Abstract
Objective
Activation of the coagulation cascade leading to generation of thrombin has been extensively documented in various forms of lung injury including that associated with systemic sclerosis. We previously demonstrated that direct thrombin inhibitor (DTI) dabigatran inhibits thrombin-induced profibrotic signaling in lung fibroblasts. This study tested whether dabigatran attenuates lung injury in a murine model of interstitial lung disease.
Methods
Lung injury was induced in 6–8 week old female C57BL/6 mice by a single intratracheal instillation of bleomycin. Dabigatran etexilate was given as supplemented chow beginning on day 1 (early treatment, anti-inflammatory effect) or on day 8 (late treatment, anti-fibrotic effect) following bleomycin instillation. Two and three weeks after bleomycin instillation mice were euthanized; lung tissue, bronchoalveolar lavage fluid (BALF), and plasma were investigated.
Results
Both early and late treatment with dabigatran etexilate attenuated the development of bleomycin-induced pulmonary fibrosis. Dabigatran etexilate significantly reduced thrombin activity and levels of TGF-β1 and PDGF-AA in BALF simultaneously decreasing inflammatory cells and protein concentrations. Histological lung inflammation and fibrosis were significantly decreased in dabigatran etexilate-treated mice. Additionally, dabigatran etexilate reduced collagen, CTGF, and α-SMA expression in mice with bleomycin-induced lung fibrosis, whereas it had no effect on basal levels of these proteins.
Conclusion
Inhibition of thrombin using the oral DTI dabigatran etexilate has marked anti-inflammatory and anti-fibrotic effects in a bleomycin model of pulmonary fibrosis. Our data provide preclinical information about the feasibility and efficacy of dabigatran etexilate as a new therapeutic approach for the treatment of interstitial lung diseases.
INTRODUCTION
In recent years, increasing evidence has accumulated to implicate the involvement of the coagulation system in various fibrotic diseases, including idiopathic pulmonary fibrosis (IPF) and the interstitial lung fibrosis associated with systemic sclerosis (SSc-ILD) (1, 2). Activation of the coagulation cascade is one of earliest events following tissue injury, including lung injury (3). This complex and highly regulated system leads to the generation of insoluble, cross-linked fibrin to forms plug at the site of tissue injury. This process is critically dependent on the action of the serine protease thrombin (4).
In addition to its essential role in coagulation, thrombin has several important functions at the cellular level, both in normal health and in multiple disease processes (5). The majority of the cellular responses to thrombin are mediated via the G protein-coupled receptor PAR-1 (protease-activated receptor 1) (2–6). Previously, we demonstrated that thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the PAR-1 receptor and a protein kinase C dependent pathway (7). Thrombin is mitogenic for lung fibroblasts (7 – 9) and enhances the proliferative effect of fibrinogen on fibroblasts (10). Thrombin is also a potent inducer of fibrogenic cytokines, such as transforming growth factor-β (TGF-β) (11), connective tissue growth factor (CTGF) (12, 13) and platelet-derived growth factor-AA (PDGF-AA) (9). Thrombin also increases expression of proinflammatory chemokines (14, 15) and extra-cellular matrix (ECM) proteins such as collagen, fibronectin and tenascin in various cells, including lung fibroblasts (16 – 18). Activation of these cells by thrombin is a likely mechanism for the development and progression of pulmonary fibrosis in general, and SSc-ILD in particular where endothelial injury and activation of the coagulation cascade is widespread.
Activation of the coagulation cascade with generation of thrombin has been also shown to occur in a bleomycin-induced animal model of lung injury and fibrosis (1, 2, 19). Previously, Howell et al. demonstrated in such a model that direct thrombin inhibition attenuates CTGF and lung collagen accumulation by lowering the profibrotic effects of thrombin (19). Additionally, increased thrombin activity and PAR-1 expression, similar to what we have reported in SSc-ILD (8, 9) has been observed in bleomycin-induced lung fibrosis (19, 20).
Dabigatran is a direct thrombin inhibitor (DTI) that reversibly binds to the active site of thrombin preventing the conversion of fibrinogen to fibrin (21). Recently, we have demonstrated that binding of dabigatran to thrombin prevents cleavage of the extracellular N-terminal domain of the PAR-1 receptor (22). In the absence of dabigatran, thrombin binds to PAR-1, cleaves the peptide bond between residues Arg-41 and Ser-42, thereby unmasking a new amino terminus, SFLLRN, which then can bind to the second extracellular loop of PAR-1 and initiate receptor signaling (23). Dabigatran-bound thrombin is unable to cleave and activate PAR-1 (22). Further, we have shown that dabigatran inhibits thrombin-induced differentiation of normal lung fibroblasts to the myofibroblast phenotype and decreases CTGF, α-SMA, and collagen type I in scleroderma lung fibroblasts (22).
In this study we studied dabigatran etexilate, the oral prodrug of dabigatran. The prodrug does not have antithrombin activity; however, after oral administration dabigatran etexilate is rapidly converted by ubiquitous esterases to the active moiety, dabigatran (21, 24). The present study was designed to determine whether the oral DTI dabigatran etexilate has any preventive and therapeutic effects on bleomycin-induced pulmonary fibrosis in mice.
MATERIALS AND METHODS
Animal model of fibrosis
Mice (n = 160), C57BL/6 female 6–8 week old were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were maintained in animal quarters specially designated for pathogen-free mice and were provided with food and water ad libitum. Lung injury was induced by intratracheal instillation of bleomycin (0.045 U/mouse) in 8–10 week old mice weighing 19–21 g. Dabigatran was administrated with food using chow supplemented with dabigatran etexilate (10mg/g) or placebo provided by Boehringer Ingelheim Pharma GmbH & Co KG on the same day as bleomycin, or 8 days following bleomycin treatment. Two and three weeks after bleomycin instillation mice were sacrificed by cervical dislocation under isoflurane anesthesia; lungs, BALF, and plasma were collected. All experimental procedures were performed according to guidelines of the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
BALF collection and analysis
Bronchoalveolar lavage was performed when mice were sacrificed with instillation of saline (1 ml) using a tracheal cannula. The BALF was centrifuged (500xg, 10 min), and supernatants were stored at −80°C until assayed. The cell pellet was removed and subjected to total and differential cell counts as previously described (25). The protein concentration in BALF was measured with BCA™ protein assay kit (Pierce, Rockford, IL).
Thrombin assay
The level of active thrombin was determined in BALF by spectrophotometric method. BALF aliquot (100µl) was mixed with 50µl of buffer containing 50mM Tris (pH 7.4) and 100mM NaCl and 50µl of thrombin substrate Boc-Val-Pro-Arg-7-amido-4-methylcoumarin hydrochloride (Sigma) at 37°C. Absorbance was read on a spectrophotometer at 405 nm and thrombin activity was determined by extrapolation from a thrombin standard curve.
Lung fixation and histological examinations
Sacrificed mice were subjected to midline thoracotomy. The trachea was cannulated, and the lungs were fixed by instillation of buffered formalin (2%) for 24 hours followed by perfusion with 70% ethanol for another 24 hours before routine processing and paraffin embedding. Multiple sections from each lung were stained with hematoxylin and eosin (H&E staining) or with trichrome staining for collagen and other extracellular matrix proteins. For the area analysis of fibrotic changes, a quantitative fibrotic scale (Ashcroft scale) was used (26). The severity of fibrotic changes in each lung section was given as the mean score from the observed microscopic fields. Ten fields within each lung section were observed at magnification of x20, and each field was assessed individually for severity and scored from 0 (normal) to 8 (total fibrosis). To avoid bias, all histological specimens were evaluated in a blinded fashion. Each specimen was scored independently by two observers, and the mean of individual scores was presented as the fibrotic score. TGF-β immunohistochemistry was performed using rabbit polyclonal anti-TGF-β1 antibody from Santa Cruz biotechnology by AEC chromogen method (Invitrogen). CTGF and α-SMA immunohistochemistry was performed by DAB substrate kit (Vector Laboratories) using anti-CTGF and anti-α-SMA rabbit polyclonal antibodies from Santa Cruz Biotechnology and Abcam Inc. respectively.
Collagen assay
Collagen assay was performed using the Sircol collagen assay method from Accurate Chemical & Scientific Corp. (Westbury, NY) in accordance with the manufacturer’s instructions.
Lung hydroxyproline assay
Hydroxyproline determination was performed according to established procedures and colorimetric measurements (27).
TGF-β1 Assay
Levels of TGF-β1 in BALF was measured using QuantikineR Mouse/Rat TGF-β1 Immunoassays from R & D Systems (Minneapolis, MN) in samples consisting of 50 µl of BALF according to manufacturer’s instructions.
Immunoblotting
CTGF was assayed as described previously (22). Smad3 was analyzed by Western blot using anti-phospho-Smad3 and anti-Smad3 rabbit monoclonal antibodies from Cell Signaling Technology; α-SMA - using rabbit polyclonal anti-α-SMA antibody (Abcam Inc). The immunoblots were then stripped and re-blotted with polyclonal anti-β-actin antibody (Santa Cruz Biotechnology) as a loading control.
Assessment of dabigatran in mice plasma
Blood was collected by cardiac puncture when mice were sacrificed. Plasma was prepared by centrifugation of citrated blood samples at 2000xg for 15 minutes. Dabigatran plasma levels were determined used validated LC-MS/MS methods described by Troconiz et al. (28). The amount of plasma obtained in this study was too little to perform both clotting tests and determine dabigatran plasma levels. Thus, plasma was obtained from separate mice (n=7) as described above. An in vitro standard curve with increasing concentrations of dabigatran was performed in order to correlate anticoagulant activity with plasma concentrations. The clotting tests, thrombin time (TT) and activated partial thromboplastin time (aPTT), were performed using a CL4 Behnk Elektronik coagulometer (Norderstedt, Germany) according to the methods of Palm et al. (29). Mouse plasma was pooled and each test was performed in duplicate. Increasing concentrations of dabigatran were pre-incubated with 500µl plasma and then used in either test. Thrombin (3 IU stock, Siemens Healthcare, Marburg, Germany) was used to initiate clotting in the TT. The aPTT reagent (Diagnostica Stago, Asnieres, France) was added to plasma and CaCl2 (Dade Behring, Marburg, Germany) was used to initiate clotting. The time until the plasma clotted was recorded in seconds.
Lung Fibroblast Isolation was performed from left mice lung by the procedures described previously (22).
Collagen Gel Contraction Assays and α-SMA immunofluorescent studies in lung fibroblasts were performed as described previously (22).
Statistical Analysis
Statistical analyses were performed using analysis of variance models followed by post hoc testing or nonparametric test as appropriate. The results were considered significant if p<0.05.
RESULTS
Bleomycin is a well-established agent for inducing pulmonary inflammation and fibrosis (30). In the present study we compared the effect of oral administration of the DTI dabigatran etexilate on the day of bleomycin instillation (day 1) and on day 8 after bleomycin instillation in mice. It is established that drugs administrated during the early injury phase predominantly act as anti-inflammatory agents and should be considered as “preventive treatment”, whereas “true” antifibrotic agents might be effective irrespective of timing, particularly if administrated during the “fibrotic” phase of the model (30). Administration of dabigatran etexilate beginning on day 1 and day 8 after bleomycin allowed us to distinguish between anti-inflammatory and antifibrotic drug effects.
Histological evaluation of lung inflammation and fibrosis
In control mice that received saline or saline and dabigatran etexilate lung histology was characterized by alveolar structures composed of septa, vascular components, and connective tissue. Alveolar septa were thin allowing maximum air to occupy the lung. Lung tissue isolated from bleomycin-treated mice indicated extensive peribronchial and interstitial infiltrates of inflammatory cells (H&E staining), thickening of the alveolar walls, and multiple focal fibrotic lesions with excessive amounts of ECM protein shown by trichrome differential staining (Figure 1A). By contrast, significantly fewer cellular infiltrates, decreased thickness of alveolar septa, and reduced accumulation of ECM proteins were noted in dabigatran etexilate treated mice. Importantly, beneficial effects of dabigatran etexilate on bleomycin-induced pulmonary fibrosis was observed not only in mice receiving dabigatran etexilate beginning on the same day as bleomycin (day 1), but also in mice that received dabigatran etexilate beginning on day 8 after bleomycin administration (Figure 1A).
Figure 1.
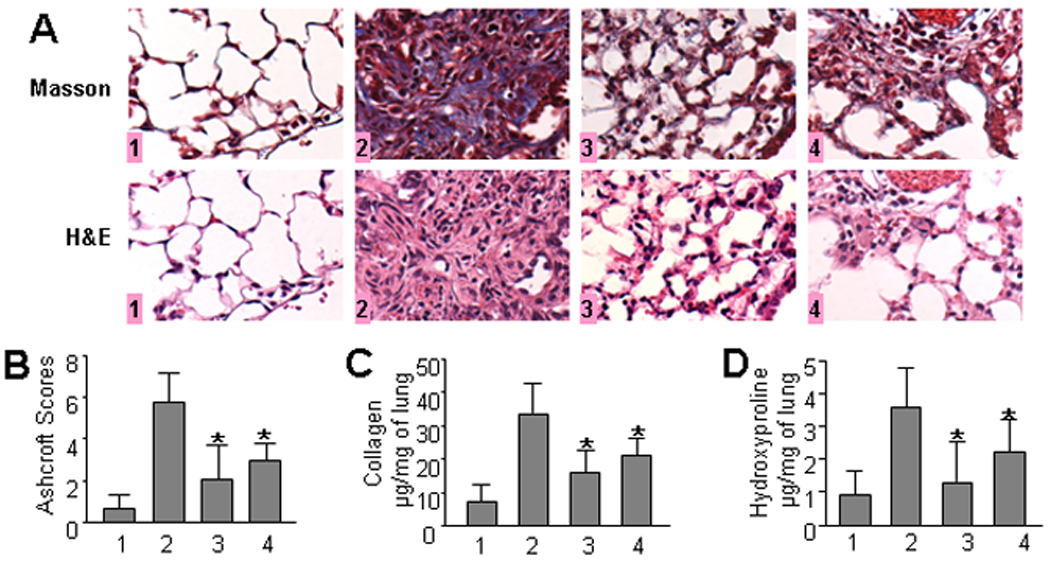
Effect of dabigatran etexilate on bleomycin-induced pulmonary fibrosis. A. Representative histological findings of lung inflammation and fibrosis. 1 – control (saline/placebo or saline/dabigatran), 2 - bleomycin/placebo, 3 – bleomycin/dabigatran etexilate (day 1), 4 – bleomycin/dabigatran etexilate (day 8), n = 40 (10 mice per group) B. Quantitative evaluation of fibrotic changes (Ashcroft scores). C. Collagen lung content determined by Sircol collagen assay, n = 24 (6 mice per group). D. Collagen lung content measured by hydroxyproline assay, n = 32 (8 mice per group). Values are the mean ± SD. * = P < 0.05 versus bleomycin/placebo-treated mice.
The overall level of fibrotic changes was quantitatively assessed based on the scoring system by Ashcroft (26). The score in mice that received bleomycin and placebo was nearly 9-fold higher compared to control mice (5.76±1.64 and 0.65±0.7 respectively). The score in mice treated with dabigatran etexilate beginning on day 1 after bleomycin instillation was significantly reduced (2.8-fold, p < 0.05) when compared to bleomycin/placebo group suggesting an anti-inflammatory effect of dabigatran (Figure 1B). Interestingly, the score in mice that received dabigatran beginning on day 8 after bleomycin instillation was also significantly reduced (1.9-fold, p < 0.05) compared to bleomycin/placebo group suggesting that in addition to anti-inflammatory properties dabigatran demonstrated a strong anti-fibrotic effect in bleomycin-induced pulmonary fibrosis.
Effect of dabigatran etexilate on collagen accumulation in bleomycin-induced pulmonary fibrosis
The most profound development of fibrosis in the bleomycin model is observed by day 21 so, therefore, we determined the effect of dabigatran on collagen accumulation in lungs 21 days after bleomycin treatment. To quantify collagen accumulation within the lungs we employed two independent methods, Sircol collagen and hydroxyproline assays. The Sircol assay is a direct quantitative method for the analysis of collagens based on the reagent Sirius Red that reacts specifically with the side chain groups of the basic amino acids present in collagen. We observed that collagen content in dabigatran etexilate-treated control mice was similar to saline-administrated control mice (data not shown). Collagen content in bleomycin-treated mice was increased by 4.4-fold compared with control. Dabigatran etexilate significantly reduced collagen by 53.0% and 37.6% in mice when administration began on day 1 and day 8, respectively (Figure 1C).
The hydroxyproline assay is based on colorimetric measurements of hydroxyproline in lung hydrolysates that reflects total collagen in lung tissue. We observed that dabigatran etexilate did not affect the basal level of hydroxyproline (data not shown). However, dabigatran etexilate significantly lowered hydroxyproline in bleomycin-treated mice by 63.9% and by 38.9% when administrated beginning on day 1 and day 8, respectively (Figure 1D). We conclude that dabigatran etexilate downregulates lung collagen consistent with an antifibrotic effect by direct thrombin inhibition.
Effect of dabigatran etexilate on BALF content
The total cell count in BALF was markedly higher in the bleomycin-treated group on day 14 as compared to saline controls (Table 1). We observed that dabigatran etexilate treatment starting on day 1 and day 8 significantly reduced total cell counts in BALF from bleomycin-treated mice (p < 0.01). Dabigatran etexilate alone did not affect BALF cell counts (data not shown). The percentage of macrophages was significantly increased on day 1, whereas changes on day 8 were not significant. The percentage of BALF neutrophils was significantly decreased in bleomycin/dabigatran etexilate-treated mice when compare to bleomycin/placebo-treated mice (p < 0.01). Total protein in BALF was increased by 7.9-fold in bleomycin-placebo treated mice when compared to control and significantly decreased by dabigatran treatment (p < 0.001). On day 21 after bleomycin instillation there was notably fewer cells in BALF in mice treated with dabigatran etexilate when compared to placebo-treated mice. However, there were no significant differences in cell numbers among studied groups (data not shown).
Table 1.
Analysis of bronchoalveolar lavage fluid
|
Parameters |
Control, n = 8 |
Bleomycin/ Placebo, n = 8 |
Bleomycin/ Dabigatran Day 1, n = 8 |
Bleomycin/ Dabigatran Day 8, n = 8 |
|---|---|---|---|---|
| Total Protein (mg/ml) | 0.24±0.09 | 1.89±0.26 | 0.87±0.41* | 0.89±0.36* |
| Total Cell (×105/ml) | 8.0±3.5 | 41.9±17.0 | 18.2±7.5* | 21.0±8.5* |
| Macrophages (%) | 97.8±1.6 | 71.6±8.3 | 84.8±9.3* | 79.4±9.0 |
| Lymphocytes (%) | 2.2±1.4 | 10.3±4.4 | 8.7±3.7 | 9.1±5.8 |
| Neutrophils (%) | 0 | 18.1±5.6 | 6.5±2.2* | 11.5±5.4* |
Bronchoalveolar lavage was performed on day 14 after bleomycin administration. Data are presented as mean ± SD.
= P < 0.01 versus bleomycin/placebo-treated mice.
Effect of dabigatran etexilate on thrombin activity in BALF
The level of active thrombin in BALF of bleomycin/placebo-treated mice was increased 35-fold when compared to saline/placebo-treated mice. Dabigatran etexilate treatment beginning on day 1 and day 8 significantly reduced active thrombin (by 74.1% in both groups, Figure 2A). Dabigatran etexilate also decreased basal level of active thrombin in BALF from 1.32±0.35ng/ml to 0.61±0.33ng/ml. The average concentration of dabigatran in plasma obtained from mice fed with dabigatran chow was 342.1 ± 90.0 ng/mL (n=21).
Figure 2.
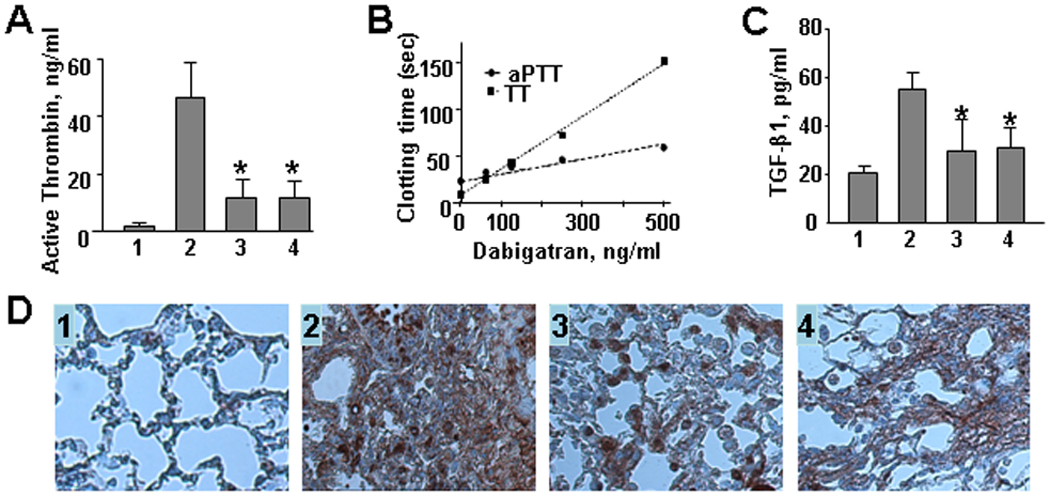
A. Effect of dabigatran etexilate on level of active thrombin in BALF. B. Effect of increasing concentrations of dabigatran on the thrombin time (TT) and activated partial thromboplastin time (aPTT) clotting times in mouse plasma in vitro. Data expressed as mean of duplicate determinations. C and D. Effect of dabigatran etexilate on TGF-β1 levels in BALF (C) and lung tissue (D). 1 – control (saline/placebo), 2 - bleomycin/placebo, 3 – bleomycin/dabigatran etexilate (day 1), 4 – bleomycin/dabigatran etexilate (day 8). Values are the mean ± SD. * = P < 0.01 versus bleomycin/placebo-treated mice.
In vitro anticoagulation
We observed a concentration-dependent increase in clotting time with increasing concentrations of dabigatran added in vitro (Figure 2B). The TT in control plasma was 7.5 seconds and the aPTT was 22.8 seconds. Mean plasma levels achieved in this study (342.1±90.0 ng/mL) resulted in an approximate 10-fold prolongation of the TT and an ~2-fold increase in aPTT over baseline levels.
Effect of dabigatran etexilate on TGF-β1 in BALF and lung tissue
We observed that TGF-β1 was up-regulated by 2.7-fold over control in bleomycin/placebo-treated mice. Dabigatran etexilate significantly reduced TGF-β1 from 54.9±6.1 pg/ml in bleomycin/placebo-treated mice to 29.9±9.3 and 31.1±8.7 pg/ml when administered beginning on day 1 and day 8, respectively (p<0.01, Figure 2C). TGF-β1 expression was also assessed by IHC in lung tissue. We observed that TGF-β1 was not detectable in control mice, while strongly positive in fibrotic areas in bleomycin/placebo-treated mice (Figure 2D). Dabigatran etexilate visibly reduced TGF-β1 accumulation when used by both early and late treatment.
Effect of dabigatran etexilate on Smad3 phosphorylation
Most profibrotic effects of TGF-β are mediated through Smad signaling pathway. Therefore, we determined the activation of Smad3 by measuring phosphorylated levels of Smad3 in lung tissue. Phosphorylated Smad3 was increased by 5.4 fold in mice treated with bleomycin/placebo as compared to control mice (Figure 3). Dabigatran etexilate significantly decreased the levels of phosphorylated Smad3 by 8.1 and 6.4 fold when administered beginning on day 1 and day 8 respectively (p<0.01, Figure 3). The basal level of Smad3 was not affected by any treatment.
Figure 3.
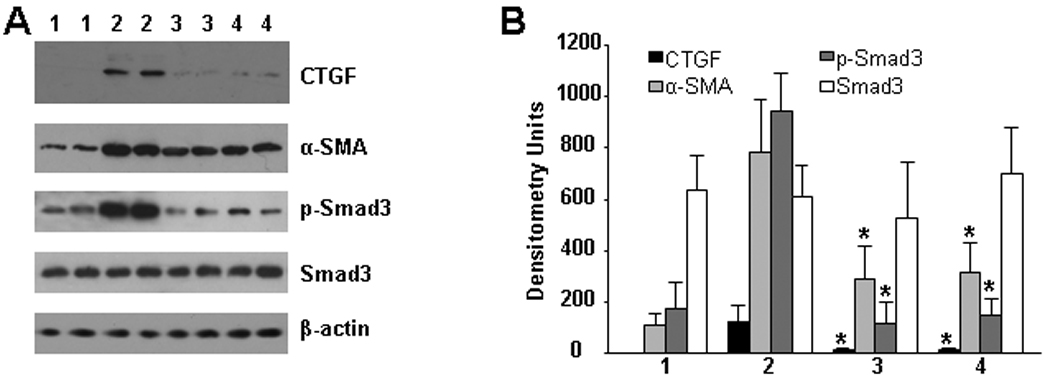
Effect of dabigatran etexilate on Smad3, CTGF and α-SMA expression in lung tissue. Representative immunoblots of phosphorylated and basal Smad3, CTGF, α-SMA, and β-actin (as loading control) from control (1), bleomycin/placebo (2), bleomycin/dabigatran etexilate (day 1) (3), bleomycin/dabigatran etexilate (day 8) (4) are presented, n = 16 (4 mice per group).
Effect of dabigatran etexilate on CTGF and α-SMA expression in bleomycin-induced pulmonary fibrosis
We could not detect CTGF in lung tissue from control mice. However, CTGF was strongly expressed in lung tissue from mice receiving bleomycin (Figure 3). Dabigatran reduced CTGF expression 10.5-fold and 9-fold when initiated on day 1 and day 8, respectively (Figure 3). Similarly, α-SMA was strongly up-regulated in lung tissue from bleomycin/placebo-treated mice, whereas it was significantly reduced in lung from both groups (day 1 and day 8) of bleomycin/dabigatran etexilate-treated mice (2.7-fold and 2.5-fold, respectively) (Figure 3). We also measured CTGF and α-SMA expression by immunohistochemistry. We observed that both proteins were strongly upregulated in lung of bleomycin-treated mice, while no visible staining was present in control lung tissue with exception for α-SMA which was restricted to smooth muscle cells located around blood vessels (Figure 4B). Dabigatran etexilate decreased bleomycin-upregulated expression of CTGF (Figure 4A) and α-SMA (Figure 4B) in lung tissue in both groups.
Figure 4.
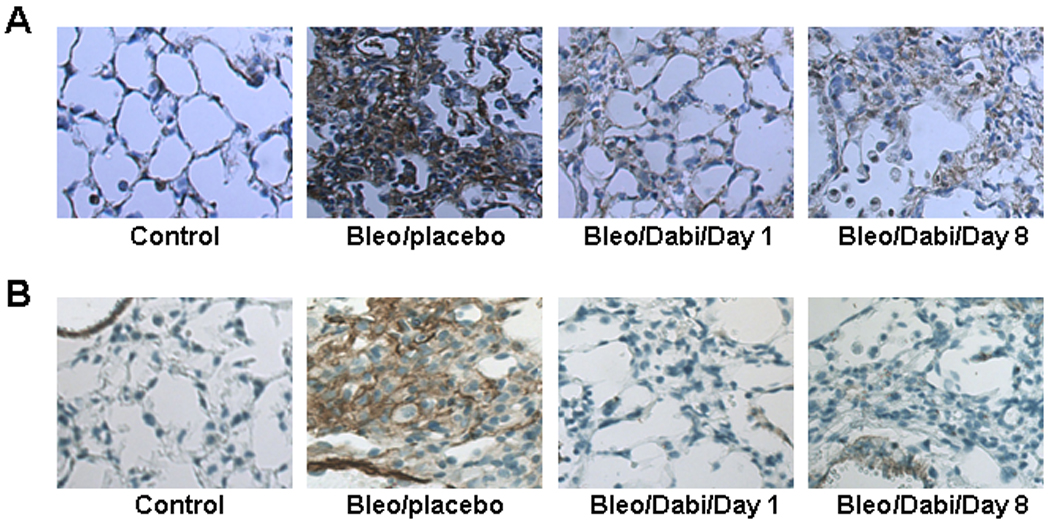
Immunohistochemical evaluation for CTGF (A) and α-SMA (B) expression in lung tissue.
Effect of dabigatran etexilate on α-SMA and collagen gel contraction in lung fibroblasts
Fibroblasts were isolated from lung tissue of mice treated with or without bleomycin or/and dabigatran etexilate. Lung fibroblasts from bleomycin-treated mice expressed high amount of α-SMA stress fibers while in control there was no visible α-SMA stress fibers (Figure 5A). Dabigatran etexilate strongly reduced α-SMA expression and organization when administrated on day 1, while the inhibition of stress fibers was observed to a lesser extend when dabigatran etexilate was administrated on day 8. Lung fibroblasts isolated from bleomycin treated mice contracted collagen gels to significantly higher extend as compared to control, while cells isolated from dabigatran etexilate-treated mice exhibited less contractile activity (Figure 5B).
Figure 5.
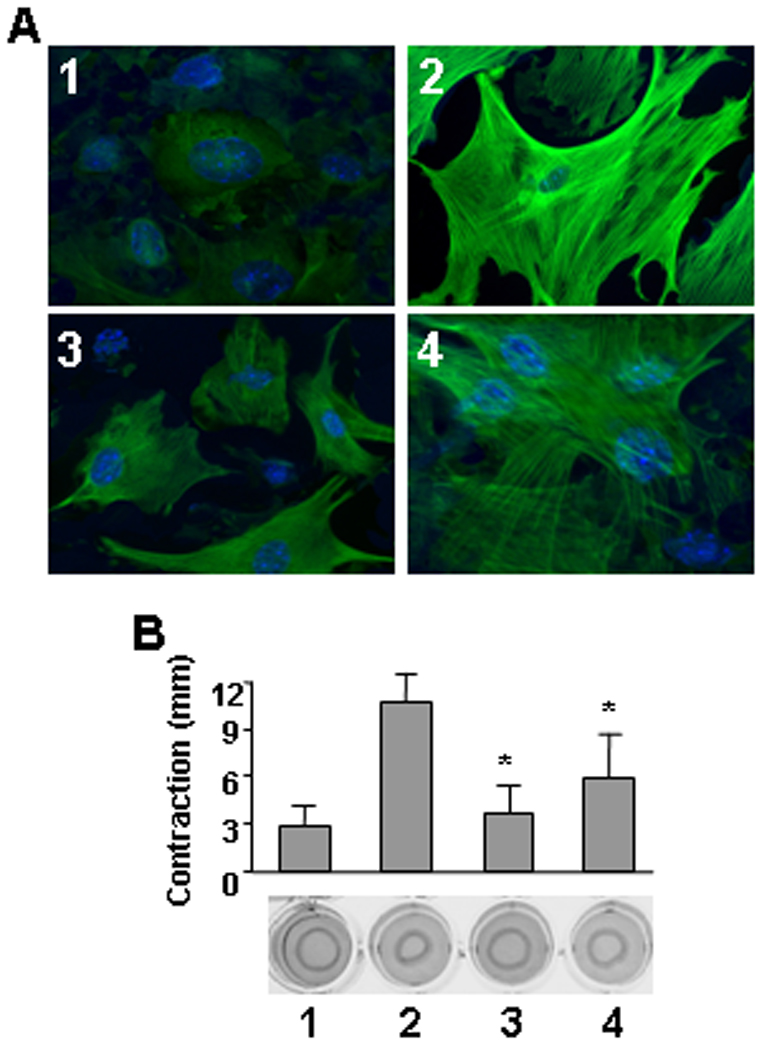
Effect of dabigatran etexilate on α-SMA expression/organization (A) and collagen gel contraction (B) in mouse lung fibroblasts. 1 – control (saline/placebo), 2 - bleomycin/placebo, 3 – bleomycin/dabigatran etexilate (day 1), 4 – bleomycin/dabigatran etexilate (day 8), n = 24 (6 mice per group). Values are the mean ± SD. * = P < 0.01 versus bleomycin/placebo-treated mice.
DISCUSSION
Our recent in vitro studies demonstrated that the direct thrombin inhibitor dabigatran blocks thrombin-induced and PAR-1-mediated profibrotic signaling (22). Dabigatran inhibited thrombin-induced collagen and CTGF in normal and SSc lung fibroblasts, blocked development of the myofibroblast phenotype from thrombin-activated normal lung fibroblasts, and reversed the myofibroblast phenotype expressed by lung fibroblasts of SSc-ILD patients (22). These data suggest that dabigatran may serve as a novel and attractive therapeutic agent for the treatment of pulmonary fibrosis. The present study was designed to determine whether dabigatran etexilate exhibits therapeutic and/or preventive effects on bleomycin-induced pulmonary fibrosis in mice. We demonstrate here for the first time that dabigatran etexilate attenuates bleomycin-induced pulmonary fibrosis by lowering thrombin activity to physiological levels and by decreasing pro-inflammatory and profibrotic factors, supporting its potential use for the treatment of pulmonary fibrosis.
Thrombin is a multifunctional protease that promotes a wide range of cellular responses in addition to its central function in thrombosis and hemostasis (1 – 6). Thrombin mediates a variety of inflammatory and tissue-repair responses associated with vascular injury (5). Additionally, thrombin stimulates secretion of mediators involved in the pathogenesis of fibrosis, such as TGF-β1 and PDGF (9, 11). TGF-β1 and PDGF are each elevated in BALF of patients with IPF and SSc-ILD, as well as in BALF in animal models of pulmonary fibrosis (9, 31 – 36). Utilizing a bleomycin model of lung injury and fibrosis we demonstrate that dabigatran etexilate significantly decreases inflammatory cells and protein content as well as levels of TGF-β1 in BALF. Dabigatran etexilate also significantly reduces CTGF and α-SMA expression in lung tissue of bleomycin-treated mice, suggesting both anti-inflammatory and antifibrotic effects of dabigatran.
There is compelling evidence that thrombin is an important mediator of interstitial lung disease including both IPF and SSc-ILD (1, 2, 5). Our laboratory and others have demonstrated dramatically increased levels of thrombin in BALF from SSc-ILD patients. BALF from normal subjects has a low level of thrombin activity, while BALF from SSc patients express significantly higher thrombin activity (9, 37). Elevated levels of thrombin activity have been also observed in bleomycin-induced pulmonary fibrosis (19). Previously, Howell et al. found that the direct thrombin inhibitor UK-156406 attenuates lung collagen accumulation by lowering the profibrotic effects of thrombin and suppressing CTGF synthesis in bleomycin-induced lung fibrosis (19). Later, the same group demonstrated that mice lacking the PAR-1 thrombin receptor are significantly protected from bleomycin-induced lung fibrosis with a reduction in CCL2 and CTGF expression and TGF-β immunoreactivity (24). Recent work of Favreau et al. demonstrated that inhibition of thrombin reduced chronic kidney graft fibrosis and significantly improved survival rate in ischemia reperfusion injury (38).
Different models of pulmonary fibrosis have been developed over the years. Most of them mimic some but never all features of IPF and SSc-ILD, especially the progressive and irreversible condition (39). The drugs effective in the treatment of bleomycin-induced pulmonary fibrosis are not always reflect the same efficacy in pulmonary fibrosis in human. In our study we employed a single intratracheal bleomycin administration, which is the most frequently utilized method for inducing pulmonary fibrosis in animal models. We used both early and late treatment with dabigatran etexilate to distinguish anti-inflammatory and antifibrotic effects of this drug. We observed that dabigatran etexilate markedly improved bleomycin-altered histopathology and decreased interstitial infiltrates. Dabigatran etexilate also reduced the thickness of alveolar septa and decreased the accumulation of ECM proteins. We observed that collagen, CTGF, and α-SMA content of the lung after bleomycin injury were significantly lower in dabigatran etexilate-treated mice than in placebo-treated mice. Importantly, we found that both early and late treatments with dabigatran etexilate were able to inhibit bleomycin-induced pulmonary fibrosis. However, early administration of dabigatran etexilate resulted in more profound inhibition of pulmonary fibrosis as compare to the late administration. Efficacy of early treatment with dabigatran etexilate was higher because it targeted inflammatory stage of fibrosis, while late treatment was introduced on more developed stage and, therefore, less effective reflecting preventive and antifibrotic actions of dabigatran etexilate. The role of inflammation in the pathogenesis of progressive pulmonary fibrosis remains a controversial question. Administration of bleomycin causes severe acute inflammatory response followed by chronic inflammation and fibrosis. It has been repeatedly shown that the degree of inflammation in bleomycin-induced lung injury is associated with the intensity of fibrosis (40).
We used a concentration of dabigatran etexilate in mouse chow to achieve plasma levels of dabigatran that are slightly higher than those achieved in patients who are being treated with dabigatran etexilate for various thrombotic diseases (~180 ng/ml peak levels achieved with 150 mg twice daily dose) (41). The dose used in this study resulted in ~2-fold elevation of the aPTT in mice and ~10-fold elevation of the TT. This trend is consistent with human plasma, where it has also been shown that the TT is more sensitive to dabigatran than the aPTT (41). Though it is not possible to directly relate plasma levels and/or anticoagulation from mice to humans, the antifibrotic effects observed in our studies were achieved at plasma levels and pharmacological effects consistent with human dosing.
It is important to note that doses of dabigatran etexilate used in this study significantly reduced but did not completely inhibit thrombin activity in BALF. We did not observe any bleeding side-effects during the study suggesting that levels of dabigatran in mouse plasma were not sufficient to completely eliminate thrombin from the normal haemostatic process. However, such doses of dabigatran etexilate ameliorated lung fibrosis even after it had been established, indicating that dabigatran etexilate could safely be administered in chronic forms of lung fibrosis, at least in mice.
Pulmonary fibrosis as seen in both IPF and SSc-ILD is often a progressive and irreversible process leading to respiratory failure and death (32, 42). There is an urgent need for new therapeutic approaches that would delay or reverse pulmonary fibrosis. Blocking a single profibrotic factor has thus far not been proven to be an effective therapeutic approach. A therapeutic strategy designed to act upstream of multiple fibrogenic pathways, e.g. altering procoagulant activity, might theoretically prove to be a more effective strategy. Indeed, anticoagulant therapy with warfarin or low-molecular-weight heparin in combination with prednisolone has been demonstrated to improve survival in IPF (43). Dabigatran etexilate represents the first synthetic oral, reversible, direct inhibitor of thrombin with a very favorable biochemical and pharmacological profile that translates into clinical efficacy and safety in patients with coagulative disorders (44). The current study provides important preclinical information about the feasibility and efficacy of dabigatran etexilate for the treatment of fibrotic diseases including IPF and SSc-ILD in which there is evidence for tissue injury with overexpression of thrombin. Any future studies of thrombin inhibition for the treatment of SSc-ILD would need to demonstrate a positive benefit/risk ratio taking into account potential risks such as gastrointestinal tract hemorrhage.
ACKNOWLEDGMENTS
The authors would like to thank C. Beth Singleton and Isaac J. Van Duys for excellent technical work.
GRANTS
This work was supported in part by a career award from National Institutes of Health K01AR051052 and Boehringer Ingelheim International GmbH (to GSB).
REFERENCES
- 1.Chambers RC. Procoagulant signaling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008;153 Suppl 1:S367–S378. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howell DC, Laurent GJ, Chambers RC. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem Soc Trans. 2002;30:211–216. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 3.Green D. Coagulation cascade. Hemodial Int. 2006;10:S2–S4. doi: 10.1111/j.1542-4758.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Mann KG. Thrombin formation. Chest. 2003;124 3 Suppl:4S–10S. doi: 10.1378/chest.124.3_suppl.4s. [DOI] [PubMed] [Google Scholar]
- 5.Ludwicka-Bradley A, Bogatkevich G, Silver RM. Thrombin-mediated cellular dysfunction in pulmonary fibrosis associated with systemic sclerosis (scleroderma) Clin Exp Rheumatol. 2004;22:S38–S46. [PubMed] [Google Scholar]
- 6.Coughlin SR. Thrombin signaling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 7.Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblast to a myofibroblast phenotype via proteolytically activated receptor-1 and protein kinase C-dependent pathway. J Biol Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- 8.Bogatkevich GS, Gustilo E, Oates J, Feghali-Bostwick C, Harley RA, Silver RM, Ludwicka-Bradley A. Distinct PKC isoforms mediate cell survival and DNA synthesis in thrombin-induced myofibroblasts. Am J Physiol: Lung Cell Mol Physiol. 2005;288:L190–L201. doi: 10.1152/ajplung.00448.2003. [DOI] [PubMed] [Google Scholar]
- 9.Ohba T, McDonald JK, Silver RM, Strange C, LeRoy EC, Ludwicka A. Scleroderma BAL fluid contains thrombin, a mediator of human lung fibroblast proliferation via induction of the PDGF-alpha receptor. Am J Resp Cell Mol Biol. 1994;10:405–412. doi: 10.1165/ajrcmb.10.4.7510986. [DOI] [PubMed] [Google Scholar]
- 10.Gray AJ, Bishop JE, Reeves JT, Laurent GJ. A alpha and B beta chains of fibrinogen stimulate proliferation of human fibroblasts. J Cell Sci. 1993;104:409–413. doi: 10.1242/jcs.104.2.409. [DOI] [PubMed] [Google Scholar]
- 11.Bachhuber BG, Sarembock IJ, Gimple LW, Owens GK. alpha-Thrombin induces transforming growth factor-beta1 mRNA and protein in cultured vascular smooth muscle cells via a proteolytically activated receptor. J Vasc Res. 1997;34:41–48. doi: 10.1159/000159200. [DOI] [PubMed] [Google Scholar]
- 12.Chambers RC, Leoni P, Blanc-Brude OP, Wembridge DE, Laurent GJ. Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem. 2000;275:35584–35591. doi: 10.1074/jbc.M003188200. [DOI] [PubMed] [Google Scholar]
- 13.Bogatkevich GS, Ludwicka-Bradley A, Paul J. Nietert, Silver RM: Scleroderma Lung Fibroblasts: Contractility and Connective Tissue Growth Factor. In: Gabbiani G, Chaponnier C, Desmouliere A, editors. Myofibroblasts. Georgetown, TX: Landes Bioscience; 2006. pp. 25–31. [Google Scholar]
- 14.Mercer PF, Deng X, Chambers RC. Signaling pathways involved in proteinase-activated receptor1-induced proinflammatory and profibrotic mediator release following lung injury. Ann N Y Acad Sci. 2007;1096:86–88. doi: 10.1196/annals.1397.073. [DOI] [PubMed] [Google Scholar]
- 15.Ludwicka-Bradley A, Tourkina E, Suzuki S, Tyson E, Bonner M, Fenton JW, 2nd, Hoffman S, Silver RM. Thrombin upregulates interleukin-8 in lung fibroblasts via cleavage of proteolytically activated receptor-I and protein kinase C-γ activation. Am J Respir Cell Mol Biol. 2000;22:235–243. doi: 10.1165/ajrcmb.22.2.3642. [DOI] [PubMed] [Google Scholar]
- 16.Tourkina E, Hoffman S, Fenton JW, 2nd, Lipsitz S, Silver RM, Ludwicka-Bradley A. Depletion of protein kinase Cε in normal and scleroderma lung fibroblasts has opposite effects on tenascin expression. Arthritis Rheum. 2001;44:1370–1381. doi: 10.1002/1529-0131(200106)44:6<1370::AID-ART230>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ. Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. Biochem J. 1998;333:121–127. doi: 10.1042/bj3330121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong MT, Fenton JW, 2nd, Andersen TT, Armstrong PB. Thrombin stimulation of matrix fibronectin. J Cell Physiol. 1996;166:112–120. doi: 10.1002/(SICI)1097-4652(199601)166:1<112::AID-JCP13>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159:1383–1395. doi: 10.1016/S0002-9440(10)62525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorbera LA, Bozzo J, Castaner J. Dabigatran/ Dabigatran etexilate. Drugs Fut. 2005;30(9):877. [Google Scholar]
- 22.Bogatkevich GS, Ludwicka-Bradley A, Silver RM. Dabigatran, a direct thrombin inhibitor, demonstrates antifibrotic effects on lung fibroblasts. Arthritis Rheum. 2009;60(11):3455–3464. doi: 10.1002/art.24935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 24.Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–162. [PubMed] [Google Scholar]
- 25.Silver RM, Metcalf JF, Stanley JH, LeRoy EC. Interstitial lung disease in scleroderma: analysis by bronchoalveolar lavage. Arthritis Rheum. 1984;27:1254–1262. doi: 10.1002/art.1780271107. [DOI] [PubMed] [Google Scholar]
- 26.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent GJ, Cockerill P, McAnulty RJ, Hasting JR. A simplified method for quantitation of the relative amounts of type I and type III collagen in small tissue samples. Anal Biochem. 1981;113:301–312. doi: 10.1016/0003-2697(81)90081-6. [DOI] [PubMed] [Google Scholar]
- 28.Troconiz IF, Tillmann C, Liesenfeld K-H, Schaefer H-G, Stangier J. Population pharmacokinetic analysis of the new oral thrombin inhibitor dabigatran etexilate (BIBR 1048) in patients undergoing primary elective hip replacement surgery. J Clin Pharmacol. 2007;47:371–382. doi: 10.1177/0091270006297228. [DOI] [PubMed] [Google Scholar]
- 29.Palm M, Frankenberg L, Johansson M, Jalkesten E. Evaluation of coagulation tests in mouse plasma. Comparative Haematology International. 1997;7:243–246. [Google Scholar]
- 30.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40(3):362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwicka A, Ohba T, Trojanowska M, Yamakage A, Strange C, Smith EA, Leroy EC, Sutherland S, Silver RM. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol. 1995;10:1876–1883. [PubMed] [Google Scholar]
- 32.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scala E, Pallotta S, Frezzolini A, Abeni D, Barbieri C, Sampogna F, De Pità O, Puddu P, Paganelli R, Russo G. Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clin Exp Immunol. 2004;138(3):540–546. doi: 10.1111/j.1365-2249.2004.02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15(4):255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Maeda A, Ishioka S, Taooka Y, Hiyama K, Yamakido M. Expression of transforming growth factor-beta1 and tumour necrosis factor-alpha in bronchoalveolar lavage cells in murine pulmonary fibrosis after intraperitoneal administration of bleomycin. Respirology. 1999;4(4):359–363. doi: 10.1046/j.1440-1843.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 36.Tani K, Ogushi F, Takahashi H, Kawano T, Endo T, Sone S. Thrombin stimulates platelet-derived growth factor release by alveolar macrophages in rats--significance in bleomycin-induced pulmonary fibrosis. J Med Invest. 1997;44(1–2):59–65. [PubMed] [Google Scholar]
- 37.Hernández-Rodríguez NA, Cambrey AD, Harrison NK, Chambers RC, Gray AJ, Southcott AM, duBois RM, Black CM, Scully MF, McAnulty RJ, et al. Role of thrombin in pulmonary fibrosis. Lancet. 1995;346:1071–1073. doi: 10.1016/s0140-6736(95)91744-6. [DOI] [PubMed] [Google Scholar]
- 38.Thuillier R, Favreau F, Celhay O, Macchi L, Milin S, Hauet T. Thrombin inhibition during kidney ischemia-reperfusion reduces chronic graft inflammation and tubular atrophy. Transplantation. 2010;90(6):612–621. doi: 10.1097/tp.0b013e3181d72117. [DOI] [PubMed] [Google Scholar]
- 39.Favreau F, Thuillier R, Cau J, Milin S, Manguy E, Mauco G, Zhu X, Lerman LO, Hauet T. Anti-thrombin therapy during warm ischemia and cold preservation prevents chronic kidney graft fibrosis in a DCD model. Am J Transplant. 2010;10(1):30–39. doi: 10.1111/j.1600-6143.2009.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 41.van Ryn J, Stangier J, Haertter S, Liesenfeld K-H, Wienen W, Feuring M, Clemens A. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 42.Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis. 2008;2(5):319–338. doi: 10.1177/1753465808096948. [DOI] [PubMed] [Google Scholar]
- 43.Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, Sasaki H. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128(3):1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 44.Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol. 2010;30(10):1885–1889. doi: 10.1161/ATVBAHA.110.203604. [DOI] [PubMed] [Google Scholar]


