Abstract
Aims
Congenital or acquired QT prolongation is a risk factor for life-threatening arrhythmias. In patients with hypertrophic cardiomyopathy (HCM), the QT interval may be intrinsically prolonged. However, the prevalence, cause, and significance of QT prolongation among patients with HCM are unknown.
Methods and results
After exclusion of patients on QT-prolonging drugs, a blinded, retrospective analysis of electrocardiograms, echocardiograms, and genotype status in 479 unrelated patients with HCM [201 females, age at diagnosis 41 ± 18 years, maximal left ventricular wall thickness (MLVWT) 22 ± 6 mm] from two independent centres was performed. The mean QTc was 440 ± 28 ms. The QTc exceeded 480 ms in 13% of patients. Age, gender, family history of HCM or sudden cardiac arrest, and genotype status had no association with QTc. Patients with a QTc over 480 ms were more symptomatic at diagnosis (P < 0.001), had a higher MLVWT (P = 0.03), were more obstructive (P < 0.001), and were more likely to have undergone septal reduction therapy (P = 0.02). There was a weak but significant direct linear relationship between QTc and peak outflow gradient (r2 = 0.05, P < 0.0001).
Conclusions
Compared with <1 in 200 otherwise healthy adults, QT prolongation (QTc > 480 ms) was present in 1 out of 8 patients with HCM. The QTc was partly reflective of the degree of cardiac hypertrophy and left ventricular outflow tract obstruction. Because of its pro-arrhythmic potential and its potential relevance to management and risk stratification, routine QTc assessment should be performed in patients with HCM, particularly when concomitant use of QT-prolonging medications is considered.
Keywords: Long QT syndrome, Hypertrophic cardiomyopathy, Sudden death, Ventricular arrhythmia, QT interval
Introduction
Affecting ∼1 in 500 persons, hypertrophic cardiomyopathy (HCM) is the most prevalent heritable cardiovascular disease and the most common cause of sudden cardiac death in young athletes.1 Clinically, HCM is characterized by unexplained and unequivocal cardiac hypertrophy in the absence of hypertrophy-inducing conditions such as hypertension and aortic stenosis. At the cellular level, HCM is characterized by cardiomyocyte hypertrophy, interstitial fibrosis, and myofibrillar disarray. To date, over 25 HCM-susceptibility genes have been implicated in the pathogenesis of this disease.2
Besides the underlying genotypic heterogeneity, HCM is characterized by profound phenotypic heterogeneity as well. Patients can display extremely varying degrees of hypertrophy, fibrosis, and left ventricular outflow tract (LVOT) obstruction.1 Similarly, the clinical presentation can vary from completely asymptomatic to end-stage heart failure or sudden death.3,4 Patients with HCM have an increased propensity for potentially life-threatening ventricular arrhythmias. The annual risk of sudden death, while initially reported by McKenna et al. in a selected population to be as high as 7%, is between 0.8 and 1.2% among unselected populations.1,5–8
Marked prolongation of the QT interval is a pro-arrhythmic risk factor in both congenital long QT syndrome (LQTS) and drug-induced LQTS.9–13 In addition, spontaneous or drug-induced QT prolongation is a risk factor in patients with coronary artery disease.14,15 While QT prolongation has been reported in small cohorts of patients with HCM,16–18 the prevalence and clinical significance of QT prolongation among a large population of patients with HCM is unknown.
Methods
Patients
In this Institutional Review Board-approved study, we retrospectively reviewed the clinical records for 531 unrelated HCM patients evaluated between 1995 and 2001. Patients were seen at one of two medical centres: (i) the Mayo Clinic Hypertrophic Cardiomyopathy Clinic, Rochester, MN, USA, and (ii) the Referral Center for Myocardial Diseases, Azienda Ospedaliero Universitaria Careggi, Florence, Italy. Clinical records were reviewed for age at diagnosis, cardiac symptoms at diagnosis, echocardiographic data, family history of HCM or sudden death, presence of an implantable cardioverter defibrillator (ICD) and/or pacemaker, and a history of surgical myectomy. Symptoms were assessed using the standard New York Heart Association (NYHA) class system. The follow-up period was calculated as the difference between the date of diagnosis at our institution(s) and the last date of available follow-up. If a patient had an ICD placed, follow-up interrogation of discharge records was performed for the presence of appropriate ventricular fibrillation (VF)-terminating interventions. Any patient on a potential QT-prolonging drug was excluded from this analysis; this predominantly consisted of patients on amiodarone, sotalol, and disopyramide. The majority of patients were on beta-blockade at the time of referral to our institutions, and these patients were included. After excluding patients with electrocardiographic (ECG) evidence of ventricular pacing or taking medications with a known QT-prolonging effect (n = 52), a cohort of 479 patients remained for analysis.
Diagnosis of hypertrophic cardiomyopathy
Diagnosis of HCM was based on the echocardiographic demonstration of a hypertrophied left ventricle (wall thickness ≥15 mm), associated with a non-dilated cavity and in the absence of another cardiac or systemic disease that could produce that magnitude of hypertrophy.
Mutational analysis
All patients had been genotyped previously for the nine most common myofilament-associated and six Z-disc-associated HCM-susceptibility genes, as previously described.19,20
Electrocardiographic analysis
We reviewed the first available ECG study for each patient performed at the time of diagnosis of HCM. Electrocardiographic analysis was performed using a 12-lead ECG and the 12SL ECG analysis programme from GE Marquette Medical Systems, or ESAOTE Organizer. Abstracted ECG measurements included the RR, QT, JT, and QRS intervals. All QT and RR intervals were confirmed manually. JT values were obtained by subtracting the QRS interval from the QT interval.21 QTc values were calculated using the standard Bazett formula.22 JTc values were calculated using a similar rate correction equation [JTc = JT/(RR1/2)]. QT intervals were measured using leads II or V5 when possible. The reviewers (J.N.J., C.G.) were blinded at all times to the patient's clinical history and genotype status. In addition, the echocardiographic and ECG analyses were performed independently to ensure a lack of bias. In patients, who had undergone surgical myectomy or septal ablation, ECGs studied were obtained prior to procedure or, if no pre-procedure ECG was available, patients were excluded from analysis.
Echocardiographic studies
Comprehensive two-dimensional and Doppler echocardiographic studies were performed in each patient using commercially available instruments at the time of diagnosis. Abstracted echocardiographic measurements included maximum left ventricular wall thickness (MLVWT), presence/absence of obstruction (a resting or provocable gradient >30 mmHg was considered obstructive), and basal LVOT gradient. Left ventricular hypertrophy (LVH) was assessed by two-dimensional echocardiography, and the site and extent of maximal wall thickness were identified. Peak instantaneous LVOT gradient, due to mitral valve systolic anterior motion and mitral–septal contact, was estimated with continuous wave Doppler under basal conditions. The left atrial dimension was measured at end-systole in the anteroposterior linear diameter from the parasternal long-axis view.
Statistical analysis
All continuous variables were reported as the mean ± SD. Proportions were analysed and compared using a two-tailed Z-test for proportions. Means were analysed using a two-tailed independent groups t-test for means. A P-value of <0.05 was considered to be statistically significant. Linear regression models were used to correlate QTc with continuous variables. Variables that were statistically significant in univariate models were included in a multivariate logistic regression model to determine whether they remained significant after adjustment for potential confounders. Population normative ECG values were obtained using available published studies.23,24 All analyses were conducted with the computer software JMP, version 8.0 (SAS Institute, Inc, Cary, NC, USA).
Results
Prevalence of QT prolongation in hypertrophic cardiomyopathy
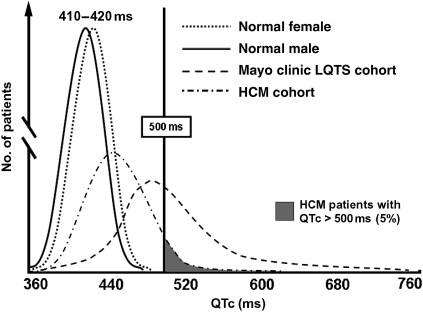
A total of 479 patients with HCM were included in the study (201 females, average age 41 ± 18 years, Table 1). Patients taking medications with a known QT-prolonging effect (www.qtdrugs.org) were excluded from the analysis. Compared with the QTc distribution reported among otherwise healthy adults23,24 (Figure 1), there was a marked right shift in the QTc distribution in this HCM cohort with a mean QTc of 440 ± 38 ms. As seen normally, females in our cohort had a slightly longer QTc (445 ± 37 ms) than males (437 ± 38 ms; P = 0.03). Compared with <0.5% of normals who have a QTc > 480 ms,23 62 HCM patients (13%) had a QTc > 480 ms and 25 of these patients (5.2% of the total) had a QTc over the pro-arrhythmic threshold of 500 ms (Figure 1). The QTc value identifying the upper quartile for our HCM cohort was 461 ms. There were no differences in age, male/female ratio, or QTc between the Mayo (n = 337) and Florence cohorts (n = 142, data not shown). Among the 62 patients with QTc > 480 ms, both prolongation of the JTc (357 ± 46 vs. 323 ± 33 ms in patients <480 ms; P < 0.0001) and prolongation of the QRS interval (138 ± 39 vs. 104 ± 22 ms; P < 0.0001) contributed to the prolonged QTc. As further confirmation, both JTc and QRS were independently strongly correlative of having a QTc > 480 ms in a logistic model of clinical correlative factors (P = 0.01).
Table 1.
Demographic variables study cohort
| Total | QTc ≤ 480 ms | QTc > 480 ms | P-value* | |
|---|---|---|---|---|
| n | 479 | 417 | 62 | — |
| Sex (male/female) | 278/201 | 244/173 | 34/28 | 0.58 |
| Age at diagnosis (years) | 41.1 ± 18 | 41.1 ± 18 | 41.1 ± 18 | 0.98 |
| Mean NYHA class at diagnosis | 1.9 ± 0.8 | 1.9 ± 0.8 | 2.4 ± 0.8 | 0.001 |
| MLVWT (mm) | 22.1 ± 6 | 21.8 ± 6 | 24.1 ± 8 | 0.03 |
| LVOT gradient (mm Hg) | 40.7 ± 40 | 38.9 ± 41 | 52.6 ± 37 | 0.01 |
| Patients w/obstruction, n (%) | 233 (49) | 188 (45) | 45 (74) | <0.0001 |
| QTc (ms) | 440.7 ± 37 | 431.1 ± 28 | 505.3 ± 25 | <0.0001 |
| JTc (ms) | 327.6 ± 37 | 323.3 ± 33 | 357.0 ± 46 | <0.0001 |
| QRS (ms) | 108.6 ± 27 | 104.3 ± 22 | 137.8 ± 39 | <0.0001 |
| Family Hx of HCM, n (%) | 146 (31) | 127 (31) | 19 (31) | 1 |
| Family Hx of sudden death, n (%) | 82 (17) | 71 (17) | 11 (18) | 0.85 |
| Surgical myectomy, n (%) | 141 (29) | 117 (28) | 24 (39) | 0.12 |
| Septal ablation, n (%) | 31 (6) | 23 (6) | 8 (13) | 0.05 |
| Myectomy or ablation, n (%) | 172 (36) | 140 (34) | 32 (52) | 0.01 |
| ICD, n (%) | 90 (19) | 72 (17) | 18 (29) | 0.04 |
| Genotype positive, n (%) | 224 (47) | 201 (48) | 23 (37) | 0.13 |
| Mutation location, n (%) | ||||
| MYBPC3 | 86 (18) | 77 (18) | 9 (15) | 0.56 |
| MYH7 | 80 (17) | 72 (17) | 8 (13) | 0.5 |
| Thin filament | 18 (4) | 17 (4) | 1 (2) | 0.55 |
| Z-disc | 12 (3) | 10 (2) | 2 (3) | 1 |
| Multiple | 28 (6) | 25 (6) | 3 (5) | 0.94 |
Shown are the total demographics for the complete study cohort as well as a comparison between patients with a QTc ≤ 480 ms and a QTc > 480 ms. All values expressed at mean ± SD. Of note, Z-disc genes were not studied in the Florence cohort.
*P-value compares the difference between cohorts with a QTc ≤ 480 and QTc > 480 ms.
Figure 1.
QTc-distribution in health and disease. Shown are the distributions of normal QTc in healthy males and females as well as the Mayo long QT syndrome cohort and the studied hypertrophic cardiomyopathy cohort. Five per cent of patients with hypertrophic cardiomyopathy had a QTc exceeding the pro-arrhythmic threshold of 500 ms.
Clinical correlates of QT prolongation
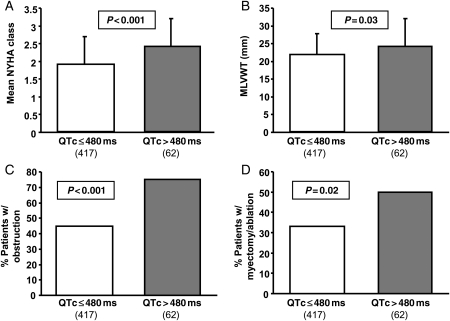
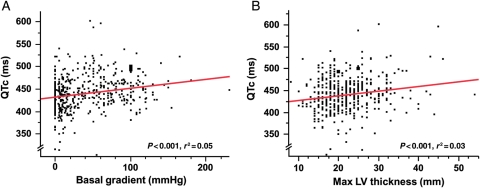
Comparing the 62 patients with a QTc > 480 ms to the rest of the HCM cohort (Table 1), patients with a QTc > 480 ms had a significantly higher mean NYHA class at diagnosis (2.4 ± 0.8 vs. 1.9 ± 0.8; P < 0.001; Figure 2A), had a higher MLVWT (24.1 ± 8 vs. 21.8 ± 6 mm; P = 0.03; Figure 2B), were more likely to be obstructive (74 vs. 45%; P < 0.001; Figure 2C), and were more likely to have undergone therapeutic septal reduction therapy by either septal ablation or surgical myectomy (52 vs. 34%; P = 0.02; Figure 2D). Patients with a QTc > 480 ms were also more likely to have a risk profile ultimately judged to require ICD for primary or secondary sudden death prevention (29 vs. 17%; P < 0.04). Overall, there was a significant but weak relationship between QTc and the LVOT basal gradient (r = 0.05; P < 0.0001; Figure 3A) and maximum MLVWT (r = 0.03; P = 0.0002; Figure 3B). Additionally, an increased NYHA class at diagnosis was related to increasing QTc values (r = 0.08; P < 0.0001). Conversely, family history of HCM, family history of sudden death, age at diagnosis, and specific genotype status had no association with the QTc duration. For the majority of patients in our cohort (90%), the hypertrophy was concentrated at the outflow tract or over the whole length of the septum; 10% of patients had an apical distribution of HCM.
Figure 2.
Bar diagrams showing significant differences between the subset of patients with a QTc ≤ 480 ms and a QTc > 480 ms. Patients with a QTc over 480 ms were more likely to be symptomatic at diagnosis (A), had a greater maximum left ventricular wall thickness (B), were more likely to be obstructive (C), and were more likely to have undergone septal reduction therapy by either septal ablation or surgical myectomy (D).
Figure 3.
QTc plotted against basal left ventricular outflow tract gradient (A) and the maximum left ventricular wall thickness (B). QTc showed a weak, but significant relationship with left ventricular outflow tract gradient and maximum left ventricular wall thickness.
The 25 patients with a QTc over the pro-arrhythmic threshold of 500 ms were significantly younger at diagnosis (34.1 ± 14 years old) compared with the remaining 37 patients (45.8 ± 19 years; P = 0.008) with a QTc between 480 and 500 ms (data not shown). Although the data trended in the same direction, no statistically significant differences were observed in the NYHA class at diagnosis, MLVWT, number of obstructive patients, or rate of therapeutic interventions for obstruction in these two subsets (data not shown). Ten of these patients (40%) ultimately underwent ICD therapy for established HCM risk factors.
Patients with unexplained QT prolongation
Although QT prolongation appeared to be generally driven by severe phenotypic expressions of HCM, such as marked hypertrophy and presence of LVOT obstruction, we were able to identify a small subset of patients in whom QT prolongation was present despite a mild HCM phenotype, and appeared to represent a primary, largely unexplained abnormality. Indeed, 9 of the 62 patients with QTc > 480 ms had no evidence of LVOT obstruction and an MLVWT <20 mm. The average age in this subset was 51 ± 20 years, three (33%) were males, and the mean MLVWT was 16.9 ± 2.6 mm. The average QTc for these patients was 504 ± 20 ms (range 482–539 ms) with a QRS duration of 148 ± 45 ms (range 88–202 ms) and a JTc duration of 345 ± 56 ms (range 268–428 ms). Two patients had a family history of sudden death and three patients underwent ICD placement. Only one of the three patients had the ICD placed for secondary prevention, after having documented sustained ventricular tachycardia. None of the three patients has had an ICD shock in the available follow-up period.
Relation to genotype
In the overall cohort, 53% of patients were genotype negative, while 47% of patients had an HCM-associated mutation discovered (Table 1). Patients in the Florence cohort were more likely to have a positive genotype (62%) compared with the Mayo cohort (40%; P < 0.001). The QTc was significantly lower among genotype-positive patients (435 ± 35 ms) when compared with genotype-negative patients (445 ± 40 ms; P = 0.006). The JTc was similarly lower among genotype-positive patients (P < 0.02). Genotype-negative patients were older in age when compared with genotype-positive patients (45.3 ± 18 vs. 36.5 ± 27 years; P < 0.0001) and had a smaller mean MLVWT (20.9 ± 6.1 vs. 23.4 ± 6.1 mm; P < 0.0001). There was no significant difference in the rate of genotype positivity between patients with a QTc over 480 ms (37% genotype positive) compared with patients with a QTc under 480 ms (48% genotype positive; P = 0.1; Table 1), nor were there any differences between the specific genetic subtypes.
Prevalence of arrhythmias and outcome
A total of 90 of 479 (19%) patients ultimately had an ICD placed based on primary or secondary prevention of sudden cardiac death. Fifteen of these patients received their device as secondary prevention after out-of-hospital cardiac arrest or sustained ventricular tachycardia (Table 2). The remaining patients had an ICD placed prophylactically based on the presence of one or more established risk factors as listed in Table 2. It must be noted that, as not always feasible in clinical practice, not all risk factors were universally assessed for each patient with Holter monitor data least available for these patients. Over a mean follow-up period of 4.9 ± 3.3 years, 11 of 90 (12%) patients with an ICD had appropriate VF-terminating device discharges; 6 of these patients had received their ICD as secondary prevention, and 5 for primary prevention. There were no characteristic differences in reason for ICD placement or specific risk factors between patients with a QTc under 480 ms when compared with patients with a QTc over 480 ms. Furthermore, there was no significant difference in the rate of appropriate VF-terminating discharges between the two groups (P = 0.1; Table 2). Appropriate ICD therapies were paradoxically only seen in the subgroup of patients with a QTc under 480 ms. Fifteen per cent of patients with an ICD in this subgroup received appropriate VF-terminating discharges, whereas no appropriate ICD discharges were observed in the 18 patients with an ICD and QTc over 480 ms. There was no significant difference in phenotypic or clinical expression of disease between patients with a QTc > 500 ms and those with a QTc between 480 and 500 ms.
Table 2.
Follow-up data for the 90 patients in the cohort who underwent implantable cardioverter defibrillator placement
| Total | QTc ≤ 480 ms | QTc > 480 ms | |
|---|---|---|---|
| n | 90 | 72 | 18 |
| Male/female | 53/37 | 41/31 | 12/6 |
| Follow-up (years) | 4.9 ± 3.3 | 5.0 ± 3.4 | 4.4 ± 3.0 |
| Secondary prevention, n (%) | 15 (17) | 12 (17) | 3 (17) |
| Mean no. of risk factors | 1.8 ± 1.0 | 1.8 ± 0.9 | 1.9 ± 1.1 |
| Presence of RF, n (%) | |||
| NSVT on Holter | 34 (38) | 29 (40) | 5 (28) |
| LVWT >30 mm | 24 (27) | 17 (24) | 7 (39) |
| ABPR on exercise | 21 (23) | 15 (21) | 6 (33) |
| (Near)syncope | 40 (44) | 30 (42) | 10 (56) |
| Family Hx of sudden death | 43 (48) | 36 (50) | 7 (39) |
| Appropriate discharges, n (%) | 11 (12) | 11 (15) | 0 (0) |
All values expressed as mean ± SD. It must be noted that as not always feasible in clinical practice, not all risk factors were routinely assessed for each patient with Holter monitor data least available for these patients. RF, risk factor; LVWT, left ventricular wall thickness; ABPR, abnormal blood pressure response.
Of note, there were 12 of 90 patients (13%) who received inappropriate discharges, of whom two had a QTc >480 ms.
Discussion
Marked prolongation of the QT interval is a pro-arrhythmic risk factor in both congenital- and drug-induced LQTS as well as in several disease states including coronary artery disease.9–14 Herein, we describe the prevalence and patterns of QT prolongation in a large cohort of patients with HCM. The QT interval was significantly prolonged (QTc >480 ms, or greater than one standard deviation above the mean QTc), even without QT-prolonging pharmacological intervention, in 13% of our HCM patients. Thus, compared with otherwise healthy adults, a patient with HCM is ∼20–25 times more likely to exhibit a QTc > 480 ms. Furthermore, 5% of these patients had a QTc over the pro-arrhythmic threshold of 500 ms, generally considered to represent a risk factor for potentially life-threatening arrhythmias irrespective of the associated condition. In most patients, the QT prolongation appeared to reflect a more generally severe phenotype in terms of LVH and presence of LVOT obstruction. In an important minority (9 of 62, 15%), however, a QTc > 480 ms was present despite mild HCM phenotype and no or only mild symptoms, suggesting that abnormalities in cardiac repolarization may represent a primary feature of the disease in selected cases, possibly subtended by specific gene defects.
The determinants of a specific individual's QTc are dependent on numerous factors, including time of day and concurrent illness or medications.15,25 In addition, if one's depolarization is abnormal, for instance, in patients with left or right bundle branch block, then the repolarization will assuredly be abnormal.26 The presence of an independent effect of both the JTc and QRS intervals on QTc suggests that abnormal hypertrophied muscle impacts both depolarization and repolarization in these patients. The JTc correlates with a risk of cardiac events in patients with coronary artery disease, even in the presence of abnormal QRS duration measurements.21 In patients with HCM, the mechanism of QT prolongation has been attributed previously to the sheer mass of ventricular myocardium.16–18 In this study, we have demonstrated that QT prolongation is associated with a higher NYHA class at diagnosis, higher frequency of obstruction, increased MLVWT, and higher rate of therapeutic septal reduction. Also, QTc weakly correlated with the magnitude of left ventricular outflow obstruction, suggesting that obstruction also influences cardiac repolarization. These findings were consistent when examining either the QTc or the JTc interval.
Several authors have reported previously QT prolongation in genotyped HCM patients.16–18 Jouven et al.16 examined 206 adult patients from 15 families. This included 112 unaffected patients, 58 affected patients with LVH, and 36 affected patients without LVH. All affected patients had mutations in either MYBPC3-encoded myosin-binding protein C or the MYH7-encoded beta-myosin heavy chain. The authors found a significant increase in QT interval measurements in the affected patients, both with and without LVH. Additionally, there were different patterns of QT prolongation found between the two genotypes, where the patients with beta-myosin heavy chain mutations had a prolonged QTc even in the absence of hypertrophy.
In our large cohort of unrelated patients, no such genotype-specific differences in QTc were detected (Table 1). This may be explained by our inclusion of only unrelated patients in the study, in an attempt to prevent a single familial phenotype from biasing the cohort at large. Uchiyama et al.18 recently published a study of 154 HCM patients of whom 111 were genotype positive and 43 were genotype negative. They found an increased QTc and QT dispersion in patients with ECG abnormalities, both with and without concurrent LVH. However, the only four patients in the study to suffer sudden death over the follow-up period all exhibited ECG abnormalities with LVH.18
Ultimately, in a small subset of patients who have had ICDs placed, we did not find a significant difference in QRS, JTc, or QTc between those who have received appropriate VF-terminating therapies and those who thus far have not. Further, a particular at risk patient subgroup was not delineated. This certainly is limited by the relatively low event rate reported in published HCM cohorts.3 Our results are consistent with a study of 277 patients with HCM published in 2001, which did not find QT dispersion or QTc predictive of a sudden death risk.27 The risk associated with QT prolongation in HCM appears to be markedly different from that of patients with LQTS. This is likely due to the expected multi-factorial origin of QT prolongation in HCM (hypertrophy, myocyte disarray, obstruction), as opposed to a specific ‘electrical' origin in LQTS, which may confer a higher arrhythmogenic potential intrinsically.
However, considering the small sample size and low event rate expected in HCM cohorts, and knowing that a QTc > 500 ms is a risk factor in multiple disease entities,9–14 it would be premature to dismiss QT prolongation in a patient with HCM as an innocuous finding. Specifically, the concomitant use of medications with known QT prolonging potential, such as the frequently employed amiodarone or sotalol, in HCM patients with baseline prolongation of the QTc may have potentially adverse consequences, by further enhancing pro-arrhythmic pre-disposition and precipitating torsades de pointes.
Our findings have important clinical implications. Routine QTc assessment should be performed in patients with HCM, particularly in those for whom the concomitant use of medications with a known QT-prolonging effect is intended. The use of QT-prolonging drugs should be weighed against potential risks, and the QT interval monitored regularly during treatment. Furthermore, these patients should be adequately counselled with regard to potential interaction with other QT-prolonging drugs (antibiotics, etc.) and of common electrolyte disturbances. Of note, in this study, a QTc cut-off value of 480 ms identified a subset of patients with clearly abnormal duration of repolarization for this specific disease. Therefore, we would propose that this value be used in clinical practice as the threshold prompting careful consideration regarding the concomitant use of medications with QT-prolonging potential. The role of QT prolongation in HCM requires further investigation with regard to a potential impact on prognosis.
Limitations
This study is limited by the low event rate as expected in most HCM cohorts. Holter recordings were not universally assessed for non-sustained ventricular tachycardia (NSVT) in the present cohort; although it might have been interesting to evaluate the relationship of this feature with QTc, it is well known that arrhythmias recorded during Holter monitoring (including primary NSVT) have poor positive predictive accuracy with regard to events in HCM. Therefore, such analysis would not have added decisive information regarding the prognostic relevance of QTc prolongation in our patients. Furthermore, in Figure 1, we compared our HCM cohort with a published historical control group,23,24 which was, therefore, not a patient-level comparison. Accordingly, there are inherent limitations to using a historical comparison group, including possible differences in the methodology used which cannot be established.
Conclusions
Compared with <1 in 200 otherwise healthy adults, QT prolongation (QTc > 480 ms) was present in 1 out of 8 patients with HCM. The QTc was partly reflective of the degree of cardiac hypertrophy and LVOT obstruction. Because of its pro-arrhythmic potential and its potential relevance to management and risk stratification, routine QTc assessment should be performed in patients with HCM, particularly when concomitant use of QT-prolonging medications is considered.
Funding
This work was supported by the following sources: M.J.A. research programme was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program. M.J.A. is also an Established Investigator of the American Heart Association and is supported by the National Institutes of Health (HD42569 and HL094291). F.C. and I.O. are supported by Ministero Istruzione Università e Ricerca (PRIN) and the European Union (STREP Project 241577 ‘BIG HEART', 7th European Framework Program).
Conflict of interest: M.J.A. is a consultant for Biotronik, Boston Scientific Corporation, Medtronic, PGxHealth, and St. Jude Medical Inc.
Acknowledgements
The investigators express their gratitude to patients who have sought clinical evaluation at their respective medical centres.
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. doi:10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201–211. doi: 10.1016/j.jacc.2009.02.075. doi:10.1016/j.jacc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA, 3rd, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–412. doi: 10.1001/jama.298.4.405. doi:10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 4.McKenna WJ, Goodwin JF. The natural history of hypertrophic cardiomyopathy. Curr Probl Cardiol. 1981;6:1–26. doi: 10.1016/0146-2806(81)90015-3. doi:10.1016/0146-2806(81)90015-3. [DOI] [PubMed] [Google Scholar]
- 5.Spirito P, Autore C, Rapezzi C, Bernabo P, Badagliacca R, Maron MS, Bongioanni S, Coccolo F, Estes NA, Barilla CS, Biagini E, Quarta G, Conte MR, Bruzzi P, Maron BJ. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314. doi:10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 6.Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT. Hypertrophic cardiomyopathy in a large community-based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol. 2003;41:987–993. doi: 10.1016/s0735-1097(02)03004-8. doi:10.1016/S0735-1097(02)03004-8. [DOI] [PubMed] [Google Scholar]
- 7.Cecchi F, Maron BJ, Epstein SE. Long-term outcome of patients with hypertrophic cardiomyopathy successfully resuscitated after cardiac arrest. J Am Coll Cardiol. 1989;13:1283–1288. doi: 10.1016/0735-1097(89)90302-1. doi:10.1016/0735-1097(89)90302-1. [DOI] [PubMed] [Google Scholar]
- 8.McKenna W, Deanfield J, Faruqui A, England D, Oakley C, Goodwin J. Prognosis in hypertrophic cardiomyopathy: role of age and clinical, electrocardiographic and hemodynamic features. Am J Cardiol. 1981;47:532–538. doi: 10.1016/0002-9149(81)90535-x. doi:10.1016/0002-9149(81)90535-X. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg I, Moss AJ, Peterson DR, McNitt S, Zareba W, Andrews ML, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Napolitano C, Priori SG, Qi M, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation. 2008;117:2184–2191. doi: 10.1161/CIRCULATIONAHA.107.701243. doi:10.1161/CIRCULATIONAHA.107.701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenberg I, Moss AJ, Bradley J, Polonsky S, Peterson DR, McNitt S, Zareba W, Andrews ML, Robinson JL, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Qi M, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long-QT syndrome after age 40. Circulation. 2008;117:2192–2201. doi: 10.1161/CIRCULATIONAHA.107.729368. doi:10.1161/CIRCULATIONAHA.107.729368. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs JB, Peterson DR, Moss AJ, McNitt S, Zareba W, Goldenberg I, Qi M, Robinson JL, Sauer AJ, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Towbin JA, Vincent GM, Zhang L. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249–1254. doi: 10.1001/jama.296.10.1249. doi:10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 12.Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ, Benhorin J, Hall WJ, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–337. doi: 10.1016/j.jacc.2006.08.057. doi:10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 13.Seth R, Moss AJ, McNitt S, Zareba W, Andrews ML, Qi M, Robinson JL, Goldenberg I, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–1098. doi: 10.1016/j.jacc.2006.09.054. doi:10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 14.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. doi:10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenigsberg DN, Khanal S, Kowalski M, Krishnan SC. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol. 2007;49:1299–1305. doi: 10.1016/j.jacc.2006.11.035. doi:10.1016/j.jacc.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Jouven X, Hagege A, Charron P, Carrier L, Dubourg O, Langlard JM, Aliaga S, Bouhour JB, Schwartz K, Desnos M, Komajda M. Relation between QT duration and maximal wall thickness in familial hypertrophic cardiomyopathy. Heart. 2002;88:153–157. doi: 10.1136/heart.88.2.153. doi:10.1136/heart.88.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin AB, Garson A, Jr., Perry JC. Prolonged QT interval in hypertrophic and dilated cardiomyopathy in children. Am Heart J. 1994;127:64–70. doi: 10.1016/0002-8703(94)90510-x. doi:10.1016/0002-8703(94)90510-X. [DOI] [PubMed] [Google Scholar]
- 18.Uchiyama K, Hayashi K, Fujino N, Konno T, Sakamoto Y, Sakata K, Kawashiri MA, Ino H, Yamagishi M. Impact of QT variables on clinical outcome of genotyped hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2009;14:65–71. doi: 10.1111/j.1542-474X.2008.00275.x. doi:10.1111/j.1542-474X.2008.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. doi: 10.4065/83.6.630. doi:10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- 20.Van Driest SL, Vasile VC, Ommen SR, Will ML, Tajik AJ, Gersh BJ, Ackerman MJ. Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:1903–1910. doi: 10.1016/j.jacc.2004.07.045. doi:10.1016/j.jacc.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: the ARIC Study with 13 years of follow-up. Circulation. 2003;108:1985–1989. doi: 10.1161/01.CIR.0000095027.28753.9D. doi:10.1161/01.CIR.0000095027.28753.9D. [DOI] [PubMed] [Google Scholar]
- 22.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. doi: [Google Scholar]
- 23.Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. doi: 10.1016/j.jelectrocard.2006.09.003. doi:10.1016/j.jelectrocard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Taggart NW, Haglund CM, Tester DJ, Ackerman MJ. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115:2613–2620. doi: 10.1161/CIRCULATIONAHA.106.661082. doi:10.1161/CIRCULATIONAHA.106.661082. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43:657–662. doi: 10.1136/bjsm.2008.054734. doi:10.1136/bjsm.2008.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot S. QT interval in right and left bundle-branch block. Br Heart J. 1973;35:288–291. doi: 10.1136/hrt.35.3.288. doi:10.1136/hrt.35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maron BJ, Leyhe MJ, 3rd, Casey SA, Gohman TE, Lawler CM, Crow RS, Maron MS, Hodges M. Assessment of QT dispersion as a prognostic marker for sudden death in a regional nonreferred hypertrophic cardiomyopathy cohort. Am J Cardiol. 2001;87:114–115, A9. doi: 10.1016/s0002-9149(00)01285-6. doi:10.1016/S0002-9149(00)01285-6. [DOI] [PubMed] [Google Scholar]