Abstract
Mitochondria are essential organelles for neuronal survival and play important roles in ATP generation, calcium buffering, and apoptotic signaling. Due to their extreme polarity, neurons utilize specialized mechanisms to regulate mitochondrial transport and retention along axons and near synaptic terminals where energy supply and calcium homeostasis are in high demand. Axonal mitochondria undergo saltatory and bidirectional movement and display complex mobility patterns. In cultured neurons, approximately one-third of axonal mitochondria are mobile, while the rest remain stationary. Stationary mitochondria near synapses serve as local energy stations that produce ATP to support synaptic function. In addition, axonal mitochondria maintain local Ca2+ homeostasis at presynaptic boutons. The balance between mobile and stationary mitochondria is dynamic and responds quickly to changes in axonal and synaptic physiology. The coordination of mitochondrial mobility and synaptic activity is crucial for neuronal development and synaptic plasticity. In this update article, we introduce recent advances in our understanding of the motor-adaptor complexes and docking machinery that mediate mitochondrial transport and axonal distribution. We will also discuss the molecular mechanisms underlying the complex mobility patterns of axonal mitochondria and how mitochondrial mobility impacts the physiology and function of synapses.
Keywords: mitochondria, axonal transport, docking, synaptic plasticity, kinesin, motor adaptor, anterograde transport, retrograde transport, stationary mitochondria, mitochondrial mobility
1. Introduction
Proper neuronal function and survival depends upon the supply of appropriate levels of ATP, approximately ninety percent of which is produced by mitochondria via oxidative phosphorylation. Neurons are highly polarized cells whose morphology precludes the efficient diffusion of somally produced ATP to distal processes. While the biogenesis of neuronal mitochondria is not well characterized, it is clear that the majority of mitochondria are produced in the cell body. Thus, distal cellular compartments such as synapses depend upon the efficient delivery of mitochondria through active transport to provide local sources of ATP. Additionally, mitochondria have been shown to aid in critical physiological processes, including the establishment of the axonal resting membrane potential required for action potential propagation, the assembly of the actin cytoskeleton within presynaptic boutons (Lee and Peng 2008), and the myosin-driven mobilization of synaptic vesicles from the reserve pool to the readily releasable pool during sustained neuronal activity (Versteken 2005). Furthermore, mitochondria’s ability to buffer Ca2+ within presynaptic terminals appears to be involved in certain types of short-term synaptic plasticity (Tang and Zucker, 1997, Billups and Forsythe, 2002, Levy et al., 2003, Kang et al., 2008). Thus, removing mitochondria from axon terminals results in aberrant synaptic transmission (Stowers et al., 2002; Guo et al., 2005; Verstreken et al., 2005; Ma et al., 2009). Many neurodegenerative diseases, including Huntington’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis, involve defects in mitochondrial function and transport (see reviews by Chan, 2006; Stokin and Goldstein, 2006).
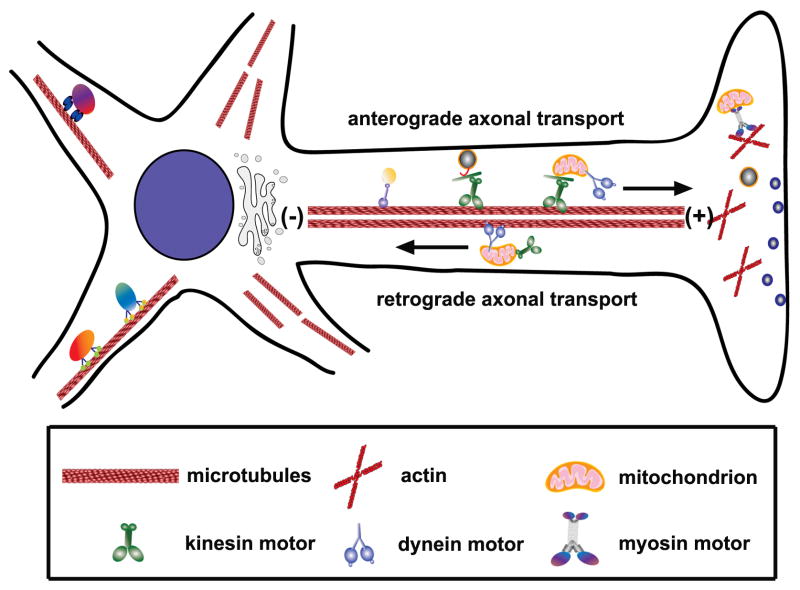
Mitochondria are transported from the cell body along axonal microtubules (MTs) by protein motors to reach areas with high ATP and calcium buffering requirements like Nodes of Ranvier, axonal branches, active growth cones, and synapses (Fabricius et al., 1993; Morris and Hollenbeck, 1993; Mutsaers and Carroll, 1998; Ruthel and Hollenbeck, 2003; Kang et al., 2008; Zhang et al., 2010). Generally, kinesin motors drive anterograde mitochondrial transport, while dyneins are responsible for retrograde transport (Fig. 1). Individual mitochondrion, however, rarely move in only one direction. Their transport along MTs typically involves pauses of short and long duration and abrupt changes in direction. At any given time, two-thirds of axonal mitochondria in cultured neurons are stationary (Hollenbeck and Saxton, 2005; Kang et al., 2008). Syntaphilin mediates axonal mitochondrial “docking” and helps establish an appropriate axonal mitochondrial distribution (Kang et al., 2008; Chen et al., 2009). The complex mobility patterns of axonal mitochondria may indicate that individual mitochondria are simultaneously coupled to kinesins, dyneins, and anchoring machinery whose actions compete or oppose one another. Averaging the bidirectional and saltatory components yields a net mitochondrial velocity that falls between fast moving vesicles and slow-moving cytoskeletal proteins: 0.3–2.0 μm second−1(Morris and Hollenbeck, 1993; Ligon and Steward, 2000).
Figure 1. Axonal mitochondrial transport.
In axons, MTs are uniformly organized with the plus (+) ends facing toward the axonal terminals and the minus (−) ends toward the cell body. While kinesin motors are mostly plus-end directed, dyneins travel toward the minus ends of MTs. Therefore, kinesin motors generally mediate anterograde axonal transport of mitochondria and dynein drives retrograde axonal transport of mitochondria.
(Adapted with permission from Qian Cai, Zu-Hang Sheng. Mitochondrial transport and docking in axons. Experimental Neurology 218, 257–267, 2009).
During development, mitochondrial mobility is tightly regulated to ensure that metabolically active areas are adequately supplied with ATP and able to respond to changes in intracellular Ca2+ levels. Later, the structural and functional plasticity characteristic of synapses and axons can drive changes in mitochondrial mobility. For example, the number of immobile mitochondria adjacent to active synapses increases in response to elevated cytosolic Ca2+ levels induced by synaptic activity (Rintoul et al., 2003; Yi et al., 2004). It is probable that neuronal development and synaptic activity are partially regulated by the mechanisms that control mitochondrial position. While these events have not been fully elucidated, two prominent proteins have been identified within the last decade. Milton, a mitochondrial motor-adaptor, provides a link between kinesin motors and mitochondria through an interaction with Miro, a calcium-sensing member of the Rho-GTPase family present in the outer mitochondrial membrane (Glater et al., 2006; Saotome et al., 2008; Macaskill et al., 2009; Wang and Schwarz, 2009; Cai and Sheng, 2009). Continued research will produce more regulatory proteins and increase our understanding of how neurons regulate mitochondrial trafficking to maintain synaptic and axonal homeostasis.
2. Motor proteins driving axonal mitochondrial transport
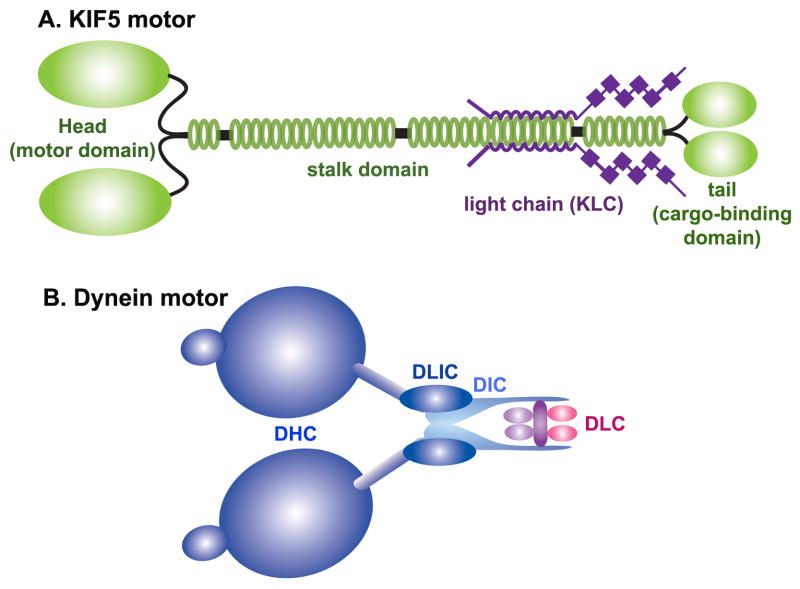
ATP-dependent kinesin motors mediates anterograde transport along MT-based tracks (Hollenbeck, 1996). Since Kinesin-1 (KIF5) was first reported to drive plus end-directed transport in vitro, at least 45 different human and mouse kinesin genes have been identified (Hirokawa and Takemura, 2004). Most members of the kinesin superfamily are structurally similar to Kinesin-1 and have two heavy chains (KHCs) and two light chains (KLCs) (Fig. 2A). The heavy chains of KIF5 contain coiled-coil domains that facilitate their association into homo- or heterodimers. Mammals have two neuron-specific KIF5s (KIF5A and –C) and another that is expressed in most cell types (KIF5B) (Kanai et al., 2000, also see review by Hirokawa and Takemura, 2005). The amino terminal domain of each KIF5 contains a MT-binding motor domain, while the carboxyl terminal domain binds to KLCs or interacts directly with cargo adaptors. Thus, through their C-terminal cargo-binding domain, kinesins attach to cargoes through either a direct interaction between their cargo-binding domains and cargo adaptor proteins or an indirect interaction via their KLCs. Regarding mitochondrial transport, recent studies demonstrate that the former situation is more likely: KIF5s appear to bind mitochondria via their adaptor proteins independent of their KLCs (Cai et al., 2005; Glater et al., 2006).
Figure 2. Structure of motor proteins.
(A) KIF5 motors form homodimers through the coiled-coil region in the stalk domains. While KIF5 possesses motor function, it also binds to the kinesin-1 light chain (KLC) through its stalk and tail domains. The specific association of KIF5 with cargoes or organelles can be mediated directly through the cargo-binding region in its tail domain or indirectly via the COOH-terminal domains of KLC, indicating the existence of two forms of KIF5 motor-cargo coupling.
(B) Cytoplasmic dyneins consist of heavy chains (DHC), intermediate chains (DIC), light intermediate chains (DLIH), and light chains (DLC). To transport cargoes, cytoplasmic dynein also bind to the dynactin complex (not shown).
Several lines of evidence demonstrate that KIF5s play a major role in the anterograde transport of axonal mitochondria (Hurd and Saxton, 1996; Tanaka et al., 1998; Stowers et al., 2002; Cai et al., 2005; Glater et al., 2006). Both imaging and biochemical analyses have confirmed that KIF5 motors associate with brain mitochondria (Hirokawa et al., 1991; Cai et al., 2005; Pilling et al., 2006; Macaskill et al., 2009; Wang and Schwarz, 2009). Furthermore, motor axons of larval Drosophila kif5 mutants display impaired mitochondrial transport and a reduced mitochondrial distribution (Pilling et al., 2006). In undifferentiated extra-embryonic cells, the targeted disruption of kif5b induces aberrant mitochondrial clustering in the perinuclear region as most mitochondria fail to undergo transport towards the cell periphery (Tanaka et al., 1998). Disrupting KIF5-mitochodria coupling in hippocampal neurons by expressing the KIF5 cargo-binding domain transgene induces impaired axonal mitochondrial transport and reduced mitochondrial density in distal axonal compartments (Cai et al., 2005).
Additionally, a member of the kinesin-3 family, KIF1Bα, is widely expressed in the brain and has been shown to interact directly with mitochondria (Nangaku et al., 1994). In vitro, KIF1Bα has been shown to transport mitochondria along MTs at 0.5 μm/sec. While the mutation of murine kif1B leads to peripheral neuropathies, its role in anterograde mitochondrial transport remains to be further characterized.
Dynein motors drive the retrograde transport of neuronal organelles, including mitochondria (Pilling et al., 2006). The dynein motor complex is comprised of two heavy chains (DHC) and several intermediate, light intermediate, and light chains (DIC, DLIC and DLC, respectively) thought to regulate the motility of the dynein motor or mediate its association with cargoes (Fig. 2B). Whereas the heterogeneity of KHCs presumably allows for the selective binding of cargoes, relatively few DHCs have been identified. Thus, the cargo selectivity of the dynein complex likely depends more heavily upon accessory proteins (Susalka and Pfister, 2000). Dynactin, though nonessential, has been shown to mediate some dynein-cargo interactions, coordinate bidirectional axonal transport, and enhance the processivity of the dynein motor (Waterman-Storer et al., 1995; Karki and Holzbaur, 1999; King and Schroer, 2000).
One of eleven different dynactin subunits, p150Glued, can bind directly to both cytoplasmic dynein and MTs. Additionally, although there is a strong directional bias towards the MT minus end, single dynein-dynactin complexes can move bidirectionally (Mallik et al., 2005). It is possible that this versatility may allow the dynein motor to backtrack in order to maneuver past physical barriers it encounters along MTs. Subsequent experiments have shown that cytoplasmic dynein is necessary for proper mitochondrial retrograde transport in the Drosophila nervous system and that dynein and dynactin components interact with purified mitochondria (Pilling et al., 2006). The mutation of dynactin p150Glued and the dynein heavy chain disrupts bidirectional fast organelle transport. The resultant phenotype is similar to those caused by kinesin mutations and is characterized by axonal swelling as retrograde and anterograde cargoes, including mitochondria, accumulate along the axon (Martin et al., 1999).
Recent evidence suggests that opposing kinesin and dynein motors localized to the same mitochondrion may be responsible for the complex mobility patterns of axonal mitochondria. As axonal mitochondria exhibit bidirectional movement and dynein can co-localize with mitochondria moving in either direction (Hirokawa et al., 1990), it is likely that kinesin and dynein coordinate the transport of individual mitochondria. A Drosophila homologue of the mammalian kinesin-interacting scaffold protein JIP-1/JNK, APLIP1, is implicated in retrograde mitochondrial transport (Horiuchi et al., 2005). More recently, it was shown that the Drosophila mitochondrial adaptor protein dMiro helps regulate both anterograde and retrograde transport of axonal mitochondria (Russo et al., 2009). Relatively little is know about the roles and mechanisms of these adaptor proteins in regulating bidirectional movement. Future experiments are necessary to determine to what extent regulatory crosstalk between kinesin, dynein, and their respective adaptors coordinate the distribution and mobility state of mitochondria in response to metabolic changes and synaptic activity.
3. Motor adaptors specific for mitochondria
Cargo identity must be preserved to permit the targeted trafficking of cellular components. Generally, this is achieved by specific linkage between cargo vesicles and motors. Regarding neuronal mitochondria, the specificity of anterograde transport is achieved by direct interactions between KIF5s and the mitochondrial membrane or by indirect coupling via an adaptor that binds KIF5s and mitochondria through lipid-binding or transmembrane domains. Several mitochondrial motor-adaptor complexes and receptors have been identified. Further characterization of these and identification of novel protein adaptors is necessary to more fully understand the complex mechanisms regulating neuronal mitochondrial distribution and transport.
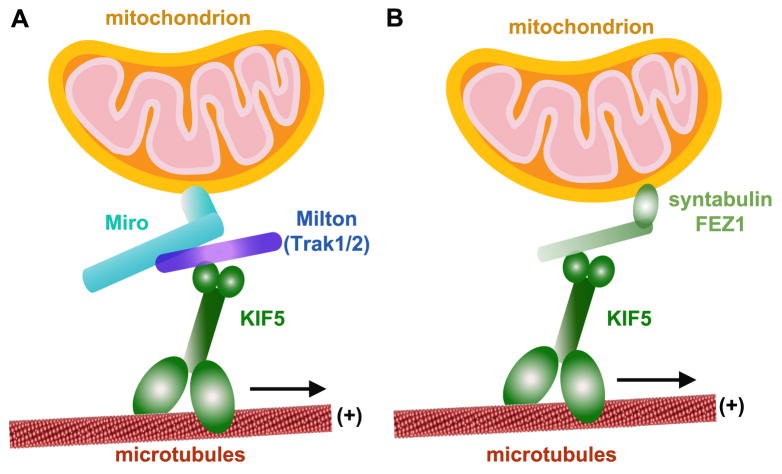
The Miro/Milton complex in Drosophila illustrates how KIF5s can utilize adaptor proteins to achieve targeted transport. Miro is a mitochondrial outer membrane protein that interacts directly with the mitochondrial transport adaptor Milton (Fig. 3A). Mutation of the Drosophila dmiro gene disrupts anterograde mitochondrial transport and inhibits their entry into the axon and synapses, which results in impairment of both neurotransmitter release and Ca2+ buffering during prolonged stimulation (Guo et al., 2005). Similarly, mutation of the Drosophila milton gene results in reduced levels of synaptic and axonal mitochondria (Stowers et al., 2002). Subsequent experiments established that the association of Milton and KIF5 with mitochondria requires membrane-bound Miro (Glater et al., 2006). Genetic and biochemical evidence indicates that mitochondrial KIF5 recruitment occurs independently of KLCs. Importantly, Miro, a member of the mitochondrial Rho-GTPase family, has two EF-hand Ca2+ binding domains that may switch the mitochondrial transport machinery on or off in response to intracellular Ca2+ levels (Fransson et al., 2003).
Figure 3. KIF5 motor adaptors for mitochondrial transport.
(A) Milton-Miro adaptor complex. Miro is a member of the mitochondrial Rho-GTPase family and a mitochondrial outer membrane protein. Milton attaches indirectly to mitochondria via an interaction with Miro and recruits KIF5 to mitochondria independently of KLC. Two mammalian Milton orthologues, TRAK1 and TRAK2, can form complexes with the two mammalian orthologues of dMiro (Miro1 and Miro2).
(B) Syntabulin is a KIF5 adaptor that targets to mitochondria via its carboxyl-terminal transmembrane domain. It provides a link between KIF5 and mitochondria that mediates mitochondrial anterograde transport. In addition, FEZ1 was reported to serve as a candidate kinesin motor adaptor.
The two mammalian orthologues of dMiro (Miro1 and Miro2) can form complexes with the two mammalian Milton orthologues (TRAK1 and TRAK2, or, OIP106 and OIP98/Grif-1) (Fig. 3A) (Fransson et al., 2003). TRAK1 and TRAK2 share 33.5% and 35.3% homology, respectively, with dMilton. Whereas dMilton serves as a mitochondria-specific adaptor that links Miro to KIF5 (Glater et al., 2006; Wang and Schwarz, 2009), mammalian TRAKs are more generally involved in the intracellular transport of cargoes and organelles, including mitochondria (Grishin et al., 2006; Kirk et al., 2006; Webber et al., 2008). While TRAKs play an important role in mitochondrial trafficking (Smith et al., 2006), whether they serve as regulators of motor activity or are adaptors that link KIF5 to Miro remains unclear.
Syntabulin was first identified in our laboratory to serves as another KIF5 motor adaptor that links KIF5 to syntaxin-containing vesicles. Syntabulin is required for the anterograde transport of presynaptic active zone components, presynaptic assembly, and activity-dependent plasticity in developing neurons (Su et al., 2004; Cai et al., 2007). Interestingly, we also found that syntabulin contains a carboxyl-terminal transmembrane domain that targets to mitochondria and plays a critical role in the anterograde transport of mitochondria (Cai et al., 2005). In cultured hippocampal neurons, knockdown of syntabulin or inhibition of syntabulin-KIF5 binding via binding-domain transgenes results in prominent mitochondrial clustering in the soma and sparse mitochondrial distribution along processes. Similarly, mobility analyses in live neurons demonstrate that syntabulin loss-of-function reduces anterograde, but not retrograde, transport of mitochondria along axonal processes. This phenotype provides evidence that syntabulin mediates mitochondrial anterograde transport by acting as an adaptor that links KIF5 to mitochondria (Fig. 3B).
Several additional proteins have also been identified as potential mitochondrial transport adaptors. One of these, the mammalian fasciculation and elongation protein zeta-1 (FEZ1) (Kuroda et al., 1999), was shown to be necessary for the anterograde transport of axonal mitochondria in hippocampal neurons (Ikuta et al., 2007). Its Caenorhabditis elegans orthologue, UNC-76, was initially identified as a brain-specific coiled-coil protein involved in axonal outgrowth (Kuroda et al., 1999). In Drosophila, UNC-76 enables axonal transport of organelles and vesicles through an association with the kinesin motor (Gindhart et al., 2003). Subsequently, the association of FEZ1 with JIP1, the c-Jun N-terminal kinase-interacting protein, was found to activate kinesin motor activity (Blasius et al., 2007). It has been proposed that FEZ1 may enable anterograde mitochondrial transport by regulating motor activity or stabilizing motor-MT binding.
4. Syntaphilin as an axonal mitochondrial docking protein
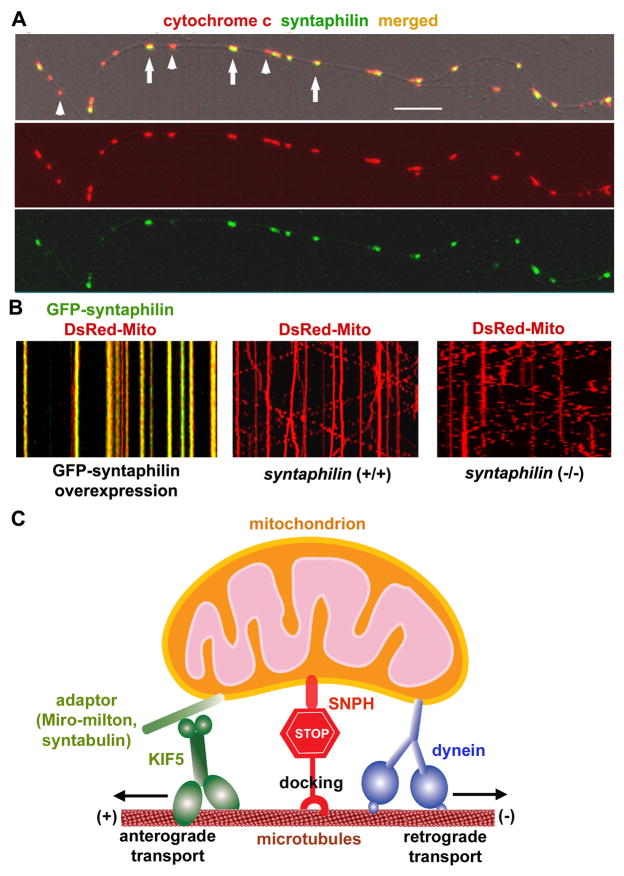
As the main goal of mitochondrial transport is to deliver organelles to areas with high metabolic and calcium-buffering requirements, it is imperative to understand the mechanisms by which mitochondrial transport terminates. A recent study in our laboratory, using genetic mouse models and time-lapse imaging, identified syntaphilin (SNPH) as a “static anchor” for axonal mitochondria (Kang et al., 2008). SNPH is neuron-specific and targets to axonal mitochondria via its carboxyl-terminal tail (Fig. 4). Mitochondrial anchoring occurs when SNPH binds both mitochondria and MTs, effectively tethering the organelles along the axon. Interestingly, there is a strong correlation between SNPH-enriched axonal mitochondria (65±14%) and the proportion of stationary mitochondria (62±15%). As predicted, mitochondrial mobility is greatly enhanced in snph knockout mice relative to wild-type controls. Furthermore, we recently showed that dynein light chain LC8 regulates the SNPH-mediated mitochondrial docking mechanism (Chen et al., 2009). LC8 binds SNPH and enhances the SNPH-microtubule interaction. These findings provide new insight into the mechanisms of mitochondrial mobility. Presumably, the SNPH-mediated anchoring mechanism allows neurons to dynamically regulate axonal mitochondrial distribution in response to changes in axonal and synaptic physiology. Mounting evidence implicates defective mitochondrial trafficking in the pathology of several major neurodegenerative diseases. Snph mutant mice provide a unique model with which to study the physiological impact of mitochondrial docking and retention on axonal homeostasis, synaptic transmission, and neurodegeneration. It will be interesting to cross snph−/− mice with neurodegenerative mouse models to test if increased mitochondrial transport may augment the transport rate of dysfunctional mitochondria to the soma for degradation, thus slowing neurodegeneration.
Figure 4. Syntaphilin acts as a receptor for docking/anchoring axonal mitochondria.
(A) Syntaphilin (SNPH) targets to axonal mitochondria in cultured hippocampal neurons. Hippocampal neurons at DIV14 were co-immunostained for SNPH and mitochondrial marker cytochrome c. Arrows point to SNPH-enriched mitochondria, and arrowheads indicate mitochondria poorly labeled by SNPH within an axonal process. Scale bars, 10 μm.
(B) SNPH immobilizes axonal mitochondria, while deletion of the snph gene in mice robustly increases axonal mitochondrial mobility. Axonal mitochondrial mobility was observed in live neurons one week after transfection. Motion data are presented in kymograph, in which vertical lines represent stationary mitochondria and slant or curved lines indicate mobile ones. Left panel: wild-type neurons co-transfected at DIV6 with DsRed-mito (red) and GFP-SNPH (green); middle panel: wild-type neurons transfected with DsRed-Mito alone; right panel: snph−/− neurons transfected with DsRed-Mito alone.
(C) SNPH acts as a receptor for docking/anchoring mitochondria in axons and is required for maintaining a large stationary axonal mitochondrial pool by interacting with the MT-based cytoskeleton.
(Images in A are adapted with permission from Jian-Sheng Kang, Jin-Hua Tian, Philip Zald, Ping-Yue Pan, Cuiling Li, Chuxia Deng, and Zu-Hang Sheng. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148, 2008. Images in B and schematic diagram in C are adapted with permission from Qian Cai, Zu-Hang Sheng. Mitochondrial transport and docking in axons. Experimental Neurology 218, 257–267, 2009).
5. Regulation of mitochondrial transport
In neurons, activity-dependent regulation of mitochondrial transport is largely due to shifts in intracellular calcium levels and ATP availability (Yi et al., 2004). The ratio of mobile and stationary mitochondrial is dynamic and responds to changes in synaptic activity. Depletion of local ATP via glutamate application, for example, reduces local mitochondrial transport velocity. Furthermore, elevated ADP levels due to increased ATP consumption appear to recruit mitochondria to synapses (Mironov, 2007; 2009).
Mitochondria buffer intracellular calcium levels. In neurons, mitochondria provide ATP to actively pump Ca2+ across the plasma membrane and sequester excess cytosolic Ca2+ during calcium influx into presynaptic terminals and postsynaptic dendritic spines. Mitochondrial mobility decreases after KCl-induced depolarization (Li et al., 2004) and NMDA receptor-mediated Ca2+ influx (Rintoul et al., 2003), and the duration of increased intracellular Ca2+ levels corresponds to the degree of mitochondrial transport reduction (Mironov, 2006). Conversely, mitochondrial mobility in cultured neurons increases after tetrodotoxin application (Li et al., 2004). These studies provide evidence that specialized activity-dependent mechanisms induce synaptic mitochondrial retention during sustained periods of synaptic activity.
Until recently, the mechanisms by which mobile mitochondria transition to a stationary state were largely unknown. Recent studies of the KIF5-Milton-Miro complex, however, have begun revealing how intracellular Ca2+ levels regulate mitochondrial mobility (Saotome et al., 2008; Wang and Schwarz, 2009; Macaskill et al., 2009; also see Cai and Sheng, 2009). Importantly, it was shown that mitochondrial mobility is arrested when the EF-hands of Miro bind Ca2+. In this fashion, Miro acts as an intracellular Ca2+ sensor. Synaptic mitochondrial recruitment induced by the activation of glutamate receptors or application of an electric field to stimulate action potentials occurs through a Miro-mediated Ca2+-sensing mechanism. For example, the Ca2+-induced reduction in mitochondrial velocity was effectively blocked in neurons expressing a mutant Miro with EF hands incapable of Ca2+ binding. Despite these advances, the precise mechanisms of Miro-mediated Ca2+-induced suppression of mitochondrial mobility are unclear.
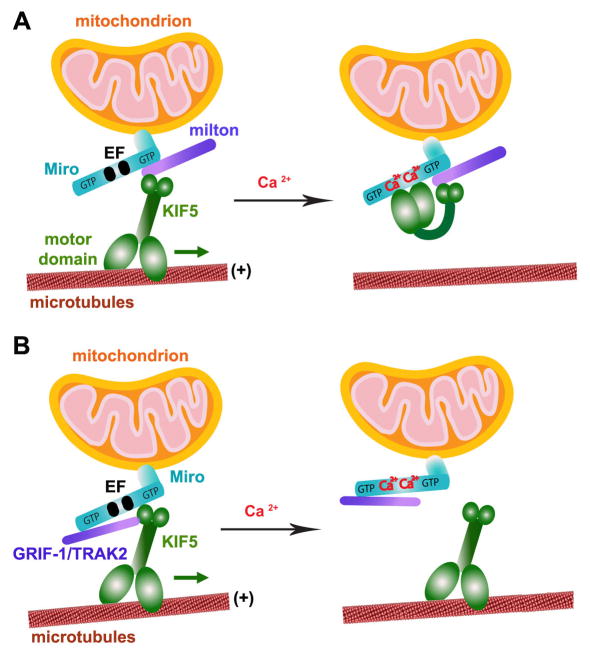
Two models have been proposed. The motor-adaptor switch model (Wang and Schwarz 2009) proposes that Miro acts as a calcium dependent on/off switch for mitochondrial transport (Fig. 5A). In the absence of Ca2+, the C-terminal tail of KIF5 binds mitochondria via an interaction with the Milton-Miro complex, while its N-terminal motor domain is free to drive anterograde transport along MTs. A conformational change driven by Ca2+ binding to Miro’s EF-hands results in a direct interaction between the motor domain of KIF5 and Miro that disrupts the KIF5-MT interaction necessary for transport. A second model (Fig. 5B), however, posits that Miro serves as an adaptor protein that indirectly links mitochondria and KIF5 (Macaskill et al. 2009). In this scenario, Ca2+ binding to Miro’s EF-hands releases Miro-bound mitochondria from KIF5. While their proposed mechanisms of action differ, both models offer possible explanations of how activity-triggered Ca2+ influx induces the recruitment of mitochondria to the synaptic stationary pool.
Figure 5. Two proposed models of Miro’s role as a Ca2+ sensor that regulates mitochondrial mobility.
Miro contains two GTPase domains and calcium-binding EF-hand motifs. Thus, Miro regulates axonal mitochondrial mobility by either GTP hydrolysis or calcium binding in response to calcium signals and synaptic activity.
(A) Ca2+-binding turns “off” KIF5 engagement with MTs. The tail of KIF5 links to Miro via Milton in a Ca2+-independent manner, thus leaving its motor domain free to engage with MTs. Ca2+-binding to the EF-hands triggers the direct interaction of the motor domain with Miro, which prevents the motor from engaging MTs (Wang and Schwarz, 2009).
(B) Ca2+-binding detaches KIF5 from mitochondria. Mitochondrial transport is mediated by Miro/KIF5 linkage. Ca2+-binding to the EF-hands dissociates Miro from KIF5 while KIF5-binding protein GRIF-1/TRAK2 (a mammalian homologue of Milton) remains bound to Miro1 (Macaskill et al., 2008; 2009).
(The model diagram is adapted with permission from Qian Cai and Zu-Hang Sheng. Moving or stopping mitochondria: Miro as a traffic cop by sensing calcium. Neuron 61, 493–496. 2009).
6. Impact of axonal mitochondrial mobility on synaptic function
Many studies have confirmed that proper axonal transport and synaptic distribution of mitochondria play a crucial role in the maintenance of synaptic homeostasis during neuronal activity. Presynaptic mitochondria within mammalian central synapses, for example, aid neurotransmission by accelerating recovery from synaptic depression after moderate neuronal activity (Billups and Forsythe, 2002). Additionally, mitochondria sequester excess calcium during tetanic stimulation and release it after stimulus removal. The loss of mitochondria from synapses also affects synaptic transmission (Stowers et al., 2002). For example, reduced synaptic mitochondria in photoreceptors expressing mutant Milton causes aberrant synaptic transmission. Similarly, a Drosophila model with defective axonal mitochondrial transport, dMiro, displays reduced mitochondrial levels at neuromuscular junctions, impaired Ca2+ buffering capacity, and aberrant neurotransmitter release during prolonged stimulation (Guo et al., 2005). During intense synaptic activity, myosin-driven mobilization of synaptic vesicles from the reserve pool depends upon mitochondrial ATP production; the loss of mitochondria from neuromuscular junctions in drp1 mutant Drosophila results in faster synaptic vesicle depletion during prolonged pulse train stimulation (Verstreken et al., 2005).
While previous studies focus on the depletion of mitochondria from synapses, recent work demonstrates that syntabulin-mediated KIF5-driven mitochondrial transport helps maintain presynaptic function. Genetic manipulation of cultured superior cervical ganglion neurons shows that syntabulin loss-of-function reduces mitochondrial density along neuronal processes. These defects are associated with delayed initiation of synaptic activity in developing neurons and impaired synaptic transmission in mature neurons, including reduced basal neurotransmission, accelerated synaptic depression during high-frequency firing, slowed recovery rates after synaptic vesicle depletion, and impaired presynaptic short-term plasticity. Importantly, these phenotypes are rescued by the introduction of ATP to presynaptic neurons (Ma et al., 2009). Thus, these findings suggest that syntabulin-mediated mitochondrial transport to presynaptic terminals plays a critical role in mobilizing synaptic vesicles to the readily releasable pool for the maintenance of synaptic function and plasticity.
Utilizing snph−/− mice, whose axonal mitochondria exhibit high mobility and reduced persistent docking, we recently provided the first direct evidence that the manipulation of mitochondrial anchoring can impact short-term presynaptic plasticity (Kang et al., 2008). Dual whole-cell patch-clamp recordings from paired hippocampal neurons in culture demonstrate that basal synaptic transmission is not significantly impacted by snph deletion. However, when short stimulus trains (20Hz, 1s) are applied to presynaptic neurons at 10-second intervals, persistent enhanced facilitation is observed in the snph−/− neurons, but not in wild-type controls. Introducing the snph transgene into mutant neurons fully rescues the phenotype, indicating that the enhanced short-term facilitation in snph−/− neurons is caused by snph deletion. We further demonstrated that snph deletion changes Ca2+ dynamics at presynaptic boutons during prolonged, intense stimulation. This phenotype could be due to an impaired ability to pump excess Ca2+ across the plasma membrane due to reduced ATP availability or a reduction in mitochondrial Ca2+ buffering capacity. These experiments provide evidence that changes in axonal mitochondrial docking status affect short-term presynaptic plasticity by altering the ability of neurons to respond to influxes of calcium at synapses.
7. Conclusion
The mechanisms that determine the balance between the mobile and stationary phases of axonal mitochondria are likely regulated by intracellular signaling and synaptic activity. How are mobile mitochondria recruited to the stationary pool (or vice versa) in response to changes in neuronal activity and synaptic physiology? While the Miro-Ca2+ models explain how Ca2+ signal and synaptic activity regulate mitochondrial transport, many questions remain unaddressed. Several lines of evidence indicate that retrograde mitochondrial transport may be an important component of the mitochondrial recruitment mechanism. For example, the Miro-mediated Ca2+-dependent mechanism suppresses mitochondrial transport in both the anterograde and retrograde directions. Thus, it is critical to assess whether dynein is mechanistically linked to Miro. Additionally, when kinesin-driven anterograde transport is disrupted, dynein-driven retrograde transport does not simply take over. Using a tug-o-war model to explain the direction of mitochondria transport is probably over-simplistic. It seems more likely that the activity of opposing motors bound to a single organelle are coordinated by motor adaptor proteins. The discovery that syntaphilin anchors axonal mitochondria also begs more questions. Is it possible that motor-adaptor complexes and docking machinery may physically displace one another? It will be important to determine if a single pathway regulates the activity of mitochondrial motor-adaptor complexes and the mechanisms of mitochondrial docking. Finally, the neuronal signals that coordinate the molecular interplay between mitochondrial transport machinery and docking receptors must be elucidated. Genetic mouse models will undoubtedly play an important role in future studies seeking to elucidate the complex mechanisms of mitochondrial transport and distribution in axons.
Acknowledgments
We thank the members of the Sheng lab for helpful discussions. The authors are supported by the Intramural Research Program of NINDS, NIH (Z.-H. Sheng) and the NIH Pathway to Independence Award K99 (Q. Cai).
Abbreviations
- SNPH
syntaphilin
- MT
microtubule
- KIFs
kinesin superfamily proteins
- KHC
kinesin heavy chains
- KLC
kinesin light chains
- DHC
dynein heavy chains
- DIC
dynein intermediate chains
- DLIC
dynein light intermediate chains
- DLC
dynein light chains
- dMiro
drosophila mitochondrial Rho-GTPase
- TRAK1
trafficking protein kinesin-binding 1
- FEZ1
fasciculation and elongation protein zeta-1
- JIP1
c-Jun N-terminal kinase (JNK)-interacting protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Pan PY, Sheng ZH. Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J Neurosci. 2007;27:7284–7296. doi: 10.1523/JNEUROSCI.0731-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Sheng ZH. Moving or stopping mitochondria: Miro as a traffic cop by sensing calcium. Neuron. 2009;61:493–496. doi: 10.1016/j.neuron.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen YM, Gerwin C, Sheng ZH. Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J Neurosci. 2009;29:9429–9438. doi: 10.1523/JNEUROSCI.1472-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius C, Berthold CH, Rydmark M. Axoplasmic organelles at nodes of Ranvier. II. Occurrence and distribution in large myelinated spinal cord axons of the adult cat. J Neurocytol. 1993;22:941–954. doi: 10.1007/BF01218352. [DOI] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Gindhart JG, Chen J, Faulkner M, Gandhi R, Doerner K, Wisniewski T, Nandlestadt A. The kinesin-associated protein UNC-76 is required for axonal transport in the Drosophila nervous system. Mol Biol Cell. 2003;14:3356–3365. doi: 10.1091/mbc.E02-12-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishin A, Li H, Levitan ES, Zaks-Makhina E. Identification of gamma-aminobutyric acid receptor-interacting factor 1 (TRAK2) as a trafficking factor for the K+ channel Kir2.1. J Biol Chem. 2006;281:30104–30111. doi: 10.1074/jbc.M602439200. [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res. 2004;301:50–59. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. Journal of Cell Science. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D, Barkus RV, Pilling AD, Gassman A, Saxton WM. APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila. Curr Biol. 2005;15:2137–2141. doi: 10.1016/j.cub.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta J, Maturana A, Fujita T, Okajima T, Tatematsu K, Tanizawa K, Kuroda S. Fasciculation and elongation protein zeta-1 (FEZ1) participates in the polarization of hippocampal neuron by controlling the mitochondrial motility. Biochem Biophys Res Commun. 2007;353:127–132. doi: 10.1016/j.bbrc.2006.11.142. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Okada Y, Tanaka Y, Harada A, Terada S, Hirokawa N. KIF5C, a novel neuronal kinesin enriched in motor neurons. J Neurosci. 2000;20:6374–6384. doi: 10.1523/JNEUROSCI.20-17-06374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Kirk E, Chin LS, Li L. GRIF1 binds Hrs and is a new regulator of endosomal trafficking. J Cell Sci. 2006;119:4689–4701. doi: 10.1242/jcs.03249. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K. Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol. 1999;144:403–411. doi: 10.1083/jcb.144.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Peng HB. The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell. 2008;19:150–158. doi: 10.1091/mbc.E07-05-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ma H, Cai Q, Lu W, Sheng ZH, Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J Neurosci. 2009;29:13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL. Spontaneous and evoked neuronal activities regulate movements of single neuronal mitochondria. Synapse. 2006;59:403–411. doi: 10.1002/syn.20256. [DOI] [PubMed] [Google Scholar]
- Mironov SL. ADP regulates movements of mitochondria in neurons. Biophys J. 2007;92:2944–2952. doi: 10.1529/biophysj.106.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL. Complexity of mitochondrial dynamics in neurons and its control by ADP produced during synaptic activity. Int J Biochem Cell Biol. 2009;41:2005–2014. doi: 10.1016/j.biocel.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Carroll WM. Focal accumulation of intra-axonal mitochondria in demyelination of the cat optic nerve. Acta Neuropathol. 1998;96:139–143. doi: 10.1007/s004010050873. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci. 2009;29:5443–5455. doi: 10.1523/JNEUROSCI.5417-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Pozo K, Brickley K, Stephenson FA. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J Biol Chem. 2006;281:27216–27228. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- Stokin GB, Goldstein LS. Axonal transport and Alzheimer’s disease. Annu Rev Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Su Q, Cai Q, Gerwin C, Smith CL, Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol. 2004;6:941–953. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- Susalka SJ, Pfister KK. Cytoplasmic dynein subunit heterogeneity: implications for axonal transport. J Neurocytol. 2000;29:819–829. doi: 10.1023/a:1010995408343. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci U S A. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber E, Li L, Chin LS. Hypertonia-associated protein Trak1 is a novel regulator of endosome-to-lysosome trafficking. J Mol Biol. 2008;382:638–651. doi: 10.1016/j.jmb.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Ho PL, Kintner DB, Sun D, Chiu SY. Activity-dependent regulation of mitochondrial motility by calcium and Na/K-ATPase at nodes of Ranvier of myelinated nerves. J Neurosci. 2010;30:3555–3566. doi: 10.1523/JNEUROSCI.4551-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]