Abstract
High-speed counter-current chromatography (HSCCC) was successfully applied for the preparative separation and purification of alkaloids from Corydalis bungeana Turcz. (Kudiding in Chinese) for the first time. After the measurement of partition coefficient of seven target alkaloids in the nine two-phase solvent systems composed of CHCl3–MeOH–(0.1 M; 0.2 M; 0.3 M) HCl (4:1.5:2; 4:2:2; 4:3:2, v/v), CHCl3–MeOH–0.2 M HCl (4:2:2, v/v) and CHCl3–MeOH–0.3 M HCl (4:3:2, v/v) were finally selected for the HSCCC separation using the first upper phase as the stationary phase and the stepwise elution of the two lower mobile phases. Consequently, sanguinarine (10 mg), corynoline (25 mg), protopine (20 mg), corynoloxine (18 mg), and 12-hydroxycorynoline (8 mg) were obtained from 200 mg of crude alkaloid extracts with purities of 94–99% as determined by HPLC. Their chemical structures were characterized on the basis of 1H-NMR, 13C-NMR, and LC-ESI-Q-TOF-MS/MS analyses.
Keywords: Alkaloids, Corydalis bungeana Turcz., ESI-MS, High-speed counter-current chromatography
1 Introduction
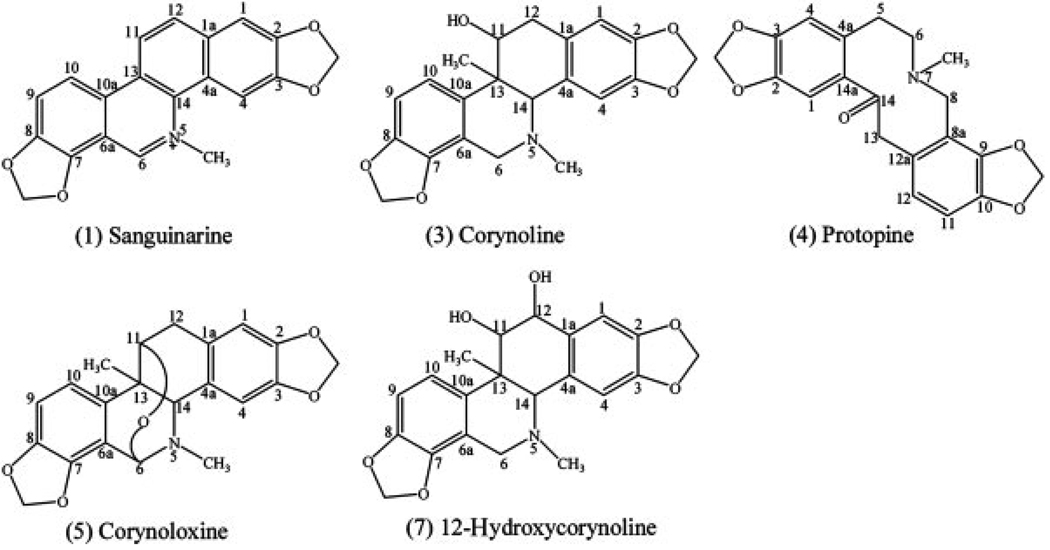
Corydalis bungeana Turcz. (Papaveraceae) is a perennial herb with violet to pink flowers distributed in the northern and eastern parts of China, the south-east of Mongolia, the northern part of the Korean peninsula and the far east of Russia [1]. The dried whole plant is referred to in traditional Chinese medicine as C. Bungeanae Herba and is officially listed in the Chinese Pharmacopoeia [2]. Alkaloids are its main components and their chemical structures are shown in Fig. 1. Some of them have many biological activities. Pharmacological experiment shows that the corynoline, acetylcorynoline, and protopine can significantly impede liver damage caused by CCl4 in mice [3]. Some isoquinoline alkaloids isolated from C. bungeana Turcz. have bacteriostatic activity [4]. Besides, it has been recorded for some local uses, such as treatment of influenza, upper respiratory tract infections, bronchitis, tonsillitis, acute nephritis, and pyelonephritis [1]. Multistep silica gel column chromatography, Sephadex LH-20 column chromatography, and recrystallization have been reported for the purification of alkaloids from C. bungeana Turcz. [1, 5]. These methods are tedious, time consuming, and require multiple chromatographic steps resulting in lower recovery and higher cost.
Figure 1.
Chemical structures of alkaloids from C. bungeana Turcz.
High-speed counter-current chromatography (HSCCC), first invented by Ito [6], is a kind of continuous liquid–liquid partition chromatography technique without using solid support matrix. The liquid stationary phase of this method is immobilized on the column by centrifugal force. HSCCC efficiently eliminates the irreversible adsorption of sample onto the solid support used in the conventional chromatographic column. As an advanced separation technique, it offers various advantages including high sample loading capacity, high sample recovery, and high purity of fractions. So, it is especially suitable for the separation and purification of active components from natural products such as alkaloids [7, 8], coumarins [9, 10], flavonoids [11], anthraquinones [12], polyphenols [13], and so on. The two-phase solvent systems, composed of CHCl3–MeOH–dilute HCl (or phosphate buffer), had been widely used for the HSCCC separation of alkaloids [7, 14]. Furthermore, stepwise elution mode in HSCCC had been frequently reported using mobile phases with pH values or different polarities for fast and efficient separation [15, 16].
The purpose of this study is to develop a method for the preparative isolation and purification of alkaloids from C. bungeana Turcz. by HSCCC. Two-phase solvent systems, CHCl3–MeOH–0.2 M HCl (4:2:2, v/v) and CHCl3–MeOH–0.3 M HCl (4:3:2, v/v), were chosen with stepwise elution. The chemical structures of the purified alkaloids were identified by 1H-NMR, 13C-NMR, and ESI-Q-TOF-MS/MS analyses.
2 Materials and methods
2.1 Apparatus
The HSCCC instrument employed in the present study was a TBE-300A HSCCC (Tauto Biotechnique, Shanghai, China) with three multilayer coil separation columns connected in series (PTFE tubing i.d.: 1.6 mm, the total volume of column: 260 mL) and a 20 mL sample loop. The revolution radius was 5 cm, and the β values of the multilayer coil varied from 0.5 at the internal terminal to 0.8 at the external terminal. The revolution speed of the apparatus can be regulated with a speed controller in the range from 0 to 1000 rpm. An HX 105 constant-temperature circulation implement (Beijing Changliu Scientific Instruments, Beijing, China) was used to control the temperature of the column oven. The solvent was pumped into the column with a Model TBP5002 constant-flow pump (Tauto Biotechnique). Continuous monitoring of the effluent was performed with a Model TBD-2000 Monitor (Tauto Biotechnique) at 254 nm. The data were recorded by WH500-USB chromatography workstation (Tauto Biotechnique).
HPLC system used was a Shimadzu LC-10AVP system consisting of two LC-10ATVP solvent delivery units, a UV–Vis photodiode array detector (SPD- M10AVP), an auto injector (SPD-10ADVP), a system controller (SCL-10AVP), a column oven (CTO-10ASVP) and a degasser (DGU-12A), and a Class-VP-LC workstation (Shimadzu, Kyoto, Japan). The column used was an Ultimate XB-C18 column (250 × 4.6 mm i.d., 5 µm; Welch Materials, USA). NMR experiments were carried out using a Bruker DMX 600 NMR spectrometer with chloroform (CDCl3) or MeOH (CD3OD) as the solvent.
The MS and MS/MS analyses of purified alkaloids were performed by ESI-Q-TOF mass spectrometer (Agilent 6530 Series, Agilent Technologies, Santa Clara, CA, USA) in a positive ionization mode. Parameters for the Agilent Jet Stream Technology are the superheated nitrogen sheath gas temperature (400°C) and flow rate (12 L/min). Electrospray conditions were the following: capillary: 4000 V; nebulizer: 40 psi; drying gas: 5 L/min; gas temperature: 325°C; skimmer voltage: 65 V; octapole RFPeak: 750 V; fragmentor: 130 V.
2.2 Reagents
All solvents used for preparation of crude sample and HSCCC separation are of analytical grade and methanol used for HPLC is of chromatography grade. They were all purchased from Beijing Yili Fine Chemical (Beijing, China). Water used was distilled. All solvents used for MS were obtained from J. T. Baker (Phillipsburg, NJ, USA). All solvents used for NMR were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.3 Extraction of crude alkaloids
The ground whole plants of C. bungeana Turcz. (2 kg) were extracted with 95% ethanol for three times. Then, the extracts were combined and evaporated to dryness under reduced pressure at 45°C. The residue obtained from the combined extracts was dissolved with 500 mL of 2% HCl. After filtration, the acidic aqueous solution was extracted three times with CHCl3 to remove the lipophilic impurities. Then the pH of the acidic aqueous solution was adjusted to about 10.0 with aqueous ammonia, and the total alkaloids were extracted with CHCl3 for three times. The CHCl3 extracts were combined and evaporated to dryness. The crude alkaloid extracts were stored in a refrigerator (−20°C) and subjected to preparative HSCCC separation.
2.4 Selection of the two-phase solvent systems
A series of two-phase solvent systems composed of CHCl3–MeOH–(0.1–0.3 M) HCl were made at three different volume ratios (4:1.5:2, 4:2:2, 4:3:2, v/v) in small amount (7–10 mL) and thoroughly equilibrated. About 3 mg of crude alkaloid sample was dissolved in a mixture of equal volume (1 mL) of the upper and lower phases of each of the nine two-phase solvent systems. The partition coefficient (K) of each alkaloid was determined by HPLC and calculated using the equation: K = Aupper phase/Alower phase (Aupper phase: peak area of one alkaloid from the upper phase; Alower phase: peak area of one alkaloid from the lower phase). The two-phase solvent systems were finally selected according to the partition coefficient (K) values of the target alkaloids.
2.5 Preparation of two-phase solvent systems and sample solution
The selected two-phase solvent systems CHCl3–MeOH–0.2 M HCl (4:2:2, v/v) and CHCl3–MeOH–0.3 M HCl (4:3:2, v/v) were prepared in a separatory funnel and thoroughly equilibrated at room temperature. Then, the upper phase and the lower phase were separated and degassed by sonication just before use. The sample solution was prepared by dissolving 200 mg of crude alkaloid sample in a mixture of 2 mL of each of the upper phase and lower phase.
2.6 HSCCC separation procedure
The HSCCC separation was performed as follows: the multilayer coiled column was first entirely filled with the upper aqueous stationary phase. Then, the apparatus was run at a revolution speed of 800 rpm. At the same time, the lower organic mobile phase was then pumped into the column at a flow rate of 2.0 or 4.0 mL/min in the head to tail mode. After the mobile phase emerged from the tail outlet of the column and hydrodynamic equilibrium was established, the sample solution was injected into the column through the sample port. The effluent from the column was continuously monitored with a UV detector at 254 nm. Peak fractions were manually collected according to the chromatogram. After the separation was completed, the apparatus was stopped and the retention of stationary phase was measured by collecting the column contents into a graduate cylinder by forcing them out of the column with pressurized nitrogen gas. Peak fractions were analyzed using HPLC.
2.7 HPLC analysis and identification of HSCCC fractions
The crude alkaloid extracts of C. bungeana Turcz. and HSCCC peak fractions were analyzed using HPLC with a reversed-phase C18 column (250 mm × 4.6 mm i.d., 5 µm, Welch Materials). Mobile phases: solvent A: methanol containing 0.05% TEA (triethylamine), solvent B: water; gradient: 0.0 min (60% A)–25 min (100% A)–35 min (100% A); flow rate: 1.0 mL/min and detection wavelength: 289 nm.
Identification of the HSCCC fractions was carried out by 1H-NMR, 13C-NMR, and ESI-Q-TOF-MS/MS analyses.
3 Results and discussion
3.1 Selection of two-phase solvent systems of HSCCC and preparative separation
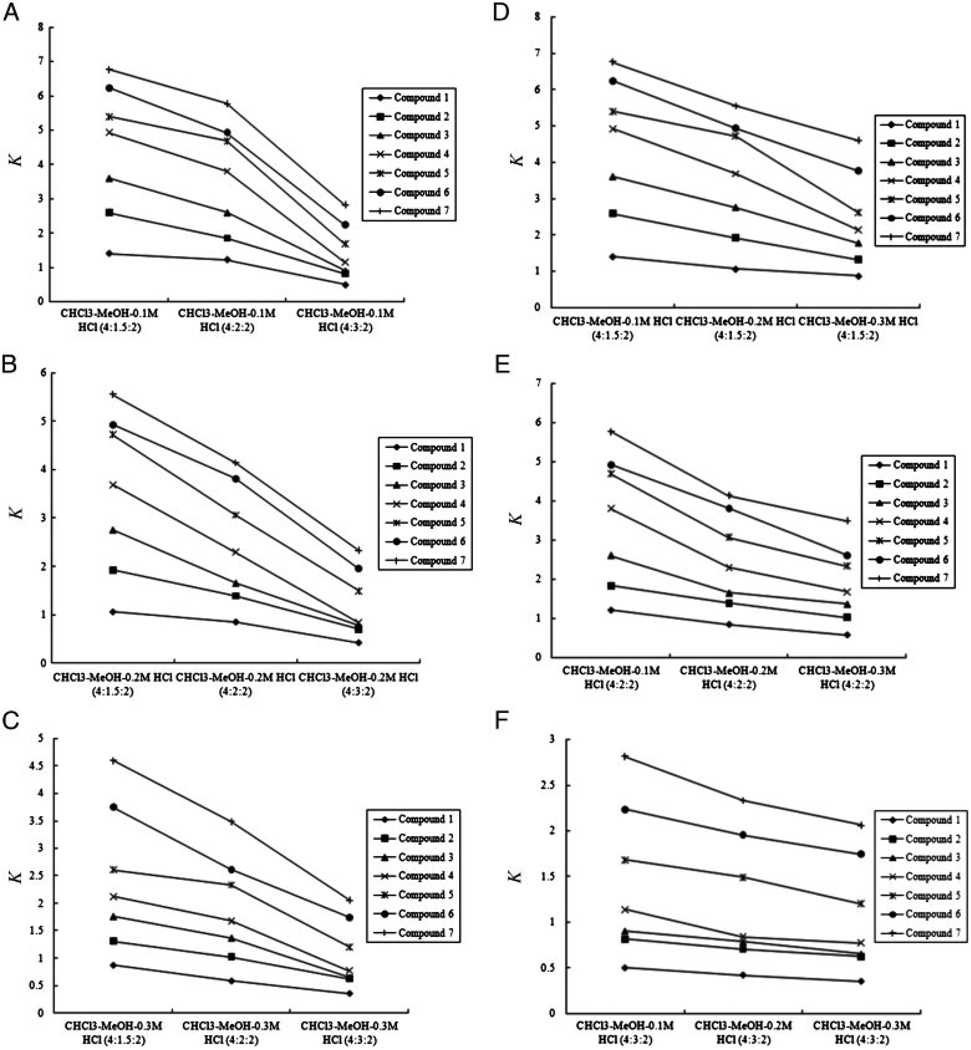
Nine two-phase solvent systems composed of CHCl3–MeOH–(0.1–0.3 M) HCl were made at three different volume ratios (4:1.5:2, 4:2:2, 4:3:2, v/v). The partition coefficient of each alkaloid was determined by HPLC and calculated using the equation (K = Aupper phase/Alower phase). The two-phase solvent systems were selected according to the difference in the partition coefficient (K) values of the seven target alkaloids. Table 1 shows the K values of the seven alkaloids in the nine two-phase solvent systems. Fig. 2(A–C) shows the effect of volume ratio on the K values of seven alkaloids at different concentrations of dilute HCl. The increase in the volume ratio of MeOH could reduce the K values of the target alkaloids. Fig. 2(D–F) shows the effect of the concentration of HCl on the K values of seven alkaloids at different volume ratios. The increase in the concentration of dilute HCl also could reduce the K values of the target alkaloids. Fig. 2 clearly indicated that a stepwise elution should be carried out in this study to purify alkaloids in reasonable time using a combination of CHCl3–MeOH–0.2 M HCl (4:2:2, v/v) or CHCl3–MeOH–0.3 M HCl (4:1.5:2, v/v) with CHCl3–MeOH–0.3 M HCl (4:3:2, v/v). The two-phase solvent systems CHCl3–MeOH–0.2 M HCl (4:2:2, v/v) and CHCl3–MeOH–0.3 M HCl (4:3:2, v/v) were finally selected for the preparative separation of alkaloids by HSCCC with stepwise elution. After the elution of the first four alkaloid peaks with the lower phase of CHCl3–MeOH–0.2 M HCl (4:2:2), the mobile phase was switched to the lower phase of CHCl3–MeOH–0.3 M HCl (4:3:2) to elute the last three alkaloids.
Table 1.
K values of the target compounds 1–7 in nine two-phase solvent systems
| Solvent systems | K1 | K2 | K3 | K4 | K5 | K6 | K7 |
|---|---|---|---|---|---|---|---|
| CHCl3–MeOH–0.1M HCl (4:1.5:2) | 1.4 | 2.59 | 3.6 | 4.93 | 5.4 | 6.24 | 6.76 |
| CHCl3–MeOH–0.1M HCl (4:2:2) | 1.22 | 1.84 | 2.6 | 3.8 | 4.69 | 4.93 | 5.76 |
| CHCl3–MeOH–0.1M HCl (4:3:2) | 0.5 | 0.81 | 0.9 | 1.14 | 1.68 | 2.23 | 2.81 |
| CHCl3–MeOH–0.2M HCl (4:1.5:2) | 1.06 | 1.92 | 2.75 | 3.69 | 4.72 | 4.93 | 5.55 |
| CHCl3–MeOH–0.2M HCl (4:2:2) | 0.85 | 1.39 | 1.65 | 2.3 | 3.06 | 3.81 | 4.14 |
| CHCl3–MeOH–0.2M HCl (4:3:2) | 0.42 | 0.7 | 0.78 | 0.84 | 1.49 | 1.95 | 2.33 |
| CHCl3–MeOH–0.3M HCl (4:1.5:2) | 0.87 | 1.31 | 1.76 | 2.13 | 2.61 | 3.75 | 4.6 |
| CHCl3–MeOH–0.3M HCl (4:2:2) | 0.58 | 1.02 | 1.37 | 1.68 | 2.33 | 2.61 | 3.49 |
| CHCl3–MeOH–0.3M HCl (4:3:2) | 0.35 | 0.62 | 0.65 | 0.77 | 1.2 | 1.74 | 2.06 |
Figure 2.
Effects of the volume ratios of MeOH (A, B, C) and the concentrations of dilute HCl (D, E, F) on the K values of the target alkaloids.
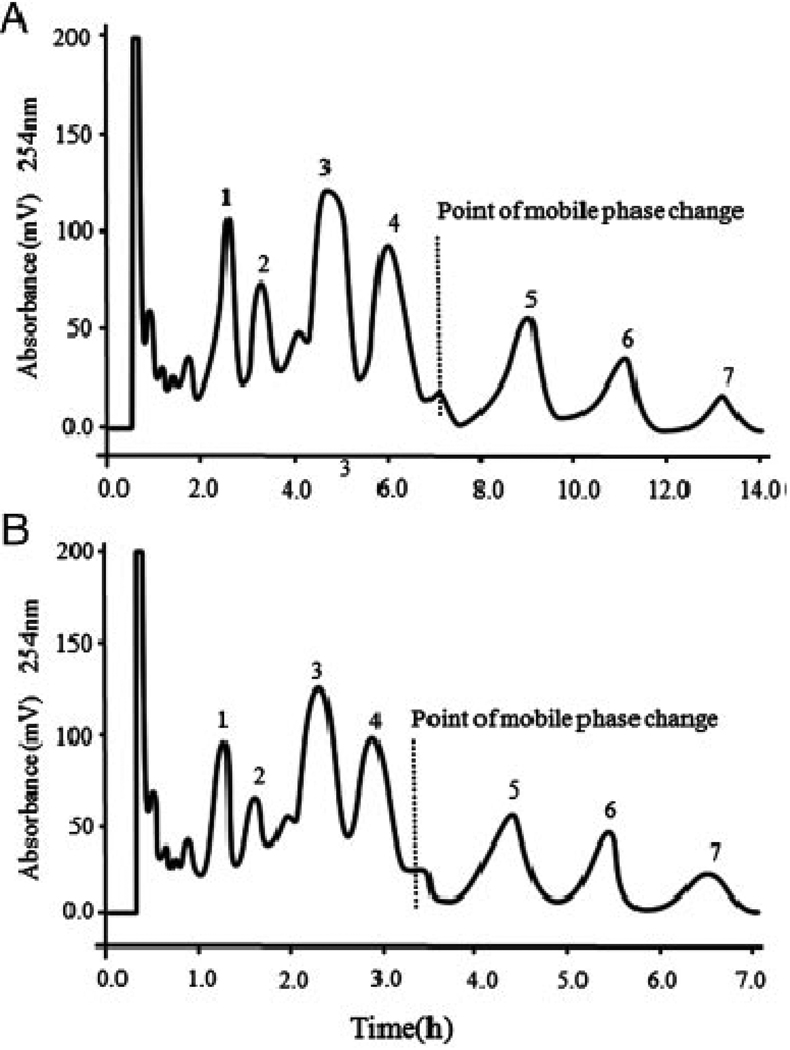
The effect of flow rate (1.0–5.0 mL/min) and the revolution speed (400–900 rpm) on the retention of the stationary phase was investigated. The results (data not shown) indicated that the retention of the stationary phase could be retained stable from 700 to 900 rpm at each flow rate. At the revolution speed of 800 rpm, when the flow rate was changed from 1.0 to 5.0 mL/min, the retention of the stationary phase was decreased from 85 to 76%. Finally, two HSCCC separations were performed at the optimized revolution speed of 800 rpm and at the flow rates of 2.0 and 4.0 mL/min. Fig. 3 shows the results of the two separations and indicated that the flow rate of 4.0 mL/min speeded up the separation without much loss of the resolution. Each separation yielded seven alkaloids in milligram amount (peak 1: 10 mg; peak 2: 5 mg; peak 3: 25 mg; peak 4: 20 mg; peak 5: 18 mg; peak 6: 2 mg; peak 7: 8 mg).
Figure 3.
Chromatograms of the separation of crude alkaloid extracts from C. bungeana Turcz. by preparative HSCCC using solvent systems of CHCl3–MeOH–0.2 M HCl (4:2:2, v/v/v) and CHCl3–MeOH–0.3 M HCl (4:3:2, v/v/v) with stepwise elution. Experimental conditions: Stationary phase: upper phase of CHCl3–MeOH–0.2 M HCl (4:2:2, v/v/v); mobile phase: lower organic phase of the two-solvent systems; flow rate: 2 mL/min (A), 4.0 mL/min (B); revolution speed: 800 rpm; temperature of column oven: 26°C; sample size: 200 mg dissolved in equal volume of lower and upper phase (each 2 mL); retention of the stationary phase: 80% (flow rate: 4.0 mL/min).
3.2 HPLC analyses results
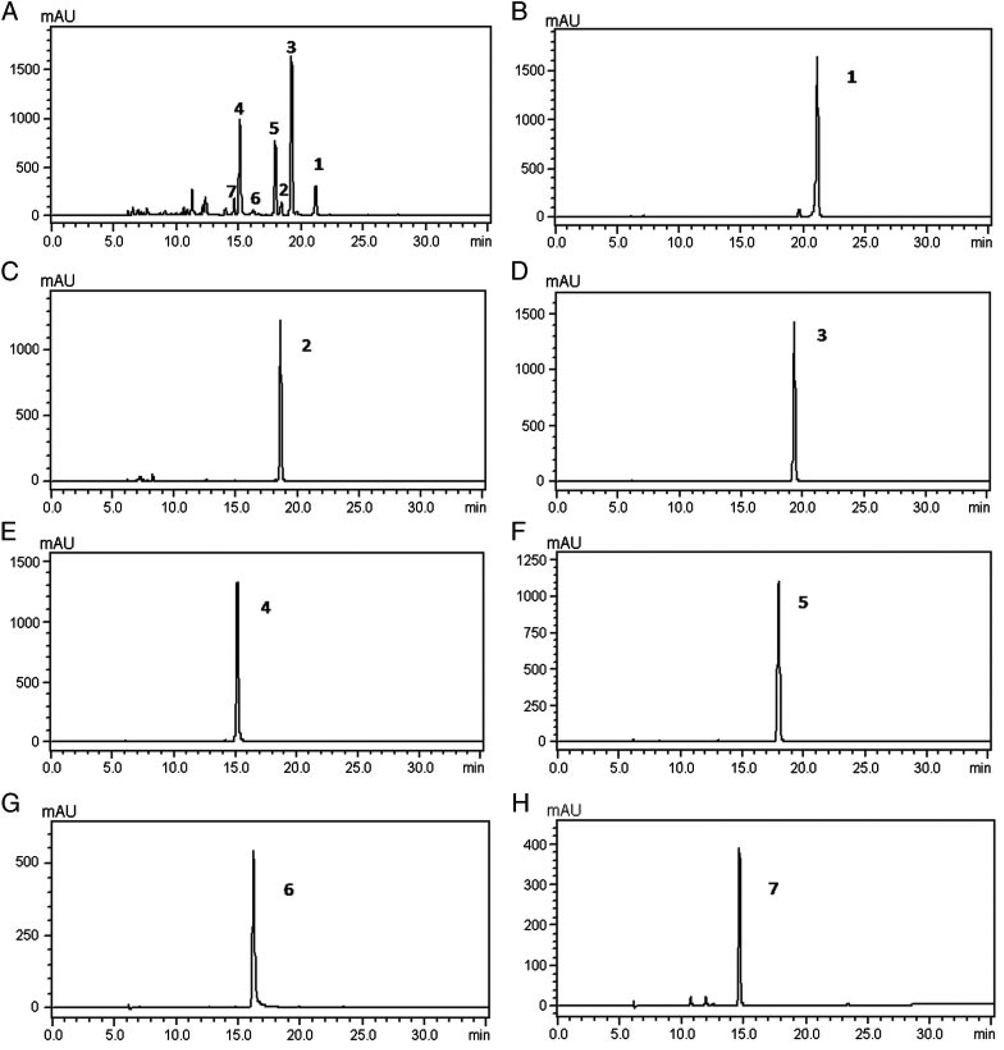
The results of HPLC analyses of crude alkaloid extracts from C. bungeana Turcz. and HSCCC fractions are shown in Fig. 4 and indicate that the purified alkaloids are of purities of 94–99%.
Figure 4.
The results of HPLC analyses of crude alkaloid extracts and HSCCC fractions from C. bungeana Turcz. (A) crude alkaloids; (B) peak 1; (C) peak 2; (D) peak 3; (E) peak 4; (F) peak 5; (G) peak 6; (H) peak 7 of the preparative HSCCC separation shown in Fig. 3; column: Ultimate RP C18 column (250 mm × 4.6 mm i.d., 5 µm, Welch Materials); mobile phase: solvent A: methanol containing 0.05% TEA; solvent B: water; gradient: 0.0 min (60% A)–25 min (100% A)–35 min (100% A); flow rate: 1.0 mL/min; UV wavelength: 289 nm.
3.3 Identification of each purified alkaloids
Compound 1 (peak 1 in Fig. 3)
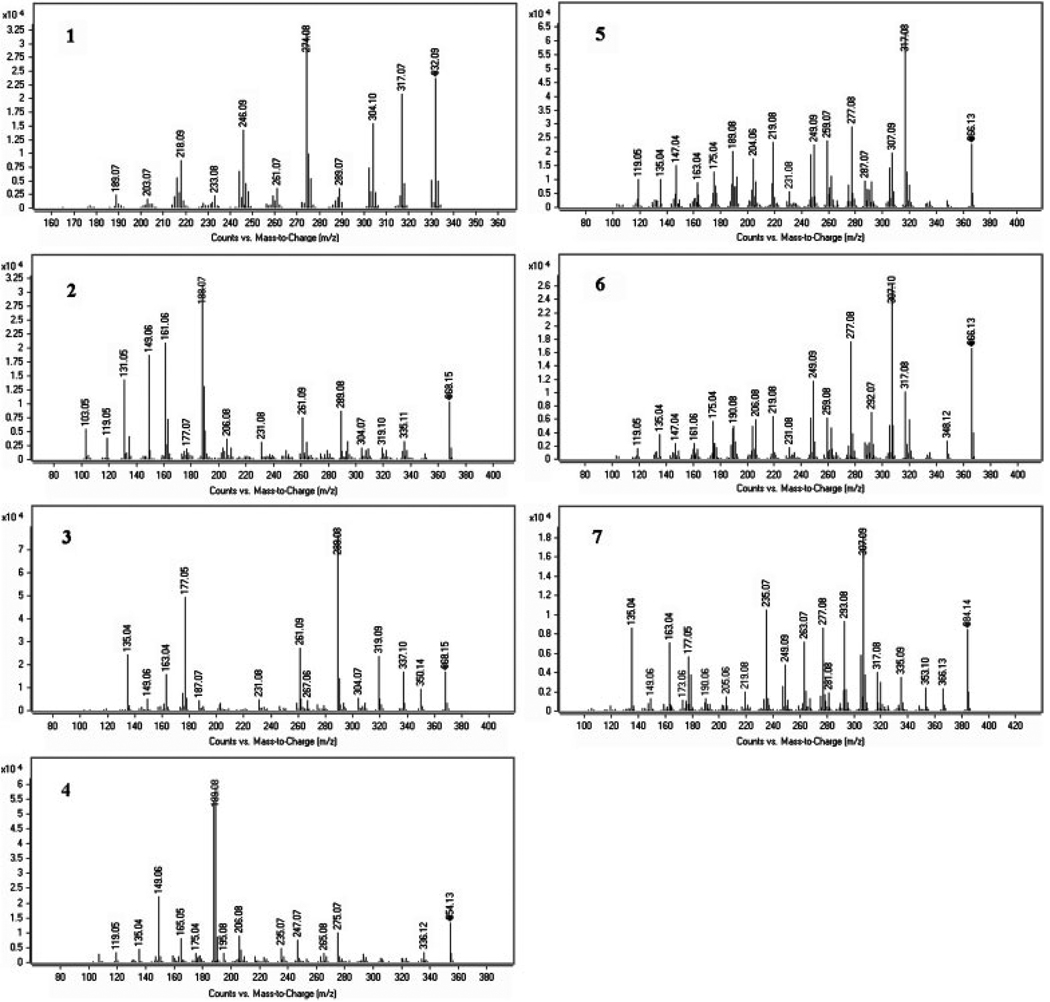
1H NMR (600 MHz, CD3OD): 7.69 (1H, s, 1-H), 6.39 (2H, s, 2-O-CH2-O-3), 8.28 (1H, s, 4-H), 5.07 (3H, s, 5-N-CH3), 10.06 (1H, s, 6-H), 6.65 (2H, s, 7-O-CH2-O-8), 8.08 (1H, d, J = 8.7, 9-H), 8.66 (1H, d, J = 8.7, 10-H), 8.76 (1H, d, J = 8.7, 11-H), 8.34 (1H, d, J = 8.7, 12-H). 13C NMR (600 MHz, CD3OD): 107.0 (C-1), 134.2 (C-1a), 150.9 (C-2), 150.8 (C-3), 105.0 (C-4), 122.0 (C-4a), 52.8 (5-N-CH3), 150.8 (C-6), 111.3 (C-6a), 148.2 (C-7), 149.5 (C-8), 121.2 (C-9), 118.3 (C-10), 129.1 (C-10a), 119.6 (C-11), 132.9 (C-12), 127.6 (C-13), 133.2 (C-14), 104.3 (2-O-CH2-O-3), 106.5 (9-O-CH2-O-10). The data were in agreement with those listed in the literature [17]. Positive ESI-Q-TOF-MS/MS (Fig. 5-1): precursor ion m/z 332.09 (M+, calc. for C20H13NO4), product ion m/z 317.07 [M-CH3]+, 304.10 [M-CO]+, 289.07 [M-CO-CH3]+, 274.08 [M-CO-CH2O]+, 246.09 [M-2CO-CH2O]+, 216.08 [M-2CO-2CH2O]+ were in good agreement with the literature [18]. So compound 1 was identified as sanguinarine.
Figure 5.
Positive ESI-Q-TOF-MS/MS analyses of purified alkaloids from C. bungeana Turcz.
Compound 3 (peak 3 in Fig. 3)
1H NMR (600 MHz, CDCl3): 6.76 (1H, s, 1-H), 6.77 (1H, s, 4-H), 2.34 (3H, s, 5-N-CH3), 3.58, 4.16 (2H, ABq, J = 15.2, 6-H), 6.91 (1H, d, J = 8.2, 9-H), 7.03 (1H, d, J = 8.2, 10-H), 4.07 (1H, s, 11-H), 3.18–3.29 (2H, m, 12-H), 1.26 (3H, s, 13-CH3), 3.43 (1H, s, 14-H), 6.06–6.10 (4H, m, 2-O-CH2-O-3, 7-O-CH2-O-8). 13C NMR (600 MHz, CDCl3): 107.7 (C-1), 125.1 (C-1a), 145.0 (C-2), 145.3 (C-3), 112.7 (C-4), 127.8 (C-4a), 43.2 (5-N-CH3), 54.2 (C-6), 116.7 (C-6a), 142.7 (C-7), 48.0 (C-8), 109.4 (C-9), 118.6 (C-10), 136.0 (C-10a), 76.1 (C-11), 36.6 (C-12), 40.7 (C-13), 23.3 (13-CH3), 69.7 (C-14), 101.0 (2-O-CH2-O-3), 101.3 (9-O-CH2-O-10). The data were in agreement with those listed in the literature [19, 20]. Positive ESI-Q-TOF-MS/MS (Fig. 5-3): precursor ion m/z 368.15 ([M+H]+, calc. for C21H22NO5), product ion m/z 350.14 [M+H-H2O]+, 337.10 [M+H-NH2CH3]+, 319.09 [M+H-H2O-NH2CH3]+, 289.08 [M+H-H2O-NH2CH3-CH2O]+, 261.09 [M+H-H2O-NH2CH3-CH2O-CO]+, 231.08 [M+H-H2O-NH2CH3-2CH2O-CO] were in agreement with the structure of corynoline. So, compound 3 was identified as corynoline.
Compound 4 (peak 4 in Fig. 3)
1H NMR (600 MHz, CDCl3): 6.75 (1H, s, 1-H), 7.02 (1H, s, 4-H), 2.48–3.98 (8H, m, 5-H, 6-H, 8-H, 13-H), 2.12 (3H, s, 7-N-CH3), 6.78 (1H, d, J = 7.8, 11-H), 6.81 (1H, d, J = 7.8, 12-H), 6.04, 6.06 (4H, each s, 2-O-CH2-O-3, 9-O-CH2-O-10). 13C NMR (600 MHz, CDCl3): 108.1 (C-1), 146.2 (C-2), 148.1 (C-3), 110.3 (C-4), 135.4 (C-4a), 31.4 (C-5), 57.6 (C-6), 41.6 (7-N-CH3), 51.1 (C-8), 117.2 (C-8a), 146.0 (C-9), 146.0 (C-10), 106.9 (C-11), 124.9 (C-12), 128.7 (C-12a), 45.8 (C-13), 132.3 (C-14a), 100.9 (2-O-CH2-O-3), 101.3 (9-O-CH2-O-10). The data were in agreement with those listed in the literature [21, 22]. Positive ESI-Q-TOF-MS/MS (Fig. 5-4): precursor ion m/z 354.13 ([M+H]+, calc. for C20H20NO5), product ion m/z 336.12 [M+H-H2O]+, 275.07 [M+H-NH2CH3-CH2(OH)2]+, 247.07 [M+H-NH2CH3-CH2(OH)2-CO]+, 206.08 [M+H-C9H8O2]+, 189.08 [M+H-C9H8O2-OH]+, 188.07 [M+H-C9H8O2-H2O]+, 149.06 [M+H-C11H11NO3]+ were in accordance with the literature [23, 24]. So, compound 4 was identified as protopine.
Compound 5 (peak 5 in Fig. 3)
1H NMR (600 MHz, CDCl3): 6.73–6.94 (4H, m, 1-H, 4-H, 9-H, 10-H), 6.02–6.13 (4H, m, 2-O-CH2-O-3, 7-O-CH2-O-8), 2.28 (3H, s, 5-N-CH3), 5.42 (1H, s, H-6), 3.80 (1H, m, H-11), 3.23 (1H, d, J = 18, H-12), 3.07 (1H, dd, J = 18, 3.2, H-12), 1.37 (3H, s, 13-CH3), 2.95 (1H, s, H-14). 13C NMR (600 MHz, CDCl3): 107.0 (C-1), 124.2 (C-1a), 146.0 (C-2), 146.7 (C-3), 109.2 (C-4), 130.9 (C-4a), 39.6 (5-N-CH3), 77.7 (C-6), 118.3 (C-6a), 141.5 (C-7), 147.4 (C-8), 109.7 (C-9), 114.5 (C-10), 134.3 (C-10a), 72.2 (C-11), 32.3 (C-12), 36.6 (C-13), 15.7 (13-CH3), 64.2 (C-14), 100.8 (2-O-CH2-O-3), 101.5 (9-O-CH2-O-10). The data were in agreement with those listed in the literature [20, 25]. Positive ESI-Q-TOF-MS/MS (Fig. 5-5): precursor ion m/z 366.13 ([M+H]+, calc. for C21H20NO5), product ions m/z 317.08 [M+H-H2O-NH2CH3]+, 307.09 [M+H-NH2CH3-CO]+, 287.07 [M+H-H2O-NH2CH3-CH2O]+, 277.08 [M+H-NH2CH3-CO-CH2O]+, 259.07 [M+H-NH2CH3-CO-CH2O-H2O]+, 249.09 [M+H-NH2CH3-2CO-CH2O]+, 231.08 [M+H-NH2CH3-2CO-CH2O-H2O]+, 219.08 [M+H-NH2CH3-2CO-2CH2O]+ were in agreement with the structure of corynoloxine. So, compound 5 was identified as corynoloxine.
Compound 7 (peak 7 in Fig. 3)
1H NMR (600 MHz, CDCl3): 6.79 (1H, s, 1-H), 7.16 (1H, s, 4-H), 2.31 (3H, s, 5-N-CH3), 3.58, 4.16 (2H, ABq, J = 15.3, 6-H), 6.94 (1H, d, J = 8.2, 9-H), 7.09 (1H, d, J = 8.2, 10-H), 4.03 (1H, s, 11-H), 5.07 (1H, s, 12-H), 1.37 (3H, s, 13-CH3), 3.45 (1H, s, 14-H), 6.08–6.13 (4H, m, 2-O-CH2-O-3, 7-O-CH2-O-8). The data were in agreement with those listed in the literature [26] and 13C NMR (600 MHz, CDCl3): 107.8 (C-1), 125.9 (C-1a), 145.1 (C-2), 147.0 (C-3), 112.0 (C-4), 128.8 (C-4a), 43.1 (5-N-CH3), 54.0 (C-6), 116.3 (C-6a), 142.7 (C-7), 148.6 (C-8), 109.9 (C-9), 118.9 (C-10), 135.5 (C-10a), 74.0 (C-11), 81.4 (C-12), 39.7 (C-13), 23.7 (13-CH3), 70.2 (C-14), 101.4 (2-O-CH2-O-3), 101.4 (9-O-CH2-O-10). Positive ESI-Q-TOF-MS/MS (Fig. 5–7): precursor ion m/z 384.14 ([M+H]+, calc. for C21H22NO6), product ions m/z 366.13 [M+H-H2O], 353.10 [M+H-NH2CH3], 335.09 [M+H-NH2CH3-H2O], 307.09 [M+H-NH2CH3-H2O-CO], 277.08 [M+H-NH2CH3-H2O-CO-CH2O], 249.09 [M+H-NH2CH3-H2O-2CO-CH2O], 219.08 [M+H-NH2CH3-H2O-2CO-2CH2O] were in agreement with the structure of 12-hydroxycorynoline. So, compound 7 was identified as 12-hydroxycorynoline.
Compound 2 (peak 2 in Fig. 3)
Positive ESI-Q-TOF-MS/MS (Fig. 5-2), precursor ion m/z 368.15 [M+H]+. It has the same molecular weight as compound 3 (corynoline). Compound 6 (peak 6 in Fig. 3): Positive Q-TOF-MS/MS (Fig. 5–6), precursor ion m/z 366.13 [M+H]+. Compound 6 has the very similar fragment pattern as that of compound 5 (corynoloxine) with slight difference in the abundance of fragmental ions. Therefore, compound 6 should be an isomer of corynoloxine. Their chemical structure determination is still going on.
4 Concluding remarks
This work presents a successful application of HSCCC to preparative separation of alkaloids from C. bungeana Turcz. Systematic measurement of K values of targets compounds is one of the best ways to select a suitable two-phase solvent system for HSCCC separation in a reasonable time. Stepwise elution mode is one of the best ways to combine different two-phase solvent systems to perform a fast and efficient HSCCC separation.
Acknowledgments
This research was supported by the National Basic Research Program of China (973) (grant no. 2010CB833703) and the National Natural Science Foundation of China (grant no. 90919047).
Abbreviation
- HSCCC
high-speed counter-current chromatography
Footnotes
The authors have declared no conflict of interest.
5 References
- 1.Chen X, Nigel CV, Peter JH, Monique SS. Phytochemistry. 2004;65:3041–3047. [Google Scholar]
- 2.China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, The first Division of 2010 Edn. Beijing: China Chemical Industry Press; 2010. p. 187. [Google Scholar]
- 3.Wei HL, Liu GT. Acta Chim. Sin. 1997;32:331–336. [PubMed] [Google Scholar]
- 4.Liu XJ, Zhang HL, Tan ZC, Han KL, Sun LX. J. Therm. Anal. Calorim. 2007;89:907–911. [Google Scholar]
- 5.Huang G, Li FM. Chin. Tradit. Herbal Drugs. 2003;34:882–883. [Google Scholar]
- 6.Ito Y. J. Chromatogr. 1981;214:122–125. [Google Scholar]
- 7.Yang FQ, Zhang TY, Zhang R, Ito Y. J. Chromatogr. A. 1998;829:137–141. doi: 10.1016/s0021-9673(98)00776-6. [DOI] [PubMed] [Google Scholar]
- 8.Tang QF, Liu JH, Xue J, Ye WC, Zhang ZJ, Yang CH. J. Chromatogr. B. 2008;872:181–185. doi: 10.1016/j.jchromb.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Liu RM, Feng L, Sun AL, Kong LY. J. Chromatogr. A. 2004;1057:89–94. doi: 10.1016/j.chroma.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y, Zhang TY, Ito Y. J. Chromatogr. A. 2004;1033:373–377. doi: 10.1016/j.chroma.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Li YN, Yin LH, Xu LN, Peng JY. J. Sep. Sci. 2010;33:2168–2175. doi: 10.1002/jssc.201000054. [DOI] [PubMed] [Google Scholar]
- 12.Zhu LC, Yu SJ, Zeng XN, Fu X, Zhao MM. Sep. Purif. Technol. 2008;63:665–669. [Google Scholar]
- 13.Cao XL, Wang C, Pei HR, Sun BG. J. Chromatogr. A. 2009;1216:4268–4274. doi: 10.1016/j.chroma.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Ling JY, Zhang GY, Cui ZJ, Zhang CK. J. Chromatogr. A. 2007;1145:123–127. doi: 10.1016/j.chroma.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 15.Yang FQ, Zhang TY, Tian GL, Cao HF, Liu QH, Ito Y. J. Chromatogr. A. 1999;858:103–107. doi: 10.1016/s0021-9673(99)00827-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang YC, Liu CM, Zhang ZK, Qi YJ, Wu GM, Li SN. J. Sep. Sci. 2010;33:2743–2748. doi: 10.1002/jssc.201000308. [DOI] [PubMed] [Google Scholar]
- 17.Sečkářová P, Marek R, Dostál J, Dommisse R, Esmans EL. Magn. Reson. Chem. 2002;40:147–152. [Google Scholar]
- 18.Hoellinger H, Re M, Deroussent A, Singh RP, Cresteil T. J. Chromatogr. B. 2004;799:195–200. doi: 10.1016/j.jchromb.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Takao N, Iwasa K, Kamigauchi M, Sugiura M. Chem. Pharm. Bull. 1978;26:1880–1889. [Google Scholar]
- 20.Kim DK, Eun JS, Shin TY, Eom DO, Lim JP. Arch. Pharm. Res. 2000;23:589–591. doi: 10.1007/BF02975246. [DOI] [PubMed] [Google Scholar]
- 21.Nonaka G, Okabe H, Nishioka I, Takao N. Yakugaku Zasshi. 1973;93:87–93. doi: 10.1248/yakushi1947.93.1_87. [DOI] [PubMed] [Google Scholar]
- 22.Tang YL, Yang AM, Zhang YS, Wang HQ. Chin. J. Chin. Mater. Med. 2005;30:195–197. [Google Scholar]
- 23.Schmidt J, Boettcher C, Kuhnt C, Kutchan TM, Zenk MH. Phytochemistry. 2007;68:189–202. doi: 10.1016/j.phytochem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Iwasa K, Cui W, Sugiura M, Takeuchi A, Moriyasu M, Takade K. J. Nat. Prod. 2005;68:992–1000. doi: 10.1021/np0402219. [DOI] [PubMed] [Google Scholar]
- 25.Choi SU, Baek NI, Kim SH, Yang JH, Eun JS, Shin TY, Lim JP, Lee JH, Jeon H, Yun MY, Leem KH, Park HW, Kim DK. Arch. Pharm. Res. 2007;30:151–154. doi: 10.1007/BF02977687. [DOI] [PubMed] [Google Scholar]
- 26.Nonaka G, Nishioka I. Chem. Pharm. Bull. 1975;23:521–526. [Google Scholar]