Abstract
The Hawaiian cricket genus Laupala (Gryllidae: Trigonidiinae) has undergone rapid and extensive speciation, with divergence in male song and female acoustic preference playing a role in maintaining species boundaries. Recent study of interspecific differences in the diel rhythmicity of singing and mating, suggests that temporal variation in behavior may reduce gene flow between species. In addition, Laupala perform an elaborate and protracted courtship, providing potential for further temporal variation. However, whether these behavioral differences have a genetic basis or result from environmental variation is unknown. We observed courtship and mating in a common garden study of the sympatric species, Laupala cerasina and Laupala paranigra. We document interspecific differences in the onset and duration of courtship, spermatophore production rate, and diel mating rhythmicity. Our study demonstrates a genetic contribution to interspecific behavioral differences, and suggests an evolutionary pathway to the origins of novel timing phenotypes.
Keywords: Laupala, Cricket, Courtship, Mating, Circadian
Introduction
Divergence in the timing of activities or life history traits can reduce competition, reproductive interference or interbreeding, and consequently promote species coexistence in sympatry (Richards 2002; Schoener 1974; Tauber and Tauber 1977). For example, temporal separation of feeding or adult emergence may partition food resources among insects (Caveney et al. 1995; Collins 2005) and daily timing differences of pollen release may partition pollinator species among plants (Stone et al. 1996), thereby reducing reproductive competition between closely related species. Reduced interbreeding may result from divergence in the timing of activities important for reproduction, such as development (Cooley et al. 2001; Feder and Filchak 1999; Lloyd and Dybas 1966), breeding season (Brophy et al. 2006; Campan and Demai 1983; Santos et al. 2007; Shine et al. 2004), daily mating activities (Miyatake and Shimizu 1999; Tauber and Tauber 1977) or ultradian (<24 h) courtship behaviors (Bennet-Clark and Ewing 1969; Popov and Shuvalov 1977; Ritchie et al. 1999).
Species of the Hawaiian swordtail cricket genus Laupala are closely related as well as morphologically and ecologically cryptic. Yet members of this group frequently live in congeneric communities of two or more species. Evidence suggests that the timing of reproductive behaviors, which vary on a number of time scales, play an important role in facilitating the coexistence of species of Laupala. For example, species differ in the song pulse rates of males and females exhibit acoustic preference for rates characteristic of their own species (Mendelson and Shaw 2002; Shaw 2000; Shaw and Herlihy 2000). Daily timing of behaviors also differs among Laupala species. Danley et al. (2007) reported that the peak singing times of the sympatric species Laupala cerasina and Laupala paranigra differ in the field, with a corresponding shift in the timing of mating in laboratory experiments among wild-caught individuals. If the timing of these daily reproductive behaviors reduces the probability of reproductive interference or interbreeding, it may have important implications for the coexistence of sympatric species. Thus, the timing per se, of reproductive behaviors becomes an important candidate phenotype in need of study.
Sexual signaling in Laupala may offer many opportunities for differentiation in reproductive behavior. The production and transfer of spermatophores is part of an elaborate and lengthy diurnal courtship lasting several hours. Following the long-distance acoustic attraction of a female by a male, a pair will engage in a number of courtship behaviors including copulation and transfer of a series of small, spermless spermatophores (microspermatophores), and culminating in the transfer of a larger, sperm-filled spermatophore (macrospermatophore) (deCarvalho and Shaw 2005; Shaw and Khine 2004; Shaw and Lugo 2001). While extensive work has examined the timing of mating-related characters in several other cricket species (French and Cade 1987; Hedwig 2000; Loher 1972, 1974; Rost and Honegger 1987; Sokolove 1975), spermless spermatophores have rarely been observed (but see: Campan and Demai 1983), and thus temporal variation in the production of sperm-less spermatophores has not been explored. The complex and temporally protracted nature of Laupala courtship behavior suggests that adaptive divergence may have caused the differences in timing of reproductive events, resulting in novel variation in timing phenotypes that apparently have not arisen in other cricket mating systems. However, the potential for selection or other evolutionary forces to shape the differential timing of reproductive and courtship events such as those observed in Laupala depends upon an underlying genetic contribution to these phenotypes.
Support for the hypothesis that genetic differences underlie variation in the daily reproductive rhythms between the sympatric Laupala species discussed above would mean that these phenotypes could evolve in response to selection. Alternatively, species may show behavioral responses that differ due to variable environmental cues (e.g., light, temperature) associated, for example, with different microhabitat use. By rearing two species of Laupala under controlled laboratory conditions, we tested whether there is a genetic component to the differences in the daily timing of mating. In addition, we examined the timing of serial microspermatophore production, transfer and other courtship elements, to better characterize variation in courtship between sympatric Laupala species.
Methods
In this study we examined two Laupala species, L. paranigra and L. cerasina, which are endemic to the Big Island of Hawaii. These species have overlapping ranges and have an estimated divergence time of approximately 3.7 Myr (Mendelson and Shaw 2005). L. paranigra nymphs were collected along Kaiwiki Road (19°45′N, 155°10′W) and L. cerasina were collected at Kalopa State Park (20°02′N, 155°26′W). The nymphs were housed with damp Kimwipes (Kimberly-Clark) and Cricket Chow (Fluker Farms), and transported back to the University of Maryland.
Lab studies were conducted at the University of Maryland, College Park, MD, where all crickets were housed at 20°C on a 12:12 light:dark cycle. Wild-caught nymphs were kept in plastic cups with damp Kimwipes and fed Cricket Chow (Fluker Farms) treated with methyl paraben (Methyl Paraben; USB Corporation) to inhibit mold growth. Upon maturing, male/female pairs were housed together in cups to allow mating and oviposition into moistened Kimwipes. Kimwipes with eggs were collected, placed in clean cups, and kept moist. As F1 nymphs emerged they were housed with siblings under similar conditions as the parental individuals. Once the sex of the nymphs could be determined males and females were separated and reared to sexual maturity, approximately 5 months post hatching. Sexually mature pairs of virgin F1 males and females were placed in plastic Petri dishes with moistened Kimwipes within 15 min of the lights coming on. The Petri dishes were separated from one another with cardboard dividers such that each mating pair within a Petri dish was visually isolated from adjacent mating pairs. Pair establishment time was recorded to ensure that there was no significant difference between species in the starting time.
Courtship is initiated via long distance acoustic communication, followed by the approach of the female and mutual antennation. Upon contact, a mating pair will typically engage in a face-to-face positioning with antennation, while the male produces a microspermatophore. Several minutes later, the male typically transfers the microspermatophore to the female via copulation. After a variable period of time following transfer, the female eats the microspermatophore. This process of transferring microspermatophores is repeated several times before the male produces and transfers a single, sperm-filled, macrospermatophore (deCarvalho and Shaw 2005; Shaw and Khine 2004; Shaw and Lugo 2001). Because the transfer of a macrospermatophore typically results in insemination, we considered macrospermatophore transfer to indicate a successful mating.
Mating pairs were observed from the time they were established through mating (the transfer of the macrospermatophore) or until the onset of the dark cycle for pairs that did not court. In each trial we recorded the times of (1) first song production; (2) initial face-to-face positioning; (3) each spermless microspermatophore production; (4) each microspermatophore transfer; (5) macrospermatophore production, and (6) macrospermatophore transfer. In many cricket species males produce a distinct courtship song when in close proximity or antennal contact with a female (reviewed in: Huber et al. 1989; Zuk and Simmons 1997), which could be considered the initiation of courtship. Laupala possess only one song type, which is expressed in several contexts (Mendelson and Shaw 2006). Shaw and Khine (2004) use the onset of male–female circling behavior as the onset of courtship; however, we did not consistently observe this behavior. Therefore, we report both the first face-to-face positioning with antennal contact and the initial onset of song as proxies of courtship initiation.
We set up equal numbers of each species on each day that observations were performed, randomizing the order in which pairs were established to eliminate effects of timing or order of set up. We used the Shapiro–Wilk test to establish normality of the data and the residuals were examined visually for homogeneity of variance. Student’s two-tailed t-tests or ANOVAs were used to determine the significance of differences in timing of behaviors and the number of microspermatophores produced and transferred. For data that were not normally distributed, such as the timing of first occurrence of song and of face-to-face positioning, we used permutation randomization tests (Manley 1997) with 10,000 iterations to assess species differences.
We investigated both the overall rate of microspermatophore production and transfer as well as the change in rate throughout mating. The overall rate was calculated by dividing the number of microspermatophores produced or transferred by the time from the first to the final microspermatophore. The change in rate of microspermatophore production during courtship was examined by determining the duration of the interval from the production of one microspermatophore to the next. We performed a log transformation of the microspermatophore interval data to homogenize the variance in interval durations. We then used one-way ANOVAs to determine whether the time between productions of successive microspermatophores varied within species over the duration of courtship. Tukey corrected pairwise comparisons were employed to examine the significance of variation in the periods from one microspermatophore to the next within species over the duration of courtship. Differences between species in mean microspermatophore production rates as well as variation in the periods between microspermatophores throughout courtship were examined with a two-way ANOVA. Unless otherwise noted, means are reported ± SE.
Results
There was no significant difference between species in the time that pairs were set up (mean times were 0 h 6 min ± 1.4 min and 0 h 5 min ± 1.5 min after lights on for L. cerasina and L. paranigra, respectively). In L. cerasina, approximately 82% (14/17) of pairs that produced microspermatophores went on to successfully produce and transfer a macrospermatophore, while in L. paranigra approximately 93% (13/14) of pairs that produced microspermatophores successfully produced and transferred a macrospermatophore. The data reported below, as well as in the table and figures, are based solely on observations from those pairs that successfully mated.
We observed a significant difference between species in the timing of both the initiation of the first song (randomization test: p ≪ 0.01) and of the first instance of face-to-face positioning (randomization test: p ≪ 0.01). The timing of both of these behaviors was earlier in L. cerasina than in L. paranigra. There was a significant difference between L. cerasina and L. paranigra in the time of production (t = 6.51, df = 24, p ≪ 0.01) and transfer (t = 6.97, df = 24, p ≪ 0.01) of the first microspermatophore (Table 1), with L. cerasina performing these behaviors approximately 4 h earlier than L. paranigra (Fig. 1). The macrospermatophores were also produced (t = 12.53, df = 25, p ≪ 0.01) and transferred (t = 10.39, df = 25, p ≪ 0.01) approximately 2–2.5 h earlier in L. cerasina than L. paranigra. The timing of the production and transfer of macrospermatophores were highly stereotyped within each species, with intraspecific standard errors of less than 10 min.
Table 1.
Timing of courtship and mating behaviors of L. cerasina and L. paranigraa
| 1st Song | 1st Facing | 1st Microspermatophore |
Macrospermatophore |
|||
|---|---|---|---|---|---|---|
| Production | Transfer | Production | Transfer | |||
| L. cerasina | 0:15 ± 0:02 | 0:19 ± 0:02 | 0:59 ± 0:11 | 1:37 ± 0:13 | 7:17 ± 0:09 | 8:28 ± 0:09 |
| L. paranigra | 1:14 ± 0:21 | 2:12 ± 0:37 | 5:06 ± 0:38 | 6:02 ± 0:35 | 9:52 ± 0:07 | 10:38 ± 0:07 |
Times are in hrs:min after lights on and are displayed as mean times ± SEM. The timing of each behavior differed significantly between L. cerasina and L. paranigra
Fig. 1.

Spermatophore production times. Productions of spermatophores are plotted against time after lights on. Each column displays data from a single individual. Smaller filled dots represent microspermatophores while larger unfilled dots represent macrospermatophores. Gray dots represent microspermatophores for which production time was not observed, but times were estimated for this graph based on transfer times. These estimates were excluded from spermatophore rate calculations
There were no significant differences between species in the number of microspermatophores produced or transferred. L. cerasina produced 8.0 ± 0.28 and transferred 6.3 ± 0.57 microspermatophores while L. paranigra produced 8.3 ± 0.43 and transferred 6.9 ± 0.45 microspermatophores. It was not unusual that some microspermatophores failed to be transferred to the female. In these cases the male generally made several unsuccessful transfer attempts, after which he ate the microspermatophore himself. When microspermatophore transfer did occur, there was negligible intraspecific variation in the time between production and transfer of microspermatophores (L. cerasina: 0 h 25 min ± 0.4 min; L. paranigra: 0 h 19 min ± 0.6 min). Thus, we only analyzed the time between production, and not transfer, of successive microspermatophores in the analyses described below.
We found a significant difference between species in the length of time between the transfer of the first microspermatophore and the transfer of the macrospermatophore (t = 3.87, df = 24, p ≪ 0.01). This interspecific difference in the duration of courtship is reflected in the overall rates of microspermatophore production, which also differed significantly between L. cerasina and L. paranigra (t = 4.02, df = 22, p ≪ 0.01). The period from production of one microspermatophore to the next varies as courtship proceeds (Fig. 2) in both L. cerasina (F = 23.38, df = 9, 95, p ≪ 0.01) and L. paranigra (F = 5.35, df = 9, 82, p ≪ 0.01). Additionally, there was a significant interaction effect of species and number of microspermatophores produced on the rate of microspermatophore production (F = 2.56, df = 9, 178, p < 0.01). In L. cerasina there was a significant decrease in the period between successive microspermatophore until the fourth microspermatophore, while in L. paranigra there was an initial non-significant increase in the period of successive microspermatophores rate from the first to second period, followed by a significant decrease in the period from the second to forth interval between microspermatophores (Fig. 2). Thus, we found a difference between species in both the rate of microspermatophore production and in the change in rate of microspermatophore production as courtship proceeds.
Fig. 2.
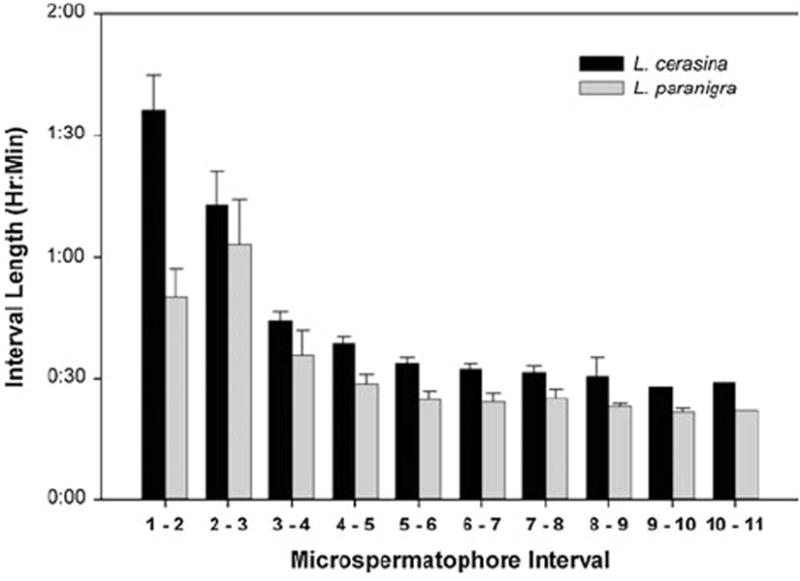
Microspermatophore production rates. The time period between productions of successive microspermatophores is potted for successive pairs of microspermatophores. The small standard errors in the last two periods for L. cerasina and last three periods for L. paranigra are due to very small sample sizes for those periods
Discussion
Establishing that temporal patterns of particular behaviors are phenotypes per se should enable tests of hypotheses about (1) whether adaptive differentiation may have generated species differences in such phenotypes, (2) how variation in such phenotypes contributes to reproductive barriers, and (3) how timing phenotypes deployed at different time scales may be correlated or pleiotropically controlled (Kyriacou and Hall 1980; Miyatake 1997; Miyatake and Kanmiya 2004; Sakai and Ishida 2001). For example, in D. melanogaster, variation in the period gene has been shown to pleiotropically regulate both circadian rhythms and the ultradian cycling of courtship song interpulse intervals (Kyriacou and Hall 1980). In addition, variation in timing has been implicated as a barrier to gene flow and a potential factor in speciation on ultradian (Kyriacou and Hall 1982, 1986; Shaw 2000), circadian (Guldemond et al. 1994; Miyatake et al. 2002; Tauber et al. 2003), seasonal (Alexander and Bigelow 1960; Brophy et al. 2006; Feder and Filchak 1999; Moore et al. 2005; Santos et al. 2007; Toro et al. 2002), and multi-year time scales (Cooley et al. 2001; Simon et al. 2000).
Here we document variation between species in the temporal expression of Laupala courtship and mating behavioral elements, establishing that these temporal patterns are phenotypes per se. L. cerasina produced the first microspermatophore in the courtship series approximately 4 h earlier in the day than did L. paranigra. Likewise, the final sperm-containing macrospermatophore was produced and transferred at a significantly earlier time of day in L. cerasina than L. paranigra (Table 1). In addition, while there was an overall acceleration in the microspermatophore production rates of both species, we also observed an interspecific difference in the rates of microspermatophore production. The difference in the rates is reflected in the fact that periods between the productions of successive microspermatophores are shorter in L. paranigra than in L. cerasina to a statistically significant degree (Fig. 2).
Because we examined these behaviors in first generation, lab reared individuals, we can conclude that there is a genetic basis to the observed differences in timing phenotypes between species. The use of such common garden experiments dates back Mendel’s pioneering work with garden peas in the mid nineteenth century (reprinted as: Mendel 1951). While molecular technology can be useful for examining the genes underlying behavior (Robinson et al. 2008), the common garden experiment is the most direct means of establishing a genetic basis to population level behavioral variation (Dingle 1991). This approach remains a useful technique with which to establish a genetic contribution to natural or mutagenically induced behavioral variation (Baldwin et al. 2010; Boake et al. 2002; Lutterschmidt and Mason 2008; Shaw 1996; Simmons 2004; Wong et al. 1995) because environmental differences in rearing and phenotypic expression are controlled. Our design used crickets reared within acoustic range of several congeners, but interspecific song differences are known to have a strong genetic basis (Shaw et al. 2007) that is not affected by learning. Likewise, because virgin crickets were used in experiments that had no prior exposure to conspecific or congeneric sexual activity, any influence of learning on the timing of behaviors can be ruled out. Our design does not allow us to fully distinguish phenotypic variation resulting from genotypic variation of the individuals examined and maternal or paternal effects (reviewed in: Wolf and Wade 2009). However, the parental crickets, themselves collected as nymphs and reared primarily in the lab, did not interact with their offspring. Thus, parental effects would be limited and interspecific differences likely the result of species-specific genotypic variation. Establishing a genetic contribution indicates that the timing of these courtship and mating behaviors can evolve in response to selection or other evolutionary forces, provided there is an additive genetic component to the genetic differences. Future work attributing variation to additive and non-additive genetic components should be productive given our observations of intraspecific variation in the described behaviors.
So far as is known, differentiation in the diel timing of courtship and mating has not occurred in most other cricket taxa. Courtship and mating in crickets typically includes female orientation to male calling song, pair formation and a brief courtship, followed by the transfer of a spermcontaining spermatophore (i.e., mating). In addition, the presence of a sperm-containing spermatophore is associated with the expression of the calling song (e.g., Acheta domesticus, Loher 1974; Teleogryllus commodus, McFarlane 1968). Furthermore, in T. commodus, species typical daily mating time is also correlated with the timing of the calling song. However, mating time appears unrestricted in the laboratory, as evidenced by pairs of T. commodus housed together that readily mate throughout the day, with only the rate at which males produce new spermatophores varying by time of day (Huber et al. 1989). In contrast, the expression of calling song and the production of a sperm-containing spermatophore exhibit a diel pattern and are temporally decoupled in Laupala (Shaw and Khine 2004; Danley et al. 2007); calling song and mating (i.e., macrospermatophore production and transfer) are interspersed by the lengthy courtship and transfer of multiple spermless microspermatophores. The present study demonstrates interspecific differences on the order of minutes in the rate of microspermatophore production, to hours for both the duration of an entire courtship and, finally, the time of day at which mating (macrospermatophore transfer) occurs. The production and transfer of multiple sperm-less spermatophores appears to be relatively common in endemic Hawaiian swordtail crickets (deCarvalho and Shaw 2005, 2010a; Shaw and Khine 2004). The transfer of a sperm-less spermatophore has been reported in just one species outside of this group, Nemobius sylvestris (Campan and Demai 1983), where a single microspermatophore is transferred before mating. In Laupala, the protracted courtship prior to mating provides a novel context for genetically-based expression and evolution of temporal differences in mating-related behaviors between species.
Our demonstration of a genetic contribution to the interspecific differences in these novel temporal phenotypes, coupled with the functional context of their expression, suggests a potential role for selection in their origins or their future evolutionary potential. However, some of the traits examined here are necessarily correlated, and any future study of selection acting of these traits should take this into account. For example, directional selection to increase microspermatophore number could be achieved by increasing the rate of microspermatophore production or prolonging the duration of courtship. Furthermore, whether there is genetic variance for variation within species, and how it might be partitioned (e.g., additive, dominant, epistasic variance) is not yet clear, but would play a critical role in the efficacy of selection. An examination of additional species with known phylogenetic relationships will help elucidate which of these characters (courtship duration, microspermatophore number, or microspermatophore rate) has diverged, while focused studies within species will reveal the nature of the genetic underpinnings of genetic variation visible to selection.
Regardless of which behavioral phenotype might be the direct target of selection, several selective pressures could potentially contribute to the patterns of divergence we have observed. For example, selection that serves to reduce reproductive interference between sympatric species could explain some of the observed differences, resulting in reproductive character displacement (Brown and Wilson 1956; Dayan and Simberloff 2005). However, a comparison of the daily timing of singing between sympatric and allopatric populations of L. cerasina (with respect to L. paranigra) by Danley et al. (2007) found no significant difference between sympatric and allopatric localities and thus character displacement of singing time was not supported. Character displacement of the other mating-related behaviors observed here therefore seems unlikely. It may be the case that the differences in timing between L. cerasina and L. paranigra characterize the deeper split, evident in the phylogenetic history of Laupala (Mendelson and Shaw 2005), between the cerasina and pacifica species groups. If so, testing the hypothesis of reproductive interference and character displacement may be addressed more appropriately through comparative analyses of a broader array of species.
Alternatively, sexual selection irrespective of the interspecific community might have shaped the timing of reproductive phenotypes in Laupala. If females have a species-specific window of receptivity and/or timing preferences, female choice could exert divergent selective pressure on the timing of male behaviors. In addition, male–male competition, ultimately driving males to sequester females for longer periods, could play a causal role in timing differentiation between L. cerasina and L. paranigra. Male Laupala are typically restricted to one macrospermatophore transfer relatively late in the day (Shaw and Khine 2004; deCarvalho and Shaw 2006, this study) and appear to require a minimum number of microspermatophore transfers for successful insemination (deCarvalho and Shaw 2010b). We found that males of L. paranigra transfer microspermatophores at a faster rate than do L. cerasina males. Thus a L. paranigra male can potentially court and mate more rapidly than a L. cerasina male. Therefore, the more rapid courtship in L. paranigra could result in directional selection on males for later macrospermatophore transfer, if males who transfer macros earlier are less able to defend against the immediate remating of their female partners. More generally, the late-day transfer of the macrospermatophore may be a form of mate guarding to reduce the probability of immediate female remating.
The unusual courtship system of Laupala presents an opportunity to express novel temporal phenotypes that differentiate species. Based on the results of this study, it is clear that there is a genetic contribution to the interspecific timing of mating related behaviors. Taken together with previous work in Laupala, behavioral timing appears to be evolutionarily labile across time scales and behaviors, from how fast a male sings or provides microspermatophores on the ultradian scale, to the time of day of singing or mating on the circadian scale. With such a wide range and array of variation in temporal phenotypes across the genus, Laupala is developing into a useful model for examining how temporal divergence affects mate choice and for understanding how temporal variation is controlled across different time scales and behaviors.
Acknowledgments
DJF was supported by an NIH training grant (No. 5T32GM007469 to the Cornell graduate field of Neurobiology and Behavior). We would like to thank Chris Wiley, Holly Menninger, Brian Coyle, Chris Ellison, and Elizabeth Turnell for valuable discussion and feedback. We thank the BEES graduate program at the University of Maryland for support in the early phases of this project.
Contributor Information
Daniel J. Fergus, Email: djf87@cornell.edu, Department of Neurobiology and Behavior, Cornell University, Seeley G Mudd Hall, Ithaca, NY 14853, USA.
Tagide N. deCarvalho, Department of Embryology, Carnegie Institution for Science, Baltimore, MD 21218, USA
Kerry L. Shaw, Department of Neurobiology and Behavior, Cornell University, Seeley G Mudd Hall, Ithaca, NY 14853, USA
References
- Alexander RD, Bigelow RS. Allochronic speciation in field crickets, and a new species, Acheta veletis. Evolution. 1960;14:334–346. [Google Scholar]
- Baldwin MW, Winkler H, Organ CL, Helm B. Wing pointedness associated with migratory distance in common-garden and comparative studies of stonechats Saxicola torquata. J Evol Biol. 2010;23:1050–1063. doi: 10.1111/j.1420-9101.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC, Ewing AW. Pulse interval as a critical parameter in the courtship song of Drosophila melanogaster. Anim Behav. 1969;17:755–759. [Google Scholar]
- Boake CRB, Arnold SJ, Breden F, Meffert LM, Ritchie MG, Taylor BJ, Wolf JB, Moore AJ. Genetic tools for studying adaptation and the evolution of behavior. Am Nat. 2002;160:S143–S159. doi: 10.1086/342902. [DOI] [PubMed] [Google Scholar]
- Brophy D, Danilowicz BS, King PA. Spawning season fidelity in sympatric populations of Atlantic herring (Clupea harengus) Can J Fish Aquat Sci. 2006;63:607–616. [Google Scholar]
- Brown WL, Wilson EO. Character displacement. Syst Zool. 1956;5:49–64. [Google Scholar]
- Campan M, Demai F. The sexual-behavior of Nemobius sylvestris (Orthoptera, Gryllidae). 1. Male and female behavioral repertory. Biol Behav. 1983;8:185–204. [Google Scholar]
- Caveney S, Scholtz CH, McIntyre P. Patterns of daily flight activity in onitine dung beetles (Scarabaeinae, Onitini) Oecologia. 1995;103:444–452. doi: 10.1007/BF00328682. [DOI] [PubMed] [Google Scholar]
- Collins PA. A coexistence mechanism for two freshwater prawns in the Parana River floodplain, Argentina. J Crust Biol. 2005;25:219–225. [Google Scholar]
- Cooley JR, Simon C, Marshall DC, Slon K, Ehrhardt C. Allochronic speciation, secondary contact, and reproductive character displacement in periodical cicadas (Hemiptera: Magicicada spp.): genetic, morphological, and behavioural evidence. Mol Ecol. 2001;10:661–671. doi: 10.1046/j.1365-294x.2001.01210.x. [DOI] [PubMed] [Google Scholar]
- Danley PD, deCarvalho TN, Fergus DJ, Shaw KL. Reproductive asynchrony and the divergence of Hawaiian crickets. Ethology. 2007;113:1125–1132. [Google Scholar]
- Dayan T, Simberloff D. Ecological and community-wide character displacement: the next generation. Ecol Lett. 2005;8:875–894. [Google Scholar]
- deCarvalho TN, Shaw KL. Nuptial feeding of spermless spermatophores in the Hawaiian swordtail cricket, Laupala pacifica (Gryllidae: Triginodiinae) Naturwissenschaften. 2005;92:483–487. doi: 10.1007/s00114-005-0023-8. [DOI] [PubMed] [Google Scholar]
- deCarvalho TN, Shaw KL. Divergence of courtship and mating behaviors among endemic Hawaiian swordtail crickets. Behaviour. 2010a;147:479–504. [Google Scholar]
- deCarvalho TN, Shaw KL. Elaborate courtship enhances sperm transfer in the Hawaiian swordtail cricket, Laupala cerasina. Anim Behav. 2010b;79:819–826. [Google Scholar]
- Dingle H. Evolutionary genetics of animal migration. Am Zool. 1991;31:253–264. [Google Scholar]
- Feder JL, Filchak KE. It’s about time: the evidence for host plant-mediated selection in the apple maggot fly, Rhagoletis pomonella, and its implications for fitness trade-offs in phytophagous insects. Entomol Exp Appl. 1999;91:211–225. [Google Scholar]
- French BW, Cade WH. The timing of calling, movement, and mating in the field crickets Gryllus veletis, Gryllus pennsylvanicus, and Gryllus integer. Behav Ecol Sociobiol. 1987;21:157–162. [Google Scholar]
- Guldemond JA, Tigges WT, Devrijer PWF. Circadian rhythm of sex pheromone production and male activity of coexisting sibling species of Cryptomyzus aphids (Homoptera, Aphididae) Eur J Entomol. 1994;91:85–89. [Google Scholar]
- Hedwig B. Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol. 2000;83:712–722. doi: 10.1152/jn.2000.83.2.712. [DOI] [PubMed] [Google Scholar]
- Huber F, Moore TE, Loher W. Cricket behavior and neurobiology. Comstock Publishing Associates; Ithaca: 1989. [Google Scholar]
- Kyriacou CP, Hall JC. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the males’ courtship song. Proc Natl Acad Sci USA. 1980;77:6729–6733. doi: 10.1073/pnas.77.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. The function of courtship song rhythms in Drosophila. Anim Behav. 1982;30:794–801. doi: 10.1006/anbe.1998.0976. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. Interspecific genetic control of courtship song production and reception in Drosophila. Science. 1986;232:494–497. doi: 10.1126/science.3083506. [DOI] [PubMed] [Google Scholar]
- Lloyd M, Dybas HS. Periodical cicada problem II Evolution. Evolution. 1966;20:466–505. doi: 10.1111/j.1558-5646.1966.tb03381.x. [DOI] [PubMed] [Google Scholar]
- Loher W. Circadian control of stridulation in the cricket Teleogryllus commodus Walker. J Comp Physiol. 1972;79:173–190. doi: 10.1016/0022-1910(74)90221-2. [DOI] [PubMed] [Google Scholar]
- Loher W. Circadian control of spermatophore formation in the cricket Teleogryllus commodus Walker. J Insect Physiol. 1974;20:1155–1172. doi: 10.1016/0022-1910(74)90221-2. [DOI] [PubMed] [Google Scholar]
- Lutterschmidt DI, Mason RT. Geographic variation in time-keeping systems among three populations of garter snakes (Thamnophis sirtalis) in a common garden. Physiol Biochem Zool. 2008;81:810–825. doi: 10.1086/589900. [DOI] [PubMed] [Google Scholar]
- Manley BFJ. Randomization, bootstrap and Monte Carlo methods in biology. 2. Chapman and Hall/CRC; New York: 1997. [Google Scholar]
- McFarlane JE. Diel periodicity in spermatophore formation in house cricket Acheta domesticus (L) Can J Zool. 1968;46:695–698. [Google Scholar]
- Mendel G. Versuche uber pflanzen-hybriden. J Hered. 1951;42:3–47. [Google Scholar]
- Mendelson TC, Shaw KL. Genetic and behavioral components of the cryptic species boundary between Laupala cerasina and L. kohalensis (Orthoptera: Gryllidae) Genetica. 2002;116:301–310. [PubMed] [Google Scholar]
- Mendelson TC, Shaw KL. Sexual behaviour: rapid speciation in an arthropod. Nature. 2005;433:375–376. doi: 10.1038/433375a. [DOI] [PubMed] [Google Scholar]
- Mendelson TC, Shaw KL. Close-range acoustic signaling and mate choice in Hawaiian crickets (Gryllidae: Laupala) Behav Ecol Sociobiol. 2006;59:770–776. [Google Scholar]
- Miyatake T. Correlated responses to selection for developmental period in Bactrocera cucurbitae (Diptera: Tephritidae): time of mating and daily activity rhythms. Behav Genet. 1997;27:489–498. doi: 10.1023/a:1025682618895. [DOI] [PubMed] [Google Scholar]
- Miyatake T, Kanmiya K. Male courtship song in circadian rhythm mutants of Bactrocera cucurbitae (Tephritidae: Diptera) J Insect Physiol. 2004;50:85–91. doi: 10.1016/j.jinsphys.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Miyatake T, Shimizu T. Genetic correlations between life-history and behavioral traits can cause reproductive isolation. Evolution. 1999;53:201–208. doi: 10.1111/j.1558-5646.1999.tb05345.x. [DOI] [PubMed] [Google Scholar]
- Miyatake T, Matsumoto A, Matsuyama T, Ueda HR, Toyosato T, Tanimura T. The period gene and allochronic reproductive isolation in Bactrocera cucurbitae. Proc Natl Acad Sci USA. 2002;269:2467–2472. doi: 10.1098/rspb.2002.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore IT, Bonier F, Wingfield JC. Reproductive asynchrony and population divergence between two tropical bird populations. Behav Ecol. 2005;16:755–762. [Google Scholar]
- Popov AV, Shuvalov VF. Phonotactic behavior of crickets. J Comp Physiol. 1977;119:111–126. [Google Scholar]
- Richards SA. Temporal partitioning and aggression among foragers: modeling the effects of stochasticity and individual state. Behav Ecol. 2002;13:427–438. [Google Scholar]
- Ritchie MG, Halsey EJ, Gleason JM. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou & Hall cycles in D. melanogaster song. Anim Behav. 1999;58:649–657. doi: 10.1006/anbe.1999.1167. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost R, Honegger HW. The timing of premating and mating behavior in a field population of the cricket Gryllus campestris L. Behav Ecol Sociobiol. 1987;21:279–289. [Google Scholar]
- Sakai T, Ishida N. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc Natl Acad Sci USA. 2001;98:9221–9225. doi: 10.1073/pnas.151443298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos H, Rousselet J, Magnoux E, Paiva MR, Branco M, Kerdelhue C. Genetic isolation through time: allochronic differentiation of a phenologically atypical population of the pine processionary moth. Proc R Soc Lond B Biol Sci. 2007;274:935–941. doi: 10.1098/rspb.2006.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- Shaw KL. Polygenic inheritance of a behavioral phenotype: interspecific genetics of song in the Hawaiian cricket genus Laupala. Evolution. 1996;50:256–266. doi: 10.1111/j.1558-5646.1996.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Shaw KL. Interspecific genetics of mate recognition: inheritance of female acoustic preference in Hawaiian crickets. Evolution. 2000;54:1303–1312. doi: 10.1111/j.0014-3820.2000.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Shaw KL, Herlihy DP. Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proc R Soc Lond B Biol Sci. 2000;267:577–584. doi: 10.1098/rspb.2000.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KL, Khine AH. Courtship behavior in the Hawaiian cricket Laupala cerasina: males provide spermless spermatophores as nuptial gifts. Ethology. 2004;110:81–95. [Google Scholar]
- Shaw KL, Lugo E. Mating asymmetry and the direction of evolution in the Hawaiian cricket genus Laupala. Mol Ecol. 2001;10:751–759. doi: 10.1046/j.1365-294x.2001.01219.x. [DOI] [PubMed] [Google Scholar]
- Shine R, Phillips B, Waye H, Lemaster M, Mason RT. Species-isolating mechanisms in a mating system with male mate choice (garter snakes, Thamnophis spp.) Can J Zool. 2004;82:1091–1098. [Google Scholar]
- Simmons LW. Genotypic variation in calling song and female preferences of the field cricket Teleogryllus oceanicus. Anim Behav. 2004;68:313–322. [Google Scholar]
- Simon C, Tang JM, Dalwadi S, Staley G, Deniega J, Unnasch TR. Genetic evidence for assortative mating between 13-year cicadas and sympatric “17-year cicadas with 13-year life cycles” provides support for allochronic speciation. Evolution. 2000;54:1326–1336. doi: 10.1111/j.0014-3820.2000.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Sokolove PG. Locomotory and stridulatory circadian rhythms in the cricket, Teleogryllus commodus. J Insect Physiol. 1975;21:537–558. doi: 10.1016/0022-1910(75)90159-6. [DOI] [PubMed] [Google Scholar]
- Stone G, Willmer P, Nee S. Daily partitioning of pollinators in an African Acacia community. Proc R Soc Lond B Biol Sci. 1996;263:1389–1393. [Google Scholar]
- Tauber CA, Tauber MJ. A genetic model for sympatric speciation through habitat diversification and seasonal isolation. Nature. 1977;268:702–705. doi: 10.1038/268702a0. [DOI] [PubMed] [Google Scholar]
- Tauber E, Roe H, Costa R, Hennessy JM, Kyriacou CP. Temporal mating isolation driven by a behavioral gene in Drosophila. Curr Biol. 2003;13:140–145. doi: 10.1016/s0960-9822(03)00004-6. [DOI] [PubMed] [Google Scholar]
- Toro JE, Thompson RJ, Innes DJ. Reproductive isolation and reproductive output in two sympatric mussel species (Mytilus edulis, M. trossulus)and their hybrids from Newfoundland. Mar Biol. 2002;141:897–909. [Google Scholar]
- Wolf JB, Wade MJ. What are maternal effects (and what are they not)? Philos Trans R Soc Lond B Biol Sci. 2009;364:1107–1115. doi: 10.1098/rstb.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Boutis P, Hekimi S. Mutations in the Clk-1 Gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1995;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, Simmons LW. Reproductive strategies of the crickets (Orthoptera: Gryllidae) In: Choe JC, Crespi BJ, editors. The evolution of mating systems in insects and arachnids. Cambridge University Press; Cambridge: 1997. [Google Scholar]


