Abstract
Previous studies demonstrated that melittin, the main peptide in bee venom, could cause persistent spontaneous pain, primary heat and mechanical hyperalgesia, and enhance the excitability of spinal nociceptive neurons. However, the underlying mechanism of melittin-induced cutaneous hypersensitivity is unknown. Effects of melittin applied topically to acutely dissociated rat dorsal root ganglion neurons were studied using whole-cell patch clamp and calcium imaging techniques. Melittin induced intracellular calcium increases in 60% of small (<25 µm) and medium (<40 µm) diameter sensory neurons. In current clamp, topical application of melittin evoked long-lasting firing in 55% of small and medium-sized neurons tested. In voltage clamp, melittin evoked inward currents in sensory neurons in a concentration-dependent manner. Repeated application of melittin caused increased amplitude of the inward currents. Most melittin-sensitive neurons were capsaicin-sensitive, and 65% were isolectin B4 positive. Capsazepine, the TRPV1 receptor inhibitor, completely abolished the melittin-induced inward currents and intracellular calcium transients. Inhibitors of signaling pathways showed that phospholipaseA2, but not phospholipase C, was involved in producing the melittin-induced inward currents. Inhibitors of cyclooxygenases (COX) and lipoxygenases (LOX), two key components of the arachidonic acid metabolism pathway, each partially suppressed the inward current evoked by melittin. Inhibitors of protein kinase A (PKA), but not of PKC, also abolished the melittin-induced inward currents. These results indicate that melittin can directly excite small and medium-sized sensory neurons at least in part by activating TRPV1 receptors via PLA2-COXs/LOXs cascade pathways.
Keywords: melittin, bee venom test, TRPV1, phospholipase A2, cyclooxygenases, lipoxygenases
Introduction
The bee venom test, as a tonic pain test in rats, was introduced in 1996 by Lariviere and Melzack [1]. Our group has demonstrated that subcutaneous injection of bee venom induced primary hyperalgesia to mechanical and heat stimuli, and secondary heat hyperalgesia, but not secondary mechanical hyperalgesia [1, 2]. Bee venom is a complex mixture of polypeptides, enzymes, amines, lipids, and amino acids. with various biological, pharmacological, and toxicological activities[2]. Melittin is the principal polypeptide in bee venom [2]. Previously [3, 4] we found that melittin played a central role in the production of nociceptive responses and cutaneous hypersensitivity after whole bee venom injection. In humans, intradermal injection of melittin induced spontaneous pain and increased skin temperature around the injection site, and subcutaneous injection of melittin induced primary hyperalgesia to mechanical and heat stimuli together with neurogenic inflammation [5, 6].
Dorsal root ganglion (DRG) primary sensory neurons responding to noxious stimuli are generally characterized by broader action potentials (AP) with an inflection on the falling phase, larger overshoot, longer duration, and larger amplitude of after-hyperpolarization (AHP) [7]. Melittin-induced behavioral changes and responses of spinal dorsal horn neurons [3, 8] appeared to be mediated by capsaicin-sensitive primary afferent Aδ and C fibers [9–11] via mechanisms involving the transient receptor potential vanilloid receptor 1 (TRPV1) [4]. However, it is currently unknown if melittin can alter the excitability of sensory neurons.
The soma of DRG neurons are commonly used as models for their own peripheral terminals [12] which are inaccessible for adequate electrophysiological studies. Here we examined effects of acute topical application of melittin on the excitability of acutely dissociated DRG neurons. Since melittin is known as an in vitro activator of secreted phospholipase A2 (sPLA2) [13, 14], we further examined the role of sPLA2 in modulating melittin-induced neuronal responses.
Material and methods
Animals
The study protocol was approved by the institutional animal care and use committees of the University of Cincinnati, Capital Medical University (CCMU), and The Fourth Military Medical University (FMMU). The ethical guidelines of the European Communities Council Directive of 24 November 1986 (86/609/EEC) were followed. Experiments were performed on acutely dissociated DRG neurons from male Sprague-Dawley rats (Laboratory Animal Centers of CCMU, Beijing and FMMU, Xi’an, China or Harlan, Indianapolis, IN, USA) weighing 80–100 g. Animals had free access to water and food and were maintained at room temperature (22–24 °C) with a 12 h light/dark cycle.
Cell preparation
Animals were anesthetized with pentobarbital sodium and decapitated. L4–L6 DRG were dissected out and cut in half in a Petri dish at 4 °C in oxygenated DMEM solution. The ganglia were then incubated at 37°C for 40 min in 1mg/ml collagenase (type IA) and 0.4 mg/ml trypsin (type I). After three washes in standard external solution (see below), cells were triturated with a fire-polished Pasteur pipette and plated on glass cover slips coated with poly-D-lysine, incubated in standard external solution at room temperature for 0.5–1 h then stored at 4°C. When used within 8 hrs, cells retained a healthy appearance, negative resting potentials and overshooting APs.
Electrophysiological recordings and calcium imaging
Recordings were made at room temperature (20–22°C) with an EPC10 amplifier and Pulse software (HEKA, Electronik, Germany). Patch electrodes fabricated with PB-7 Puller (Narishige, Tokyo, Japan) had resistances of 2–4 MΩ. The internal solution contained (in mM): 150 KCl, 1 MgCl2, 10 HEPES, and 4 Mg-ATP, adjusted to pH 7.4 with KOH. The capacitance transient was cancelled, series resistance was compensated (>80%), and leak current was subtracted digitally. The liquid junction potential (approximately −10 mV) was corrected. Data were low-pass filtered at 10 kHz and obtained only from small and medium-sized neurons (10 to 40 µm in diameter) with resting membrane potentials < −50 mV and AP overshoot above 0 mV. In some patch clamp experiments, isolectin B4 (IB4) conjugated to fluorescein isothiocyante (FITC) (1 µg/µl) was added to the culture medium for 30–60 min prior to recording to identify IB4-positive and IB4-negative neurons. Calcium imaging was performed using an upright Olympus IX81microscope with Olympus Fluociew ver.1.7a software (Olympus, Tokyo, Japan). Intracellular Ca2+ level ([Ca2+]i) was represented by the Fluo-3 fluorescence intensity as described previously [15]. Briefly, cells were preloaded with media containing 5 µM Fluo-3/AM for 30 min at room temperature. Images were collected at 2 Hz with excitation at 488 nm and emission at 530 nm. Data are presented as Fluo-3 fluorescence intensity increase ratio: R=ΔF/F0, where ΔF = F − F0, and F and F0 are the maximum and minimum fluorescence values, respectively.
Chemicals and Solutions
Melittin was isolated and purified from whole bee venom using gel chromatography and reverse-phase high pressure liquid chromatography. Melittin stock solution was 2 mM in water. Capsaicin (8-methyl-N-vanillyl-6-nonenamide, Sigma) stock solution was10 mM in ethanol. Capsazepine, Antiflammin (Anti), U73122, Indomethacin (Indo), NDGA, H-89, and Bisindolylmaleimide (BIM) stock solutions (DMSO) were 5, 5, 2, 10, 10, 1, and 1 mM, respectively. All chemicals were purchased from Sigma-Aldrich, Inc., St. Louis, MO, USA. Stock solutions were stored at −20°C and diluted to final concentrations prior to use in standard external solution, which contained (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2.5 CaCl2, 10 HEPES and 10 glucose (pH 7.4 adjusted with NaOH). Test solutions were topically applied to DRG neurons using the DAD-VC Voltage Command Valve Control System (ALA Science, New York, USA), which can quickly change the local solution around individual cells.
Statistical analysis
All results were expressed as mean ± S.E.M. Nonparametric statistics were used for data not normally distributed. The specific test used in each case is indicated in the figure legends or text. P value < 0.05 was considered to be statistically significant. For each cell, if the calcium ratio increased more than 0.20 (20%) above baseline after melittin or capsaicin application, it was counted as melittin-sensitive or capsaicin-sensitive, respectively. In patch clamp recording experiments, a cell was defined as melittin-sensitive if melittin-induced firing persisted more than 10 seconds in current clamp mode.
Results
Acute topical application of melittin excited DRG neurons
We first measured calcium responses in acutely dissociated DRG neurons. In most experiments, a melittin concentration of 2 µM was chosen based on literature review [16, 17]. We found that topical application of melittin increased [Ca2+]i by at least 20% in 93 of 134 (69%) cells tested (Figure 1A). In melittin-sensitive cells, the average response latency was 124±57 (s) and the average [Ca2+] increase ratio was 1.5±0.1.
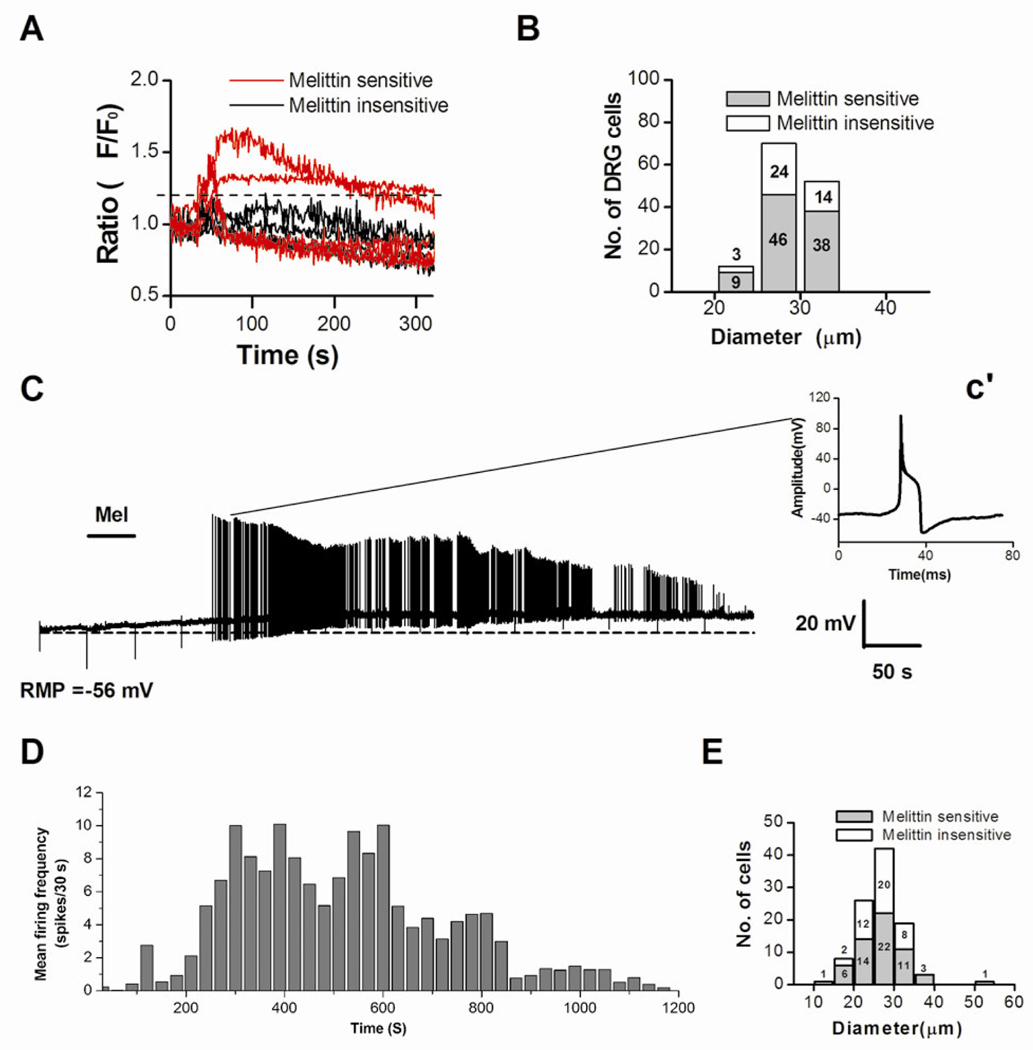
Figure 1. Melittin (Mel)-induced rise in intracellular Ca2+ concentration ([Ca2+]i) and action potential firing in DRG neurons.
A: Graph shows that a subpopulation of acutely isolated rat DRG neurons were able to respond to Mel (2.0 µM) with a [Ca2+]i rise (red vs. black). Typical traces of [Ca2+]i changes were simultaneously recorded during the application of Mel for 30 s. B: Distribution histogram of the cell body sizes for Mel sensitive (n=93) and Mel insensitive (n=41) DRG neurons. C: Application of Mel (2.0 µM, for 50 s) to a DRG cell evoked a slow membrane depolarization (resting membrane potential, RMP = −56 mV before melittin application; dotted line) and a period of tonic AP discharge. Inset c’ shows the waveform of the AP recorded at the indicated time, 133 seconds after the melittin application. D: Histogram showing time course of firing frequency averaged from 45 melittin-sensitive DRG neurons. E. Distribution histogram of cell body diameters for Mel-sensitive (n=56) and Mel-insensitive (n=46) DRG neurons as measured in current clamp experiments.
Examples of DRG cells responding to melittin are given in Figure 1A in which of 9 cells tested, 5 responded to melittin, and 4 did not show any responses. The melittin-sensitive cells ranged from 20 to 35µm in diameter, which is the size range for small and medium-sized cells (Figure 1B).
Whole-cell patch clamp technique was used to measure the electrophysiological responses of DRG neurons after melittin application. In current clamp mode, 55% (56/102) of recorded DRG neurons responded to melittin. A typical response to a 50-s application of melittin (2 µM) is shown in Figure 1C. The cell responded with a slow depolarization (average value, 18 ± 2 mV) and long lasting discharges. The amplitude of the APs decreased dramatically over time after melittin application, possibly caused by accumulation of inactivated sodium channels. The duration of melittin-induced firing ranged from 70 s to 1200 s (Figure 1D) and was most intense during the 200–800 s time period. However, there was no correlation between the size of DRG neurons and the latencies of melittin-induced responses, which ranged from 33 s to 826 s. The right inset (Figure 2c’) represents a single AP observed at 133s after the melittin was applied. The AP exhibited typical electrophysiological characteristics of nociceptive cells: a long AP duration and a prolonged after- hyperpolarization. There was an inflection on its falling phase, which is generally considered characteristic of nociceptors [7]. Among all the melittin-sensitive cells, 20 were small- (<25µm) and 36 were medium-sized (<40µm) (Figure 1E). Thus, many of the melittin-sensitive cells we recorded are likely nociceptive. This was confirmed in a different experiment using the calcium imaging technique, in which we examined the capsaicin-sensitivity in DRG cells that responded to melittin. Of the 14 melittin-sensitive cells tested, 13 responded to a subsequent topical application of capsaicin (1 µM).
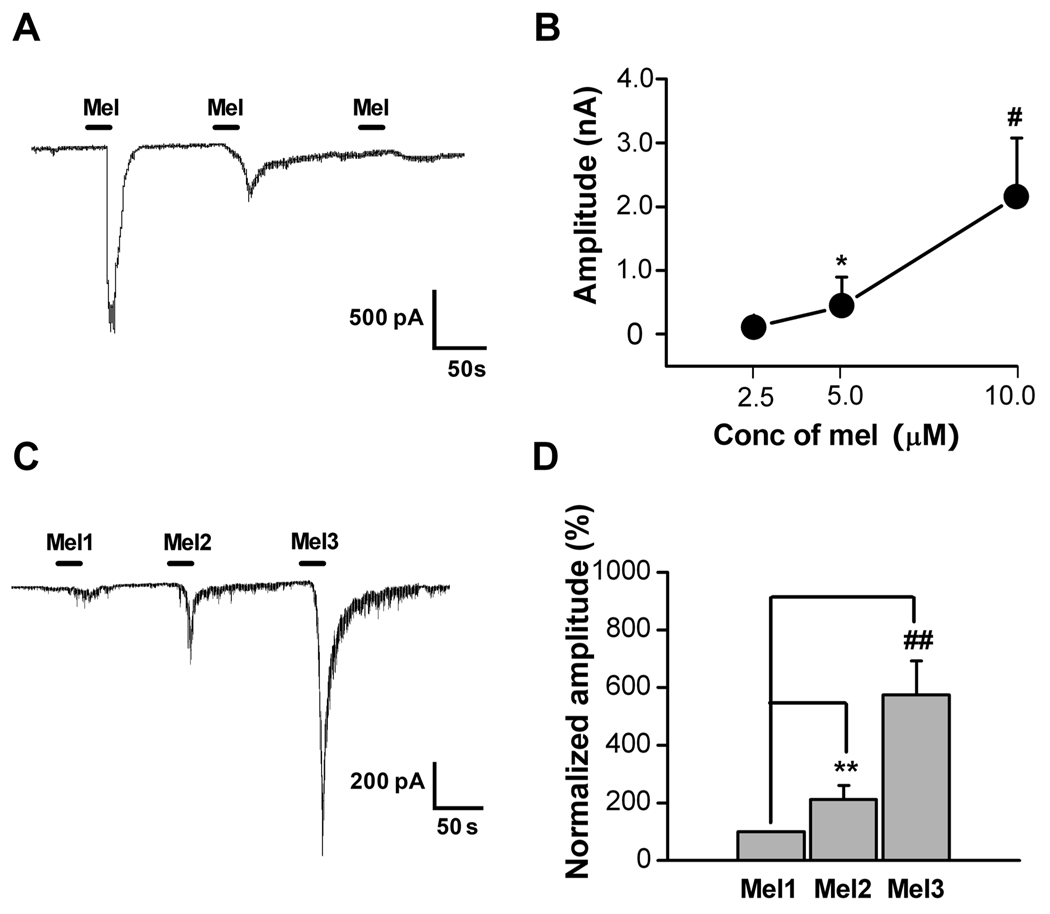
Figure 2. Dose-related and sensitization effects of melittin (Mel) on DRG cell currents.
A: Amplitude of current evoked from a DRG cell following successive applications of Mel (10, 5, 1 µM) showed dose-dependent decreases in evoked current. B: Plot of mean inward current amplitude evoked by different doses of melittin (2.5, 5, 10µM) in DRG cells (n=17, 15, 7, respectively). *, # p<0.05 compared to lowest dose (Kruskal-Wallis ANOVA on Ranks with Dunn’s post test). For this experiment each cell was exposed to only one dose. C: Sample trace of a current recording from a DRG cell following three repeated application of 2 µM Mel (Mel1, Mel2 and Mel3). There was a clear sensitization of the Mel-evoked inward current following repeated applications. D: Average responses to successive applications of 2 µM Mel (protocol as in C), normalized to the amplitude of the first response. ##,** P<0.01 vs. Mel1(Wilcoxon Signed Rank Test with Bonferroni correction, n=20). Cells with no response to the first application excluded from the analysis.
Next we tested melittin-sensitivity in both IB4-positive and IB4-negative neurons using patch clamp recording combined with IB4 labeling. Among 52 cells recorded, 37 were melittin-sensitive. Twenty-four (65%) of the 37 melittin-sensitive cells were IB4-positive and the rest were IB4-negative.
Using voltage clamp recordings at a holding potential of −70 mV, we found that melittin evoked large inward currents in 39/86 neurons (42%) in a concentration-dependent manner. Figure 2A shows an example of a neuron in which the amplitude of inward currents decreased with decreasing concentrations of melittin (10, 5, 1 µM) applied sequentially to the same neuron at 2-min intervals. Figure 2B shows the average concentration-response relationship for melittin-induced inward currents; in these experiments each cell was exposed to only one dose of melittin. Repeated application of 2 µM melittin (20 s interval, 200–300 s duration) had sensitizing effects on the inward currents, as evidenced by an increased current amplitude following the second (Mel2) and the third (Mel3) melittin application compared to the first response (Figure 2C, D).
Melittin-induced activation of DRG neurons was blocked by a TRPV1 antagonist and PLA2-COX/LOX inhibitors
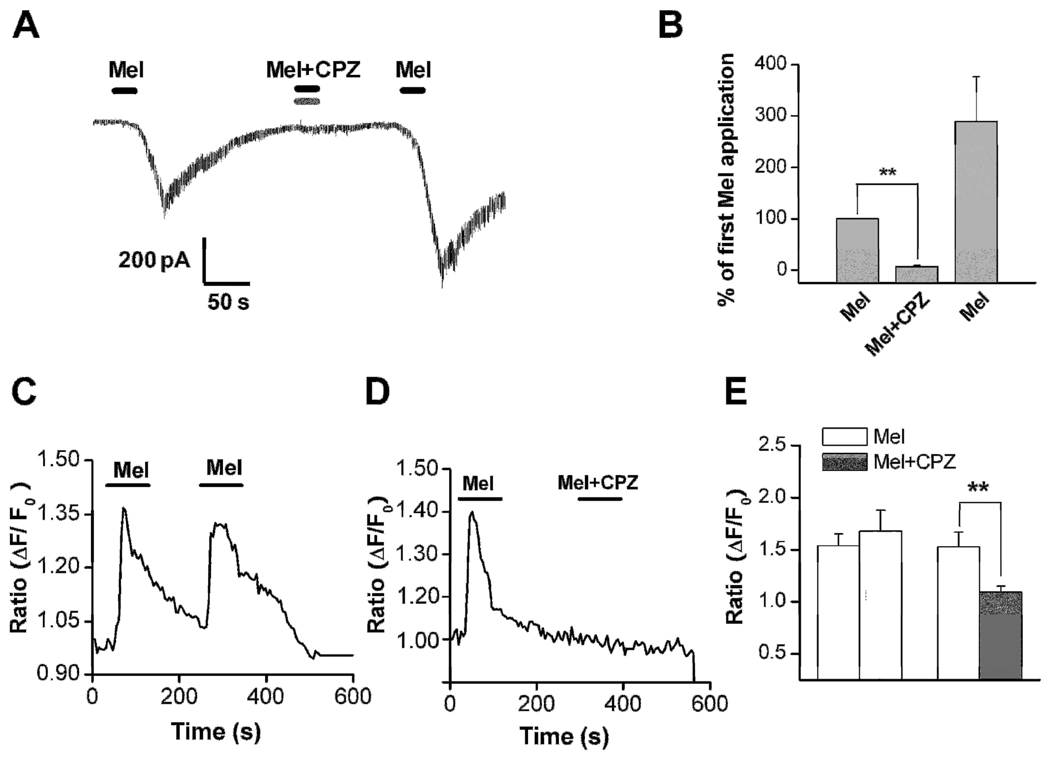
Capsazepine, an inhibitor of the TRPV1 receptor, blocked the inward current induced by 2 µM melittin in a reversible manner (Figure 3A,B). In this series of experiments, melittin plus the capsazepine vehicle (DMSO, 0.1%) was first applied to the recorded cells followed by melittin plus capsazepine, followed by a third application of Melittin plus vehicle in the absence of capsazepine. As shown in figure 3B, the melittin response in the presence of capsazepine (“Mel + CPZ”) was significantly reduced to almost zero. After washing out of the capsazepine, the melittin response was largely restored. The above results were further confirmed using the calcium imaging technique. It was found that capsazepine application blocked the [Ca2+]i rise induced by melittin: the average calcium ratio was 1.1±0.06 during the second application of melittin in the presence of capsazepine, compared to 1.5±0.14 for the first application in the absence of capsazepine (n = 56; Figure 3D). In most cells (n=46) the capsazepine completely blocked the calcium response; in 10 cells partial block was observed. This result cannot be explained by a desensitization of the melittin-evoked calcium response since a second application of melittin (2 µM) in the absence of the blocker consistently produced an increase in the intracellular calcium level in the absence of the TRPV1 antagonist (Figure 3C). In these experiments the first application gave an average calcium ratio increase of 1.4±0.1 from baseline, while the second application gave an increase of 1.7±0.2, n= 52; p = 0.004 comparing the second application in the presence vs. absence of capsazepine.
Figure 3. The blocking effect of capsazepine (CPZ), a TRPV1 antagonist.
A: Co-application of capsazepine (CPZ, 5 µM) (Vh = −70 mV) resulted in a significant blockade of the 2.0 µM Mel-evoked inward currents from a holding potential of −70 mV (n=5). Protocol was as in Fig. 2C except that capsazepine was co-applied during the second melittin application. B: Statistical analysis of inhibitory effects of CPZ on the melittin-induced inward current. **P<0.01, Mel+CPZ vs. Mel (first application) (Students paired t-test). C: Repeated application of melittin evoked rises in intracellular Ca2+ concentration ([Ca2+]i) measured by calcium imaging. D: Blocking effects of capsazepine (CPZ) on Melittin-induced rise in intracellular Ca2+ concentration ([Ca2+]i) measured by calcium imaging. E. Statistical analysis of inhibitory effects of CPZ on the melittin-induced rises in [Ca2+]i. **P<0.01, Mel+CPZ vs. Mel (paired t-test). Left pair of bars corresponds to the protocol shown in C (first and second applications of melittin); right pair of bars corresponds to protocol shown in D (first melittin application followed by second application of melittin + CPZ).
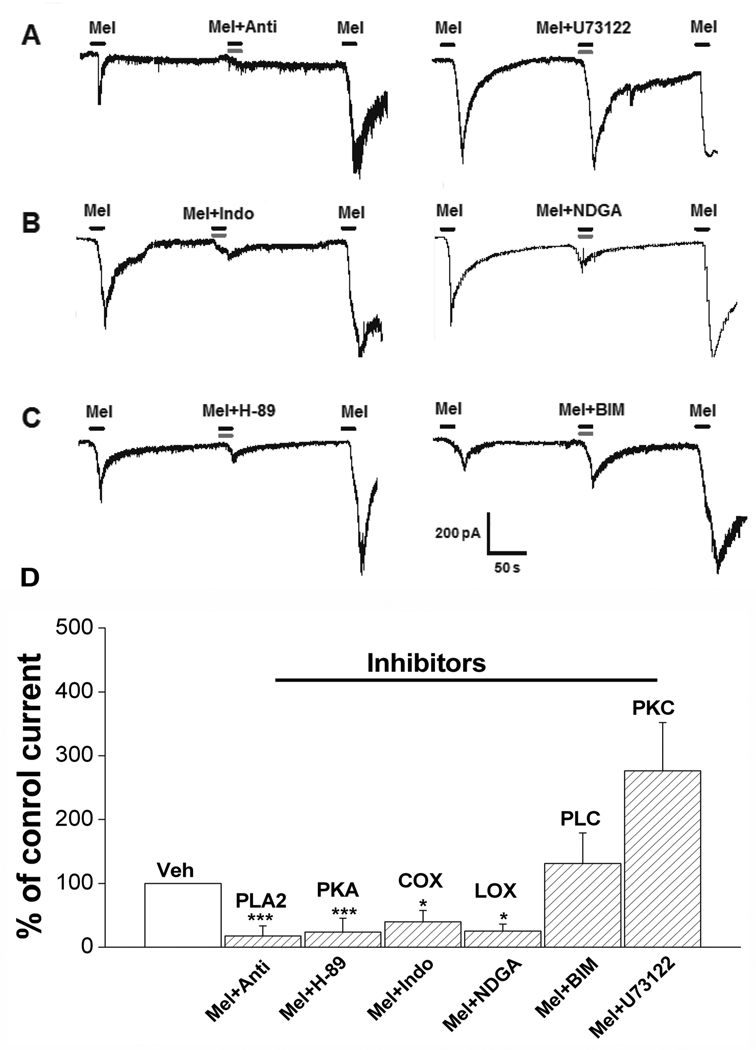
In the next study, melittin plus the antiflammine vehicle was first applied to the dissociated DRG cells followed by co-treatment of the same cells with 2 µM melittin and 1 µM antiflammine, a PLA2 inhibitor. As shown Figure 4A (left) and D, the inward current induced by melittin alone was significantly reduced when combined with antiflammine (n=9). Melittin sensitivity was restored after a 2-min washout with normal external solution. In contrast, 1µM U-73122, a PLC inhibitor, failed to block melittin-induced responses (n=7), using the same experimental protocol (Figure 4B right, D). Furthermore, melittin-induced inward current was blocked when combined with nordihydroguaiaretic acid (NDGA), a non-specific LOX inhibitor (n=7). Similarly, indomethacin (10 µM), a cyclooxygenase inhibitor, also reduced melittin-induced inward current (n=5; Figure 4B, D). These results indicate that melittin evokes inward currents in sensory neurons by stimulating PLA2 and LOX/COX, but not PLC.
Figure 4. The effect of inhibitors of PLA2-LOX/COX metabolites on the inward current induced by melittin.
A: PLA2 but not PLC is involved in the generation of the inward current induced by melittin. Anti (1 µM), Antiflammine, a blocker of PLA2. U73122 (1 µM) is a blocker of PLC. B: Both COX and LOX are partially involved in the inward current induced by melittin. Indo (10 µM), Indomethacin, a blocker of COX. NDGA is a blocker of LOX. C: PKA but not PKC is involved the inward current induced by melittin. H-89 (1µM) is a blocker of PKA. BIM (1µM) is a blocker of PKC. For all inhibitor experiments, the first and third application of melittin included the appropriate vehicle control for that inhibitor, while the second application was of melittin plus the indicated inhibitor. D. Statistical analyses of the effect of inhibitors of PLA2-LOX/COX metabolites on the inward current. *, P<0.05; **, P<0.01, *** P<0.001 compared with normalized control current during the first Mel application (Paired t-test or Wilcoxon signed rank test, as appropriate). Cells not responding to the first application of melittin excluded from the inhibitor analysis data.
Application of 1 µM H-89, a PKA inhibitor, blocked the melittin-induced inward current in 9 cells recorded (p<0.001). In contrast, 1µM BIM, a PKC inhibitor, failed to block the melittin-induced responses in all 5 cells tested (Figure 4C and D).
Discussion
In this study, we demonstrated for the first time that topical application of melittin, the main active component of whole bee venom, caused long-lasting discharges and calcium responses in a subset of small and medium-sized DRG neurons in vitro. Melittin also evoked a large inward current that was blocked by inhibitors of the PLA2-LOX/COX pathway. Previously, we have found that subcutaneous injection of melittin in rats caused spontaneous pain and robust mechanical and thermal hyperalgesia beginning in less than 5 min. Results from this study suggest that melittin causes pain by directly activating nociceptive sensory neurons. The long-lasting discharge as observed in the current study may contribute to the onset of spontaneous pain and the initiation of primary and secondary cutaneous hyperalgesia.
We also found that the melittin-evoked inward current was blocked by the TRPV1 receptor antagonist capsazepine. Capsazepine, in addition to being a competitive TRPV1 blocker [18], has been reported to block voltage-gated calcium channels at higher doses or longer exposures than used here [19], but this seems unlikely to explain the present results, especially since the melittin-evoked current was observed at a holding potential −70 where calcium channels would not be activated. This suggests that the effects of melittin on the excitability of DRG neurons were mainly mediated by the TRPV1 receptor, rather than voltage-gated calcium channels. It is known that capsaicin binds to the intracellular domain of the capsaicin-activated ion channels [20], which makes it plausible that certain endogenous substances may also activate TRPV1 receptor at an intracellular site, and lipoxygenase products can directly activate TRPV1 receptors [21].
Results from our current study indicated that melittin-evoked inward currents are mediated by the PLA2-COX/LOX pathway. Melittin is a powerful PLA2 activator [22, 23] and releases arachidonic acid from plasma membrane [24, 25]. It is most likely that topical application of melittin caused the release of COX/LOX metabolites such as prostaglandins and HPETEs by activating the PLA2-COX/LOX pathway. As mentioned earlier, HPETEs, as lipoxygenase products, are able to activate TRPV1 receptors. A similar pathway, of PLA2 activating lipoxygenases which then activate TRPV1 receptors, has been proposed to account for the effects of histamine[26] and bradykinin[27].
Our results indicate that the PLC and PKC pathways are not involved in melittin-induced excitatory effects on sensory neurons. However, blocking the PKA pathway can effectively reduce melittin-evoked inward currents. The PKA pathway can regulate the TPRV1 receptor via receptor phosphorylation [28].
Perhaps the most striking observation in our patch clamp studies is the sensitizing effects of melittin on sensory neurons during repeated applications. The response to a brief application of melittin is long-lasting. In some cells, melittin-induced firing may last for up to 20 min following a 50-s treatment. Although melittin-induced inward currents are blocked by a TRPV1 receptor antagonist, there are distinct differences between melittin- and capsaicin-induced responses. Repeated application of capsaicin often leads to desensitization rather than sensitization of nociceptive neurons [29]. In addition, capsaicin-evoked responses in nociceptive neurons only last a few seconds.
In summary, this study demonstrates that melittin can activate and/or sensitize small- and medium-sized sensory neurons by activating TRPV1 receptors via the PLA2-COX/LOX pathway. Many of the melittin-sensitive neurons are capsaicin-sensitive nociceptive neurons. Results of this study will help in developing new strategies in treating acute inflammatory pain.
Acknowledgement
The authors thank Mark Baccei, Ph.D., University of Cincinnati, for comments on the manuscript.
Funding: This work was supported in part by NSFC (30770668, 81070899) (J Chen), the Natural Science Foundation of Beijing (KZ200510025016) (J Chen), NIH grants NS55860 (J-M Zhang), and NS45594 (J-M Zhang).
Abbreviations
- AP
action potential
- COX
cyclooxygenase
- DRG
dorsal root ganglion
- IB4
isolectin B4
- LOX
lipoxygenase
- PKA
protein kinase A
- PKC
protein kinase C
- PLA2
phospholipase A2
- PLC
phospholipase C
- TRPV1
transient receptor potential vanilloid receptor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the funding sources played any additional roles in the work.
References
- 1.Lariviere WR, Melzack R. The bee venom test: a new tonic-pain test. Pain. 1996;66:271–277. doi: 10.1016/0304-3959(96)03075-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Lariviere WR. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog Neurobiol. 2010;92:151–183. doi: 10.1016/j.pneurobio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li KC, Chen J. Altered pain-related behaviors and spinal neuronal responses produced by s.c. injection of melittin in rats. Neuroscience. 2004;126:753–762. doi: 10.1016/j.neuroscience.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Chen YN, Li KC, Li Z, Shang GW, Liu DN, Lu ZM, Zhang JW, Ji YH, Gao GD, Chen J. Effects of bee venom peptidergic components on rat pain-related behaviors and inflammation. Neuroscience. 2006;138:631–640. doi: 10.1016/j.neuroscience.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Koyama N, Hirata K, Hori K, Dan K, Yokota T. Computer-assisted infrared thermographic study of axon reflex induced by intradermal melittin. Pain. 2000;84:133–139. doi: 10.1016/s0304-3959(99)00192-x. [DOI] [PubMed] [Google Scholar]
- 6.Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. A comparison of hyperalgesia and neurogenic inflammation induced by melittin and capsaicin in humans. Neurosci Lett. 2003;337:147–150. doi: 10.1016/s0304-3940(02)01325-3. [DOI] [PubMed] [Google Scholar]
- 7.Todd AJ, Koerber HR. Neuroanatomical substrates of spinal nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. Elsevier; 2006. pp. 73–90. [Google Scholar]
- 8.Yu YQ, Chen J. Activation of spinal extracellular signaling-regulated kinases by intraplantar melittin injection. Neurosci Lett. 2005;381:194–198. doi: 10.1016/j.neulet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Chen HS. Pivotal role of capsaicin-sensitive primary afferents in development of both heat and mechanical hyperalgesia induced by intraplantar bee venom injection. Pain. 2001;91:367–376. doi: 10.1016/S0304-3959(00)00458-9. [DOI] [PubMed] [Google Scholar]
- 10.Shin HK, Kim JH. Melittin selectively activates capsaicin-sensitive primary afferent fibers. Neuroreport. 2004;15:1745–1749. doi: 10.1097/01.wnr.0000135919.37807.a7. [DOI] [PubMed] [Google Scholar]
- 11.Chen HS, He X, Wang Y, Wen WW, You HJ, Arendt-Nielsen L. Roles of capsaicin-sensitive primary afferents in differential rat models of inflammatory pain: a systematic comparative study in conscious rats. Exp Neurol. 2007;204:244–251. doi: 10.1016/j.expneurol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Vyklicky L, Knotkova-Urbancova H. Can sensory neurones in culture serve as a model of nociception? Physiol Res. 1996;45:1–9. [PubMed] [Google Scholar]
- 13.Hassid A, Levine L. Stimulation of phospholipase activity and prostaglandin biosynthesis by melittin in cell culture and in vivo. Res Commun Chem Pathol Pharmacol. 1977;18:507–517. [PubMed] [Google Scholar]
- 14.Steiner MR, Bomalaski JS, Clark MA. Responses of purified phospholipases A2 to phospholipase A2 activating protein (PLAP) and melittin. Biochim Biophys Acta. 1993;1166:124–130. doi: 10.1016/0005-2760(93)90292-h. [DOI] [PubMed] [Google Scholar]
- 15.Merritt JE, McCarthy SA, Davies MP, Moores KE. Use of fluo-3 to measure cytosolic Ca2+ in platelets and neutrophils. Loading cells with the dye, calibration of traces, measurements in the presence of plasma, and buffering of cytosolic Ca2+ Biochem J. 1990;269:513–519. doi: 10.1042/bj2690513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Chen T, Zhang N, Yang M, Li B, Lu X, Cao X, Ling C. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J Biol Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- 17.Yang Shen LJ-E, Ao-zhen Zhang, Ming-Hua Jiang. Biphasic manner of melittin on isolated guinea pig atria. Acta Pharmacol Sin. 2000;21:221–224. [PubMed] [Google Scholar]
- 18.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shier WT, Trotter JT. Phospholipase A2 electrophoretic variants and their detection after polyacrylamide gel electrophoresis. Anal Biochem. 1978;87:604–611. doi: 10.1016/0003-2697(78)90711-x. [DOI] [PubMed] [Google Scholar]
- 23.Yalcin M, Ak F, Erturk M. The role of the central thromboxane A2 in cardiovascular effects of a phospholipase A2 activator melittin administrated intracerebroventricularly in normotensive conscious rats. Neuropeptides. 2006;40:207–212. doi: 10.1016/j.npep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Metz SA. Lack of specificity of melittin as a probe for insulin release mediated by endogenous phospholipase A2 or lipoxygenase. Biochem Pharmacol. 1986;35:3371–3381. doi: 10.1016/0006-2952(86)90438-7. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen SF, Poulsen KA, Lambert IH. Roles of phospholipase A2 isoforms in swelling- and melittin-induced arachidonic acid release and taurine efflux in NIH3T3 fibroblasts. Am J Physiol Cell Physiol. 2006;291:C1286–C1296. doi: 10.1152/ajpcell.00325.2005. [DOI] [PubMed] [Google Scholar]
- 26.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 28.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 29.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]