Abstract
To test the hypothesis that tumor-associated macrophages (TAMs) enhance the growth and metastasis of human prostate cancer in the bone, we evaluated the effects of decreasing interleukin-6 (IL-6) production by tumor cells and TAMs in a mouse model of bone metastasis. Human PC-3MM2 cells that produce IL-6 were transfected with lentivirus containing IL-6 small hairpin RNA (shRNA) or nonspecific RNA and injected into the tibias of nude mice treated intraperitoneally every 5 days for 5 weeks with phosphate-buffered saline (PBS), liposomes containing PBS, or liposomes containing clodronate (to decrease the number of macrophages). Transfection of PC-3MM2 cells with IL-6 shRNA significantly decreased cellular expression of IL-6 and the number of TAMs and osteoclasts in bone tumors, which correlated with significant decreases in tumor size, bone lysis, and incidence of lymph node metastasis. Treatment of mice with clodronate liposomes significantly decreased the number of TAMs and osteoclasts in the bone tumors, the expression of IL-6 in the PC3-MM2 cells, and the production of tumor necrosis factor (TNF)-α by TAMs. These findings correlated with a significant decrease in tumor size, bone lysis, and lymph node metastasis. Knocking down IL-6 in tumor cells and decreasing TAMs was associated with the lowest incidences of bone tumors and lymph node metastasis. These results suggest that TAMs enhance the growth of prostate cancer cells in the bone.
Keywords: macrophages, prostate cancer, bone metastasis
1. Introduction
A common site for prostate cancer metastasis is the bone [1,2]. Although prostate cancer cells in the bone microenvironment respond to the hormone ablation and taxane-based chemotherapy, the emergence of androgen-independent and chemoresistant metastatic lesions is responsible for cancer-related morbidity and mortality [2–4]. Since the outcome of metastasis is regulated by the interaction of tumor cells with the organ microenvironment [4,5], attempts to interrupt these interactions have led to the development of antibodies and small molecules that target tumor cells and host cells [6–8].
Interleukin-6 (IL-6) was originally discovered as an immune modulator [9]. IL-6 can also stimulate tumor cell proliferation, reduce apoptosis, induce drug resistance, and promote the production of bone metastasis [10–12]. As in pleural mesothelioma [13], glioblastoma [14], and ovarian cancer [15], the production of IL-6 has been shown to correlate with progressive disease in prostate cancer [10,16]. Increased expression of IL-6 and its receptor has been documented in human prostate cancer cell lines, surgical specimens, and benign prostate hyperplasia [17–19]. Clinically, the level of IL-6 in serum is significantly elevated in many patients with advanced hormone-refractory prostate cancer [12,20], and study of circulating prediagnostic IL-6 suggests that IL-6 may potentially be involved in the development or progression of prostate cancer [21]. To date, however, the mechanism by which IL-6 promotes the resistance and progressive growth of prostate cancer is unclear [11,22–25], and clinical in vivo experiments targeting IL-6 with the anti-IL-6 monoclonal antibody CNTO 328 have produced inconsistent responses [26–28].
Macrophages are known to accumulate in tumors which can usurp their trophic roles [29–34]. Since tumor necrosis factor (TNF)-α and IL-1 secreted by macrophages are strong stimulators of IL-6 production by tumor cells [35], we hypothesized that tumor-associated macrophages (TAMs) enhance the growth of human prostate cancer in the bone. To test this hypothesis, we evaluated whether a decrease in IL-6 production by tumor cells and/or a decrease in the number of TAMs can decrease the progressive growth of human prostate cancer implanted into the tibias of nude mice and the formation of lymph node metastasis.
2. Materials and methods
2.1 PC-3MM2, metastatic variant cell line of human prostate cancer
The metastatic PC-3MM2 cell line was established as described previously [36] and maintained as monolayer cultures in Eagle’s minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Inc., Grand Island, NY), L-glutamine, pyruvate, nonessential amino acids, vitamins, and penicillin-streptomycin (Invitrogen, Carlsbad, CA) and incubated in 5% CO2 with balance of air at 37°C. All reagents used for tissue culture were free of endotoxin, mycoplasma, and the following viral pathogens: reovirus type 3, pneumonia virus, K virus, Theiler’s encephalitis virus, Sendai virus, min virus, mouse adenovirus, mouse hepatitis virus, lymphocytic choriomeningitis virus, ectromelia virus, and lactate dehydrogenase virus (assayed by M. A. Bioproducts, Walkersville, MD). Macrophages were isolated from the H-2Kb-tsA58 mouse.
2.2 Animals
Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research Facility (Frederick, MD). The mice were housed and maintained in specific pathogen-free conditions. The facilities were approved by the American Association for Accreditation of Laboratory Animal Care and met all current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and National Institutes of Health. The mice were used in accordance with institutional guidelines when they were 8 to 12 weeks old.
2.3 Immorto-mouse peritoneal macrophages
Macrophages were isolated from the H-2Kb-tsA58 mouse [37]. In brief, the mice were injected in the peritoneal cavity with 1.5 ml of thioglycollate broth (BD, Franklin Lakes, NJ). Three days later, the peritoneal cavity was irrigated with 5 ml of PBS, and the peritoneal fluid was collected, plated into a culture dish, and cultured in 10% MEM. Primary cultures were supported at 33°C in a mixture of 5% carbon dioxide and 95% oxygen, and the media was replaced as needed. Cells were collected by a brief exposure to 0.25% trypsin, hybridized FITC-conjugated F4/80 (eBioscience, San Diego, CA), and sorted by Beckman Epics Elite flow cytometer (Beckman Coulter, Miami, FL) equipped with an air-cooled argon ion laser. Gating parameters were adjusted based on the fluorescence histograms for the positive and negative controls. Cells were maintained at 33°C and. For the co-culture experiment, cells were transferred and maintained at 37°C and incubated for 72 hours before use.
2.4 IL-6 knockdown by short hairpin RNA
The Lentilox 3.7 lentiviral short hairpin RNA (shRNA) vector was used to knock down IL-6 in PC-3MM2 cells. On the basis of the reported human IL-6 cDNA sequence reported in the database (GeneBank™ accession number M14584), the sequence of 5’-TGATGGATGCTACCAAACT-3’ was selected for shRNA-mediated knockdown of human IL-6 (IL-6 shRNA) [38]. The duplex DNA (5’-T TGATGGATGCTACCAAACTTTCAAGAGAAGTTTGGTAGCATCCATCA-3’) was made and cloned into the Lentilox 3.7 vector; shRNA for the Lentilox vector were designed as described previously [39]. An irrelevant sequence corresponding to nt 153–173 of the firefly luciferase mRNA was used for negative control (NS shRNA). Lentiviral particles were produced by transfection of 293T cells with 10 µg of vector DNA combined with the ViralPower™ Packaging Kit (containing pLP1, pLP2, and pLP/VSVG DNAs, providing necessary proteins for virus production, Invitrogen). Seventy-two hours after transfection, supernatants of virus-containing cell cultures were collected, passed through a 0.45-µm filter, and then concentrated by centrifugation in a swinging bucket for 90 minutes at 83,000 g. The virus pellet was subsequently resuspended in 30 µl of PBS with 0.1% BSA and titrated by serial dilutions on PC-3MM2 cells. Viral titers were typically about 2 × 108 transfection units/ml. PC-3MM2 cells were transfected with lentivirus with NS shRNA or IL-6 shRNA to produce PC-3MM2-NS shRNA or PC-3MM2-IL-6 shRNA, respectively. Green fluorescent protein-positive populations were enriched to 100% by fluorescence-activated cell sorting. Sorted cells were expanded, and single-cell sorting yielded clonal colonies. Cells were grown to log density, harvested, lysed, and analyzed for IL-6 knockdown by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). The clones with the most efficient knockdown of IL-6 were selected and stored in liquid nitrogen.
2.5 Determination of IL-6 and TNF-α levels by enzyme-linked immunosorbent assay
In the first set of experiments, the production and secretion of IL-6 by PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA cells were measured by ELISA (Human IL-6 Quantitative ELISA Kit, D6050, R&D Systems, Minneapolis, MN). Cells (1 × 105) were plated in 200 µl medium with or without 10% FBS into 38-mm2 wells (96-well plates). Twenty-four hours later, PBS (control), TNF-α (10 ng/ml, rhTNF-α, R&D Systems), or IL-1 (100 ng/ml, rhIL-1B, R&D Systems) was added and cells were incubated for 24 hours.
In the second set of experiments, PC-3MM2 cells (1 × 105) and macrophages (1 × 105) alone or mixed (cell ratio, 1:1; total, 1 × 105), were plated in 200 µl of MEM with 10% FBS into 38-mm2 wells (96-well plates) with or without TNF-α antibody (0.5 µg/ml, R&D System). Cells were incubated for 24 hours. To investigate whether IL-6 was produced by tumor cells or macrophages, co-localization immunofluorescence analysis of IL-6 and F4/80 was performed.
Supernatants were collected, and the level of IL-6 was measured by ELISA. The number of metabolically active cells was determined using the tetrazolium salt (MTT) assay (M2128; Sigma Chemical Co., St. Louis, MO), and the level of IL-6 was adjusted to the number of cells in the well (adjusted ELISA = ELISA/MTT). In the last set of experiments, the levels of IL-6 and TNF-α (Mouse TNF-α Quantitative ELISA Kit, MTA00, R&D Systems) in mouse serum were determined by ELISA.
2.6 Expression of p44/p42 and phosphorylated p44/p42 by Western blot and immunohistochemical analyses
To determine whether IL-6-mediated tumor progression is mediated by p44/p42 mitogen activated protein kinase pathway, we carried out Western blot and immunohistochemical analyses using LNCaP (STAT-3-positive) [40,41] and the variants of PC3-MM2 (STAT-3-negative). Cells cultured in minimal essential medium supplemented with 10% FBS were lysed, and 20 µg of whole-cell lysates were separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Blots were blocked with 5% nonfat dry milk for 1 hour at room temperature and then incubated overnight at 4°C with 1:1,000 dilution of polyclonal rabbit anti-phosphorylated p44/p42 antibody (Cell Signaling, Danvers, MA), 1:1,000 dilution of polyclonal rabbit anti-p44-/p42 antibody (Cell Signaling), followed by incubation with 1:3,000 dilution of horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. The signal was visualized with an enhanced chemiluminescence detection reagent (Amersham Biosciences, Piscataway, NJ). β-actin was detected simultaneously using 1:10,000 dilution of monoclonal mouse anti-β-actin antibody (Sigma) as a loading control. Immunohistochemical analysis was performed using formalin-fixed and paraffin-embedded tissue with the same antibodies used for Western blot.
2.7 Preparation of clodronate liposomes
Clodronate liposomes were prepared as described previously [42,43]. A total of 11 mg of cholesterol (Sigma) and 75 mg of phosphatidylcholine (Sigma) were combined with 10 ml of 0.7 M clodronate solution (Sigma) and gently sonicated. The resulting liposomes were washed three times to eliminate any unused drug. Control liposomes containing PBS were prepared under the same conditions.
2.8 In vivo depletion of macrophages by intraperitoneal administrations of liposomes containing clodronate
To determine the optimal dose of liposomes containing clodronate for depletion of macrophages, we injected 800 µl, 400 µl, 200 µl, and 100 µl of liposomes containing clodronate or PBS into the peritoneal cavities of 20 mice (n=5). This injection was repeated every 5 days for a total of 3 injections. Two days after the last liposome injection, the mice were injected in the peritoneal cavity with 1.5 ml of thioglycollate broth (BD, Franklin Lakes, NJ) [44]. Three days later, the peritoneal cavity was irrigated with 5 ml of PBS, and the peritoneal fluid was collected. The number of macrophages in 1 ml of peritoneal fluid was determined by Wright-Giemsa staining. The number of macrophages in mice injected with 800 µl (1.2 ± 0.7 × 105/ml, p<0.01), 400 µl (2.6 ± 0.6 × 105/ml, p<0.01), or 200 µl (5.8 ± 2.1 × 105/ml, p<0.05) of clodronate liposomes was significantly lower than that in mice injected intraperitoneally (i.p.) with PBS (23 ± 16.1 × 105/ml) or 100 µl (27.7 ± 18.7; p>0.05) of clodronate liposomes. Since injection of 800 µl of clodronate liposomes produced lethal pneumonitis (3 of 5 mice became moribund), in all subsequent experiments, mice were injected i.p. with 400 µl of clodronate liposomes.
2.9 In vivo study
Injection of Human Prostate Cancer Cells into the Tibia of Male Nude Mice
In the first set of experiments, we determined whether expression of IL-6 by PC-3MM2 cells correlates with their growth in the bone and production of metastasis. PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Medium containing 10% FBS was added, and the cells were washed once in serum-free medium and resuspended in Ca++- and Mg++-free Hank’s balanced salt solution (HBSS). Cell viability was determined by trypan blue exclusion, and only single-cell suspensions with >95% viability were used for the intratibial injections. Male nude mice were anesthetized with Nembutal (0.5 mg/g body weight, Abbott Laboratories, North Chicago, IL), and 2 × 105 PC-3MM2, PC-3MM2-NS shRNA, or PC-3MM2-IL-6 shRNA cells in 20 µl of Ca++- and Mg++-free HBSS were injected interosseously into the tibia of nude mice (n=10) as described previously [45]. Four weeks later, bone lesions were examined by digital radiography (Faxitron MX-20, Faxitron X-ray Corp., Wheeling, IL). The mice were euthanized by injection of Nembutal (1 g/kg body weight), blood (for serum) was collected by cardiac puncture, and the mice were necropsied. Body weight, bone tumor incidence, tumor weight, and incidence of lymph node metastasis were recorded.
In the second set of experiments, we determined whether depletion of TAM alone or in combination with decreased production of IL-6 by PC-3MM2 cells can inhibit tumor growth in the bone and production of lymph node metastasis. Mice (n=10) were injected i.p. with PBS, control liposomes, or clodronate liposomes. One day later, the mice were injected in the tibia with 2 × 105 cells of PC-3MM2, PC-3MM2-NS shRNA, or PC-3MM2-IL-6 shRNA [45]. Intraperitoneal injections of PBS, control liposomes, or clodronate liposomes were repeated every 5 days for 5 weeks, after which bone lesions were examined by digital radiography. The mice were euthanized by injection of Nembutal (1 g/kg body weight), blood (for serum) was collected by cardiac puncture, and the mice were necropsied. Body weight, bone tumor incidence, tumor weight, and incidence of lymph node metastasis were recorded.
2.10 Preparation of tissues
Tissues were processed for immunohistochemical analyses of IL-6, F4/80 (frozen blocks), and TNF-α (paraffin blocks). Bone tumors were harvested and fixed in 10% buffered formalin for 24 hours at 4°C, washed with PBS for 30 minutes, and decalcified with 10% EDTA (pH 7.4) for 10 days at 4°C. For paraffin blocks, tissues were washed in PBS for 10 minutes and then embedded in paraffin. For frozen blocks, tissues were fixed in PLP solution (4% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodata) for 24 hours at 4°C and decalcified in 10% EDTA (pH 7.4). The EDTA was removed by dipping tissues in solutions of serially increased concentrations of sucrose (10% sucrose for 4 hours, 15% sucrose for 4 hours, and 20% sucrose for 16 hours), and the tissues were then embedded in optimal cutting temperature (OCT; Miles, Inc., Elkhart, IN).
2.11 Immunohistochemical analysis
The expression of IL-6 and TNF-α in tissues was determined by immunohistochemical analyses using mouse anti-human monoclonal anti-IL-6 antibody (1:200, Invitrogen, Carlsbad, CA) and rabbit anti-mouse polyclonal TNF-α antibody (1:200, Genzyme, Cambridge, MA). Mouse macrophages were detected by staining and counted with rat anti-mouse monoclonal F4/80 antibody (1:1000, Abd Serotec, Raleigh, NC). In brief, paraffin-embedded tissues were sectioned (4–6 µm), mounted on positively charged Superfrost slides (Fisher Scientific Co., Houston, TX), and warmed on a heating pad. The sections were deparaffinized in xylene, dehydrated in a graded alcohol series, and rehydrated with PBS (pH 7.5). Frozen blocks were sectioned (4–6 µm), mounted on positively charged Superfrost slides (Fisher Scientific), and dried in air. Tissues were fixed in acetone and stained. Nonspecific binding was blocked with 4% fish gel (Cold Water Fish Skin Gelatin, 40% Aurion; Electron Microscopy Sciences, Fort Washington, PA) in PBS. After incubation with primary antibodies, slides were incubated with one of the following secondary antibodies: HRP-conjugated goat anti-rabbit IgG, HRP-conjugated goat anti-rat IgG (Jackson Research Laboratories, West Grove, PA), or HRP-conjugated rat anti-mouse IgG2a (Abd Serotec, Harlan Bioproducts for Science, Inc., Indianapolis, IN). Positive antibody reactions were visualized by incubating the slides with stable 3,3’-diaminobenzidine for TNF-α F4/80, and with 3-amino-9-ethylcarbazole (AEC) chromogen B (IHC Select® AEC Chromogen B; Millipore, Temecula, CA) for IL-6. The slides were rinsed with distilled water, counterstained with Gill’s hematoxylin for 1 minute and mounted with Universal Mount (Research Genetics, Pittsburgh, PA) [45–47].
2.12. Determination of tartrate-resistant acid phosphatase-positive cells
Paraffin-embedded tissue sections mounted on slides were fixed for 30 seconds at room temperature in fixative solution (25 ml citrate solution, 65 ml acetone, and 8 ml formaldehyde) and then incubated for 1 hour at 37°C with protection from light in reaction solution (45 ml deionized water, 1 ml diazotized fast Garnet GBC solution-0.5 ml fast Garnet GBC solution, 0.5 ml fast Garnet GBC base solution, 0.5 ml sodium nitrite solution, 0.5 ml naphtol AS-BI phosphate solution, and 2 ml acetate solution) with or without 1 ml tartrate solution. Slides were thoroughly rinsed in deionized water and then counterstained for 2 minutes in hematoxylin solution (Gill No. 3). To color the nuclei, the slides were soaked in alkaline tap water. Cells with purple or red cytoplasmic staining were counted as tartrate-resistant acid phosphatase (TRAP) positive [48–50].
2.13. Statistical analyses
Tumor incidence (x2 test), tumor weight (Mann-Whitney t test), incidence of lymph node metastasis (x2 test), and level of IL-6 and TNF-α and number of F4/80- and TRAP-positive cells in 10 random 0.159-mm2 fields (unpaired Student’s t test) were analyzed.
3. Results
3.1 In vitro production of IL-6
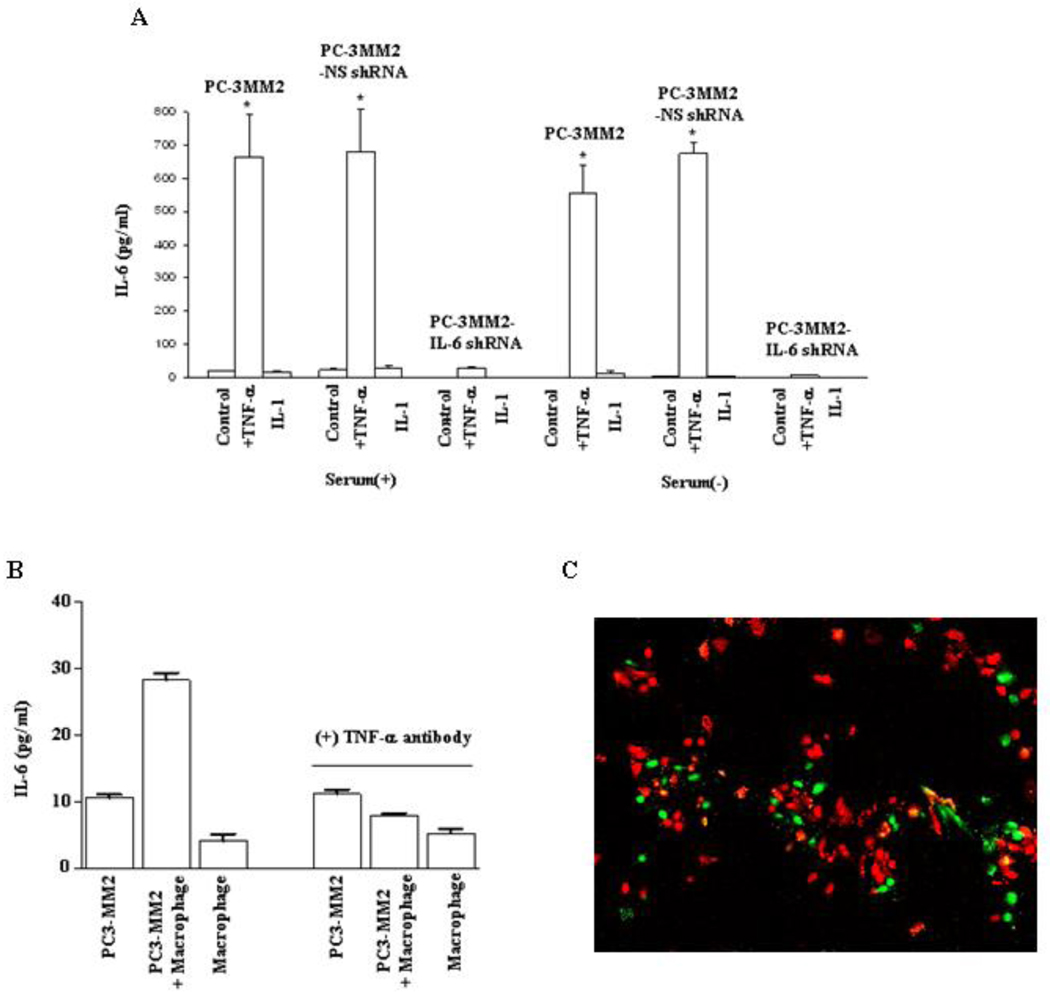
The levels of IL-6 in PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA cells cultured in MEM with 10% FBS were 18.2 ± 1.1 pg/ml, 21.5 ± 3.8 pg/ml, and 0.0 pg/ml, respectively. Stimulation with exogenous TNF-α significantly increased the levels of IL-6 to 665.5 ± 126.1 pg/ml (p<0.001), 680.3 ± 127.3 pg/ml (p<0.001), and 27.7 ± 3.7 pg/ml (from an undetectable level), respectively (Figure 1). IL-6 was not detectable in any of the cells cultured in serum-free MEM. Exogenous stimulation with TNF-α significantly increased the levels of IL-6 to 555.3 ± 82.4 pg/ml and 674 ± 36 pg/ml for PC-3MM2 and PC-3MM2-NS shRNA cells, respectively. In contrast, PC-3MM2-IL-6 shRNA cells had minimal response to exogenous stimulation with TNF-α. The addition of IL-1 to the cultures (under any condition) did not increase production of IL-6 (Fig. 1A).
Fig. 1.
Enzyme-linked immunosorbent assay. A: PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA human prostate cancer cells (1 × 105) were plated into 96-well plates and cultured in MEM with or without 10% fetal bovine serum. Cells were treated with PBS (control), TNF-α (10 ng/ml), or IL-1 (100 ng/ml) for 24 hours. Supernatants were collected and measured for the level of IL-6 by ELISA. Transfection of PC-3MM2 cells with IL-6 shRNA significantly decreased the production of IL-6 and exogenous TNF-α did not lead to increased production of IL-6. Stimulation with IL-1 did not affect the production of IL-6. B: PC-3MM2 cells (1 × 105), immorto-mouse peritoneal macrophages (1 × 105), or PC-3MM2 cells and immorto-mouse peritoneal macrophages (cell ratio, 1:1; total 1 × 105) were plated into 96-well plates and cultured in MEM with 20% FBS. Cells were treated with PBS or anti-TNF-α antibody (0.5 µg/ml) for 24 hours. Co-cultures of PC-3MM2 with immorto-mouse peritoneal macrophages significantly increased the production of IL-6, and the stimulatory effect of macrophages on the production of IL-6 was neutralized by anti-TNF-α antibody (0.5 µg/ml). C: Co-localization of IL-6 and F4/80 by immunofluorescence analysis. Tumor cells (F4/80-negative) were positive for IL-6 staining (green). Macrophages (F4/80-positive; red) were IL-6-negative.
The levels of IL-6 in PC-3MM2 co-culture of PC-3MM2 and macrophages or macrophages alone were 10.6 ± 0.8 pg/ml, 28.2 ± 1.9 pg/ml, and 4.1 ± 1.8 pg/ml, respectively. These data demonstrate that co-culturing PC-3MM2 with macrophages significantly increased the production of IL-6 (p<0.01, as compared with the level of the PC-3MM2 culture). Macrophages are a major source of TNF-α [34,35]. Neutralization of the effect of TNF-α with anti-TNF-α antibody significantly decreased the production of IL-6 in the co-culture of PC-3MM2 and macrophages (28.2 ± 1.9 pg/ml vs 7.9 ± 0.4 pg/ml, p>0.05) (Fig. 1B). Co-localization immunofluorescence analysis of IL-6 (green) and F4/80 (red) showed that IL-6 was produced by tumor cells (Fig. 1C).
3.2 Tumorigenicity, tumor growth, bone lysis, and lymph node metastasis
In mice treated i.p. with PBS, the median weight of the bone lesions produced by PC-3MM2-IL-6 shRNA cells was 0.5 g (range 0–1 g), which was significantly less than the weight of bone lesions produced by PC-3MM2 cells (median, 1.1 g; range, 0–1.4 g; p<0.05) and PC-3MM2-NS shRNA cells (median, 1.1 g; range, 0–1.3g; p<0.05). The incidence of lymph node metastasis for mice injected with PC-3MM2-IL-6 shRNA was 45% (9 of 20, p<0.012), compared with 95% for PC-3MM2 and PC-3MM2-NS shRNA lesions. In mice treated with PBS, there was no difference in tumor incidence among the three groups (Table 1), but the tibias of mice injected with PC-3MM2-IL-6 shRNA cells were significantly better preserved (Figure 2).
Table 1.
Tibia tumors produced by PC-3MM2 cells are dependent on production of IL-6 by tumor cells and macrophages
| Experiment groups | Tumor incidence | Tumor size (g) Median (range) |
Incidence of lymph node metastasis |
|---|---|---|---|
| PBS | |||
| PC-3MM2 | 19/20 | 1.1 (0–1.4) | 19/20 |
| PC-3MM2-NS shRNA | 19/20 | 1.1 (0–1.3) | 19/20 |
| PC-3MM2-IL-6 shRNA | 19/20 | 0.5 (0–1)* | 9/20* |
| PBS Liposome | |||
| PC-3MM2 | 20/20 | 1.2 (0.6–1.2) | 20/20 |
| PC-3MM2-NS shRNA | 20/20 | 1.2 (0.6–1.6) | 20/20 |
| PC-3MM2-IL-6 shRNA | 19/20 | 0.4 (0–1.1)* | 11/20* |
| Clodronate Liposome | |||
| PC-3MM2 | 20/20 | 0.6 (0.2–1.1)† | 13/20† |
| PC-3MM2-NS shRNA | 20/20 | 0.6 (0.2–1)† | 10/20† |
| PC-3MM2-IL-6 shRNA | 20/20 | 0.3 (0.1–0.8)*,† | 1/20† |
Statistically significant as compared with untreated mice bearing PC-3MM2 bone tumors in the same treatment groups (p<0.05).
Statistically significant as compared with mice bearing bone tumors produced by PC-3MM2, or PC-3MM2-NS shRNA, or PC-3MM2-IL-6 shRNA in the different treatment groups (p<0.05)
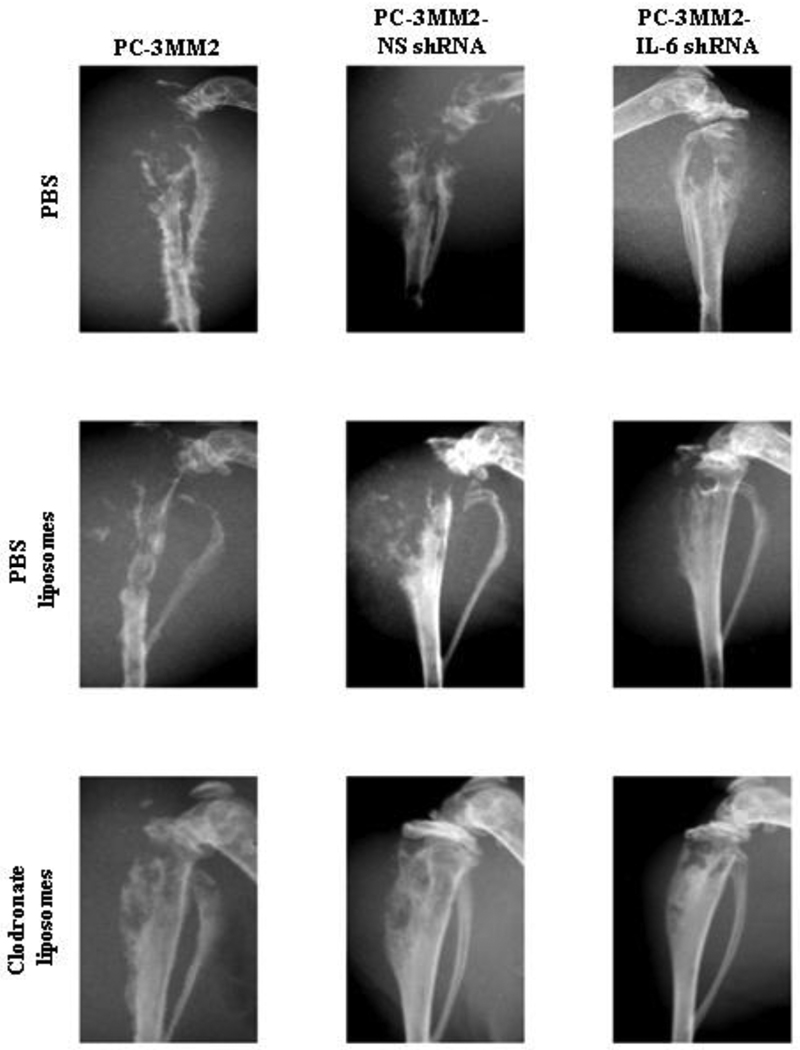
Fig. 2.
Digital radiography of tumors in the hind legs of nude mice. Mice were injected intraperitoneally with PBS, PBS liposomes, or clodronate liposomes one day prior to the intratibial injection of 2 × 105 PC-3MM2, PC-3MM2-NS shRNA, or PC-3MM2-IL-6 shRNA cells. Extensive bone lysis was found in mice with PC-3MM2 and PC-3MM2-NS shRNA tumors treated with PBS or PBS liposomes. There was significant preservation of bone in mice injected with cells transfected with IL-6 shRNA (PC-3MM2-IL-6 shRNA) and mice treated with clodronate liposomes. The most important outcome (no lysis) was achieved by the combination of knockdown of IL-6 in tumor cells and treatment of mice with clodronate liposomes.
In mice treated with control PBS liposomes, the PC-3MM2-IL-6 shRNA cells produced significantly smaller tumors (median, 0.4 g; range, 0–1.1 g; p<0.05), and a lower incidence of lymph node metastasis (11 of 20; p<0.01). Bone lysis was also decreased as compared with mice injected with PC-3MM2 and PC-3MM2-NS shRNA cells. No significant differences in tumor incidence were found among the 3 groups.
When mice were treated with clodronate liposomes, tumor size (median, 0.3 g; range, 0–0.8 g; p<0.05), the incidence of lymph node metastasis (1 of 21; p<0.01), and bone lysis were all significantly decreased in mice with PC-3MM2-IL-6 shRNA cells growing in the tibia when compared with mice injected with PC-3MM2 cells (median, 0.6g; range, 0.2–1.1g; 13 of 20), or PC-3MM2-NS shRNA cells (median, 0.6g; range, 0.2–1 g; 10 of 20).
In all mice treated with clodronate liposomes, tumor size and the incidence of lymph node metastasis were significantly decreased compared to treatment with PBS or PBS liposomes (Table 1). The tibias of mice injected with clodronate liposomes were also significantly preserved (Fig, 2). Treatment with PBS or PBS liposomes did not produce significant differences in tumor size, bone lysis, or incidence of lymph node metastasis.
3.3 In vitro and In vivo expression of p44/p42 and phosphorylated p44/p42
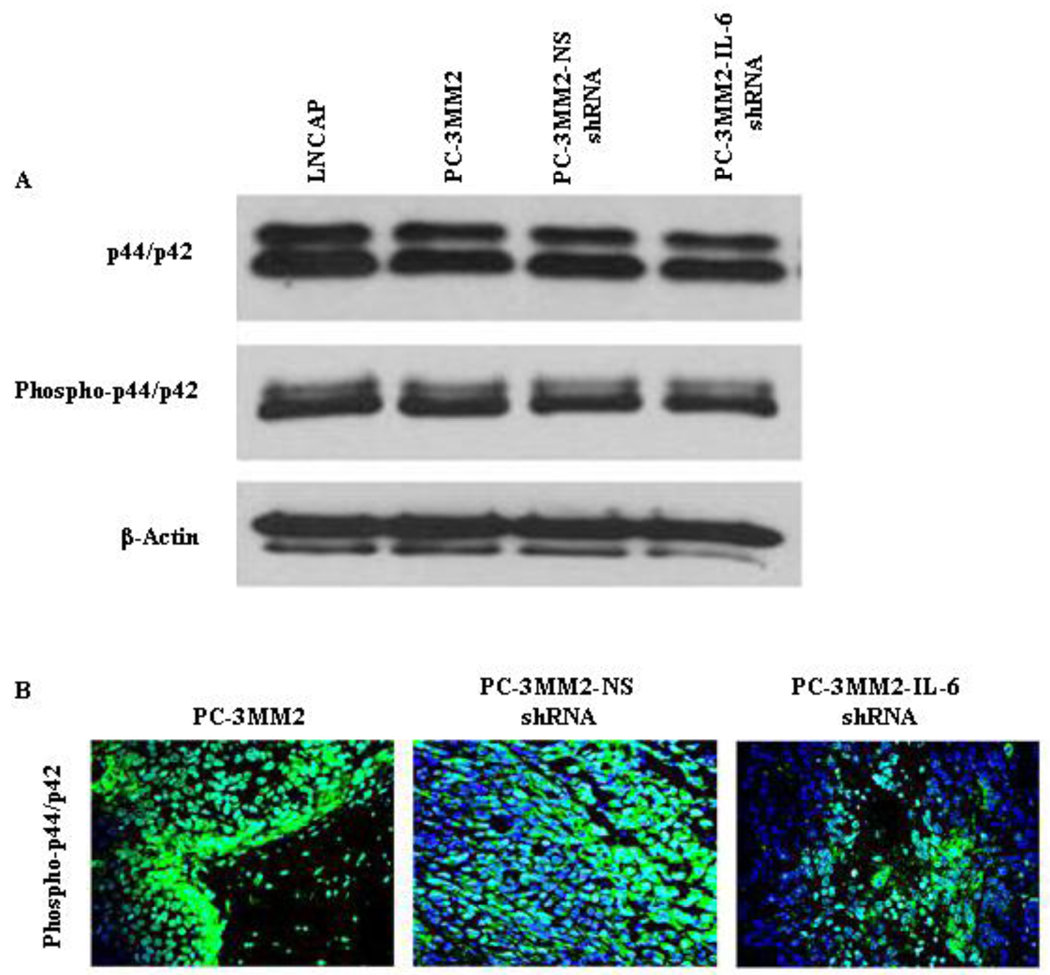
No significant differences in the expression of p44/p42 or phosphorylated p44/p42 were found among PC-3MM2, PC-3MM2-NS shRNA, or PC-3MM2-IL-6 shRNA cells growing in culture. Moreover, the expression of p44/p42 or phosphorylated p44/p42 did not differ between androgen-dependent/PSA producing cells (LNCaP) and androgen-independent/PSA non-producing cells (PC-3MM2) growing in culture (Fig, 3A). Knockdown of IL-6 did not affect the cellular proliferation rate (measured by MTT; data not shown). In contrast, expression of phosphorylated p44/p42 was significantly reduced in PC-3MM2-IL-6 shRNA cells growing in vivo (Fig, 3B).
Fig. 3.
Western blot and immunohistochemical analyses. A: Cell lysates were harvested from LNCaP, PC3-MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA cells and analyzed by Western blot for the expression of p44/p42 and phosphorylated p44/p42, and tumor tissues were stained with anti-phosphorylated antibody to determine the expression of phosphorylated p44/p42. There was no significant difference in the expression of p44/p42 or phosphorylated p44/p42 among cultured cells PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA. The expression of p44/p42 or phosphorylated p44/p42 did not differ between androgen-dependent/PSA producing cells (LNCaP) and androgen-independent/PSA non-producing cells (PC-3MM2) growing in culture. B: The expression of phosphorylated p44/p42 was significantly reduced in PC-3MM2-IL-6 shRNA tumors growing in vivo.
3.4 Expression of IL-6 and TNF-α and number of macrophages and osteoclasts in bone lesions
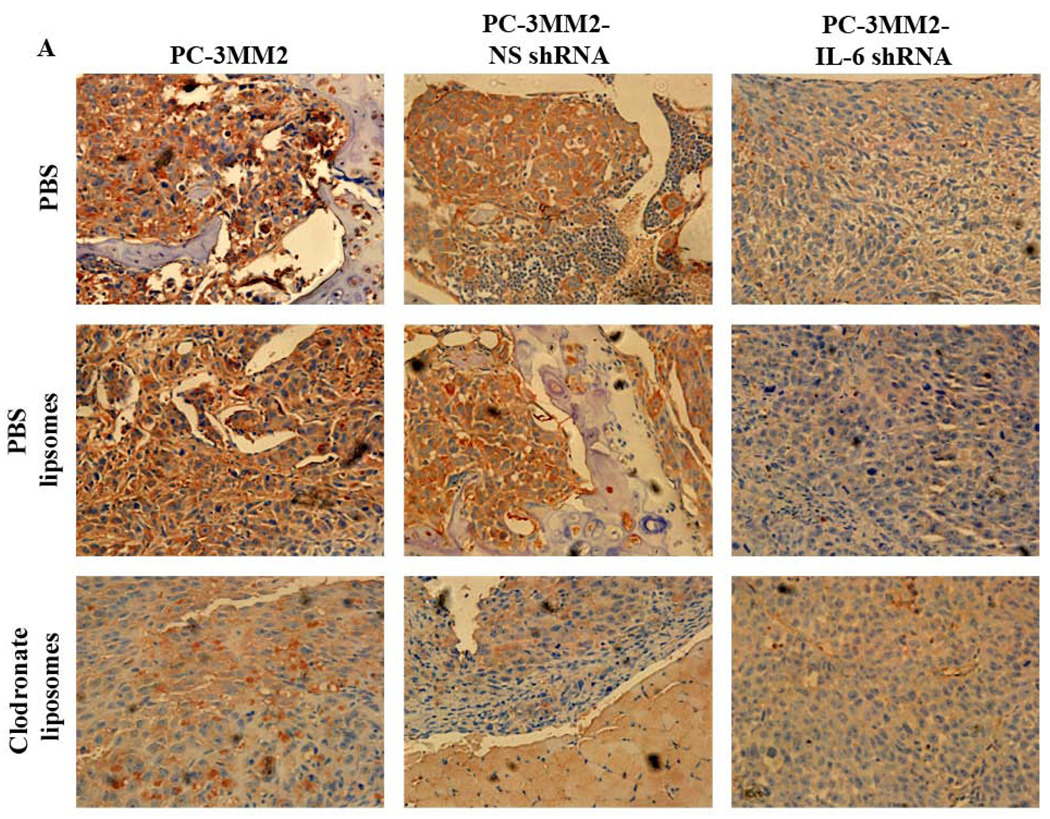
Expression of IL-6 and TNF-α was significantly decreased in bone tumors produced by PC-3MM2-IL-6 shRNA cells. Treatment of mice with clodronate liposomes also decreased the expression of IL-6 and TNF-α in bone lesions produced by PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA cells. Transfection of PC-3MM2 cells with NS shRNA or treatment of mice with PBS liposomes did not affect the expression of IL-6 and TNF-α in bone lesions (Figs. 4A and 4B).
Fig. 4.
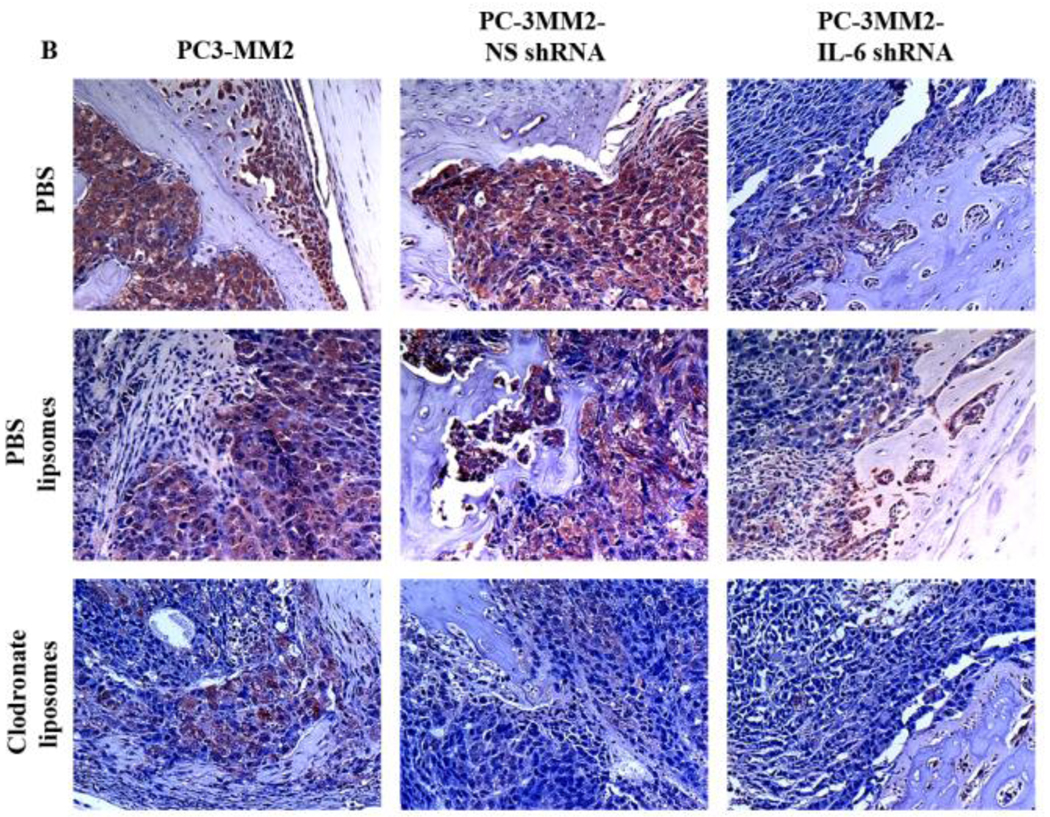
Immunohistochemical analyses. Tissues were harvested and processed for immunohistochemical analysis of the expression of IL-6 (using 3-amino-9-ethylcarbazole chromogen B) and TNF-α (using 3,3’-diaminobenzidine). A: Expression of IL-6 (red) was significantly decreased by transfection of PC-3MM2 with IL-6 shRNA. Treatment with clodronate liposomes also decreased the expression of IL-6 in bone tumors. B: Expression of TNF-α (brown) was significantly decreased by knock down of IL-6 by IL-6 shRNA as well as by treatment with clodronate liposomes. Positive cells are recognized as brown color with 3,3’-diaminobenzidine.
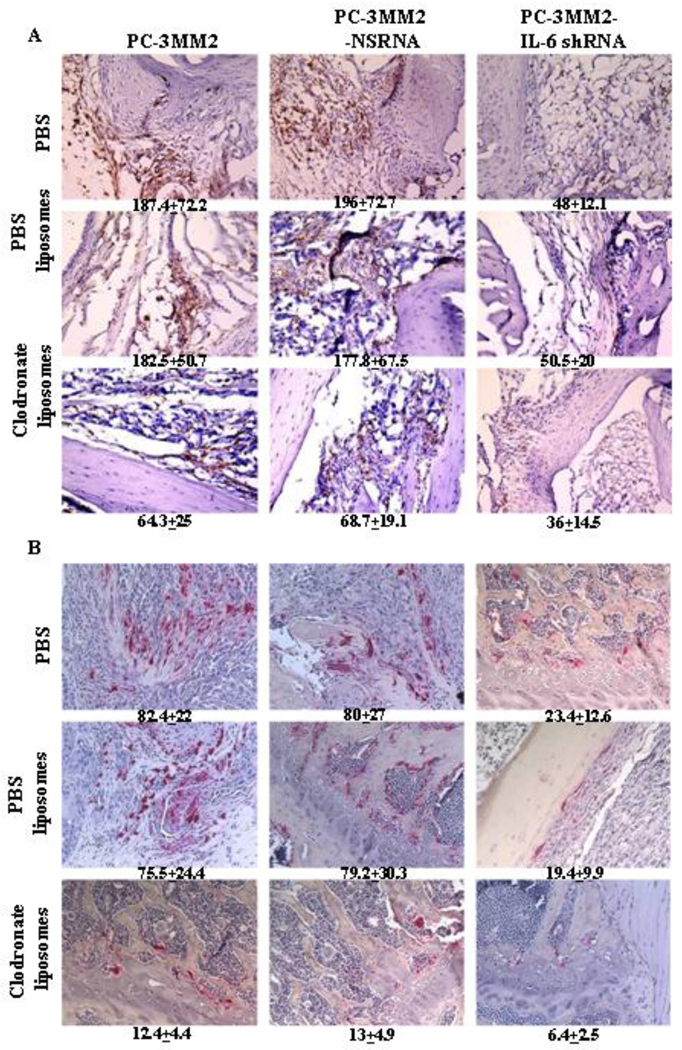
Compared with bone tumors produced by PC-3MM2 cells, bone tumors produced by PC-3MM2-IL-6 shRNA cells exhibited a significant decrease in F4/80-positive cells (TAMs) in mice treated with PBS (187.4 ± 72.2 vs 48 ± 12.1, p<0.01), PBS liposomes (182.5 ± 50.7 vs 50.5 ± 20, p<0.01), and clodronate liposomes (64.3 ± 25 vs 36 ± 14.5, p<0.01). Treatment of mice with clodronate liposomes, as compared with PBS, also significantly decreased the number of TAMs in mice bearing PC-3MM2 (187.4 ± 72.2 vs 64.3 ± 25, p<0.01), and PC-3MM2-NS shRNA (196 ± 72.7 vs 68.7 ± 19.1, p<0.01) bone tumors. The reduction of TAMs in mice bearing PC-3MM2-IL-6 shRNA was not significant (48 ± 12.1 vs 36 ± 14.5, p=0.06) (Fig. 5A).
Fig. 5.
Infiltrating macrophages (TAMs) and osteoclasts in bone tumors. A: Tissues were immunohistochemically analyzed for F4/80 to detect infiltrating macrophages (brown). F4/80-positive cells counted in 10 random 0.159-mm2 fields (unpaired Student’s t test) were statistically analyzed. Compared with PC-3MM2 tumors, bone tumors of PC-3MM2-IL-6 cells showed a significant decrease in F4/80-positive cells in mice treated with PBS (187.4 ± 72.2 vs 48 ± 12.1, p<0.01), PBS liposomes (182.5 ± 50.7 vs 50.5 ± 20, p<0.01), and clodronate liposomes (64.3 ± 25 vs 36 ± 14.5, p<0.01). Treatment of mice with clodronate liposomes also significantly decreased the number of TAMs in mice bearing PC-3MM2 (187.4 ± 72.2 vs 64.3 ± 25, p<0.01), and PC-3MM2-NS shRNA (196 ± 72.7 vs 68.7 ± 19.1, p<0.01) cells. However, reduction of the number of TAM in mice bearing PC-3MM2-IL-6 shRNA tumors was not significant (48 ± 12.1 vs 36 ± 14.5, p=0.06). B: Tissues were stained with tartrate-resistant acid phosphatase-positive (TRAP) cells to detect osteoclasts. TRAP-positive cells (red) counted in 10 random 0.159-mm2 fields (unpaired Student’s t test) were statistically analyzed. Knock-down of IL-6 with IL-6 shRNA significantly decreased the number of TRAP-positive cells in mice treated with PBS (82.4 ± 22 vs 23.4 ± 12.6, p<0.01), PBS liposomes (75.5 ± 24.4 vs 19.4 ± 9.9, p<0.01), and clodronate liposomes (12.4 ± 4.4 vs 6.4 ± 2.5, p<0.01). Treatment of mice with clodronate liposomes also significantly decreased the number of osteoclasts in mice bearing PC-3MM2 (82.4 ± 22 vs 12.4 ± 4.4, p<0.01), PC-3MM2-NS shRNA (80 ± 27 vs 13 ± 4.9, p<0.01), and PC-3MM2-IL-6 shRNA (23.4 ± 12.6 vs 6.4 ± 2.5, p<0.01) tumors.
Knock-down of IL-6 with IL-6 shRNA significantly decreased the number of TRAP-positive cells (osteoclasts) in bone tumors of mice treated with PBS (82.4 ± 22 vs 23.4 ± 12.6, p<0.01), PBS liposomes (75.5 ± 24.4 vs 19.4 ± 9.9, p<0.01), and clodronate liposomes (12.4 ± 4.4 vs 6.4 ± 2.5, p<0.01). Treatment of mice with clodronate liposomes also significantly decreased the number of osteoclasts in PC-3MM2 (82.4 ± 22 vs 12.4 ± 4.4, p<0.01), PC-3MM2-NS shRNA (80 ± 27 vs 13 ± 4.9, p<0.01), and PC-3MM2-IL-6 shRNA (23.4 ± 12.6 vs 6.4 ± 2.5, p<0.01) tumors (Figure 5B). Transfection with NS shRNA and treatment with PBS liposomes did not produce significant differences in TAM as compared to control mice.
3.5 Serum levels of IL-6 and TNFα
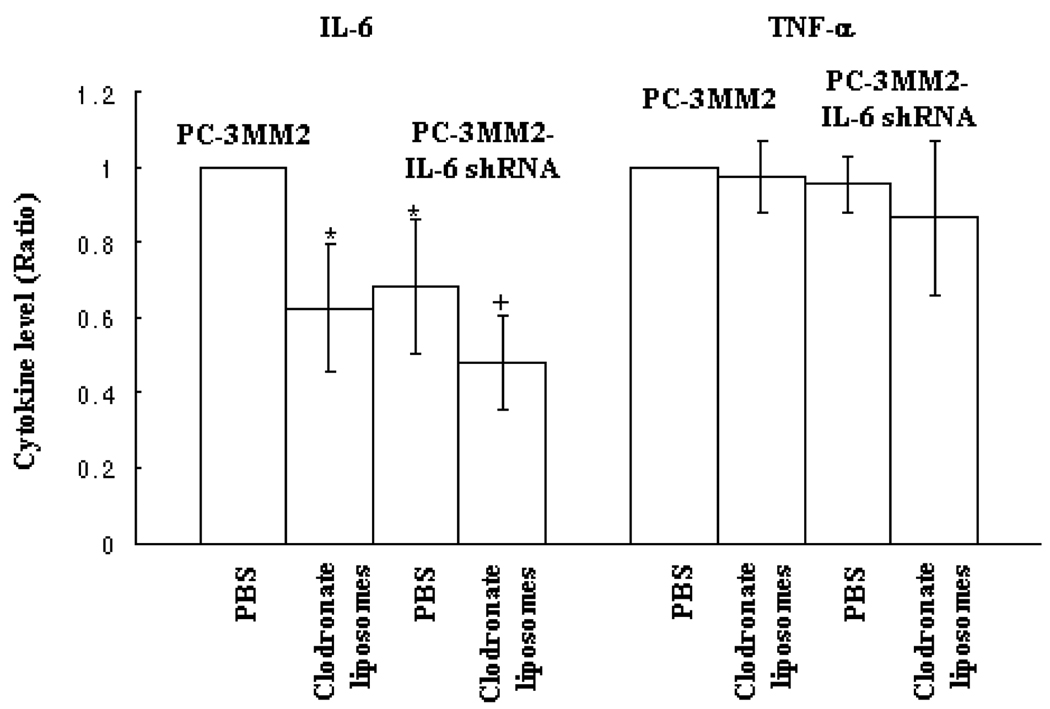
In the last set of experiments, we measured the levels of IL-6 and TNF-α in the serum of control mice or mice treated with clodronate liposomes and injected in the tibia with prostate cancer cells. Since the ranges of IL-6 and TNF-α levels vary depending on the tumor burden and presence of inflammation [9,16,51], we report the levels of IL-6 and TNF-α as ratio compared to control mice with similar tumor burden. Transfection of PC-3MM2 cells with IL-6 shRNA in control mice treated i.p. with PBS significantly decreased the level of IL-6 in the serum (0.68 ± 0.18 as compared with PC-3MM2 treated with PBS; p<0.05). Treatment with clodronate liposomes significantly decreased the level of serum IL-6 in mice with PC-3MM2 tumors (0.63 ± 0.17, as compared with mice bearing PC-3MM2 tumors treated with PBS; p<0.05) and in mice with PC-3MM2-IL-6 shRNA tumors (0.48 ± 0.12, as compared with PC-3MM2 tumors treated with PBS; p<0.05). Treatment with clodronate liposomes also significantly decreased the level of serum IL-6 in mice bearing PC-3MM2-IL-6 shRNA bone tumors compared with the serum IL-6 level in PC-3MM2-IL-6 shRNA tumors treated with PBS (0.74 ± 0.22; p<0.05). The serum level of TNF-α was not affected by either transfection of IL-6 shRNA or treatment with clodronate liposomes (Fig. 6). Treatment of mice bearing any of the three tumor lines with PBS liposomes did not affect the level of IL-6 or TNF-α.
Fig. 6.
Enzyme-linked immunosorbent assay. Sera were collected from mice bearing PC-3MM2 or PC-3MM2-IL-6 shRNA bone tumors and measured for the levels of IL-6 and TNF-α by ELISA. There was a significant decrease in the level of IL-6 in mice bearing PC-3MM2-IL-6 shRNA bone tumors as compared with mice bearing PC-3MM2 bone tumors. Treatment with clodronate liposomes produced an additional significant decrease in the level of serum IL-6 in mice bearing either PC-3MM2 or PC-3MM2-IL-6 shRNA bone tumors.
4. Discussion
Our data show that the level of IL-6 produced by PC-3MM2 cells correlated with aggressive tumor growth in the tibia and the production of lymph node metastasis. Knocking down IL-6 expression with shRNA in tumor cells significantly decreased bone tumor size, bone lysis, and the incidence of lymph node metastasis, but it did not decrease tumor incidence. In vitro, PC-3MM2 cells responded to exogenous TNF-α by producing increased amounts of IL-6, and the knockdown of the IL-6 in PC-3MM2 by IL-6 shRNA resulted in s significant reduction in IL-6 production in the absence or presence of exogenous TNF-α. This decreased production of IL-6 by the tumor cells correlated with a significant decrease in the size of bone lesions, bone lysis, and the incidence of lymph node metastasis. Lower expression of IL-6 by tumor cells was also correlated with a decrease in the number of TAMs (F4/80-positive cells) and osteoclasts (TRAP-positive cells).
Macrophages are recruited in great numbers by many solid tumors and can be the source of cytokines, such as TNF-α and IL-1 [29–32,35,51,52]. In vitro, PC-3MM2 cells responded to TNF-α by producing significantly higher amounts of IL-6. To test this association in vivo, we depleted the macrophages from mice by treatment with clodronate liposomes [43]. This allowed us to investigate the reciprocal role of tumor cells and tumor-associated macrophages in the progressive growth of prostate cancer bone metastasis. Treatment of mice with liposomes containing clodronate significantly reduced the number of macrophages in the peritoneal cavity, and within the bone tumors. Treatment with clodronate liposomes also significantly decreased the size of bone lesions, bone lysis, and the incidence of lymph node metastasis in mice bearing PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 shRNA bone tumors. Mice treated with clodronate liposomes (low TAM) and injected with PC-3MM2-IL-6 shRNA into the tibia had the smallest bone tumors, the least bone lysis, and the lowest incidence of lymph node metastasis.
Immunohistochemical analyses revealed that the expression of IL-6 and TNF-α and the number of TAM and osteoclasts (TRAP-positive cells) in bone tumors were significantly decreased in mice treated with clodronate liposomes and injected with PC-3MM2, PC-3MM2-NS shRNA, and PC-3MM2-IL-6 cells into the tibia. However, the reduction in the number of TAMs was not significant in mice bearing PC-3MM2-IL-6 shRNA bone tumors, although it was significant in mice bearing PC-3MM2 and PC-3MM2-NS shRNA tumors. From these observations, it can be postulated that IL-6 promotes the aggressiveness of cancer cells through both autocrine and paracrine loops to recruit TAM that enhance disease progression. IL-6 is a strong chemotactic factor that recruits macrophages to the tumor lesion [52], and TNF-α produced by the macrophages stimulates the prostate cancer cells to produce additional IL-6 capable of attracting additional macrophages [52]. IL-6 and TNF-α also activate osteoclasts which destroy the bone [53,54]. Furthermore, macrophages are a source of osteoclast ontogeny, and human TAMs differentiate into osteoclastic bone-resorbing cells [55,56]. Therefore, decreasing the number of TAMs by either knocking down IL-6 in tumor cells or depleting macrophages in the host may significantly suppress prostate cancer disease progression.
The effect of IL-6 on cell proliferation has been shown to be related to activation of STAT3 pathway [40]. LNCaP cells express STAT3, and treatment of these cells with IL-6 can lead to suppressed growth [41]. Since PC3-MM2 cells are independent of STAT3 pathway, we determined the expression of p44/p42 and phosphorylated p44/p42 tumor cells growing in culture or in the tibia. The expression of phosphorylated p44/p42 did not differ among the various groups of PC-3MM2 cells growing in culture, but there was a significant difference in the expression of phosphorylated p44/p42 by tumor cells growing in vivo, suggesting that knockdown of IL-6 affects the progression of PC-3MM2 bone tumors by modulating the interaction between tumor cells and the host microenvironment rather than by a direct effect on tumor cells.
In conclusion, tumor-associated macrophages enhance the progressive growth of prostate cancer bone metastasis via a vicious cycle of production of IL-6 by tumor cells that can be chemotactic to macrophages which, in turn, produce TNF-α leading to increased production of IL-6 by tumor cells. These data clearly reaffirm the venerable “seed and soil” hypothesis of Paget [4,5,57] and prove that tumor cells can exploit host factors for progressive growth and spread.
Acknowledgments
Supported in part by Cancer Center Support Core Grant CA16672 and Grant 1U54-CA143837 from the National Cancer Institute, National Institutes of Health. We thank Dawn Chalaire for critical editorial review and Lola López for expert assistance with the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Koutsilieris M. Osteoblastic metastasis in advanced prostate cancer. Anticancer Res. 1993;13:443–449. [PubMed] [Google Scholar]
- 3.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth GHA Clowes Memorial Award Lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 4.Fidler IJ. Modulation of the organ microenvironment for the treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 5.Talmadge JE, Fidler IJ. AACR Centennial Series: The biology of cancer metastasis: historical perspective. Cancer Res. 2010;70(14):5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol. 2003;30:39–50. doi: 10.1016/s0093-7754(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Uehara H, Yazici S, Busby JE, Nakamura T, He J, et al. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. J Natl Cancer Inst. 2006;98:783–793. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9:1200–1211. [PubMed] [Google Scholar]
- 9.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 10.Corcoran NM, Costello AJ. Interleukin-6: minor player or starring role in the development of hormone-refractory prostate cancer? BJU Int. 2003;91:545–553. doi: 10.1046/j.1464-410x.2003.04025.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee SO, Lou W, Johnson CS, Trump DL, Gao AC. Interleukin-6 protects LNCaP cells from apoptosis induced by androgen deprivation through the Stat3 pathway. Prostate. 2004;60:178–186. doi: 10.1002/pros.20045. [DOI] [PubMed] [Google Scholar]
- 12.Domingo-Domenech J, Oliva C, Rovira A, Codony-Servat J, Bosch M, Filella X, et al. Interleukin 6, a nuclear factor-κB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear docetaxel antitumor activity. Clin Cancer Res. 2006;12:5578–6686. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- 13.Nakano T, Chahinian AP, Shinjo M, Tonomura A, Miyake M, Togawa N, et al. Interleukin 6 and its relationship to clinical parameters in patients with malignant pleural mesothelioma. Br J Cancer. 1998;77:907–912. doi: 10.1038/bjc.1998.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami S, Gupta A, Sharma SK. Interleukin-6-mediated autocrine growth promotion in human glioblastoma multiform cell line U87MG. J Neurochem. 1998;71:1837–1845. doi: 10.1046/j.1471-4159.1998.71051837.x. [DOI] [PubMed] [Google Scholar]
- 15.Foti E, Ferrandina G, Martucci R, Romanini ME, Benedetti PP, Testa U, et al. IL-6, M-CSF and IAP cytokines in ovarian cancer: simultaneous assessment of serum levels. Oncology. 1999;57:211–215. doi: 10.1159/000012033. [DOI] [PubMed] [Google Scholar]
- 16.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 17.Siegall CB, Schwab G, Nordan RP, Fitzgerald DJ, Pastan I. Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res. 1990;50:7786–7788. [PubMed] [Google Scholar]
- 18.Siegsmund MJ, Yamazaki H, Pastan I. Interleukin 6 receptor mRNA in prostate carcinoma and benign prostate hyperplasia. J Urol. 1994;151:1396–1399. doi: 10.1016/s0022-5347(17)35267-9. [DOI] [PubMed] [Google Scholar]
- 19.Hobisch A, Rogatsch H, Hittmair A, Fuchs D, Bartsch G, Jr, Klocker H, et al. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191:239–244. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41:127–133. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Stark JR, LI H, Kraft P, Kurth T, Giovannucci EL, Stampfer MJ, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009;124:2683–2689. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paule B, Terry S, Kheuang L, Soyeux P, Vacherot F, de la Taille A. The NF-κB/IL-6 pathway in metastatic androgen-independent prostate cancer: new therapeutic approach? World J Urol. 2007;25:477–489. doi: 10.1007/s00345-007-0175-6. [DOI] [PubMed] [Google Scholar]
- 23.Malinowska K, Neuwirt H, Cavarretta IT, Bektic J, Steiner H, Dietrich H, et al. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocrine-Related Cancer. 2009;16:155–169. doi: 10.1677/ERC-08-0174. [DOI] [PubMed] [Google Scholar]
- 24.Xie S, Lin HK, Ni J, Yang L, Wang L, di Sant’Agnese PA, et al. Regulation of interleukin-6 mediated PI3K activation and neuroendocrine differentiation by androgen signaling in prostate cancer LNCaP cells. Prostate. 2004;60:61–67. doi: 10.1002/pros.20048. [DOI] [PubMed] [Google Scholar]
- 25.Cavarretta IT, Neuwirt H, Untergasser G, Moser PL, Zaki MH, Steiner H, et al. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer in mediated by Mcl-1. Oncogene. 2007;26:2822–2832. doi: 10.1038/sj.onc.1210097. [DOI] [PubMed] [Google Scholar]
- 26.Smith PC, Keller ET. Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenograft in nude mice. Prostate. 2001;48:47–53. doi: 10.1002/pros.1080. [DOI] [PubMed] [Google Scholar]
- 27.Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;40:1066–1072. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
- 28.Steiner H, Canarretta IT, Moser PL, Berger AP, Bektic J, Dietrich H, et al. Regulation of growth of prostate cancer cells selected in the presence of interleukin-6 by the anti-interleukin-6 antibody CNTO 328. Prostate. 2006;66:1744–1752. doi: 10.1002/pros.20492. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 30.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunol. 2004;351:2817–2826. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 33.Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extramural macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizutani K, Sud S, McGregor NA, Martinivski G, Rice BT, Craig MJ, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Meir E, Sawamura Y, Diserens AC, Hamou MF, de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990;50:6683–6688. [PubMed] [Google Scholar]
- 36.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 37.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2Kb-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2972–2976. [PubMed] [Google Scholar]
- 38.Baeza-Raja B, Munoz-Canoves P. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell. 2004;15:2013–2026. doi: 10.1091/mbc.E03-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 40.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of Stat 3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Horiatis D, Pinski J. Interleukin-6 inhibits the growth of prostate cancer xenograft in mice by the process of neuroendocrine differentiation. Int J Cancer. 2004;111:508–513. doi: 10.1002/ijc.20286. [DOI] [PubMed] [Google Scholar]
- 42.Nakao S, Kuwano T, Tsusumi-Miyahara C, Ueda S, Kimura YN, Hamano S, et al. Infiltration of COX-2 expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. J Immunol Meth. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 44.Bianco C, Griffin FM, Silverstein SC. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975;141:1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SJ, Uehara H, Yazici S, Langley RR, He J, Tsan R, Fan D, et al. Simultaneous blockade of platelet-derived growth factor receptor and epidermal growth factor receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res. 2004;64:4201–4208. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch S, Austyn JM, Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981;154:713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chun L, Yoon J, Song Y, Huie P, Regula D, Goodman S. The characterization of macrophages and osteoclasts in tissues harvested from revised total hip prostheses. J Biomed Master Res. 1999;48:899–903. doi: 10.1002/(sici)1097-4636(1999)48:6<899::aid-jbm20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 49.Kim SJ, Uehara H, Yazici S, He J, Langley RR, Mathew P, et al. Modulation of bone microenvironment with zoledronate enhances the therapeutic effects of STI571 and paclitaxel against experimental bone metastasis of human prostate cancer. Cancer Res. 2005;65:3707–3715. doi: 10.1158/0008-5472.CAN-04-3601. [DOI] [PubMed] [Google Scholar]
- 50.Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclasts function. Calcif Tissue Int. 1982;34:285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 51.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-α correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horowitz SM, Purdon MA. Mechanisms of cellular recruitment in aseptic loosening of prosthetic joint implants. Calcif Tissue Int. 1995;57:301–305. doi: 10.1007/BF00298886. [DOI] [PubMed] [Google Scholar]
- 53.Black K, Garrett IR, Mundy GR. Chinese hamster ovarian cells transfected with the murine interleukin-6 gene causes hypercalcemia as well as cachexia, leukocytosis, and thrombocytosis in tumor-bearing nude mice. Endocrinology. 1991;128:2657–2659. doi: 10.1210/endo-128-5-2657. [DOI] [PubMed] [Google Scholar]
- 54.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNF-α potently activates osteoclasts through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143:1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 55.Steven L, Teitelbaum M, Tondravi M, Ross FP. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J Leukocyte Biol. 1997;61:381–388. doi: 10.1002/jlb.61.4.381. [DOI] [PubMed] [Google Scholar]
- 56.Quinn JM, McGee JO, Athanasou NA. Human tumour-associated macrophages differentiate into osteoclastic bone-resorbing cells. J Pathol. 1998;184:31–36. doi: 10.1002/(SICI)1096-9896(199801)184:1<31::AID-PATH962>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 57.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]