Abstract
Aims
To evaluate arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) in affected families with desmosome mutations on the basis of the recently revised Task Force Criteria (TFC).
Methods and results
One hundred and three consecutive carriers of pathogenic desmosome mutations and 102 mutation-negative relatives belonging to 22 families with dominant and 14 families with recessive ARVC/D were evaluated according to the original and revised TFC. Serial cardiac assessment with 12-lead, signal-averaged, and 24 h ambulatory ECG and two-dimensional echocardiography was performed. Clinical events and outcome were prospectively analysed up to 24 years (median 4 years). With the revised criteria, 16 carriers were newly diagnosed on the basis of ECG abnormalities in 100%, ventricular arrhythmias in 79%, and functional/structural alterations in 31%, increasing diagnostic sensitivity from 57 to 71% (P = 0.001). Task Force Criteria specificity improved from 92 to 99% (P = 0.016). In dominant mutation carriers, penetrance changed significantly (61 vs. 42%, P = 0.001); no changes were observed in recessive homozygous carriers (97 vs. 97%, P = 1.00). Affected carriers according to the revised TFC (n = 73) had 12-lead ECG abnormalities in 96%, ventricular arrhythmias in 91%, and functional/structural alterations fulfilling echocardiographic criteria in 76%. Cumulative and event-free survival did not differ significantly between dominant and recessive affected carriers, being at 78.6 vs. 76 and 51.7 vs. 55.4%, respectively, by the age of 40 years.
Conclusion
Revised TFC increased diagnostic sensitivity particularly in dominant ARVC/D. Serial family evaluation may rely on electrocardiography which seems to have the best diagnostic utility particularly in early disease that is not detectable by two-dimensional echocardiography.
Keywords: Cardiomyopathy, Arrhythmogenic right ventricular cardiomyopathy/dysplasia, Diagnostic criteria, Desmosome mutations
See page 1049 for the editorial comment on this article (doi:10.1093/eurheartj/ehr088)
Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a primary heart muscle disorder presenting clinically with ventricular arrhythmias, sudden death, or heart failure and characterized pathologically by progressive myocardial loss with fibrous or fibrofatty replacement mostly of the right ventricle.1,2 In the absence of a clinical gold standard for the diagnosis of ARVC/D, in 1994, the International Task Force Criteria (TFC) was established.3 The original TFC focused on the diagnosis of overt and severe disease and lacked sensitivity for early forms common among family members. In a recent modification which included quantitative parameters, it was aimed to improve diagnostic sensitivity with the important requisite of maintaining specificity.4
Arrhythmogenic right ventricular cardiomyopathy/dysplasia is a genetically determined disorder of the desmosomal plaque which in ∼50% of probands is associated with mutations of desmosomal proteins plakoglobin (JUP), desmoplakin (DSP), plakophilin-2 (PKP2), desmoglein-2, and desmocollin-2 (DSC2).5–9 The mode of inheritance is usually dominant with reduced penetrance.6–9 A recessive form due to JUP mutation associated with a cutaneous phenotype (Naxos disease) has been reported to show full penetrance by adolescence.5,10 This form of the disease permits testing of TFC diagnostic sensitivity and studying the mode of disease onset.11
This study evaluated the recently revised TFC for the diagnosis of ARVC/D comparing them with the original ones in affected families with dominant and recessive desmosome gene mutations. Age-related penetrance and disease expression were assessed by applying the revised TFC.
Methods
The study population included 103 consecutive carriers of pathogenic desmosome mutations and 102 mutation-negative relatives (first- and second-degree) belonging to 36 families with dominant (n = 22) and recessive (n = 14) ARVC/D (members per family: median 3.5, inter-quartile range: 3–5) (see Supplementary material online, Figure S1). The proband in each family met TFC for the diagnosis of ARVC/D; 31 probands met the original plus revised TFC, whereas five met only the revised TFC. Of the 36 probands, 22 were carriers of dominant mutations (11 of PKP2, 4 of DSP, 6 of DSC2, and 1 of PKP2 plus DSP) and 14 were homozygous carriers of the known recessive JUP mutation (Naxos disease).5 All family members were screened for the probands’ mutations by direct sequencing as described previously.9 None of the identified sequence variants were present in a cohort of 400 control chromosomes. Sequence variants were defined as pathogenic as detailed in the revised TFC.4
All individuals underwent cardiac assessment including a detailed history of cardiac events, physical examination, resting 12-lead ECG, 24 h ambulatory ECG, signal-averaged ECG (SAECG), and two-dimensional echocardiography. These investigations were performed every 12 months or more frequent in those with clinical events in a prospective follow-up of up to 24 years (median 4 years, inter-quartile range: 3–6). Myocardial tissue was available in 13 patients (4 endomyocardial, 3 surgical, and 6 post-mortem). The original and revised TFC (see Supplementary material online, Table S1) were applied to all examined individuals.3,4 The diagnosis of ARVC/D was fulfilled by the presence of two major or one major plus two minor or four minor criteria from different categories. The study complies with the Declaration of Helsinki, the locally appointed Ethics Committee approved the research protocol, and informed consent was obtained from all individuals. Clinical events and outcome were recorded by age. Detailed historical data were available from medical records.
Electrocardiography and echocardiography
All ECGs were recorded at rest (10 mm/mV in speed 25 mm/s) at the standard lead position. No individual was receiving antiarrhythmic or other drugs known to affect the QRS complex at the time of acquisition of the ECG trainings. To increase the accuracy of measurements, ECGs were enlarged three times. Digital callipers capable of measuring to within 0.1 mm (4 ms) in the horizontal axis and 0.1 mm (0.01 mV) in the vertical axis were used to determine the intervals (Adobe Photoshop CS3). QRS complex duration in leads V1 to V6 and terminal activation duration (TAD) of QRS complex in leads V1 to V3 (from the nadir of the S-wave to the end of the QRS, including R′) were measured in three consecutive beats in each lead according to the protocol described by Nasir et al.12; the mean value of the three beats was used. Epsilon waves and T-wave inversion were studied on precordial leads.4 Signal-averaged ECG was performed using time-domain analysis with a band-pass filter of 40 Hz in individuals with a QRS complex duration of <110 ms on standard ECG. It was considered positive for late potentials if at least two of the three parameters for original TFC or one for revised TFC was abnormal.4 A 24 h ambulatory ECG was recorded on an outpatient basis. Ventricular extrasystoles and episodes of ventricular tachycardia (three or more consecutive ventricular complexes at a rate of ≥100 b.p.m.) were noted. The morphology and axis of non-sustained or sustained (lasting >30 s) ventricular tachycardia was determined when three of more lead ECG recordings were available.
Echocardiography was performed with a 2.5 MHz transducer. Right ventricular size was estimated in two-dimensional echocardiographic recordings at the end of diastole. In particular, right ventricular outflow tract diameter was measured on the parasternal long-axis view (RVOT-PLAX) and right ventricular inflow tract (RVIT) diameter on apical four-chamber view according to the protocol by Foale et al.13 Wall motion abnormalities of the right ventricle (hypokinesia, akinesia, dyskinesia, and aneurysm) were documented. Severe hypokinesia was classified as akinesia. Aneurysm was defined as an akinetic or dyskinetic area with diastolic bulging.3
All measurements were performed by the same investigator (N.P.), before the result of mutation analysis was known. The repeatability of all measurements was tested in random samples of 25 ECG and 25 echocardiographic recordings; the 95% Bland–Altman limits ranged from −6.4 to 3.9 ms for QRS complex width, from −4.1 to 3.7 ms for TAD, from −1.6 to 1.8 mm for RVOT-PLAX, and from −2.2 to 3.1 mm for RVIT. Normal limits of RVOT-PLAX and RVIT were defined by the assessment of 140 healthy Greek volunteers (70 men and 70 women) aged 39 ± 13 years (range: 13–66 years). Right ventricular dilatation was classified as minor (2–3 SD from normal values) or major (more than 3 SD from normal values) according to the original TFC (see Supplementary material online, Table S2).3 Applying the revised TFC, right ventricular dilatation was classified as minor (RVOT-PLAX ≥29 to <32 mm) and major (RVOT-PLAX ≥32 mm) according to the reported cut-off points.4
Statistical analysis
Summary descriptive statistics are reported as mean ± SD, or frequency counts and %, as appropriate. Intra-observer variability for randomly selected ECG/echocardiographic recordings was assessed with the Bland–Altman method. Sensitivity and specificity were compared using the McNemar test. The Kaplan–Meier event-free survival curves were constructed and compared with the log-rank test. SPSS 17 was used for all analyses. All statistical tests were performed at the two-sided 5% level of significance.
Results
Revised vs. original Task Force Criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia
Fifty-nine of 103 (57%) mutation carries (see Supplementary material online, Table S3) and 8 of 102 (8%) mutation-negative family members (see Supplementary material online, Table S4) fulfilled the original TFC. Applying modified criteria one by one, the rate of diagnosis significantly changed by modifying Criterion VI (family history/genetics) (Table 1). Applying proposed modifications of all six TFC, 16 additional carriers were diagnosed, but 2 other carriers fulfilling the original TFC were missed, increasing diagnostic sensitivity from 57 to 71% (P = 0.001) (Tables 1 and 2). In particular, diagnostic sensitivity was significantly increased in carriers of dominant mutations (61 vs. 42%, P = 0.001), whereas no significant change was observed for recessive homozygous carriers (97 vs. 97%, P = 1.000) (Table 2). Among mutation-negative family members, application of revised TFC excluded from diagnosis seven individuals by quantitative parameters for functional/structural and depolarization abnormalities, increasing specificity from 92 to 99% (P = 0.016) (Tables 1 and 2).
Table 1.
Changes in diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia applying modifications of Task Force Criteria
| Modifications of Task Force Criteria | Mutation carriers (n = 103) |
Mutation-negative (n = 102) |
||||
|---|---|---|---|---|---|---|
| New diagnosed | Missed diagnosis | P-valuea | New diagnosed | Missed diagnosis | P-valuea | |
| Criterion I (functional/structural alterations) | 2 | 6 | 0.289 | 0 | 3 | 0.250 |
| Criterion II (tissue characterization) | 0 | 0 | 1.000 | 0 | 0 | 1.000 |
| Criterion III (repolarization abnormalities) | 4 | 0 | 0.125 | 0 | 0 | 1.000 |
| Criterion IV (depolarization abnormalities) | 4 | 2 | 0.688 | 0 | 4 | 0.125 |
| Criterion V (arrhythmias) | 4 | 0 | 0.125 | 0 | 0 | 1.000 |
| Criterion VI (family history/genetics) | 13 | 0 | <0.001 | 0 | 0 | 1.000 |
| Criteria I to VI (all modifications) | 16 | 2 | 0.001 | 0 | 7 | 0.016 |
aTask Force Criteria with modifications vs. original ones.
Table 2.
Comparison of revised to original Task Force Criteria to diagnose arrhythmogenic right ventricular cardiomyopathy/dysplasia in 205 individuals
| Demographics |
Individuals fulfilling TFC |
P-value | |||
|---|---|---|---|---|---|
| Age (years) | Male:female | Original TFC | Revised TFC | ||
| Mutation carriers (n = 103) | 41 ± 18 | 52:51 | 59 (57) | 73 (71) | 0.001 |
| Dominant mutation carriers (n = 74) | 42 ± 17 | 37:37 | 31 (42) | 45 (61) | 0.001 |
| Recessive mutation carriers (n = 29) | 41 ± 18 | 15:14 | 28 (97) | 28 (97) | 1.000 |
| Mutation-negative family members (n = 102) | 41 ± 16 | 47:55 | 8 (8) | 1 (1) | 0.016 |
Values are n (%). TFC, Task Force Criteria.
Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia according to revised Task Force Criteria
Seventy-three mutation carriers (55% male) aged 40 ± 18 years (range: 12–77) fulfilled the revised TFC (affected carriers) (see Supplementary material online, Table S3): 10 fulfilled one major plus at least two minor criteria, whereas the other 63 fulfilled at least two major criteria. Forty-five were carriers of dominant mutations and 28 were homozygous carriers of the recessive JUP mutation. All 16 of the newly diagnosed individuals had ECG abnormalities, whereas 79% also had ventricular arrhythmias; functional/structural alterations fulfilling echocardiographic criteria were detected in 31%.
Thirty mutation carriers (43% male) aged 48 ± 17 years (range: 16–84) at last follow-up did not fulfil the revised TFC (non-affected carriers). Twelve of them fulfilled one minor criterion besides family history/genetics: 10 had minor depolarization abnormalities and two had ventricular extrasystoles >500/24 h. None developed symptoms during follow-up. Two carriers met the original TFC, but were excluded from diagnosis by the revised quantitative echocardiographic criteria (see Supplementary material online, Table S3, Cases 38 and 53).
Among 102 mutation-negative family members (46% male) aged 41 ± 16 years (range 13–88), 27 had minor repolarization (n = 6) or depolarization (n = 21) abnormalities. Another one, a 71-year-old woman, first-degree relative, fulfilled the revised TFC showing T-wave inversion in leads V1, V2, and V3 (see Supplementary material online, Table S4, Case 1). None developed arrhythmias or symptoms during follow-up.
Clinical features of affected carriers
Clinical characteristics of 73 affected carriers fulfilling the revised TFC were analysed. Seventy individuals (96%) fulfilled 12-lead ECG criteria. In particular, 32 (44%) had repolarization plus depolarization abnormalities, 22 (30%) had repolarization abnormalities alone, and 16 (22%) had depolarization abnormalities alone. Three individuals (see Supplementary material online, Table S3, Cases 14, 34, and 45) who did not meet 12-lead ECG criteria fulfilled all three parameters for late potentials on SAECG. Ventricular extrasystoles >500/24 h were recorded in 64 out of 70 individuals (91%) who underwent 24 h ambulatory ECG. Non-sustained (n = 15) or sustained (n = 23) ventricular tachycardia of left bundle branch block configuration on either resting ECG, 24 h ambulatory ECG, or stress testing was recorded in 48% of the affected. Fifty of 66 individuals (76%) with quantitative echocardiographic data showed functional/structural abnormalities which fulfilled the revised echocardiographic criteria. In the remaining 16 individuals, right ventricular functional/structural alterations on two-dimensional echocardiography were subdiagnostic (n = 12) or absent (n = 4) (Figures 1–3). Left ventricular functional/structural alterations on two-dimensional echocardiography were detected in 35% of the affected.
Figure 2.
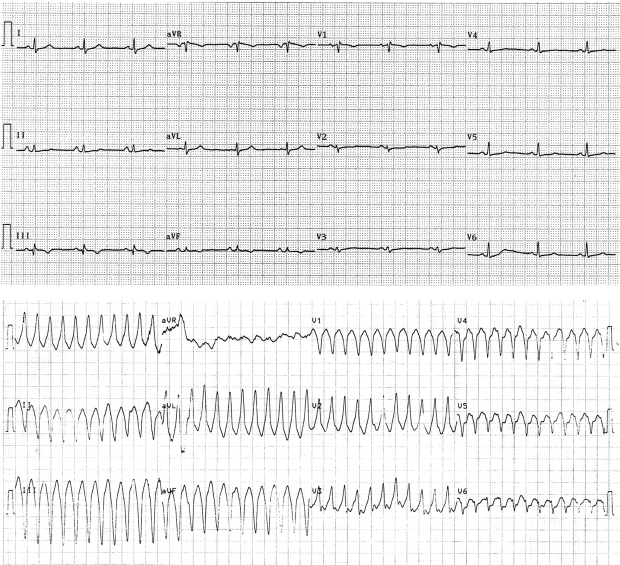
Recordings from 12-lead ECG (25 mm/s, 10 mm/mV) of a 24-year-old desmoplakin mutation carrier (see Supplementary material online, Table S3, Case 29) not diagnosed with original Task Force Criteria. During sinus rhythm (top) there is low voltage, T-wave inversion in leads V1 (−0.7 mm) and V2 (−0.2 mm), normal QRS complex width (≤100 ms) in leads V1 to V3, and prolonged TAD (56 ms) in lead V2. She presented sustained ventricular tachycardia of left bundle branch block morphology with superior axis (bottom) but she did not fulfil echocardiographic criteria.
Figure 1.
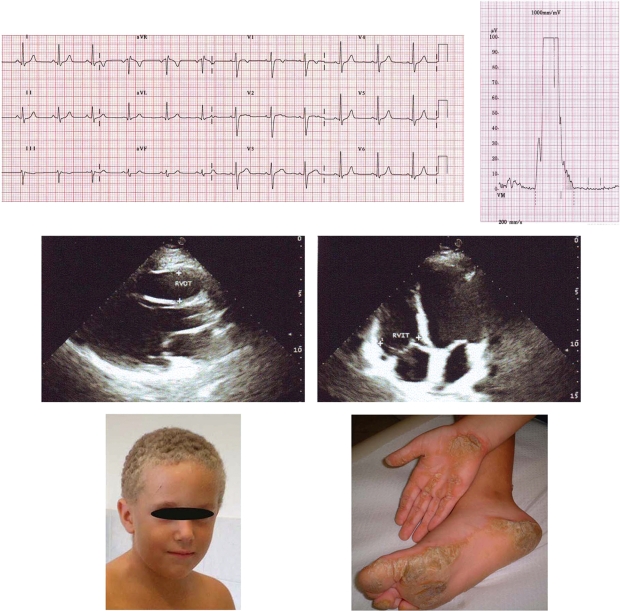
Recordings from 12-lead ECG (25 mm/s, 10 mm/mV) (top left) and signal-averaged ECG (filter 40–250 Hz) (top right) of a 12-year-old JUP homozygous carrier (see Supplementary material online, Table S3, Case 73) presenting >1000/24 h ventricular extrasystoles. There is prolonged terminal activation duration (58 ms) in lead V1 and all three parameters for late potentials are abnormal (filtered QRS duration = 125 ms, LAS = 42 ms, RMS = 11 μV). Two-dimensional echocardiographic images on parasternal long axis view (middle left) and apical four-chamber view (middle right). There are no morphological/functional abnormalities of the right and left ventricle; right ventricular end-diastolic diameters at outflow tract (RVOT) and inflow tract (RVIT) were within normal limits (corrected for body surface area, 14 and 21 mm/m2, respectively). The boy shows the Naxos cutaneous phenotype (woolly hair and palmoplantar keratoderma) (bottom).
Figure 3.
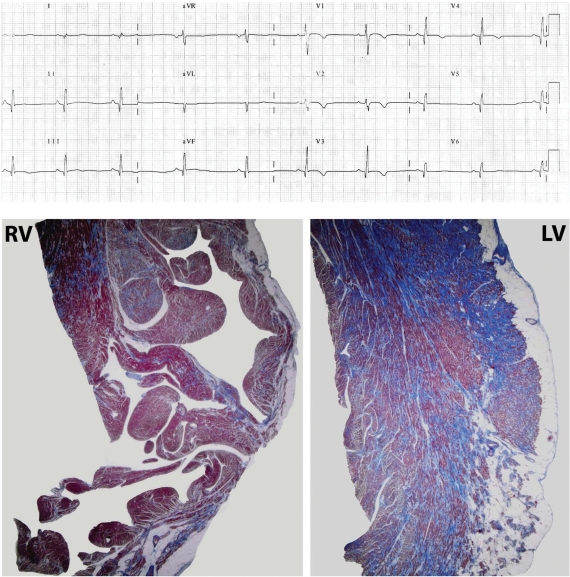
Recording from 12-lead ECG (25 mm/s, 10 mm/mV) (top) of a 18-year-old PKP2 mutation carrier (see Supplementary material online, Table S3, Case 13) shortly before he died suddenly during sport activity. He presented >1000/24 h ventricular extrasystoles but he did not fulfil echocardiographic criteria. There is T-wave inversion in leads V1 to V4 and flattening in V5 and V6; abnormal Q waves are observed in inferolateral leads. Autopsy samples (bottom) reveal mild fibro-fatty replacement of the right ventricular myocardium (RV), with predominant involvement of the left ventricular myocardium (LV), with a subepicardial and midmural distribution and a preserved wall thickness (Heidenhain's trichrome stain).
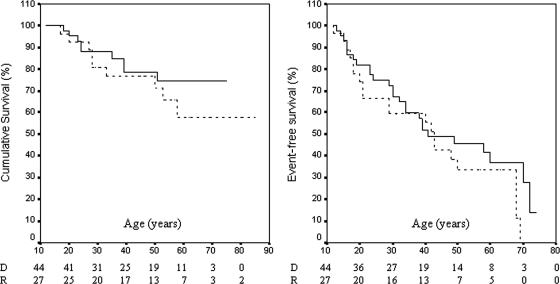
Clinical events occurred in 46 of the 73 affected (63%) (see Supplementary material online, Table S3). The initial presenting event was sustained ventricular tachycardia in 20, syncope without documented sustained ventricular tachycardia in 19, symptoms of heart failure in 2, and sudden death in 5 at age 33 ± 18 years (range: 12–72). Oral antiarrhythmic treatment was applied in 39 patients, surgical ablation was performed in 3, and an antitachycardia pacemaker defibrillator was implanted in 20. Twelve affected carriers (five carriers of dominant mutations and seven homozygous carriers of the recessive mutation) developed heart failure at age 36 ± 15 years (range: 15–60). Eighteen patients died prematurely from a cardiac cause (14 suddenly and 4 from heart failure). The mean age at death was 33 ± 13 years (range: 17–58). The Kaplan–Meier curves did not differ significantly between dominant and recessive affected carriers for cumulative survival (P = 0.37) and event-free survival (P = 0.24) (Figure 4). By the age of 40 years, cumulative survival for dominant and recessive affected carriers was 78.6 ± 6.8 and 76 ± 8.3%, respectively, whereas event-free survival was 51.7 ± 7.9 and 55.4 ± 9.6%, respectively.
Figure 4.
The Kaplan–Meier survival curves for cumulative survival (left) and event-free survival (right) in dominant (D) (solid line) and recessive (R) (dotted line) affected carriers.
Discussion
The diagnosis of ARVC/D is challenging, particularly in relatives of affected individuals in whom disease expression is often incomplete. Task Force Criteria established in 1994 were not sensitive enough to diagnose ARVC/D at early stages of disease when only a subtle phenotype may have developed, although sudden death might be the initial presentation.3,4 The recently modified TFC aimed to increase diagnostic sensitivity with the important requisite of maintaining specificity by including quantitative parameters.4 Cox et al.14 published recently on the sensitivity of modified TFC but not in a genotyped population. However, evaluation of specificity needs a gold standard for diagnosis to exclude false-positive results. In the present study, the pathogenic mutation was considered as a gold standard for the risk of disease development and enabled testing of specificity among mutation-negative family members. Also, the upper limit of diagnostic sensitivity was tested among recessive homozygous carriers in whom ARVC/D shows full penetrance by adolescence.10
In 36 genotyped families affected by dominant and recessive ARVC/D, modification of TFC significantly increased diagnostic sensitivity without a decrease in specificity, which also improved. In dominant ARVC/D that shows incomplete penetrance harbouring the risk of missing mild forms of the disease, the revised criteria improved diagnostic accuracy and increased penetrance from 42 to 61%. In recessive ARVC/D showing a more homogeneous and severe clinical expression, modification of TFC did not alter the existing high penetrance of 97% which implies the upper limit of diagnostic sensitivity. The increased diagnostic sensitivity is mostly due to modified Criterion VI (family history/genetics) supported by modifications in Criteria III (repolarization abnormalities) and V (arrhythmias). Improvement of specificity mostly resulted from modified Criterion IV (depolarization abnormalities) followed by modified Criterion I (functional/structural alterations). False-positive diagnosis using the original TFC was mainly related to right precordial lead QRS prolongation and mild right ventricular dilatation (see Supplementary material online, Table S4).
In this study, 12-lead ECG showed the highest diagnostic sensitivity exceeding 95%. A recent study demonstrated perfect reproducibility for repolarization abnormalities but moderate reproducibility for depolarization abnormalities.15 The vast majority of our patients presented right precordial lead T-wave inversion, whereas epsilon waves and/or terminal activation delay was the only ECG criteria in less than one-forth of cases.
Data presented suggest that electrocardiographic and arrhythmic manifestations of disease precede structural abnormalities. The practical implication of this is that serial evaluation of asymptomatic family members will produce a higher yield when focused on serial ECG and arrhythmia detection. Failure of two-dimensional echocardiography to detect early disease may relate to no full thickness involvement of the myocardium that does not result in detectable change in wall motion or dimensions of the chamber.16 It is possible that delayed enhancement imaging using cardiac magnetic resonance (CMR) may be informative in this arena, particularly in the detection of early left ventricular involvement.17 The finding of electrical abnormalities preceding structural abnormalities has been noted previously and is supported by the case of the 12-year-old boy described in Figure 1, whose initial disease manifestation was the development of ECG/arrhythmia abnormalities in the absence of functional/structural alterations. This confirms an analogous disease presentation of another Naxos child which has been published previously.11
Conclusions
This study demonstrates that revised TFC are more sensitive to original ones for the diagnosis of familial ARVC/D, particularly the dominant form. The data suggest that electrical/arrhythmic abnormalities precede the development of morphological/functional alterations on two-dimensional echocardiography; this will have practical significance for the serial assessment of family members at risk of disease development.
Study limitations
There are several limitations in this study. Although ARVC/D due to desmosome mutations represents more than 50% of ARVC/D probands and the majority of familial cases, other genetic or acquired causes might exist. A standard and accurate protocol was followed for measurement of QRS complex duration; however, in other studies, this electronic method has not been proved to be of higher reproducibility.15 In addition, since CMR was not achievable for all patients, functional/structural alterations were evaluated by two-dimensional echocardiography. Although delayed enhancement in CMR might be proved superior to two-dimensional echocardiography in detecting early left ventricular involvement, it has not yet been included in established criteria for the diagnosis of ARVC/D.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Genetic analysis in this study was supported by the British Heart Foundation (programme grant RG/04/010 to P.S. and W.J.M.).
Conflict of interest: none declared.
Supplementary Material
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. doi:10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 3.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. doi:10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox M, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Yoerger Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D): proposed modification of the task force criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. doi:10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. doi:10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 6.Bauce B, Basso C, Rampazzo A, Beffagna G, Daliento L, Frigo G, Malacrida S, Settimo L, Danieli GA, Thiene G, Nava A. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J. 2005;16:1666–1675. doi: 10.1093/eurheartj/ehi341. doi:10.1093/eurheartj/ehi341. [DOI] [PubMed] [Google Scholar]
- 7.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breidhardt G, Schulze-Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. doi:10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 8.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. doi:10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 9.Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S, McKenna WJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. doi:10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protonotarios N, Tsatsopoulou A, Anastasakis A, Sevdalis E, McKoy G, Stratos K, Gatzoulis K, Tentolouris K, Spiliopoulou C, Panagiotakos D, McKenna W, Toutouzas P. Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J Am Coll Cardiol. 2001;38:1477–1484. doi: 10.1016/s0735-1097(01)01568-6. doi:10.1016/S0735-1097(01)01568-6. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Prost Squarcioni C, Mckenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz J. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. doi:10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, Prakasa K, Tichnell C, James C, Jspevak P, Marcus F, Calkins H. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation. 2004;110:1527–1534. doi: 10.1161/01.CIR.0000142293.60725.18. doi:10.1161/01.CIR.0000142293.60725.18. [DOI] [PubMed] [Google Scholar]
- 13.Foale R, Nihoyannopoulos P, McKenna W, Klienebenne A, Nadazdin A, Rowland E, Smith G. Echocardiographic measurements of the normal adult right ventricle. Br Heart J. 1986;56:33–44. doi: 10.1136/hrt.56.1.33. doi:10.1136/hrt.56.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox MG, van der Smagt JJ, Noorman M, Wiesfeld AC, Volders PGA, vanLangen IM, Atsma DE, Dooijes D, Houweling AC, Loh P, Jordaens L, Arens Y, Cramer MJ, Doevendans PA, vanTintelen JP, Wilde AAM, Hauer RNW. ARVC/D diagnosis: impact of new Task Force Criteria. Circ Arrhythm Electrophysiol. 2010;3:126–133. doi: 10.1161/CIRCEP.109.927202. doi:10.1161/CIRCEP.109.927202. [DOI] [PubMed] [Google Scholar]
- 15.Jain R, Tandri H, Daly A, Tichnell C, James C, Abraham T, Judge DP, Calkins H, Dalal D. Reader-and instrument-dependent variability in the electrocardiographic assessment of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2010 doi: 10.1111/j.1540-8167.2010.01961.x. doi:10.1111/j.1540-8167.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- 16.Basso C, Ronco F, Marcus F, Abudurheman A, Rizzo S, Frigo AC, Bauce B, Maddalena F, Nava A, Corrado D, Grigoletto F, Thiene G. Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria. Eur Heart J. 2008;29:2760–2771. doi: 10.1093/eurheartj/ehn415. doi:10.1093/eurheartj/ehn415. [DOI] [PubMed] [Google Scholar]
- 17.Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. doi: 10.1161/CIRCULATIONAHA.106.660241. doi:10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.