Abstract
To test the effect of alternative bases at the distal histidine position, four CcP variants have been constructed that substitute the two basic residues, aspartate and glutamate, and their amides, asparagine and glutamine, for histidine-52, i.e., CcP(H52D), CcP(H52E), CcP(H52N), and CcP(H52Q). All four mutants catalyze oxidation of ferrocytochrome c by H2O2 with steady-state activities that are between 250 and 7,700 times slower than wild-type CcP at pH 6.0, 0.10 M ionic strength, 25 °C. The rate of Compound I formation is decreased between 3.5 and 5.4 orders of magnitude for the mutants compared to wild-type CcP, with the rate of the reaction between CcP(H52Q) and H2O2 the slowest yet observed for any CcP mutant. A correlation between the rate of Compound I formation and the rate of HCN binding for CcP and various CcP distal pocket mutants provides strong evidence that the rate-limiting step in CcP Compound I formation is deprotonation of H2O2 within the distal heme pocket under the experimental conditions employed in this study. While CcP(H52E) reacts stoichiometrically with H2O2 to form Compound I, only ~36% of CcP(H52D), ~21% of CcP(H52Q) and ~8% of CcP(H52N) appear to be converted to Compound I during their respective reactions with H2O2. This is partially due to the slow rate of Compound I formation and the rapid endogenous decay of Compound I for these mutants. The pathways for the endogenous decay of Compound I for the four mutants used in this study are distinct from that of wild-type CcP Compound I.
Keywords: Cytochrome c peroxidase, distal histidine, H2O2 reactivity, Compound I decay, cyanide binding
1. Introduction
The term ‘distal histidine’ was first used to describe a histidine residue identified in the x-ray crystal structure of sperm whale metmyoglobin that was within hydrogen-bonding distance of the water molecule coordinated to the heme iron [1]. This histidine residue was on the opposite (distal) side of the heme from a histidine residue directly coordinated to the heme iron, the proximal histidine [2]. Since that time, distal histidine residues have been found in a large number of heme proteins including myoglobins, hemoglobins, peroxidases, and catalases [3–5]. The distal histidine can affect ligand binding to the heme iron and thus the functional properties of the protein. One of the first studies to directly demonstrate the influence of a distal histidine residue made use of a naturally occurring variant of hemoglobin, hemoglobin MRadom [6]. The distal histidine in the β-chains of hemoglobin MRadom is replaced by a tyrosine residue and significantly affects the autoxidation rate of the heme iron. The development of site-directed mutagenesis in the early 1980s opened the door for systematic studies on the role of specific amino acid residues in determining protein reactivity. The distal histidine has been of particular interest and it now appears that the distal histidine plays different roles in the oxygen transport proteins, such as myoglobin and hemoglobin, and in heme enzymes such as the peroxidases and catalase [7–21]. The distal histidine in myoglobin helps discriminate between CO and O2 binding by both sterically hindering bound CO and stabilizing bound O2 through hydrogen bonding [9] and by gating access from the solvent to the distal heme pocket [10, 12]. In the peroxidases, it has been suggested that the distal histidine catalyzes deprotonation of the incoming H2O2 facilitating binding of the peroxide anion to the heme iron [19–21].
One of the more interesting distal histidine mutants in cytochrome c peroxidase (CcP)1 is the H52Q mutant [22], which has been reported to have no detectable peroxidase activity but does catalyze the electrochemical oxidation of H2O2. These data differ from two similar horseradish peroxidase (HRP) mutants, H42E and H42Q [23]. The HRP mutants retain peroxidase activity and this activity decreases in order of decreased basicity of the distal residue as expected if the distal residue primarily functions as a base catalyst in the peroxidases.
In this study we extend the studies on CcP His-52 variants to those that have different basicity and reexamine the properties of CcP(H52Q). We report on the spectroscopic properties, steady-state activity, rate of Compound I formation, stability of Compound I, and the cyanide binding properties of four CcP variants: CcP(H52D), CcP(H52E), CcP(H52N), and CcP(H52Q). The first two variants place alternative bases at position 52 while the latter two have the corresponding amides at the critical distal residue position. All four variants have detectable peroxidase activity and all four react with hydrogen peroxide to form Compound I but at significantly slower rates compared to wild-type CcP.
2. Materials and Methods
2.1. Cloning, Expression, and Purification of CcP Mutants
James Satterlee, Washington State University, kindly provided the expression system for the recombinant CcP (rCcP) used in this study [24]. The CcP gene was modified to include an N-terminal methionine for bacterial expression and the exact amino acid sequence of mature baker’s yeast CcP [25]. The CcP gene is inserted into the multiple cloning site of the Novagene vector pET24a(+) under control of the T7 promoter. The CcP mutants were created using the Stratagene QuikChange mutagenesis kit and sequenced in both directions to assure that, except for the intended mutations, the gene was identical to the published sequence. Expression and purification of rCcP and the CcP mutants were performed as previously described [26, 27]. The expression system removes the N-terminal methionine and the isolated rCcP has the exact amino acid sequence as baker’s yeast CcP, yCcP.
2.2. Other Materials
Yeast iso-1 cytochrome c(C102T) was purified as described previously [28, 29]. Potassium cyanide, potassium phosphate salts, and hydrogen peroxide (30%) were obtained from Fisher Scientific. Hydrogen peroxide solutions were standardized by titration with Ce(IV) as described previously [30]. Buffers were 0.010 M acetate with sufficient KH2PO4 to adjust the ionic strength to 0.100 M between pH 4.0 and 5.5. Between pH 5.5 and 8.0, the buffers were mixtures of KH2PO4 and K2HPO4 with a total ionic strength of 0.100 M.
2.3. Spectroscopic Measurements and Protein Concentration Determination
Protein spectra were determined using either a Varian/Cary Model 3E or a Hewlett Packard Model 8452A spectrophotometer. The extinction coefficients of the four CcP mutants were determined using the pyridine hemochromogen method of Berry and Trumpower [31].
2.4. Transient-State Kinetic Measurement
The rates of Compound I formation and of cyanide binding were determined using stopped-flow techniques with an Applied Photophysics Ltd. Model SX.17MV stopped-flow instrument. Reactions were carried out under pseudo-first order conditions with excess hydrogen peroxide or cyanide. Protein concentrations were typically ~1 μM. Observed rate constants were determined at a minimum of five different H2O2 or cyanide concentrations with the concentrations varying by at least a factor of five for each experiment. A minimum of 10 individual traces of absorbance change versus time were acquired at each experimental condition allowing the mean value of the observed rate constant and its standard deviation to be determined. Kinetic studies were carried out in 0.10 M ionic strength potassium phosphate buffer, pH 6.0, 25 °C.
2.5. Steady-State Kinetic Measurements
Steady-state velocities for the enzyme-catalyzed oxidation of yeast iso-1 ferrocytochrome c(C102T) by hydrogen peroxide were determined in 0.10 M ionic strength potassium phosphate buffer, pH 6.0, 25 °C. The reaction was monitored at 416 nm. Relative steady-state activities of the mutants were determined using ~8 μM ferrocytochrome c and ~200 μM H2O2.
2.6. Equilibrium Cyanide Binding
Spectroscopic changes associated with the formation of the cyanide complex enabled monitoring of complex formation. Determination of the equilibrium constants was done by titrating ~10 μM protein with increasing concentrations of buffered cyanide solution until saturation with cyanide was reached. The spectrum of the solution was determined after each cyanide addition. Equilibrium constants were determined from changes in absorbance as a function of the cyanide concentration. Equilibrium studies were carried out in 0.10 M ionic strength potassium phosphate buffer, pH 6.0, 25 °C.
3. Results
3.1. Spectroscopic Properties of CcP Variants
CcP and its mutants are generally stable between pH 4 and 8. Spectra of CcP(H52E) and CcP(H52Q) at pH 4.0, 6.0, and 8.0 are shown in Figure 1, while the spectra of CcP(H52D) and CcP(H52N) are shown in Figures S1 and S2 in the supplementary material accompanying this article. Spectroscopic parameters for the four mutants at pH 6.0 are collected in Table S1 of the supplementary material along with those of yCcP and rCcP for comparison.
Figure 1.
Panel A. Spectra of CcP(H52E) at pH 4.0 (thin line), pH 6.0 (thick line) and pH 8.0 (dashed line). Panel B. Spectra of CcP(H52Q) at pH 4.0 (thin line), pH 6.0 (thick line) and pH 8.0 (dashed line). Experimental conditions: 0.10 M ionic strength, 25 °C.
The spectra of CcP(H52E) and CcP(H52D), Figures 1A and S1, are pH dependent with spectra characteristic of five-coordinate, high-spin hemes at pH 4 and 6 but having some six-coordinate, low-spin character at pH 8.0 as seen by the loss of absorptivity in the two charge transfer bands near 500 nm and 640 nm. The spectra of CcP(H52Q) and CcP(H52N), Figure 1B and S2 are essentially independent of pH between 4 and 8 and both have the spectral features characteristic of a predominantly five-coordinate high-spin heme.
3.2. Enzyme-Catalyzed Steady-State Oxidation of Ferrocytochrome c by H2O2
All four mutants retain catalytic activity, although significantly less than that of wild-type CcP. Figure S3 (supplementary material) shows the CcP(H52Q)-catalyzed oxidation of recombinant yeast iso-1-ferrocytochrome c(C102T) by H2O2. Figures S4 to S7 show the dependence of the initial steady-state velocity on the enzyme concentration for all four mutants. Turnover numbers (v0/e0) of the four mutants are collected in Table 1 and show activities that range between 0.4% and 0.013% that of wild-type CcP under the conditions of the experiment.
Table 1.
Steady-State Activity and Kinetic Parameters for the H2O2 Reaction for CcP, HRP, and Selected Distal Histidine Mutantsa
| Enzyme | v0/e0b (s−1) | Relative Activity | k1app (Steady-State) c (M−1 s−1) | k1app (Stopped-Flow) d (M−1 s−1) | Intercept e (s−1) | Δε424 (Max) f (mM−1 cm−1) |
|---|---|---|---|---|---|---|
| wt-CcP g | (6.20 ± 0.2) × 102 | 1.0 | (4.8 ± 0.2) ×107 | 49 ± 2 | ||
| CcP(H52D) a | (4.9 ± 0.1) ×10−1 | 7.9 ×10−4 | (3.2 ± 0.3) ×103 | (3.7 ± 0.3) ×103 | 0.14 ± 0.02 | 11 ± 1 |
| CcP(H52E) a | (2.4 ± 0.3) ×100 | 4.0 ×10−3 | (1.4 ± 0.1) ×104 | (1.7 ± 0.1) ×104 | 0.09 ± 0.02 | 37 ± 2 |
| CcP(H52N) a | (1.1 ± 0.1) ×10−1 | 1.8 ×10−4 | (2.1 ± 0.2) ×102 | (2.8 ± 0.2) ×102 | 0.4 ± 0.1 | 3 ± 4 |
| fast phase | (6.4 ± 0.9) ×103 | -2.2 ± 3.0 | 3 ± 4 | |||
| CcP(H52Q) a | (7.8 ± 1.3) ×10−2 | 1.3 ×10−4 | (2.3 ± 0.3) ×102 | (1.8 ± 0.1) ×10 2 | 0.13 ± 0.01 | 13 ± 1 |
| CcP(H52L) h | (7.3 ± 0.4) ×102 | |||||
| wt-HRP i | 1.4 ×107 | |||||
| HRP(H42E) i | 4.9 ×103 | |||||
| HRP(H42L) j | 1.4 ×102 | |||||
| HRP(H42Q) i | 9.6 ×101 | |||||
| HRP(H42A) k | (1.9 ± 1.2) ×101 | |||||
| HRP(H42V) k | (1.0 ± 2.0) ×101 |
This work - pH 6.0, 0.10 M ionic strength potassium phosphate buffer,
slope of plots of v0 vs e0,
slope of plots of v0/e0 vs [H2O2],
slope of plots of k1obs vs [H2O2] from stopped-flow experiments,
intercept of plots of k1obs vs [H2O2] from stopped-flow experiments,
maximum observed ΔA424/e0 value from stopped-flow experiments of enzyme/[H2O2] reaction,
reference [19],
reference [23],
reference [21],
reference [20].
The steady-state studies can be used to determine the rate of reaction between H2O2 and the mutants to form an intermediate capable of oxidizing cytochrome c. Under experimental conditions where the H2O2/mutant reaction is the rate-limiting step in the catalytic cycle, the normalized initial velocity is given by eq 1. The factor of 2 in the denominator of eq 1 is
| (1) |
required since H2O2 oxidizes two molecules of ferrocytochrome c during each catalytic cycle. v0/e0 for the CcP(H52E)-catalyzed oxidation of yeast iso-1 ferrocytochrome c(C102T) as a function of the hydrogen peroxide concentration is shown in Figure 2A. Similar plots for the H52D, H52Q, and H52N mutants are shown in Figures S8–S10 of the supplementary material. The slope of a plot of v0/e0 as a function of [H2O2], under limiting H2O2, gives the apparent bimolecular rate constant, k1app, for the reaction between H2O2 and the mutant enzyme. Values of k1app, determined from steady-state kinetics for all four mutants, are collected in Table 1.
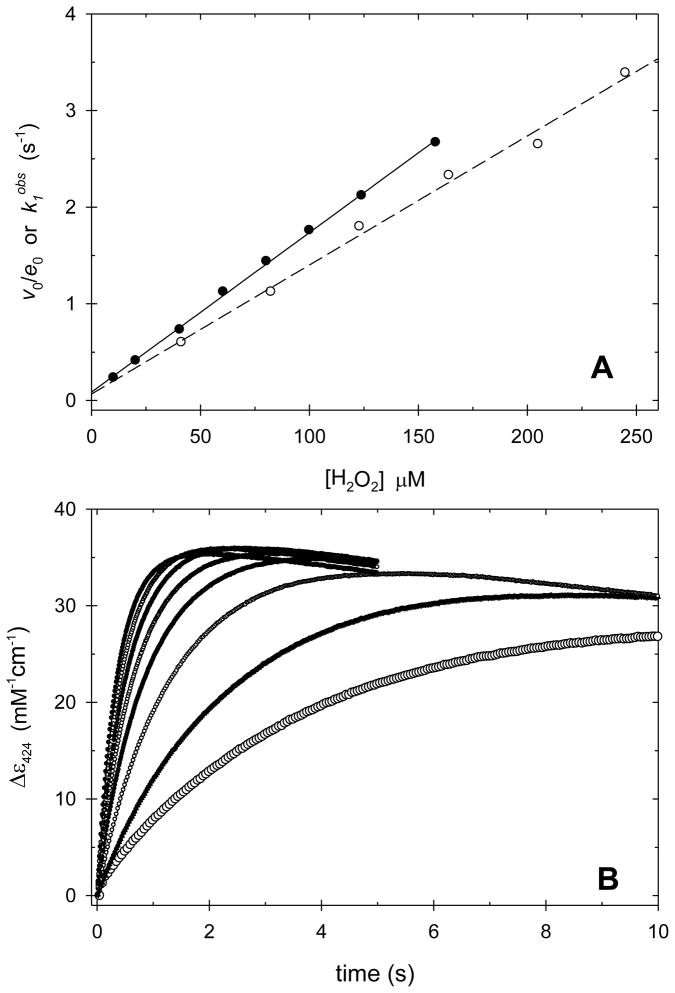
Figure 2.
Panel A. Plots of the normalized initial steady-state velocity, v0/e0 (open circles), and the observed pseudo-first order rate constant for CcP(H52E) Compound I formation, k1obs (solid circles), as a function of the H2O2 concentration. Panel B. Change in absorbance at 424 nm upon mixing H2O2 and CcP(H52E). The traces represent reactions with increasing concentrations of H2O2 from 10.2 μM (lowest trace) to 158 μM H2O2 (highest trace) with intermediate concentrations of 20.3, 40.5, 60.5, 80.3, 100 and 124 μM. The absorbances are normalized by dividing by the concentration of CcP(H52Q) for each trace. The increase in absorbance corresponds to Compound I formation and the subsequent decrease is due to the beginning of Compound I decay. Experimental conditions: [CcP(H52E)] = 0.99 μM, pH 6.0, 0.100 M ionic strength potassium phosphate buffer, 25 °C.
3.3. Rate of Compound I Formation for the CcP Mutant
All four mutants react with hydrogen peroxide to form an intermediate with increased absorbance at 424 nm (A424). Figure 2B shows the rate of change of A424 upon mixing CcP(H52E) and excess hydrogen peroxide in the stopped-flow instrument. Similar data are shown for CcP(H52D), CcP(H52Q), and CcP(H52N) in Figures S11–S13 of the supplementary material. In all cases the initial increase in A424 is followed by a decrease suggesting rapid decay of Compound I for these mutants.
The time dependence of A424, Figure 2B, was fit to a one-exponential function for Compound I formation with a slope to correct for Compound I decay. Best-fit values for the observed rate constant, k1obs, are shown as a function of [H2O2] in Figure 2A for CcP(H52E). For this mutant, the plot of k1obs as a function of hydrogen peroxide is linear with a slope of (1.7 ± 0.1) × 104 M−1 s−1 and an intercept of 0.09 ± 0.02 s−1, Table 2. The slope gives the apparent bimolecular rate constants for Compound I formation, k1app. Plots of k1obs versus [H2O2] for CcP(H52D), CcP(H52Q), and CcP(H52N) are shown in Figures S8–S10. Slope (k1app) and intercept values for these three mutants are included in Table 1. The formation of CcP(H52N) Compound I is biphasic and data for both the fast and slow phases of the reaction are included in Table 1. There is good agreement between the steady-state and transient-state determination of k1app for the H52E, H52D, and H52Q mutants, while the steady-state determination of k1app for CcP(H52N) correlates with the slow phase of the transient-state kinetic studies.
Table 2.
Equilibrium and Rate Constants for Cyanide Binding to CcP(H52D), CcP(H52E), CcP(H52N), and CcP(H52Q)a
| Mutant | ka (M−1 s−1) | kd (s−1) | kd/ka = KDkin (M) | KD (M) |
|---|---|---|---|---|
| yCcPb | (1.1 ± 0.1) ×105 | 0.39 ± 0.05 | (3.6 ± 0.6) ×10−6 | (6 ± 3) ×10−6 |
| CcP(H52D) c | (1.9 ± 0.5) ×103 | 3.7 ± 0.2 | (1.9 ± 0.5) ×10−3 | (3.0 ± 0.7) ×10−3 |
| CcP(H52E) | (4.0 ± 0.1) ×103 | 0.36 ± 0.02 | (0.90 ± 0.06) ×10−4 | (1.0 ± 0.1) ×10−4 |
| CcP(H52N) | (9.4 ± 1.2) ×10−3 | |||
| fast | (3.4 ± 0.1) ×103 | 3.3 ± 0.1 | (0.97 ± 0.04) ×10−3 | |
| slow | (9 ± 2) ×102 | 0.3 ± 0.2 | (3 ± 2) ×10−3 | |
| CcP(H52Q) c | (8.4 ± 2.5) ×101 | (4.9 ± 0.5) ×10−2 | (5.8 ± 1.9) ×10−3 | (6.1 ± 0.8) ×10−3 |
pH 6.0, 0.10 M ionic strength with potassium phosphate buffer.
from reference [53].
The rate constants for the H52D and H52Q mutants are from the major phase of the cyanide binding reaction.
The change in absorbance at 424 nm, ΔA424, during the reaction with H2O2 is much smaller for CcP(H52D), CcP(H52Q), and CcP(H52N) compared to CcP(H52E), Figures 2B, S11–S17. The maximum observed values of ΔA424, normalized by dividing by the initial enzyme concentration, vary between ~37 mM−1 for CcP(H52E) to ~3 mM−1 for each phase of the CcP(H52N) reaction, Table 1, compared to a value of 49 mM−1 for yCcP. There are several possibilities to explain the low yield of Compound I. The lower amounts of Compound I observed during the reaction between hydrogen peroxide and the four mutants compared to yCcP could be due to a competition between the rate of formation and decay of Compound I for the mutants such that the maximum concentration of Compound I observed is determined by the relative rates. Another possibility is that the spectra of the H2O2-oxidized mutants differ in some respect from the spectrum of yCcP Compound I and the absorbance change at 424 nm is smaller than expected.
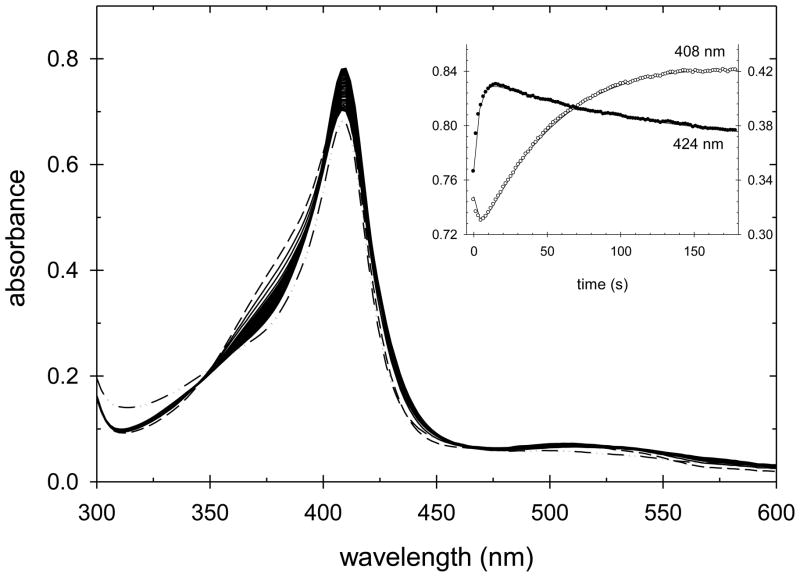
In order to determine if the intermediate formed during the initial phase of the reaction of each of these mutants with H2O2 has a spectrum similar to that of yCcP Compound I, a second series of experiments was performed using manual mixing of the two reactants and acquiring transient spectra with a diode-array spectrophotometer. Figure 3 shows the spectra obtained during the first 60 seconds of a reaction between CcP(H52E) and a 10-fold excess H2O2. The inset in Figure 3 shows the absorbance changes at both 424 and 408 nm. The absorbance at 424 nm increases rapidly, reaching a maximum value at ~3 s after which A424 decreases. The absorbance at 408 nm shows the opposite behavior, decreasing rapidly, reaching a minimum at ~3 s followed by an increase to a plateau value after ~60 s. At longer times, the absorbance at both 408 and 424 nm decrease (see Figures S18 and S19 in the supplementary material).
Figure 3.
CcP(H52E) Compound I formation and decay. A 10-fold excess of H2O2 was added to CcP(H52E) and spectra acquired every 2 s for 1 minute. The spectrum of native CcP(H52E) is shown by the dashed line. CcP(H52E) Compound I is formed within 3 s of mixing and is rapidly converted to an Fe(III) species with a Soret maximum at 408 nm. The time dependence of the absorbance changes at 408 and 424 nm are shown in the inset. Singular value decomposition (SVD) and non-linear least squares regression was used to fit the data to a biphasic kinetic process, with the observed rates of Compound I formation and formation of the 408 nm species equal to 1.3 ± 0.2 s−1 and 0.06 ± 0.04 s−1, respectively. Experimental conditions: [CcP(H52E)] = 7.65 μM, [H2O2] = 76.1 μM, pH 6.0, 0.100 M ionic strength potassium phosphate buffer, 25 °C.
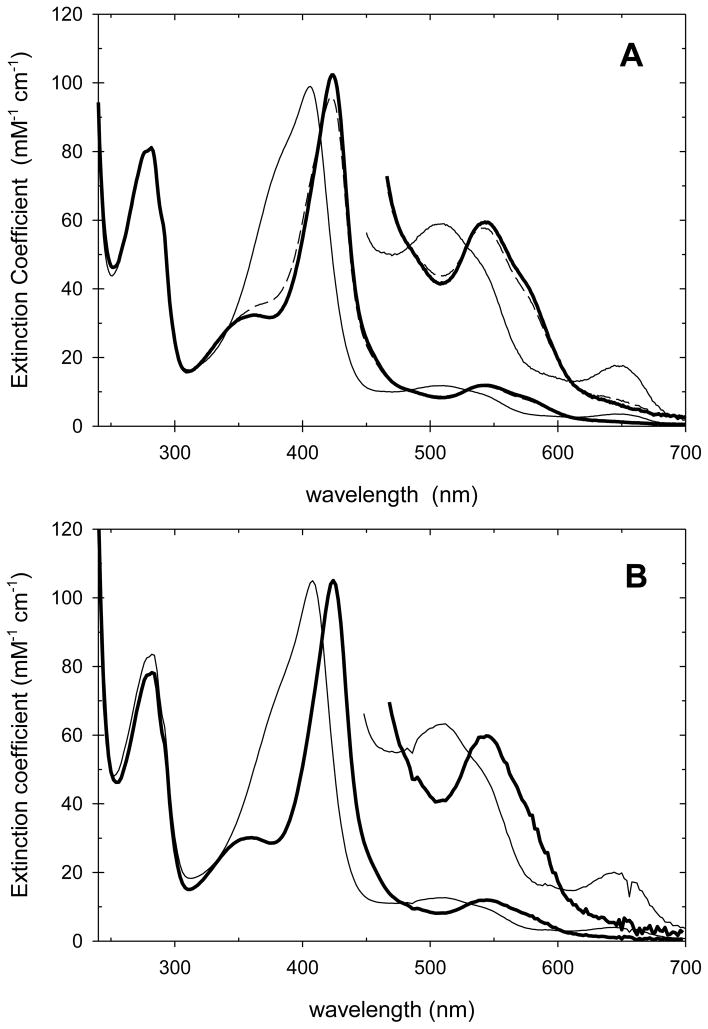
The maximum amount of Compound I formed is observed when A424 reaches its maximum value during the reaction. The details of the spectral changes when A424 reaches its maximum value can be seen in the difference spectrum between the reaction mixture at maximum A424 and native CcP(H52E), Figure 4. The difference spectrum for CcP(H52E) is very similar to that of the difference spectrum for authentic yCcP Compound I formation, also shown in Figure 4, and we conclude that CcP(H52E) is oxidized to Compound I by H2O2 with a spectrum that is similar to that of authentic CcP Compound I. Difference spectra for the other three mutants, obtained in a similar manner are also included in Figure 4. Although the absolute amplitudes of the difference spectra are much smaller for the H52D, H52Q, and H52N mutants than for the H52E mutant, the shapes of the difference spectra are similar. All four mutants show a decrease in absorbance near 380 nm and increases in absorbance at 424, 530, and 560 nm, characteristic of the spectral changes associated with formation of yCcP Compound I [32]. We conclude that each of the four mutants form an oxidized intermediate with spectral characteristics similar to those of yCcP Compound I.
Figure 4.
Difference spectra between the enzyme/H2O2 reaction mixture at the time with a maximum value of A424 and native enzyme for the four CcP mutants compared to the difference spectrum between yCcP Compound I and yCcP. Labels identify each of the difference spectra. The difference spectrum between yCcP Compound I and yCcP is shown by the small open circles. Experimental conditions: [CcP(H52E)] = 7.65 μM, [H2O2] = 76.1 μM, spectrum 3 s after mixing; [CcP(H52Q)] = 9.47 μM, [H2O2] = 498 μM, spectrum 30 s after mixing; [CcP(H52D)] = 6.04 μM, [H2O2] = 498 μM, spectrum 3.4 s after mixing; [CcP(H52N)] = 6.82 μM, [H2O2] = 4.77 mM, spectrum 18 s after mixing; pH 6.0, 0.100 M ionic strength potassium phosphate buffer, 25 °C.
An estimate of the maximum amount of Compound I formed in each of the mutants by H2O2 was made by comparing the maximum absorbance change at 424 nm in the difference spectra for each of the mutants and comparing it to that of yCcP. Estimated maximum concentrations of Compound I are approximately 92%, 36%, 21%, and 8% for H52E, H52D, H52Q, and H52N, respectively, Figure 4.
3.4. Endogenous Decay of CcP(H52E) Compound I
The non-stoichiometric formation of Compound I for the four mutants could be due to the slow formation of Compound I followed by rapid decay such that complete conversion of the mutants to Compound I is never observed under our experimental conditions. In order to determine if rapid decay of Compound I can account for the low levels of Compound I observed during the reaction between the mutants and H2O2, we have explored the endogenous decay of Compound I for all four CcP mutants. We initially assume that the mechanism for formation and decay of Compound I for the mutants is similar to that for yCcP Compound I. The mechanism includes irreversible oxidation of CcP to Compound I, a species that is oxidized two equivalents above the native Fe(III) state of the enzyme and contains an oxyferryl-Fe(IV) heme and a Trp-191 radical. Formation of Compound I is followed by two sequential decay processes ultimately reducing both the oxyferryl-Fe(IV) and Trp-191 radical sites at the expense of other residues within the protein, Mechanism 1 [33]. In yCcP, the first decay appears to be
| Mechanism 1 |
migration of the radical from Trp-191 to other locations in the protein while the second decay reaction is reduction of the oxyferryl-Fe(IV) to Fe(III). Reduction of Fe(IV) to Fe(III) causes the largest change in absorbance in the Soret region and can be used to distinguish the two decay processes. The decay products in Mechanism 1 are generically designated DP1 and DP2. If high concentrations of H2O2 are used to oxidize the enzyme, generally greater than a 10-fold excess of H2O2, additional reactions may be observed as the porphyrin ring begins to be oxidized. At [H2O2]/[CcP] > 10, we have detected up to four decay reactions and designate the additional decay products as DP3 and DP4 and the additional rate constants as kd3 and kd4. At very high ratios of [H2O2]/[CcP] (>100), the heme group in yCcP is completely oxidized at the end of the reaction, with no detectable absorbance in the visible region of the spectrum.
In the reaction between H2O2 and CcP(H52E) shown in Figure 3, the Soret band shifts from 406 nm to longer wavelengths during the early part of the reaction, characteristic of Compound I formation, followed by a return to 408 nm as Compound I decays. CcP(H52E) Compound I is much less stable than yCcP Compound I with detectable decay beginning at ~3 s after mixing CcP(H52E) and H2O2 in the reaction shown in Figure 3. The reaction shown in Figure 3 was monitored for an additional 4 hours and three kinetic processes were detected during the decay of Compound I (See Figures S18 and S19 in the supplementary material). The values of kd1, kd2, and kd3 are (2.7 ± 0.1) × 10−2 s−1, (5.0 ± 0.2) × 10−3 s−1 and (5.3 ± 0.5) × 10−4 s−1, respectively, and are collected in Table S2 of the supplementary material.
The spectral changes shown in Figures 3, S18, and S19 were analyzed according to Mechanism 1 (with the inclusion of a third irreversible decay step to DP3) using a combination of singular value decomposition (SVD) and non-linear least squares regression. Analysis shows that maximum formation of Compound I occurs 2.7 s after mixing and that 89% of CcP(H52E) is converted to Compound I, consistent with the estimate of 92% made from the maximum absorbance change at 426 nm, Figure 4. Spectra of Compound I, DP1, DP2, and DP3 were calculated from the analysis and these are shown in Figures S20 and S21 of the Supporting Information. Selected spectral parameters for CcP(H52E) Compound I are included in Table S1. The spectrum of CcP(H52E) Compound I is similar to that of yCcP Compound I, with Soret maximum at 420 nm and α and β bands at 568 and 532 nm, respectively.
The relative rates of Compound I formation and decay are sufficient to account for about 90% of the Compound I formed in the reaction between CcP(H52E) and H2O2. However, there are some differences in the decay reactions for CcP(H52E) and yCcP. yCcP Compound I decays by initial reduction of the Trp-191 radical site followed by reduction of the oxyferryl-Fe(IV) group and returns to an Fe(III) species with a spectrum similar to that of native CcP [34]. In CcP(H52E), the Fe(IV) site in Compound I is reduced prior to the radical site and the spectrum of the Fe(III) decay product is quite different from that of native CcP(H52E). The spectrum of wild-type CcP(H52E) is that of a five-coordinate, high-spin heme group with Soret maximum at 406 nm, The Fe(III) decay product has a spectrum that appears to be that of a six-coordinate, high-spin heme with a Soret maximum at 408 nm and significantly larger extinction coefficient than the wild-type form of CcP(H52E). The decay product may have a water molecule or a hydroxide ion coordinated to the heme iron. We will refer to the Fe(III) decay product as the “408 nm” species due to its altered spectrum compared to wild-type CcP(H52E).
3.5. Endogenous Decay of CcP(H52D) Compound I
The normalized A424 values for CcP(H52D) Compound I formation, Figure S11, are about three times smaller that those for CcP(H52E), Figure 3, and estimates of Compound I formation suggest that a maximum of 36% of the enzyme is observed as Compound I during the reaction. The initial stages of the reaction between CcP(H52D) and a ~12-fold excess of H2O2 are shown in Figure 5. Formation of Compound I is demonstrated by the initial increase in A424 and decrease in A408. However, the amount of Compound I formed is insufficient to shift the Soret maximum, which remains at 408 nm throughout the reaction. Compound I formation is followed by rapid reduction of the Fe(IV) site to an Fe(III) form of the enzyme with Soret maximum at 408 nm, similar to that observed during the decay of CcP(H52E) Compound I, Figure 3. In the case of the H52D mutant, both formation and decay of Compound I are slower than for the H52E mutant, with maximum Compound I formation occurring ~15 s after mixing and maximum “408 nm” species formation occurring at ~170 s after mixing.
Figure 5.
Transient absorption spectra during the reaction of 6.02 μM CcP(H52D) and 74.5 μM H2O2. The spectrum of the CcP(H52D) is shown by the dashed line. The solid lines show spectra acquired over the first 180 seconds of the reaction and the dashed-dotted line shows the spectrum after 5 hours, essentially that of the final decay product. The inset shows the absorbance changes at 408 nm (open circles and left-hand axis of inset) and at 424 nm (solid circles and right-hand axis of inset). Experimental conditions: pH 6.0, 0.100 M ionic strength potassium phosphate buffer, 25 °C.
The reaction shown in Figure 5 was monitored for an additional 5 hours and two additional kinetic processes were detected, both associated with decreases in absorbance throughout the Soret band, Figure S22. The spectral changes collected over 5 hours, Figures 5 and S22, were analyzed according to Mechanism 1 with the inclusion of a third irreversible decay step to DP3. A combination of singular value decomposition (SVD) and non-linear least squares regression gave global best-fit values for k1obs, kd1, kd2, and kd3 are (2.8 ± 0.3) × 10−1 s−1, (1.4 ± 0.2) × 10−2 s−1, (1.7 ± 0.1) × 10−3 s−1 and (9.5 ± 0.3) × 10−5 s−1, respectively, and are collected in Table S3 of the supplementary material. Figure S23 shows the calculated spectra of CcP(H52D) Compound I, DP1, DP2, and DP3.
The calculated spectrum of CcP(H52D) Compound I, Figure S23, very different than that of yCcP Compound I [32] or of CcP(H52E) Compound I, Figure S20. More detailed analysis (see supplementary material) suggests that the calculated spectrum of CcP(H52D) Compound I resembles a linear combination of 24% of the spectrum of yCcP Compound I and 76% of the spectrum of native CcP(H52D). Using higher H2O2 concentrations, as much as 36% of CcP(H52D) can be converted to species with a yCcP Compound I-like spectrum, Figure 4. It appears that Mechanism 1 is insufficient to account for the formation and decay of CcP(H52D) Compound I. Alternative interpretations of the data will be discussed below.
3.6. Endogenous Decay of CcP(H52Q) Compound I
CcP(H52Q) has the slowest rate of reaction with H2O2 of the four mutants under study and should be subject to the strongest competition between formation and decay of Compound I for the four mutants. In order to detect significant CcP(H52Q) Compound I formation, high concentrations of H2O2 were used. Figures S24 and S25 in the supplementary material show the spectral changes as a function of time for the reaction between 9.47 μM CcP(H52Q) and 498 μM H2O2. Figure S24 shows spectra acquired at 2 s intervals for the first 60 seconds of the reaction and Figure S25 shows spectra acquired at 1-minute intervals for 2 hours. The insets in both figures show the absorbances at 408 and 424 nm as a function of time. Maximum CcP(H52Q) Compound I occurs at ~30 s after mixing. As with the reaction between CcP(H52D) and H2O2, the Soret maximum remains at 408 nm throughout the reaction between CcP(H52Q) and H2O2 indicating that insufficient Compound I is formed to shift the Soret band to the red as seen for native CcP [32] and CcP(H52E), Figure 3. Note that, in contrast to the reactions of H2O2 with both CcP(H52E) and CcP(H52D), the absorbance at 408 nm decreases continually throughout the reaction indicating that a “408 nm” species is not formed during the endogenous decay of CcP(H52Q) Compound I.
The data in Figures S24 and S25 were analyzed by SVD and non-linear least squares regression. Three decay rate processes were observed along with formation of Compound I. The global best-fit values for k1obs, kd1, kd2, and kd3 are (1.6 ± 0.1) × 10−1 s−1, (4.8 ± 0.2) × 10−3 s−1, (1.1 ± 0.1) × 10−3 s−1 and (2.8 ± 0.1) × 10−4 s−1, respectively, and are collected in Table S4 of the supplementary material. Figure S26 shows the calculated spectra of CcP(H52Q) Compound I, DP1, DP2, and DP3, along with the spectrum of CcP(H52Q), when the data are analyzed according to Mechanism 1.
The calculated spectrum of CcP(H52Q) Compound I, Figure S26, looks more like that of CcP(H52D) Compound I, Figure S23, than that of yCcP Compound I [32] or CcP(H52E) Compound I, Figure S20. The calculated spectrum of CcP(H52Q) Compound I is a linear combination of about 19% of the yCcP Compound I and 81% of the native CcP(H52Q), suggesting that Mechanism 1 is insufficient to account for the formation and decay of CcP(H52Q) Compound I.
3.7. Endogenous Decay of CcP(H52N) Compound I
The reaction between CcP(H52N) and H2O2 may be the most complex of all four mutants under study. The increase in A424 is biphasic, Figure S13, and the amplitude of the reaction is the smallest of the four mutants, Figure 4. The initial estimate is that only ~8% of the H52N mutant is converted to Compound I. The same questions arise for the very low yields of CcP(H52N) Compound I as have for the H52D and H52Q mutants. We have investigated CcP(H52N) Compound I decay to determine if the relative rate of Compound I formation and decay can account for the very low levels of Compound I observed for this mutant.
Figure S27 (supplementary material) shows the spectral changes occurring during the first 10 minutes of the reaction between 7.10 μM CcP(H52N) and 72.7 μM H2O2. The reaction was monitored for a total of 6 hours and analyzed according to Mechanism 1. Mechanism 1 predicts that 77% of CcP(H52D) should be converted to Compound I within 14 s after mixing the two reactants while the absorbance change at A424 indicates a maximum ~4% conversion, Figure S27. Again, Mechanism 1 is insufficient to account for the data.
3.8. Cyanide Binding to the CcP Mutants – Spectroscopic Properties and Equilibrium Constants
In order to further characterize the reactivity of the four mutants under study, we have investigated the equilibrium binding and kinetics of the reaction with hydrocyanic acid, HCN, at pH 6.0. Addition of buffered cyanide solutions to the CcP mutants causes large changes in the absorption spectrum of the enzyme as cyanide binds. Spectral changes associated with cyanide binding to CcP(H52E) and CcP(H52Q) at pH 6.0 are shown in Figure 6. Spectra of the cyano forms of CcP(H52D) and CcP(H52N) at pH 6.0 are shown in Figures S28 and S29 of the supplementary material. Spectral parameters for the cyanide complexes of all four CcP mutants are collected in Table S1. The spectra of the cyanide complexes of the four mutants are similar suggesting that each of the mutants react stoichiometrically with cyanide.
Figure 6.
Panel A. Spectrum of the CcP(H52E)/cyanide complex. Three spectra are shown, the native enzyme (thin solid line), the spectrum at 0.743 mM cyanide (dashed line), and the calculated spectrum for 100% complex formation (thick solid line). Panel B. Calculated spectrum of the CcP(H52Q)/cyanide complex (thick solid line. The spectrum of unligated CcP(H52Q) is shown by the thin solid line. Experimental conditions: [CcP(H52E)] = 4.8 to 5.5 μM, [CcP(H52Q)] = 4.5 to 6.7 μM, [KCN] = 0.0 to 95 mM, pH 6.0, 0.100 M ionic strength potassium phosphate buffer, 25 °C.
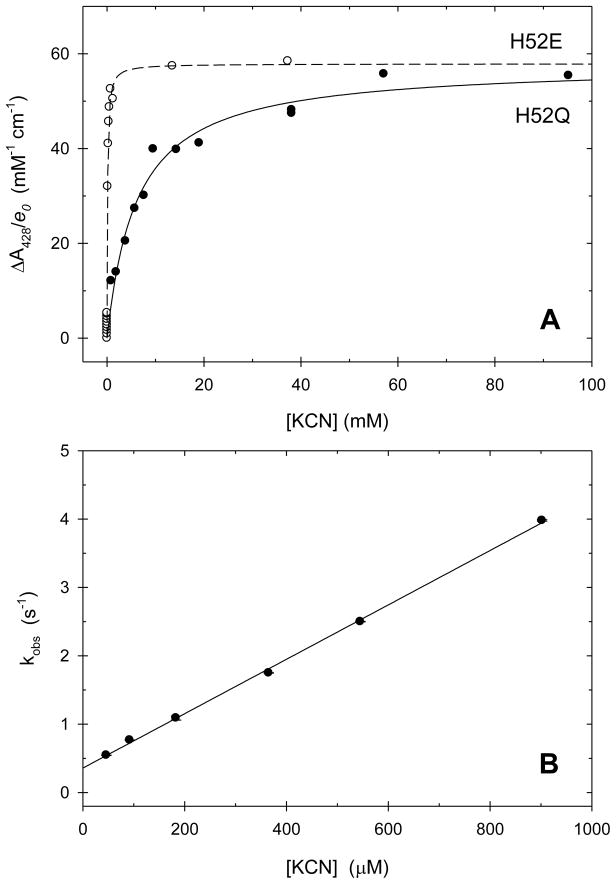
The large spectral changes associated with cyanide binding can be used to monitor the binding equilibria and to determine the equilibrium dissociation constants for the cyano complexes. Figure 7A shows spectral titrations of both CcP(H52E) and CcP(H52Q) with HCN. Figures S30 and S31 show similar titrations for CcP(H52D) and CcP(H52N). At pH 6.0, cyanide binding to all four mutants fit a simple hyperbolic binding equation with the absorbance change given by eq 2. In eq 2, ΔAobs is the observed change in absorbance upon cyanide addition, ΔAmax is the
Figure 7.
Panel A. Titration of CcP(H52E) and CcP(H52Q) with KCN at pH 6.0. The change in absorbance at 428 nm is normalized by dividing by the enzyme concentration. Both data sets were fit to a simple hyperbolic equation with using non-linear least squares regression. CcP(H52E): The data are shown with the open circles and the best-fit line is dashed. Best-fit values for ΔA428/e0 and KD are 58 ± 1 mM−1 cm−1 and 0.10 ± 0.01 mM, respectively. CcP(H52Q): The data are shown with the solid circles and the best-fit line is solid. Best-fit values for ΔA428/e0 and KD are 58 ± 2 mM−1 cm−1 and 6.1 ± 0.8 mM, respectively. Panel B. Dependence of the observed rate constant for cyanide binding to CcP(H52E). The slope and intercept of the plot give the apparent cyanide association rate constant, ka and dissociation rate constant, kd, respectively, which are collected in Table 3. Experimental conditions: [CcP(H52E)] = 1.1 to 5.5 μM, [CcP(H52Q)] = 4.5 to 6.7 μM, [KCN] = 0.0 to 95 mM, pH 6.0, 0.100 M ionic strength potassium phosphate buffer, 25 °C.
| (2) |
maximum absorbance change for 100% formation of the cyanide complex, KD is the equilibrium dissociation constant, and [L] is the total cyanide concentration. Values of KD are collected in Table 2. Binding of cyanide to the four mutants range from 17 to 2,300 times weaker than to wild-type CcP with CcP(H52E) being most similar to wild-type enzyme.
3.9. Kinetics of Cyanide Binding to the CcP Mutants
The binding of cyanide to the four CcP mutants was monitored by observing changes in the absorbance at 428 nm upon rapid mixing of the reactants in a stopped-flow apparatus. The reaction between excess cyanide and CcP(H52E) was monophasic. The time dependence of A428 was fit to a single-exponential equation to obtain the observed pseudo-first order rate constant, kobs, for the reaction. A plot of kobs as a function of the cyanide concentration is linear, Figure 7B. The slope of the plot in Figure 7B gives the apparent bimolecular rate constant for cyanide binding, ka, and the intercept gives the apparent dissociation rate constant, kd. Best-fit values are collected in Table 2. There is good agreement between the kinetically-determined equilibrium dissociation constant, KDkin = kd/ka, and the equilibrium dissociation constant determined from the spectrophotometric titration, KD, Figure 7A.
The reactions between cyanide and the other three mutants, CcP(H52D, CcP(H52Q), and CcP(H52N), are biphasic. The two phases are characterized by two first-order rate constants designated kslow and kfast. Each of the three mutants with biphasic cyanide binding has unique characteristics. The major phase of the reaction between CcP(H52D) and cyanide is the slow phase and the slow phase accounts for 80% of the reaction amplitude, on average. The rate constant for the slow phase of the reaction, kslow, is linearly dependent on the cyanide concentration, Figure S32, while kfast is a hyperbolic function of the cyanide concentration, inset in Figure S32. KDkin, from the slow phase of the reaction, is about 40% smaller than KD, Table 2.
The major phase of the reaction between cyanide and the H52Q mutant is also the slow phase of the binding reaction, accounting for about 83% of the reaction amplitude. The rate constant for the slow phase, kslow, is linearly dependent on the cyanide concentration, Figure S33, with the slope equal to 8.4 ± 2.5 M−1 s−1 and the intercept equal to 0.049 ± 0.007 s−1, giving a value of (5.8 ± 1.9) × 10−3 M for KDkin, which agrees quite well with the equilibrium titration value for KD of (6.1 ± 0.8) × 10−3 M, Table 2. The rate constant for the fast phase of the reaction, kfast, is independent of the cyanide concentration with a value of 0.38 ± 0.21 s−1.
The amplitudes of the fast and slow phases of the reaction between cyanide and the H52N mutant are 71% and 29%, respectively and both kfast and kslow are linearly dependent on the cyanide concentration, Figure S34. The calculated KDkin values of (0.96 ± 0.04) × 10−3 M and (0.37 ± 0.19) × 10−3 M for the fast and slow phases, respectively, are considerably smaller than the value of (9.4 ± 1.2) × 10−3 M for KD determined from the equilibrium titration experiments
4. Discussion
4.1. Spectroscopic Properties of the CcP His-52 Mutants
The spectra of CcP(H52Q) and CcP(H52N) are essentially independent of pH between 4 and 8 and have spectra characteristic of five-coordinate, high-spin (5c,hs) heme groups over the entire pH region, Figures 1B and S2. The crystal structure of CcP(H52Q) confirms the presence of a five-coordinate heme in this mutant [22]. The spectra of CcP(H52E) and CcP(H52D) are 5c, hs at pH 4 and 6 but show the presence of a small amount of six-coordinated, low-spin heme (6c, ls) at pH 8.0, Figures 1A and S1. It is assumed that hydroxide begins to bind to the heme iron near pH 8 in yCcP and most of the CcP mutants studies to date [35].
4.2. Catalytic Activity
All four mutants retain the ability of catalyze the oxidation of ferrocytochrome c by H2O2 although the activity levels are significantly lower than that of wild-type CcP. The reduction in catalytic activity is related to the slower rates of reaction between the mutants and H2O2 to form Compound I as is to be expected from replacing the distal histidine with less basic residues.
4.3. Compound I Formation
Yeast CcP and the native forms of the recombinant enzymes, CcP(MI) and rCcP react stoichiometrically and rapidly with H2O2 with k1app = (4.8 ± 0.3) × 107 M−1 s−1 [36]. His-52 is a critical residue for facilitating the reaction with H2O2 as demonstrated by an H52L mutation in CcP(MI), which reduces the H2O2 reaction rate by about five orders of magnitude, with reported values of 236 M−1 s−1 in a nitrate-containing buffer and 731 M−1 s−1 in potassium phosphate buffer at pH 6 [18, 19], the slowest reaction observed between any CcP mutant and H2O2 prior to this study. Interestingly, the reaction between CcP(H52Q) and H2O2 is slower than the reaction between CcP(H52L) and H2O2 with a rate constant of 180 M−1 s−1 in 0.10 M potassium phosphate buffer, pH 6, Table 1. The HRP analog, HRP(H42Q) has an even slower rate in its reaction with H2O2, a value of 96 M−1 s−1 at pH 7 [23].
There is a general correlation between the base strength of the residue at position 52 and the rate of formation of Compound I. The solution pKAs for histidine and the side-chain carboxylates are about 6 and 4, respectively, [37] and it is estimated that the pKA for a protonated amide side chain has a upper limit near 0 [38], giving a range of at least 6 log units in base strength between these mutants and wild-type CcP. The rates of Compound I formation for the reactive forms of the four mutants and wild-type CcP span a range of 5.4 orders of magnitude, Table 1. The data in this report suggest that, although the base strength of residue 52 is an important factor in determining the rate of Compound I formation, other factors such as the position and orientation of residue 52 relative to the heme iron, and perhaps the redox potential of the mutant/Compound I couple, can also affect the rate of reaction. For example, the reaction between CcP(H52N) and H2O2 is biphasic suggesting two conformational states of the enzyme with differing reactivity. If base strength were the only consideration in accelerating the rate of Compound I formation, the rate of the CcP(H52N) reaction should be similar to that of CcP(H52Q). The slow phase of the CcP(H52N)/H2O2 reaction is similar to that of CcP(H52Q) but the H52N mutant also has a faster reaction, with rate constant intermediate in value between that of the H52D and H52E mutants. In addition, ~90% of CcP(H52N) does not appear to react with H2O2 to form Compound I.
It is generally accepted that the primary role of the distal histidine in the peroxidases is to serve as a base catalyst, facilitating deprotonation of the H2O2 within the distal heme pocket and promoting rapid binding of the peroxide anion to the heme iron. This is the same role postulated for the distal histidine in binding of cyanide to the peroxidases. At neutral pH, HCN diffuses into the distal heme pocket followed by deprotonation of HCN within the interior of the protein, and subsequent binding of the cyanide anion binding to the heme iron. The entry of HCN into the distal heme pocket is not rate limiting since the observed rates are significantly slower than a diffusion-limited process, even diffusion within the interior of a protein matrix [39]. Using alternative bases at position 52 should alter the efficiency of deprotonation and modulate cyanide binding to the heme iron [40]. If the distal histidine serves as a base catalyst for both Compound I formation and HCN binding, then the rates of these two reactions should change in a similar manner for all of the CcP mutants that alter the basicity of the residue at position 52 in CcP.
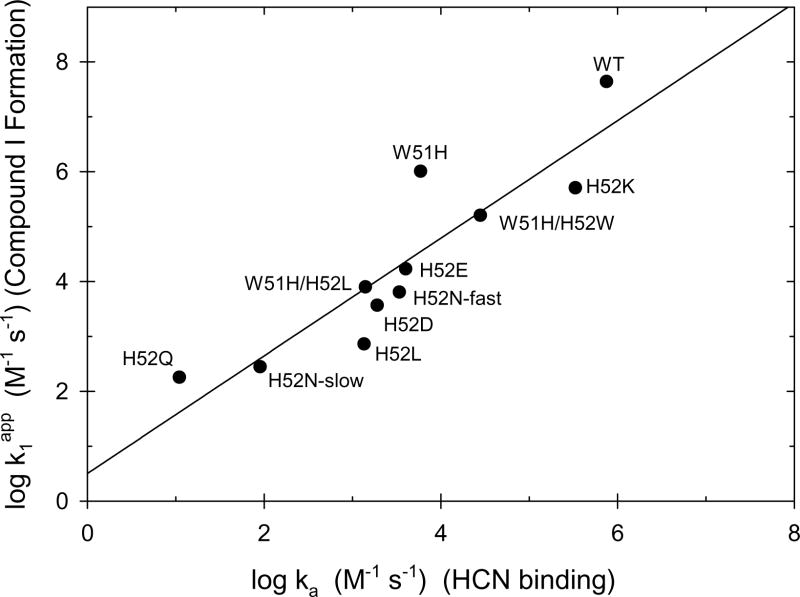
Figure 8 shows a correlation plot of the rate of Compound I formation and the rate of HCN binding for wild-type CcP and nine distal pocket mutants, including the fast and slow phases of the two reactions for CcP(H52N). For CcP(H52D) and CcP(H52Q), the rate constant for the major cyanide binding phase was used in the correlation. There is a reasonably good correlation, with the rate of the H2O2 reaction decreasing in concert with the decrease in the rate of HCN binding for the CcP mutants. The slope of the log/log plot is 1.07 ± 0.17 and the intercept is 0.50 ± 0.66. Under the conditions used in this study, we believe that the correlation shown in Figure 9 provides strong support for two hypotheses: (1) that H2O2 binding to the heme iron is the rate-limiting step for Compound I formation and (2) that the primary role of His-52 in CcP is to catalyze deprotonation of H2O2 and facilitate peroxy anion binding to the heme iron [41].
Figure 8.
Correlation between the rates of reaction of CcP and CcP mutants with H2O2 and with HCN. The logarithm of the rate constant for the reaction between H2O2 and CcP variants, k1app, is plotted as a function of the logarithm of the association rate constant for HCN binding to the same CcP variants, ka. Data from this study as well as from references [19, 54–57]
4.4. Endogenous Decay of Compound I
While not a central focus of this study, the very different modes of Compound I decay for these four mutants in comparison to wild-type CcP are quite remarkable and a good demonstration of the role of the distal pocket residues in stabilizing Compound I as well as in directing its endogenous reduction back to the Fe(III) state.
CcP Compound I is not thermodynamically stable and will decay back to the Fe(III) state of the heme in the absence of added reducing agent. This is attributed to the presence of endogenous reductants in the protein that can reduce both the Fe(IV) and Trp-191 radical in Compound I, ultimately forming stable 2-electron oxidation products [34, 42–44]. The endogenous decay of yCcP Compound I is a biphasic kinetic process when monitored at 424 nm and using H2O2 to CcP ratios of ≤10. The Fe(IV) site is reduced at a rate of (2.9 ± 0.6) × 10−5 s−1 (half-life of 6.6 hr) at pH 6.0 when Compound I is formed with a stoichiometric addition of H2O2 [33]. The rate of Fe(IV) reduction increases when excess H2O2 is used to form Compound I, increasing to (1.6 ± 0.4) × 10−4 s−1 (half-life of 1.2 hr) at an H2O2/CcP ratio of 10, Table S2. A faster decay process, (1.3 ± 0.3) × 10−3 s−1, is attributed to a change in the perturbation of the heme absorbance by the radical site, possibly related to migration of the radical away from its original location at Trp-191 to a site located further from the heme. Yeast CcP can react with up to 10 equivalents of H2O2 before there is significant oxidation of the heme group. However, the heme is completely oxidized in the presence of 100 equivalents of H2O2.
There are several differences observed in the Compound I decay process for the four His-52 mutants compared to the endogenous decay of yCcP Compound I. The first is that the oxyferryl Fe(IV) site is reduced prior to the radical site in the mutants. In addition, the reduction of the oxyferryl group in the H52E, H52D, and H52N mutants produces an Fe(III) state that is quite distinct from the original native state of the mutants the “408 nm” species. We postulate that the oxygen atom of the oxyferryl group remains bound to the heme iron, either as a hydroxide ion or a water molecule, when the Fe(IV) is reduced to Fe(III). It is not clear why a six-coordinate Fe(III) heme would be favored in the Compound I decay product when the five-coordinate heme is produced during isolation of the mutants. It should be noted that the decay of CcP(H52Q) Compound I does not produce the 408 nm species. In this case, we have observed a verdoheme-type intermediate in the decay of CcP(H52Q) Compound I, which is not observed for the other three mutants nor for yCcP, indicating that oxidation of the porphyrin ring in the presence of excess H2O2 can progress along alternative pathways depending upon the residues in the distal heme pocket.
Of the four mutations investigated in this study, the H52E mutation causes the least perturbation in the properties of CcP. This may be a consequence of the similar sizes of the glutamate and histidine side chains and the potential of the oxygen atoms of the glutamate side chains to occupy similar positions as the two nitrogen atoms of the imidazole side chain of His-52 within the distal heme pocket. Although a crystal structure of CcP(H52E) is not available, crystal structures of CcP(H52Q) and of the horseradish peroxidase analog, HRP(H42E), have been determined [22, 45]. Figure 2 of reference [22] shows a comparison of the distal heme pockets in wild-type CcP and CcP(H52Q). In CcP(H52Q) the amide carbonyl oxygen is within 0.75 Å of the position of the ε-nitrogen in the imidazole side chain of His-52 [34]. In HRP(H52E) the two carboxylate oxygens of Glu-42 occupy similar positions as the two nitrogen atoms in the imidazole side chain of His-42. The structure of the distal pocket is not significantly perturbed by the glutamate substitution for His-52. The carboxylate group of Glu-52 is a weaker base than the imidazole group of His-52 and, although CcP(H52E) reacts stoichiometrically with H2O2 to form Compound I, it does so at a rate that is ~2,800 times slower than wild-type enzyme. The glutamate substitution also destabilizes CcP(H52E) Compound I relative to the wild-type enzyme and the oxyferryl-Fe(IV) site reduced ~280 times faster in the mutant. The CcP(H52E) data are almost identical to data reported for HRP(H42E). HRP(H42E) reacts with H2O2 ~2,900 times more slowly than does HRP and HRP(H42E) Compound II is reported to be less stable than wild-type HRP Compound II [44]. Chloroperoxidase has a glutamate residue in a similar location as the His-52 residue in CcP [45] and its reaction with H2O2 is about 10-times slower than that of CcP but faster than that of CcP(H52E) [46]
4.5. Non-Stoichiometric Formation of Compound I for CcP(H52D), CcP(H52Q), and CcP(H52N)
The properties of CcP(H52D), CcP(H52Q), and CcP(H52N) are more difficult to interpret, primarily because these three mutants do not appear to react stoichiometrically with H2O2. Each produce significantly less Compound I than can be accounted for based on Mechanism 1 and taking into account the slower formation and faster decay of Compound I compared to yCcP and CcP(H52E). Two alternative hypotheses can be offered to rationalize the low yields of Compound I in these three mutants but neither of the two interpretations is completely satisfactory. The first, and simplest, hypothesis is that there are multiple conformational states with differential reactivities toward H2O2 in each of these mutants. This is certainly the case for CcP(H52N) where the reaction with H2O2 is biphasic and both observed pseudo-first order rate constants are linear functions of the H2O2 giving apparent second-order rate constants differing by a factor of 23. It is not difficult to anticipate that there may be conformations of the mutants that do not react with H2O2 at all. In addition, the observation that the rate of cyanide binding to these three mutants is biphasic supports the idea of multiple conformational states with different reactivities toward HCN and to H2O2 as well.
In order to account for the maximum observed conversion of each of the mutants to Compound I, taking into account the competition between formation and decay of Compound I, we estimate that 57%, 78% and 90% of the H52D, H52Q, and H52N mutants, respectively, would need to be in H2O2-unreactive conformations at pH 6.0
The second hypothesis to explain the low yields of Compound I is that there is an equilibrium between the initial reactants and Compound I such that only partial conversion enzyme to Compound I occurs under the experimental conditions used in these studies. We discuss the evidence for equilibration between CcP, H2O2 and Compound I in the Supplementary Material.
In order to observe reversible formation of Compound I, the reduction potentials of the CcP Compound I/CcP and H2O2/H2O half-reactions would have to be similar. We estimate that the reduction potential for the CcP Compound I/CcP half-reaction, eq 3, would have to be between
| (3) |
1.27 and 1.31 V in order to account for the low levels of compound I observed for CcP(H52D) and CcP(H52Q).
The estimated reduction potential of ~1.3 V is substantially more positive than previous estimates of the reduction potential for CcP Compound I. Purcell and Erman [47] estimate a value of 1.087 V for the one-electron reduction of Fe(IV) in CcP Compound II at pH 5.26, 25 °C while Mondal et al. [48] determine a potential of 0.740 V for the two-electron reduction of CcP Compound I adsorbed on an edge-oriented pyrolytic graphite electrode at pH 6.1, 4°C. In HRP, the one-electron reduction potentials for the Compound I/Compound II and Compound II/HRP couples are 0.99 and 0.96 V at pH 6.06, with a calculated two-electron reduction potential for the Compound I/HRP couple of 0.98 V [49]. The question arises as to whether the H52D and H52Q mutations could increase the Compound I reduction potential by at least 0.2 V.
The redox chemistry of the Fe(III)/Fe(II) couple in these mutants is not unusual. DiCarlo et al. [50] have determined the reduction potential of the Fe(III) state for a large number of CcP mutants, including those used in this study. The E0′ values for reduction of CcP(H52D), CcP(H52E), CcP(H52N), and CcP(H52Q) are −221, −183, −259, and −224 mV, respectively, compared to a value of −191 mV for wild-type CcP. Bateman and colleagues [22] have observed unusual electrochemical behavior by CcP(H52Q). In the presence of 160 μM H2O2 at pH 5.4, 0° C, Bateman et al. observe that CcP(H52Q), adsorbed on a pyrolytic graphite electrode, catalyzes the electrochemical oxidation of H2O2 whereas wild-type CcP catalyzes the reduction of H2O2 under the same conditions. The voltammetric peak potential varies from 0.95 V to 0.80 V over the pH range 4.6 to 7.5. None of these data suggest that the CcP Compound I/CcP reduction potential could be increased to values needed to support an equilibrium between Compound I and the Fe(III) state in the presence of H2O2 for the H52D and H52Q mutants.
In light of the significant increase in reduction potential that would be required to permit equilibration between the CcP mutants, H2O2, and Compound I to occur, the initial explanation that H2O2-unreactive conformations causes the non-stoichiometric formation of Compound I seems the more likely interpretation for CcP(H52D), CcP(H52Q), and CcP(H52N).
4.6. Differential Role of the Distal Histidine in CcP and Metmyoglobin
Differential roles of the distal histidine in metmyoglobin and the peroxidases is amply demonstrated by looking at the properties of mutants in which the distal histidine is replaced by a glutamine residue in both classes of protein. In the peroxidases, the distal histidine/glutamine switch causes a five order of magnitude decrease in the rate of reaction with H2O2 while the same amino acid substitution in metmyoglobin either has no effect on the H2O2 reaction rate [16] or slightly increases the rate [13]. The role of the distal histidine in the peroxidases appears to be base catalysis of H2O2 binding to the heme iron while the distal histidine appears to have a gating function in metmyoglobin, controlling access to the distal heme pocket [10–12].
Supplementary Material
Footnotes
Abbreviations: CcP, generic abbreviation for cytochrome c peroxidase; yCcP, authentic yeast cytochrome c peroxidase isolated from S. cervisiae; rCcP, recombinant cytochrome c peroxidase expressed in E. coli with an amino acid sequence identical to that of yCcP; HRP, horseradish peroxidase.
Supplementary material associated with this article can be found in the online version at doi:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kendrew JC. Myoglobin and the structure of proteins. Science. 1963;139:1259–1266. doi: 10.1126/science.139.3561.1259. [DOI] [PubMed] [Google Scholar]
- 2.Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR. Structure of myoglobin. A three-dimensional fourier synthesis at 2 Å resolution. Nature (London) 1960;185:422–427. doi: 10.1038/185422a0. [DOI] [PubMed] [Google Scholar]
- 3.Finzel BC, Poulos TL, Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-Å resolution. J Biol Chem. 1984;259:13027–13036. [PubMed] [Google Scholar]
- 4.Bolton W, Perutz MF. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 angstom units resolution. Nature. 1964;228:551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- 5.Fita I, Rossman MG. The active center of catalase. J Mol Biol. 1985;185:21–37. doi: 10.1016/0022-2836(85)90180-9. [DOI] [PubMed] [Google Scholar]
- 6.Murawski K, Carta S, Sorcini M, Tentori L, Vivaldi G, Antonini E, Brunori M, Wyman J, Bucci E, Rossi-Fanelli A. Observations on the structure and behavior of hemoglobin MRadom. Arch Biochem Biophys. 1965;111:197–201. doi: 10.1016/0003-9861(65)90340-1. [DOI] [PubMed] [Google Scholar]
- 7.Braunstein D, Ansari A, Berendzen J, Cowen BR, Egeberg KD, Frauenfelder H, Hong MK, Ormos P, Suake TB. Ligand binding to synthetic mutant myoglobin (His-E7 → Gly): role of the distal histidine. Proc Natl Acad Sci U S A. 1988;85:8497–8501. doi: 10.1073/pnas.85.22.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JS, Mathews AJ, Rohlfs RJ, Springer BA, Egeberg KD, Sligar SG, Tame J, Renaud JP, Nagai K. The role the distal histidine in myoglobin and hemoglobin. Nature. 1988;336:265–266. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- 9.Springer BA, Egeberg KD, Sligar SG, Rohlfs RJ, Mathews AJ, Olson JS. Discrimination between oxygen and carbon monoxide and inhibition of autoxidation by myoglobin. Site-directed mutagenesis of the distal histidine. J Biol Chem. 1989;264:3057–3060. [PubMed] [Google Scholar]
- 10.Rohlfs RJ, Mathews AJ, Carver TE, Olson JS, Springer BA, Egeberg KD, Sligar SG. The effects of amino acid substitution at position E7 (residue 64) on the kinetics of ligand binding to sperm whale myoglobin. J Biol Chem. 1990;265:3168–3176. [PubMed] [Google Scholar]
- 11.Carver TE, Rohlfs RJ, Olson JS, Gibson QH, Blackmore RS, Springer BA, Sligar SG. Analysis of the kinetic barriers for ligand binding to sperm whale myoglobin using site-directed mutagenesis and laser photolysis techniques. J Biol Chem. 1990;265:20007–20020. [PubMed] [Google Scholar]
- 12.Brancaccio A, Cutruzzolá F, Allocatelli CT, Brunori M, Smerdon SJ, Wilkinson AJ, Dou Y, Keenan D, Ikeda-Saito M, Brantly RE, Jr, Olson JS. Structural factors governing azide and cyanide binding to mammalian metmyoglobins. J Biol Chem. 1994;269:13843–13853. [PubMed] [Google Scholar]
- 13.Brittain T, Baker AR, Butler CS, Little RH, Lowe DJ, Greenwood C, Watmough NJ. Reaction of variant sperm-whale myoglobins with hydrogen peroxide: the effects of mutating a histidine residue in the haem distal pocket. Biochem J. 1997;326:109–115. doi: 10.1042/bj3260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan KK, Mondal MS, Padhy L, Mitra S. The role of distal histidine in peroxidase activity of myoglobin. Transient-kinetic study of the reaction of H2O2 with wild-type and distal-histidine-mutated recombinant human myoglobin. Eur J Biochem. 1998;257:547–555. doi: 10.1046/j.1432-1327.1998.2570547.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsui T, Ozaki S, Liong E, Phillips GN, Jr, Watanabe Y. Effects of the location of distal histidine in the reaction of myoglobin with hydrogen peroxide. J Biol Chem. 1999;274:2838–2844. doi: 10.1074/jbc.274.5.2838. [DOI] [PubMed] [Google Scholar]
- 16.Alayash AL, Ryan BAB, Eich RF, Olson JS, Cashon RE. Reactions of sperm whale myoglobin with hydrogen peroxide. Effects of distal pocket mutations on the formation and stability of the ferryl intermediates. J Biol Chem. 1999;274:2029–2037. doi: 10.1074/jbc.274.4.2029. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki S, Roach MP, Matsui T, Watanabe Y. Investigations of the roles of the distal heme environment and the proximal heme iron ligand in peroxide activation by heme enzymes via molecular engineering of myoglobin. Acc Chem Res. 2001;34:818–825. doi: 10.1021/ar9502590. [DOI] [PubMed] [Google Scholar]
- 18.Erman JE, Vitello LB, Miller MA, Kraut J. Active-site mutations in cytochrome c peroxidase: a critical role for histidine-52 in the rate of formation of compound I. J Amer Chem Soc. 1992;114:6592–9593. [Google Scholar]
- 19.Erman JE, Vitello LB, Miller MA, Shaw A, Browne KA, Kraut J. Histidine 52 is a critical residue for rapid formation of cytochrome c peroxidase compound I. Biochemistry. 1993;32:9798–9806. doi: 10.1021/bi00088a035. [DOI] [PubMed] [Google Scholar]
- 20.Newmyer SL, Ortiz de Montellano PR. Horseradish peroxidase His-42 → Ala, His-42 → Val, and Phe-41 → Ala mutants. Histidine catalysis and control of substrate access to the heme iron. J Biol Chem. 1995;270:19430–19438. doi: 10.1074/jbc.270.33.19430. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Lopez JN, Smith AT, Thornley RNF. Recombinant horseradish peroxidase isoenzyme C: the effect of distal haem cavity mutations (His42 → Leu and Arg38 → Leu) on compound I formation and substrate binding. J Biol Inorg Chem. 1996;1:136–142. [Google Scholar]
- 22.Bateman L, Leger C, Goodin DB, Armstrong FA. A distal histidine mutant (H52Q) of yeast cytochrome c peroxidase catalyzes the oxidation of H2O2 instead of its reduction. J Amer Chem Soc. 2001;123:9260–9263. doi: 10.1021/ja0158612. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Ishimori K, Mukai M, Kitagawa T, Morishima I. Catalytic activities and structural properties of horseradish peroxidase distal His42 → Glu or Gln mutant. Biochemistry. 1997;36:9889–9898. doi: 10.1021/bi970906q. [DOI] [PubMed] [Google Scholar]
- 24.Savenkova MI, Satterlee JD, Erman JE, Siems WF, Helms GL. Expression, purification, characterization, and NMR studies of highly deuterated recombinant cytochrome c peroxidase. Biochemistry. 2001;40:12123–12131. doi: 10.1021/bi0111000. [DOI] [PubMed] [Google Scholar]
- 25.Takio K, Titani K, Ericsson LH, Yonetani T. Primary structure of yeast cytochrome c peroxidase II. The complete amino acid sequence. Arch Biochem Biophys. 1980;203:615–6299. doi: 10.1016/0003-9861(80)90219-2. [DOI] [PubMed] [Google Scholar]
- 26.Teske JG, Savenkova MI, Mauro JM, Erman JE, Satterlee JD. Yeast cytochrome c peroxidase expression in Escherichia coli and rapid isolation of various highly purified holoenzymes. Protein Expression Purif. 2000;19:139–147. doi: 10.1006/prep.2000.1220. [DOI] [PubMed] [Google Scholar]
- 27.Fishel LA, Villafranca JE, Mauro JM, Kraut J. Yeast cytochrome c peroxidase: mutagenesis and expression in Escherichia coli show tryptophan-51 is not the radical site in compound I. Biochemistry. 1987;26:351–360. doi: 10.1021/bi00376a004. [DOI] [PubMed] [Google Scholar]
- 28.Morar AS, Kakouras D, Young GB, Boyd J, Pielak GJ. Expression of 15N-labeled eukaryotic cytochrome c in Escherichia coli. J Biol Inorg Chem. 1999;4:220–222. doi: 10.1007/s007750050307. [DOI] [PubMed] [Google Scholar]
- 29.Pollock WBR, Rosell FI, Twitchett MB, Dumont ME, Mauk AG. Bacterial expression of a mitochondrial cytochrome c. Trimethylation of Lys72 in yeast iso-1-cytochrome c and the alkaline conformational transition. Biochemistry. 1998;37:6124–6131. doi: 10.1021/bi972188d. [DOI] [PubMed] [Google Scholar]
- 30.Kolthoff IM, Belcher R. Volumetric Analysis. Vol. 3. Interscience; New York: 1957. Hydrogen peroxide; pp. 75–76. [Google Scholar]
- 31.Berry EA, Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- 32.Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2. and ferrocytochrome c and enzymic determination of the extinction coefficients of cytochrome c. J Biol Chem. 1965;240:4509–4514. [PubMed] [Google Scholar]
- 33.Erman JE, Yonetani T. A kinetic study of the endogenous reduction of the oxidized sites in the primary cytochrome c peroxidase-hydrogen peroxide compound. Biochem Biophys Acta. 1975;393:350–357. doi: 10.1016/0005-2795(75)90061-6. [DOI] [PubMed] [Google Scholar]
- 34.Erman JE, Yonetani T. The oxidation of cytochrome c peroxidase by hydrogen peroxide: Characterization of products. Biochim Biophys Acta. 1975;393:343–349. doi: 10.1016/0005-2795(75)90060-4. [DOI] [PubMed] [Google Scholar]
- 35.Vitello LB, Erman JE, Miller MA, Mauro JM, Kraut J. Effect of Asp-235 → Asn substitution on the absorption spectrum and hydrogen peroxide reactivity of cytochrome c peroxidase. Biochemistry. 1992;31:11524–11535. doi: 10.1021/bi00161a034. [DOI] [PubMed] [Google Scholar]
- 36.Pearl NM, Jacobson T, Arisa M, Vitello LB, Erman JE. Effect of single-site charge-reversal mutations on the catalytic properties of yeast cytochrome c peroxidase: Mutations near the high-affinity cytochrome c binding site. Biochemistry. 2007;46:8263–8272. doi: 10.1021/bi700623u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasman GD. Handbook of biochemistry and molecular biology. 3. I. CRC Press; Cleveland, Ohio: 1976. pp. 318–320. [Google Scholar]
- 38.Adelman RL. Studies on the base strengths of N,N-disubstituted amides. J Org Chem. 1964;29:1837–1844. [Google Scholar]
- 39.Lakowski JR, Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973;12:4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bidwai A, Witt M, Foshay M, Vitello LB, Erman JE. Cyanide binding to cytochrome c peroxidase(H52L) Biochemistry. 2003;42:10764–19771. doi: 10.1021/bi034632k. [DOI] [PubMed] [Google Scholar]
- 41.Coulson AFW, Yonetani T. Oxidation of cytochrome c peroxidase with hydrogen peroxide: Identification of the ‘endogenous donor’. Biochem Biophys Res Comm. 1972;49:391–398. doi: 10.1016/0006-291x(72)90423-8. [DOI] [PubMed] [Google Scholar]
- 42.Tsaprailis G, English AM. A. M. Different pathways of radical translocation in yeast cytochrome c peroxidase and its W191F mutant on reaction with H2O2 suggest an antioxidant role. J Biol Inorg Chem. 2003;8:248–255. doi: 10.1007/s00775-002-0407-6. [DOI] [PubMed] [Google Scholar]
- 43.Spangler BD, Erman JE. Cytochrome c peroxidase compound I: Formation of covalent protein crosslinks during the endogenous reduction of the active site. Biochim Biophys Acta. 1986;872:155–157. doi: 10.1016/0167-4838(86)90159-7. [DOI] [PubMed] [Google Scholar]
- 44.Meno K, Jennings S, Smith AT, Henriksen A, Gajhede M. Structural analysis of the two horseradish peroxidase catalytic residue variants H42E and R38S/H42E: implications for the catalytic cycle. Acta Crystal. 2001;D58:1803–1812. doi: 10.1107/s090744490201329x. [DOI] [PubMed] [Google Scholar]
- 45.Sundaramoorthy M, Terner J, Poulos TL. The crystal structure of chloroperoxidase: A heme peroxidase-cytochrome P450 functional hybrid. Structure. 1995;3:1367–1377. doi: 10.1016/s0969-2126(01)00274-x. [DOI] [PubMed] [Google Scholar]
- 46.Dunford HB. Heme Peroxidases. Wiley-VCH; New York: 1999. pp. 323–339. [Google Scholar]
- 47.Purcell WL, Erman JE. Cytochrome c peroxidase catalyzed oxidations of substitution inert iron(II) complexes. J Amer Chem Soc. 1976;98:7033–7037. doi: 10.1021/ja00438a049. [DOI] [PubMed] [Google Scholar]
- 48.Mondal M, Fuller HA, Armstrong FA. Direct measurement of the reduction potential of catalytically active cytochrome c peroxidase compound I: Voltammetric detection of a reversible, cooperative two-electron transfer reaction. J Amer Chem Soc. 1996;118:263–264. [Google Scholar]
- 49.Hayashi Y, Yamazaki I. The oxidation-reduction potentials of Compound I/Compound II and Compound II/Ferric couples of horseradish peroxidases A2 and C. J Biol Chem. 1979;254:9101–9106. [PubMed] [Google Scholar]
- 50.DiCarlo CM, Vitello LB, Erman JE. Effect of active site and surface mutations on the reduction potential of yeast cytochrome c peroxidase and spectroscopic properties of the oxidized and reduced enzyme. J Inorg Biochem. 2007;101:603–613. doi: 10.1016/j.jinorgbio.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller MA. A complete mechanism for steady-state oxidation of yeast cytochrome c by yeast cytochrome c peroxidase. Biochemistry. 1996;35:15791–15799. doi: 10.1021/bi961488c. [DOI] [PubMed] [Google Scholar]
- 52.Loo S, Erman JE. A kinetic study of the reaction between cytochrome c peroxidase and hydrogen peroxide. Dependence on pH and ionic strength. Biochemistry. 1975;14:3467–3470. doi: 10.1021/bi00686a027. [DOI] [PubMed] [Google Scholar]
- 53.Erman JE. Kinetic and equilibrium studies of cyanide binding to cytochrome c peroxidase. Biochemistry. 1974;13:39–44. doi: 10.1021/bi00698a007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.