9.5749. [DOI] [PubMed] [Google Scholar]
- 31.Pivarcsi A, Gombert M, Dieu-Nosjean MC, et al. CC chemokine ligand 18, an atopic dermatitis-associated and Dendritic Cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol. 2004;173:5810–5817. doi: 10.4049/jimmunol.173.9.5810. [DOI] [PubMed] [Google Scholar]
- 32.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/S1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Yoneyama H, Wang Y, et al. Mobilization of Dendritic Cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004;96:201–209. doi: 10.1093/jnci/djh024. [DOI] [PubMed] [Google Scholar]
- 34.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/S1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 35.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandal M, Borowski C, Palomero T, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201:603–614. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera PY, Qureshi N, Christ WJ, Stutz P, Vogel SN. Lipopolysaccharide and its analog antagonists display differential serum factor dependencies for induction of cytokine genes in murine macrophages. Infect Immun. 1998;66:2562–2569. doi: 10.1128/iai.66.6.2562-2569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, Yee E, Harlan JM, Wong F, Karsan A. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood. 1998;92:2759–2765. [PubMed] [Google Scholar]
- 40.Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 41.Wehrwein G, Neumeier M, Schaffler A, et al. Lipopolysaccharide regulated protein expression is only partly impaired in monocytes from patients with type I diabetes. Cardiovasc Diabetol. 2006;5:5. doi: 10.1186/1475-2840-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Shikh ME, El Sayed RM, Wu Y, Szakal AK, Tew JG. TLR4 on follicular Dendritic Cells: an activation pathway that promotes accessory activity. J Immunol. 2007;179:4444–4450. doi: 10.4049/jimmunol.179.7.4444. [DOI] [PubMed] [Google Scholar]
- 43.Nikulina M, Habich C, Flohe SB, Scott FW, Kolb H. Wheat gluten causes Dendritic Cell maturation and chemokine secretion. J Immunol. 2004;173:1925–1933. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- 44.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Tureci O, Bian H, Nestle FO, et al. Cascades of transcriptional induction during Dendritic Cell maturation revealed by genome-wide expression analysis. FASEB J. 2003;17:836–847. doi: 10.1096/fj.02-0724com. [DOI] [PubMed] [Google Scholar]
- 47.Bleharski JR, Niazi KR, Sieling PA, Cheng G, Modlin RL. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated Dendritic Cells and directly augments production of inflammatory cytokines. J Immunol. 2001;167:3174–3181. doi: 10.4049/jimmunol.167.6.3174. [DOI] [PubMed] [Google Scholar]
- 48.Lapteva N, Nieda M, Ando Y, et al. Expression of renin-angiotensin system genes in immature and mature Dendritic Cells identified using human cDNA microarray. Biochem Biophys Res Commun. 2001;285:1059–1065. doi: 10.1006/bbrc.2001.5215. [DOI] [PubMed] [Google Scholar]
- 49.Le NF, Hohenkirk L, Grolleau A, et al. Profiling changes in gene expression during differentiation and maturation of monocyte-derived Dendritic Cells using both oligonucleotide microarrays and proteomics. J Biol Chem. 2001;276:17920–17931. doi: 10.1074/jbc.M100156200. [DOI] [PubMed] [Google Scholar]
- 50.Ju XS, Hacker C, Madruga J, et al. Towards determining the differentiation program of antigen-presenting Dendritic Cells by transcriptional profiling. Eur J Cell Biol. 2003;82:75–86. doi: 10.1078/0171-9335-00294. [DOI] [PubMed] [Google Scholar]
- 51.Cattaruzza M, Slodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J Biol Chem. 2003;278:37874–37880. doi: 10.1074/jbc.M301670200. [DOI] [PubMed] [Google Scholar]
- 52.Wang YC, Hu XB, He F, et al. Lipopolysaccharide-induced maturation of Bone Marrow-derived Dendritic Cells is regulated by Notch signaling through the up-regulation of CXCR4. J Biol Chem. 2009;284:15993–16003. doi: 10.1074/jbc.M901144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satyam A, Khandpur S, Sharma VK, Sharma A. Involvement of T(H)1/T(H)2 cytokines in the pathogenesis of autoimmune skin disease-pemphigus vulgaris. Immunol Invest. 2009;38:498–509. doi: 10.1080/08820130902943097. [DOI] [PubMed] [Google Scholar]
- 54.Decker WK, Li S, Xing D, et al. Deficient T(H)-1 responses from TNF-alpha-matured and alpha-CD40-matured Dendritic Cells. J Immunother. 2008;31:157–165. doi: 10.1097/CJI.0b013e31815eb0df. [DOI] [PubMed] [Google Scholar]
- 55.Severa M, Remoli ME, Giacomini E, et al. Differential responsiveness to IFN-alpha and IFN-beta of human mature DC through modulation of IFNAR expression. J Leukoc Biol. 2006;79:1286–1294. doi: 10.1189/jlb.1205742. [DOI] [PubMed] [Google Scholar]
- 56.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic Cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santini SM, Lapenta C, Santodonato L, D’Agostino G, Belardelli F, Ferrantini M. IFN-alpha in the generation of Dendritic Cells for cancer immunotherapy. Handb Exp Pharmacol. 2009;188:295–317. doi: 10.1007/978-3-540-71029-5_14. [DOI] [PubMed] [Google Scholar]
- 58.Pan J, Zhang M, Wang J, et al. Interferon-gamma is an autocrine mediator for Dendritic Cell maturation. Immunol Lett. 2004;94:141–151. doi: 10.1016/j.imlet.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 Dendritic Cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4 + T cell responses in vitro. J Immunother. 2007;30:75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 60.Nagai T, Devergne O, Mueller TF, Perkins DL, van Seventer JM, van Seventer GA. Timing of IFN-beta exposure during human Dendritic Cell maturation and naive Th cell stimulation has contrasting effects on Th1 subset generation: a role for IFN-beta-mediated regulation of IL-12 family cytokines and IL-18 in naive Th cell differentiation. J Immunol. 2003;171:5233–5243. doi: 10.4049/jimmunol.171.10.5233. [DOI] [PubMed] [Google Scholar]
- 61.Severa M, Remoli ME, Giacomini E, et al. Sensitization to TLR7 agonist in IFN-beta-preactivated Dendritic Cells. J Immunol. 2007;178:6208–6216. doi: 10.4049/jimmunol.178.10.6208. [DOI] [PubMed] [Google Scholar]
- 62.Schlaak JF, Hilkens CM, Costa-Pereira AP, et al. Cell-type and donor-specific transcriptional responses to interferon-alpha. Use of customized gene arrays. J Biol Chem. 2002;277:49428–49437. doi: 10.1074/jbc.M205571200. [DOI] [PubMed] [Google Scholar]
- 63.Wang E, Worschech A, Marincola FM. The immunologic constant of rejection. Trends Immunol. 2008;29:256–262. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Worschech A, Chen N, Yu YA, et al. Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy. BMC Genomics. 2009;10:301. doi: 10.1186/1471-2164-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic Cells require a systemic type I interferon response to mature and induce CD4 + Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stroncek DF, Basil C, Nagorsen D, et al. Delayed polarization of mononuclear phagocyte transcriptional program by type I interferon isoforms. J Transl Med. 2005;3:24. doi: 10.1186/1479-5876-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Longman RS, Braun D, Pellegrini S, Rice CM, Darnell RB, Albert ML. Dendritic-cell maturation alters intracellular signaling networks, enabling differential effects of IFN-alpha/beta on antigen cross-presentation. Blood. 2007;109:1113–1122. doi: 10.1182/blood-2006-05-023465. [DOI] [PubMed] [Google Scholar]
- 68.Lee AW, Truong T, Bickham K, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived Dendritic Cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/S0264-410X(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 69.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 70.Spisek R, Bretaudeau L, Barbieux I, Meflah K, Gregoire M. Standardized generation of fully mature p70 IL-12 secreting monocyte-derived Dendritic Cells for clinical use. Cancer Immunol Immunother. 2001;50:417–427. doi: 10.1007/s002620100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colic M, Mojsilovic S, Pavlovic B, et al. Comparison of two different protocols for the induction of maturation of human Dendritic Cells in vitro. Vojnosanit Pregl. 2004;61:471–478. doi: 10.2298/VSP0405471C. [DOI] [PubMed] [Google Scholar]
- 72.Liu W, Kelly KA. Prostaglandin E-2 modulates Dendritic Cell function during chlamydial genital infection. Immunology. 2008;123:290–303. doi: 10.1111/j.1365-2567.2007.02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyte JA, Mu L, Aamdal S, et al. Phase I/II trial of melanoma therapy with Dendritic Cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006;13:905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- 74.Kyte JA, Trachsel S, Risberg B, Straten PT, Lislerud K, Gaudernack G. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Cancer Immunol Immunother. 2009;58:1609–1626. doi: 10.1007/s00262-009-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soleimani A, Berntsen A, Svane IM, Pedersen AE. Immune responses in patients with metastatic renal cell carcinoma treated with Dendritic Cells pulsed with tumor lysate. Scand J Immunol. 2009;70:481–489. doi: 10.1111/j.1365-3083.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 76.Schuurhuis DH, Lesterhuis WJ, Kramer M, et al. Polyinosinic polycytidylic acid prevents efficient antigen expression after mRNA electroporation of clinical grade Dendritic Cells. Cancer Immunol Immunother. 2009;58:1109–1115. doi: 10.1007/s00262-008-0626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Felzmann T, Huttner KG, Breuer SK, et al. Semi-mature IL-12 secreting Dendritic Cells present exogenous antigen to trigger cytolytic immune responses. Cancer Immunol Immunother. 2005;54:769–780. doi: 10.1007/s00262-004-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang E, Monaco A, Monsurro V, et al. Antitumor vaccines, immunotherapy and the immunological constant of rejection. IDrugs. 2009;12:297–301. [PMC free article] [PubMed] [Google Scholar]
- 79.Jongmans W, Tiemessen DM, van Vlodrop IJH, Mulders PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade Dendritic Cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J Immunother. 2005;28:480–487. doi: 10.1097/01.cji.0000171290.78495.66. [DOI] [PubMed] [Google Scholar]
- 80.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 81.Hokey DA, Larregina AT, Erdos G, Watkins SC, Falo LD., Jr Tumor cell loaded type-1 polarized Dendritic Cells induce Th1-mediated tumor immunity. Cancer Res. 2005;65:10059–10067. doi: 10.1158/0008-5472.CAN-05-1692. [DOI] [PubMed] [Google Scholar]
- 82.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.V97.11.3466. [DOI] [PubMed] [Google Scholar]
- 83.Hashimoto SI, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. Identification of genes specifically expressed in human activated and mature Dendritic Cells through serial analysis of gene expression. Blood. 2000;96:2206–2214. [PubMed] [Google Scholar]
- 84.Baltathakis I, Alcantara O, Boldt DH. Expression of different NF-kappaB pathway genes in Dendritic Cells (DCs) or macrophages assessed by gene expression profiling. J Cell Biochem. 2001;83:281–290. doi: 10.1002/jcb.1231. [DOI] [PubMed] [Google Scholar]
- 85.Ju XS, Hacker C, Madruga J, et al. Towards determining the differentiation program of antigen-presenting Dendritic Cells by transcriptional profiling. Eur J Cell Biol. 2003;82:75–86. doi: 10.1078/0171-9335-00294. [DOI] [PubMed] [Google Scholar]
- 86.Schlaak JF, Hilkens CM, Costa-Pereira AP, et al. Cell-type and donor-specific transcriptional responses to interferon-alpha. Use of customized gene arrays. J Biol Chem. 2002;277:49428–49437. doi: 10.1074/jbc.M205571200. [DOI] [PubMed] [Google Scholar]
- 87.Moschella F, Maffei A, Catanzaro RP, et al. Transcript profiling of human Dendritic Cells maturation-induced under defined culture conditions: comparison of the effects of tumour necrosis factor alpha, soluble CD40 ligand trimer and interferon gamma. Br J Haematol. 2001;114:444–457. doi: 10.1046/j.1365-2141.2001.02953.x. [DOI] [PubMed] [Google Scholar]
- 88.Dietz AB, Bulur PA, Knutson GJ, Matasic R, Vuk-Pavlovic S. Maturation of human monocyte-derived Dendritic Cells studied by microarray hybridization. Biochem Biophys Res Commun. 2000;275:731–738. doi: 10.1006/bbrc.2000.3372. [DOI] [PubMed] [Google Scholar]