Abstract
Objective
Resveratrol, a non-toxic natural product, exhibits multifaceted biological effects including anti-mutagenic and anti-cancer properties. We examined the effect of resveratrol on the expression and activation of Akt/PKB and its impact on melanoma cell migration and invasiveness. We also explored the use of resveratrol as an anti-malignant treatment option against skin melanoma in mouse models of the disease.
Methods
Akt expression and activity were determined by a combination of real-time PCR and Western analysis. Cell lines stably expressing Akt or a dominant negative variant were used to further establish the role of Akt during the response to resveratrol. Wound healing and transwell assays were employed as in vitro correlates of melanoma cell migration and invasiveness. The efficacy of resveratrol in the treatment of melanoma was assessed in two syngeneic mouse models.
Results
Resveratrol down-regulated and inactivated Akt in B16F10 and B16BL6 melanoma cells. Resveratrol also inhibited the migratory and invasive properties of these highly malignant cells. The reduction of cell migration and invasion, however, was reversed in cell lines over-expressing Akt or following co-treatment with pharmacological inhibitors that blocked Akt degradation. Dominant-negative Akt cells were more sensitive to resveratrol and had diminished migratory properties. Oral treatment with resveratrol reduced primary tumor volume, Akt expression and the propensity for metastasis in syngeneic mouse models of melanoma.
Conclusion
These results suggest that resveratrol can reduce the malignant properties of highly invasive melanoma cells by inactivating Akt. The non-toxic targeting of Akt by resveratrol make it an attractive treatment option for melanoma.
Keywords: Resveratrol, Akt/PKB, cutaneous melanoma
INTRODUCTION
The incidence of advanced cutaneous melanoma has risen rapidly in the last decades and is now responsible for the greatest number of skin cancer related deaths (1, 2). Although it comprises only 1 percent of skin cancers, malignant melanoma accounts for over 60 percent of skin cancer deaths (3). Once metastasized to remote sites, it is characteristically unresponsive to treatment. At present, prevention and early detection are the most effective measures against melanoma-related mortality.
Resveratrol, a natural plant product with considerable health benefits (4), may be an effective non-toxic chemopreventive agent. It is anti-proliferative and pro-apoptotic for a variety of human cancer cell lines (5). Topical administration of resveratrol already has been shown to inhibit carcinogenesis in a mouse model of melanoma (6).
Conversion of early benign skin lesions to metastatic cutaneous melanoma occurs in stages (7). Akt /PKB, a serine/threonine kinase, is thought to play a significant role in this conversion (8). The importance of Akt as a potential therapeutic target is further justified from studies that correlate elevated expression of Akt with the degree of malignancy in metastatic tumors arising from cutaneous melanoma (9).
Cell migration and extracellular matrix invasion are two of the hallmarks of malignancy (10, 11). We studied the ability of resveratrol to affect these phenotypes in vitro with the highly malignant murine melanoma cell line variants B16F10, selected for its metastatic ability, and the more invasive variant B16BL6 (12, 13, 14). Using subcutaneous and tail vein injection of B16BL6 and B16F10 respectively, we further tested whether resveratrol treatment could affect tumor growth and reduce formation of lung metastasis in syngeneic mouse models of melanoma.
We show that resveratrol deactivates and attenuates Akt. It effectively reduces the migration and invasiveness of melanoma cells in vitro and reduces tumor growth and metastasis in vivo. These properties may make this readily available bioactive food component an attractive preventive or treatment option for cutaneous melanoma.
MATERIALS AND METHODS
Cell culture
B16BL6 and B16F10 cell lines were procured respectively from Dr. I.J. Fidler (Department of Cancer Biology, MD Anderson Cancer Center, Houston Texas) and Dr. Paul M. Sondel (Departments of Pediatrics & Human-Oncology, University of Wisconsin, Madison). B16BL6 cells were maintained in Earle’s MEM with 10% fetal bovine serum (FBS), 2mm L-glutamine, 1mM sodium pyruvate, 1% nonessential amino acids, and antibiotics (10,000 units/ml penicillin, 10 mg/ml streptomycin sulfate and 25 µg/ml amphotericin B solution, Sigma). B16F10 cells were maintained in RPMI media supplemented with glutamine, 10% FBS and antibiotics. Cells were kept at 37°C with 5% CO2.
Pre-Clinical Studies
Female C57 BL/6 mice, 5–6 weeks of age (Harlan Sprague-Dawley, Indianapolis, IN), were used for the in vivo studies. Mice were housed under pathogen-free conditions and fed standard chow and water ad libitum. Animal experiments followed guidelines of the University of Wisconsin-Madison Animal Care and Use Committee.
A. Primary tumor growth
B16BL6 mouse melanoma cells (1×103) suspended in 500 µl of 1:1 medium and Matrigel (Fisher) were implanted subcutaneously in the dorsal flanks of C57BL/6 mice (n=21). At day 4, when the tumors were palpable, mice were randomized into two treatment regimens. Group 1 (n=10) and group 2 (n=11) were treated with vehicle (Neobee oil) or 1mg resveratrol suspended in Neobee oil, respectively. Solutions were administered by oral gavage daily for 17 days. Tumor volumes were measured twice a week in three dimensions by means of calipers and the volume was approximated by multiplying the three values. At the end of treatment, animals were euthanized, a final measurement was made after the tumors were taken out of the animals, and tissues were processed for histology as described elsewhere (15).
B. Metastasis study
B16F10 mouse melanoma cells (1×105) suspended in 100 µl medium were injected into the tail vein (day 1) of C57 BL/6 mice (n=32). Mice were then randomized into two groups. The control group (n=16) received 100µl Neobee oil while the treated group (n=16) received 1mg of resveratrol suspended in 100 µl Neobee oil by IP injection daily for 21 days starting on day 1. Mice were euthanized on day 21 and their lungs were harvested for histological processing as previously described (15).
Wound Healing Assay
Plates containing confluent cultures of the B16F10 cell line grown in serum-containing medium were scratched with a sterile 1ml pipette tip. The medium was replaced with serum-free RPMI medium with resveratrol or DMSO and returned to 37°C for designated times. Cells were fixed (4% buffered parafomaldehyde), stained (0.5% crystal violet in 20% methanol) and their images captured (RS Imager). Distances between the gaps formed by the scratch were measured with ImageJ software. Percentages were calculated relative to the gap size immediately after scratching.
Migration and invasion assays
For migration assays, transwell inserts (Costar 3422) were coated on the lower surface with a 5µg/ml solution of fibronectin (Sigma) overnight at 4°C. In a separate series of experiments to measure invasiveness, the upper surface of the inserts were coated with 200µg/ml Matrigel at room temperature followed by 5µg/ml fibronectin at 4°C on the lower surface. Actively growing B16F10 cells were trypsinized, washed and re-suspended at 1×106 cells per ml in serum-free RPMI medium with either DMSO or resveratrol. 1×105 cells in 0.1ml were carefully placed in the upper chamber. The upper and lower chambers contained the same media as the cell suspensions. Following incubation for 3–4 hours, cells from the upper surface were wiped clear and cells on the lower surface were fixed with a 4% buffered paraformaldehyde solution. H&E stained membranes were then transferred to slides, cover slipped, photographed and cells were counted manually.
Western Analysis
Cells were treated in serum-free medium with DMSO or resveratrol for designated periods prior to their collection by scraping, washed with cold PBS and lysed in ice-cold 1X RIPA buffer supplemented with protease and phosphatase inhibitors. Equal amounts of protein were loaded onto 15% polyacrylamide gels, transferred to PVDF membrane and stained with antibodies. Mouse anti-pan-Akt, rabbit anti-phospho (S473) Akt, and mouse anti-GAPDH antibodies were obtained from R&D systems. Rabbit anti-phospho (T308) Akt antibody was purchased from Cell Signaling Technology. Antibody binding was detected using the ECL kit from Amersham as described previously (15). In experiments with Boc-D Fmk, cells were co-incubated with 50 µM Boc-D Fmk and resveratrol prior to harvest.
Generation of stable Akt cell lines
The plasmid CMV3-EE-WT-AKT-1 and the control empty vector pCMV3-EE were gifts from Dr. Karen Anderson, Babraham Institute, Babraham Research Campus, Cambridge UK (16). B16F10 cells were permeabilized with an Amaxa II nucleo-porator using cell line specific kit-V following the manufacturer’s protocol. After 36 hr, mixed populations of transfected cells were generated by pooling G418 resistant cells. The dominant-negative Akt plasmid pDSRed2 C1-Akt (PH 1–108) and the corresponding empty vector were kindly provided by Dr. Nobuyuki Takahashi, National Institute for Physiological Sciences, Japan (17). Stable transfectants were generated as described above.
RESULTS
Resveratrol reduces the migratory properties of murine melanoma cells
The ability of cancer cells to migrate is an essential event in the progression towards malignancy. Scratch assays measure the migration of cells at the leading edge of a wound and act as an in vitro correlate to cell migration in vivo.
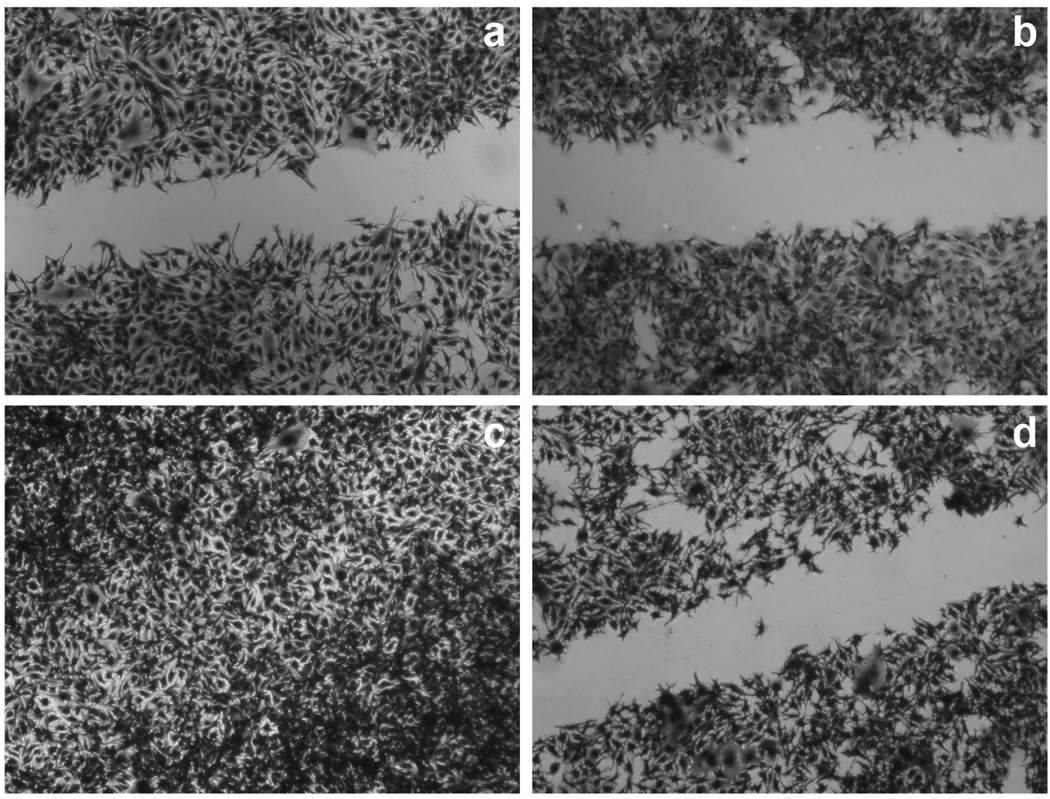
The data in Figure 1 demonstrate that resveratrol inhibits the migratory property of the highly invasive B16F10 melanoma cell line. Wound closure in the presence of DMSO begins within 6–8 hrs (Figure 1a) and is complete by 17 hrs (Figure 1c). However, treatment with resveratrol inhibited cell migration (Figures 1b and 1d). There was no significant change in B16F10 cell viability during this period; 100% and 81% of the cells were viable 4 hrs and 24 hrs post-treatment, respectively.
Figure 1.
Wound closure is inhibited by resveratrol. Confluent B16F10 cells were grown in serum-containing media, scratched and the media then replaced without serum but containing DMSO or 100 µM resveratrol. Representative images from three separate experiments show wounds at 8hrs (a, b) and 17hrs (c, d) with DMSO (a, c) or resveratrol (b, d).
Resveratrol inhibits the invasive properties of murine melanoma cells
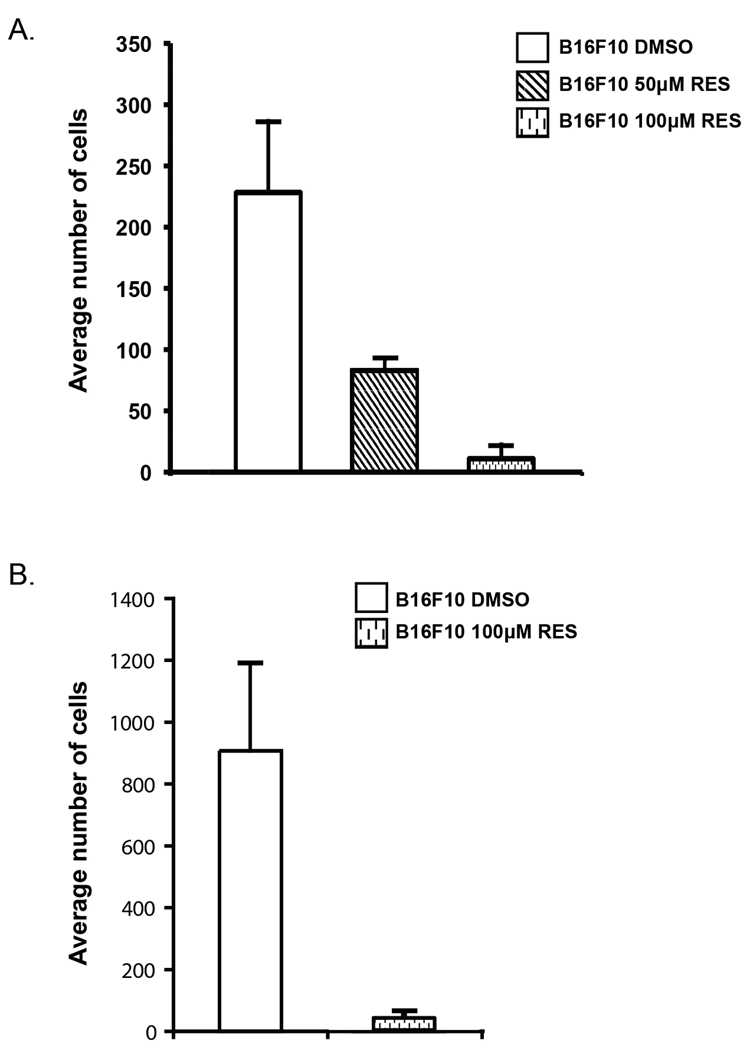
By virtue of their ability to adhere to fibronectin matrices elaborated by other cells, malignant cells have enhanced invasive potential (18). In a transwell migration assay, fibronectin serves as the chemo-attractant and as an adhesive substrate for the cells. Utilizing a filter (8 µm pore size) coated on the lower surface with fibronection and resveratrol in the medium, we show that the number of cells that migrated through the membrane decreased to nearly 40% and 5% in the presence of 50 µM and 100 µM resveratrol, respectively, compared to DMSO controls (Figure 2A).
Figure 2.
Resveratrol inhibits cell migration and invasion. A) Average number of cells migrating to the lower surface in a transwell assay in the presence of DMSO, 50 µM and 100 µM resveratrol. B) Average number of cells migrating through Matrigel in the presence of DMSO and 100µM resveratrol. Each of the figures is representative of three separate experiments. Mean values ± S.D.
Invasion then requires the migration of cells through a barrier, like basement membrane or extracellular matrix. In a similar transwell invasion assay where the upper side of the membrane was coated with Matrigel, a solubilized basement membrane matrix, and the lower surface with fibronectin, incubation with resveratrol for 3–4hrs reduced the number of cells migrating through the matrix to 4% compared to the DMSO control (Figure 2B). These data suggest that resveratrol affects at least one intrinsic property of these melanoma cells, namely their ability to migrate, prior to any changes in viability.
Resveratrol acts like an inhibitor of Akt
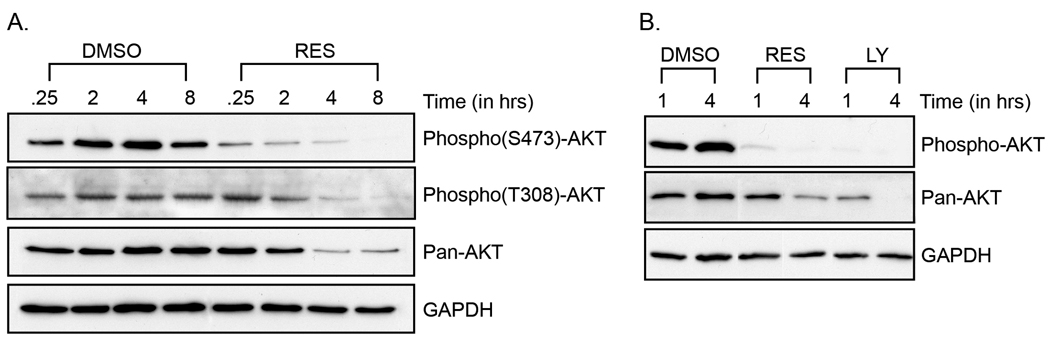
Akt is a major determinant of cell migration and invasion associated with tumor aggressiveness (19). Increased Akt activity has been reported in breast, ovarian and prostate cancers (20). Recently, a phase II clinical trial was conducted with perifosine, a drug that decreases cancer cell growth through decreased Akt phosphorylation in hormone-sensitive prostate cancer patients [14]. In order to gain insight into the mechanisms of inhibition of melanoma cell migration and invasion, we examined the status of Akt in response to resveratrol treatment. We show that resveratrol inactivated and attenuated Akt (Figure 3). B16F10 cells were treated with resveratrol for different periods and their lysates were then subjected to Western blot analysis using antibodies specific for Akt and phospho-Akt (Figure 3A). Treatment with resveratrol caused a decrease in both phospho (S473)-Akt and phospho (T308)-Akt within 1.5 hrs of resveratrol addition. Thereafter, a decline in the total amount of Akt also was observed. A parallel study was conducted with Ly294002, a known Akt inhibitor (Figure 3B). A similar deactivation and attenuation of Akt was noted for both resveratrol and Ly294002. These data demonstrate the potential of using resveratrol as a nontoxic Akt inhibitor similar to Ly294002, a drug being considered for the treatment of melanoma (21). Similar observations were made with the B16BL6 cell line (data not shown).
Figure 3.
Resveratrol deactivates and attenuates Akt. Exponentially growing B16F10 cells were treated with (A) DMSO or 100µM resveratrol (RES) and with (B) DMSO, 100µM resveratrol (RES) or 25 µM Ly294002 (LY) in serum-free media for the designated time. Equal amounts of protein were loaded onto polyacrylamide gels, transferred to PVDF membrane and immuno-stained with an anti-phospho (S473) AKT antibody, an anti-phospho (T308) AKT antibody or an anti-Pan Akt antibody that recognizes the three isoforms of Akt. GAPDH was used as a loading control. The figure is representative of three separate experiments.
Akt has three isoforms sharing a high degree of homology. All three Akt transcripts were detected in B16F10 cells. In order to discern if any one of the three isoforms underwent resveratrol-mediated changes relative to the house-keeping gene Polr2A at the transcriptional level, RNA was obtained from cells treated with resveratrol. Real time-PCR analysis of the corresponding cDNAs with isoform-specific primers was performed and compared to DMSO treated cells. No significant differences in the transcripts were observed after 4 hours of resveratrol treatment. Therefore, the decline in the amount of Akt induced by resveratrol likely reflects an effect of resveratrol on protein degradation.
Boc-D-Fmk partially rescues the effects of resveratrol
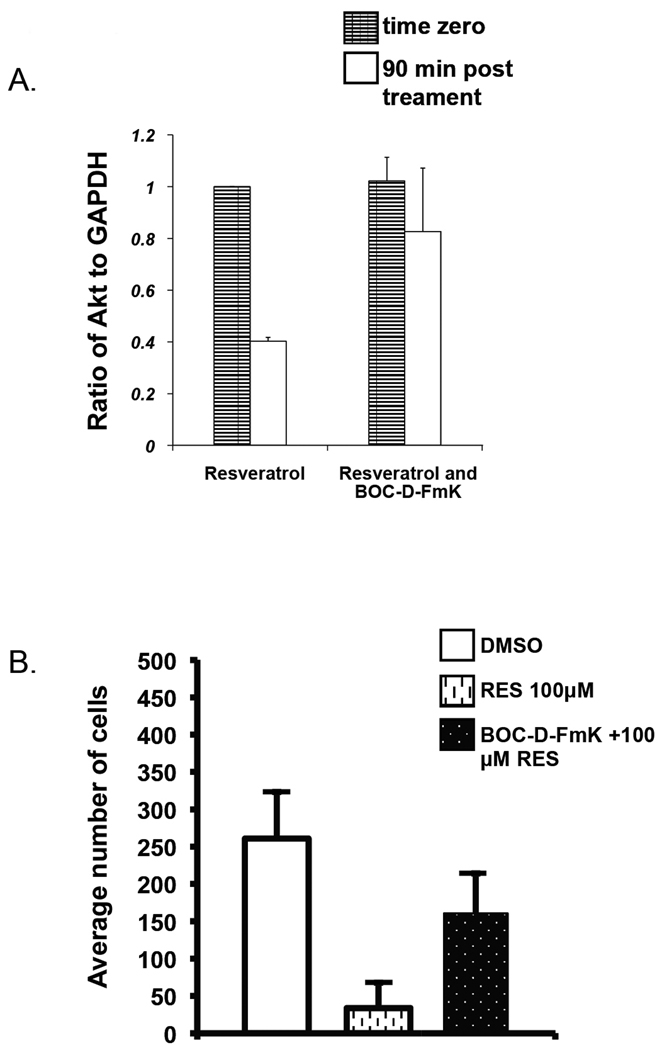
The mechanism of caspase-mediated Akt cleavage is well described (22). The pharmacological agent Boc-D-Fmk, a pan caspase inhibitor, has been used to block Akt degradation (23). We therefore tested whether Boc-D-Fmk would block Akt degradation and then interfere with resveratrol-mediated inhibition of cell migration. Co-treatment of Boc-D-Fmk with resveratrol blocked Akt degradation by 50% compared to cells treated with resveratrol alone (Figure 4A).
Figure 4.
Inhibition of Akt degradation partially rescues cell migration. A) Exponentially growing B16F10 cells were incubated with or without 100 µM resveratrol in the presence or absence of 50 µM Boc- D -Fmk for the designated time in serum-free media. Lysates were prepared and equal amounts of protein were subjected to SDS-PAGE, blotted and immuno-stained with anti-Pan Akt antibodies. GAPDH was stained as a loading control. Data from three separate experiments were quantified using Image-J software and presented relative to resveratrol at time zero. Mean values ± S.D. B) In a transwell assay, photomicrographs of 4 different fields were compiled. The number of cells were counted for the three conditions - DMSO, 100 µM resveratrol, and 100 µM resveratrol supplemented with 50 µM Boc-D –Fmk. The representative outcome from three separate experiments is shown. Mean values ± S.D.
Following the same treatment paradigm, Boc-D-Fmk blocked the resveratrol-mediated inhibition of melanoma cell migration (Figure 4B). While only 12% of cells migrated through the membrane in the presence of resveratrol, co-incubation with Boc-D-Fmk increased that value to 61%.
These initial findings prompted us to investigate further the role of Akt in melanoma cell migration.
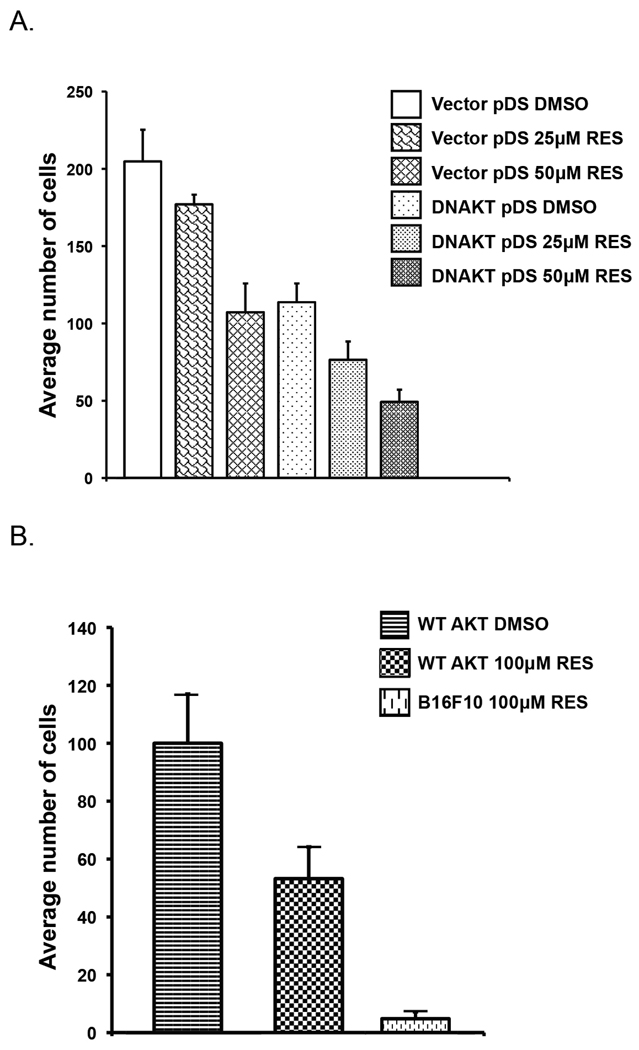
Stably transfected dominant-negative Akt cells are less invasive
Following the observations noted above, we tested whether the invasive property would be reduced in melanoma cells stably transfected with a dominant-negative Akt. To this end, melanoma cell lines were established either with a dominant-negative Akt (PH 1–108) or the corresponding empty vector control (pDS Red2-C1). Cells were then tested in transwell migration assays as previously described. As shown in Figure 5A, 25 µM and 50 µM resveratrol reduced the average number of migratory cells to 86% and 52%, respectively, in cells containing an empty vector compared to the same cells treated with DMSO. As seen in the same figure, expression of dominant-negative Akt alone reduced cell migration to levels comparable to those obtained with 50 µM resveratrol. Addition of resveratrol to these cells further suppressed melanoma cell migration to final values of 37% and 24% compared to controls. This compares with the inhibitory effect of resveratrol only achievable at much higher concentrations of the compound.
Figure 5.
Affect of cell migration in dominant-negative Akt or over-expressing stable cell lines in the presence or absence of resveratrol. A) Cells expressing dominant-negative Akt are less invasive. In a transwell assay, photomicrographs of 4 different fields per condition were used to count the number of migrating cells incubated with DMSO, 25 µM resveratrol or 50 µM resveratrol. Results are representative of three separate experiments. Mean values ± S.D. B) Over-expression of wild-type Akt1 rescues cells from resveratrol-mediated inhibition of invasiveness. In a transwell assay, photomicrographs of 4 different fields per condition were used to count the number of migrating cells incubated with DMSO or 100 µM resveratrol. Images are representative of three separate experiments. Mean values ± S.D.
These results demonstrate that inhibition of Akt, either with resveratrol or via the expression of a dominant-negative species, reduces the migratory behavior of the metastatic B16F10 melanoma cells.
In contrast, the inhibition of cell migration by resveratrol was attenuated by over-expression of wild-type Akt (Figure 5B). In the presence of 100µM resveratrol, approximately 55% of over-expressing Akt stable cells migrated through the Matrigel in a transwell assay compared to only 5% of cells containing the empty vector. The over-expression of wild-type Akt, therefore, partially mitigates the effect of resveratrol.
Similarly, the over-expression of Akt, made cells less sensitive to resveratrol in a scratch assay. At 8hrs post incubation, the gaps between cells containing the empty vector with DMSO and resveratrol were 23% and 61% open respectively, while in cells over-expressing Akt, the wounds were completely closed in the DMSO condition and only 10% open in the presence of resveratrol.
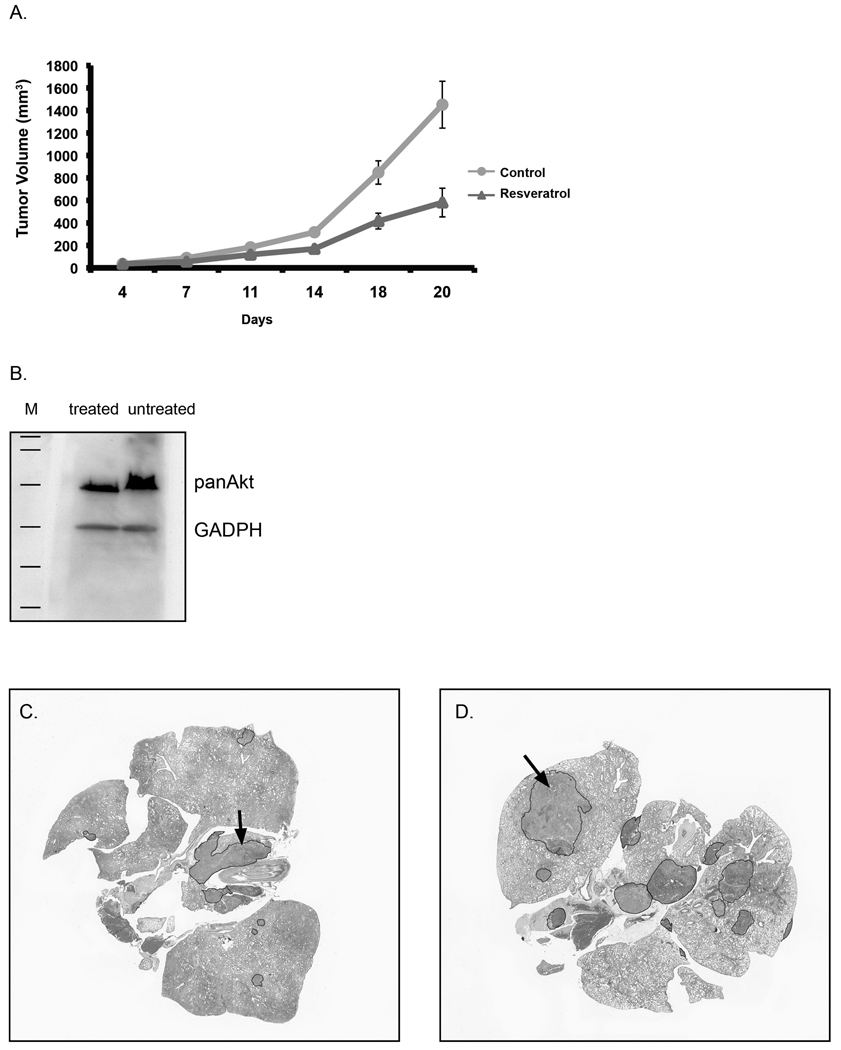
Resveratrol treatment significantly reduces tumor growth in vivo
Since migration is an essential step in the progression towards malignancy, we determined first whether resveratrol could reduce tumor growth in a syngeneic mouse model. The B16BL6 cell line was selected for its highly invasive properties (12). Cells were implanted subcutaneously, and daily treatment by oral gavage either with resveratrol or vehicle alone started 4 days later when tumors were smaller than 50 mm3. A reduction in tumor volume was then observed between the two groups (Figure 6A). Significant differences were detected after the first week of treatment, culminating at three weeks in a 60% inhibition of tumor growth (p=.001). Thus, our results demonstrate that, similar to in vitro findings, resveratrol interferes with the growth of tumors in vivo. During the treatment, there were no signs of toxicity; mice continued to gain weight, behaved normally, and there were no signs of damage to vital organs upon necropsy.
Figure 6.
Resveratrol treatment reduces tumor burden and metastasis in syngeneic mouse models of melanoma. A) B16BL6 cells were inoculated subcutaneously at day zero. Treatment with 50 mg/kg resveratrol in Neobee oil or vehicle alone by daily oral gavage began on day 4. Tumor measurements were described in Methods. Panel B illustrates the Western analysis of Akt levels in tumor specimens obtained from the mice described in (A) treated with either resveratrol or vehicle. In a separate experiment, mice were inoculated with cells through the tail vein and treated i.p. either with resveratrol or vehicle alone as described in Methods. Histological sections from the lungs of resveratrol-treated (C) and untreated (D) mice were stained with H&E, and tumors were demarcated using ImageJ software and their areas calculated.
Fresh tumor tissues also were obtained from treated and untreated mice and processed for Western analysis to assess the levels of Akt. As shown in Figure 6B, resveratrol treatment reduced the amount of Akt, thus paralleling our in vitro findings.
Resveratrol treatment reduces the propensity for lung metastasis
In addition to primary tumor inhibition, resveratrol interfered with the metastasis of melanoma cells. Mice were treated daily either with vehicle or resveratrol beginning on the same day as the inoculation of cells through the tail-vein. After 21 days, lungs were removed from euthanized mice, fixed in formalin, sectioned, and stained with H&E. The area of the lung occupied by tumor in each section was assessed by two independent observers. Representative examples of treated and untreated tissue specimens are presented in Figures 6C and 6D, respectively. Areas of tumor (blue stain) are marked in the figures. Resveratrol-treated average tumor sizes were smaller than that of the control group with a marginal significance of 10%, (p-value of 0.05, SE of mean difference 8.68). Thus, there is a general trend of reduced metastasis in resveratrol-treated mice, however, a much larger number of animals would be required to determine more accurately the percent change.
DISCUSSION
Despite intensive research, cutaneous melanoma is still a major cause of skin cancer related deaths worldwide. The need to develop an effective treatment option for melanoma is significant.
Akt/PKB is positioned at the crossroads of multiple oncogenic and tumor suppresser signaling networks. It promotes epithelial to mesenchymal transition and thus plays a pivotal role in enhancing the metastatic potential of cells (24, 25). The inhibition of Akt activity therefore may provide an effective strategy for the development of an anti-cancer drug. However, toxicity and pharmacologic variables associated with potent Akt inhibitors has been a major obstacle when considering the use of such candidate drugs for the treatment of cutaneous melanoma (26, 27).
In contrast, we and others (15, 28, 29 30) have shown that resveratrol, a natural plant product, can effect tumor cell growth in vitro and in pre-clinical studies of cancer in a non-toxic manner via attenuation of anti-apoptotic proteins, the activation of caspases and the loss of mitochondrial function specifically in tumor cells. That Akt is an additional target of resveratrol has been shown in the current study.
We demonstrate that resveratrol, a nontoxic drug similar in activity to Ly294002, inactivates Akt and decreases the migratory and hence the invasive properties of the highly malignant melanoma cell line B16F10 in vitro. We suggest that the deactivation and attenuation of Akt underlie the resveratrol-mediated decrease of melanoma cell migration and invasiveness. This contention is supported by the partial rescue of the resveratrol-mediated effects using the pharmacological inhibitor Boc-D-Fmk. The finding that the over-expression of Akt blocks the effect of resveratrol on melanoma cell migration and invasiveness provides further support. Ultimately, the combined effects of resveratrol are shown in these studies to inhibit tumor growth and reduce metastasis in mouse models of melanoma. While the bioavailability of resveratrol in serum may be low, and there is no evidence for its accumulation in tumors following oral administration or intraperitoneal injection, its relatively low level is still sufficient to cause significant inhibition of tumor growth in animal models of melanoma and other types of cancer without signs of significant cytotoxicity (31). Whether resveratrol can be effective in the treatment of melanoma now needs to be ascertained in clinical trials.
ACKNOWLEDGMENTS
The authors thank Chue Vang for excellent technical support and Dr. Abhik Bhattacharya for statistical analysis.
This work was supported by funds from the National Cancer Institute (CA103653), the Mandelbaum Cancer Therapeutics Initiative and the Retina Research Foundation (RRF). ASP is the M.D. Matthews/RRF Professor.
Abbreviations
- PKB
protein kinase B
- Ly294002
2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- RIPA buffer
Radio-Immunoprecipitation Assay buffer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: Risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005 Jul 20;23(21):4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 3.Sauer GC, Hall JC, editors. A manual of skin diseases. 7th ed. Philadelphia: Lippincot-Raven; 1996. Skin tumors; p. 342. [Google Scholar]
- 4.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006 Jun;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004 Sep–Oct;24(5A):2783–2840. [PubMed] [Google Scholar]
- 6.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997 Jan 10;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 7.Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA. 1997 May 14;277(18):1439–1444. [PubMed] [Google Scholar]
- 8.Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, et al. Overexpression of akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007 Mar;117(3):719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002 Dec 15;62(24):7335–7342. [PubMed] [Google Scholar]
- 10.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 11.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 Mar 1;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- 13.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci. 2002 Jan 4;70(7):791–798. doi: 10.1016/s0024-3205(01)01454-0. [DOI] [PubMed] [Google Scholar]
- 15.van Ginkel PR, Darjatmoko SR, Sareen D, Subramanian L, Bhattacharya S, Lindstrom MJ, et al. Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1299–1306. doi: 10.1167/iovs.07-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson KE, Coadwell J, Stephens LR, Hawkins PT. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998 Jun 4;8(12):684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 17.Subramanyam M, Takahashi N, Hasegawa Y, Mohri T, Okada Y. Inhibition of protein kinase Akt1 by apoptosis signal-regulating kinase-1 (ASK1) is involved in apoptotic inhibition of regulatory volume increase. J Biol Chem. 2010 Feb 26;285(9):6109–6117. doi: 10.1074/jbc.M109.072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3(1):43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- 19.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995 Aug 22;64(4):280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 20.Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001 Aug;159(2):431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, et al. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol. 2007 Jun;156(6):1204–1213. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 22.Liao Y, Hung MC. Physiological regulation of akt activity and stability. Am J Transl Res. 2010 Jan 1;2(1):19–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Medina EA, Afsari RR, Ravid T, Castillo SS, Erickson KL, Goldkorn T. Tumor necrosis factor-{alpha} decreases akt protein levels in 3T3-L1 adipocytes via the caspase-dependent ubiquitination of akt. Endocrinology. 2005 Jun;146(6):2726–2735. doi: 10.1210/en.2004-1074. [DOI] [PubMed] [Google Scholar]
- 24.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene. 2005 Nov 14;24(50):7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 25.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, et al. The protein kinase akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003 May 1;63(9):2172–2178. [PubMed] [Google Scholar]
- 26.Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, et al. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol. 2007 Jun;156(6):1204–1213. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 27.Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008 Jan 1;68(1):206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 28.Sareen D, van Ginkel PR, Takach JC, Mohiuddin A, Darjatmoko SR, Albert DM, et al. Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2006 Sep;47(9):3708–3716. doi: 10.1167/iovs.06-0119. [DOI] [PubMed] [Google Scholar]
- 29.Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles JM, et al. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007 Jan 8;245(1–2):144–148. doi: 10.1016/j.canlet.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y, Okuda H. Resveratrol isolated from polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in lewis lung carcinoma-bearing mice. J Nutr. 2001 Jun;131(6):1844–1849. doi: 10.1093/jn/131.6.1844. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR. Resveratrol: challenges in translation to the clinic - A critical discussion. Clin Cancer Res. 2010 Dec 15;16(24):5942–5948. doi: 10.1158/1078-0432.CCR-10-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]