Abstract
Event-related potentials (ERPs) are a direct measure of neural activity and are ideally suited to study the time-course of attentional engagement with emotional and drug-related stimuli in addiction. In particular, the late positive potential (LPP) appears enhanced following cocaine-related compared to neutral stimuli in individuals with cocaine use disorders (CUD). However, previous studies have not directly compared cocaine-related to emotional stimuli while examining potential differences between abstinent and current cocaine users. The present study examined ERPs in 55 CUD (27 abstinent and 28 current users) and 29 matched healthy controls while they passively viewed pleasant, unpleasant, neutral, and cocaine-related pictures. To examine the time-course of attention to these stimuli, we analyzed both an early and later window in the LPP as well as the early posterior negativity (EPN), established in assessing motivated attention. Cocaine pictures elicited increased electrocortical measures of motivated attention in ways similar to affectively pleasant and unpleasant pictures in all CUD, an effect that was no longer discernible during the late LPP window for the current users. This group also exhibited deficient processing of the other emotional stimuli (early LPP window: pleasant pictures; late LPP window: pleasant and unpleasant pictures). Results were unique to the LPP and not EPN. Taken together, results support a relatively early attention bias to cocaine stimuli in cocaine addicted individuals further suggesting that recent cocaine use decreases such attention bias during later stages of processing but at the expense of deficient processing of other emotional stimuli.
Keywords: Cocaine addiction, motivated attention, emotional processing, late positive potential
Drug-related compared to neutral stimuli elicit increases in physiological reactivity in drug addicted individuals (Carter & Tiffany, 1999). Similar research demonstrates unique reactions to emotional compared to neutral stimuli in healthy individuals (Lang et al., 1997; Vuilleumier, 2005; Schupp et al., 2007). Termed ‘motivated attention’, it is hypothesized that motivational systems automatically allocate attention to, and enhance the salience of, emotional stimuli (Lang et al., 1997). Two event-related potentials (ERP), the early posterior negativity (EPN) and the late positive potential (LPP), are larger for both pleasant and unpleasant compared to neutral visual stimuli, interpreted as reflecting increased attention to motivationally relevant stimuli (Cuthbert et al., 2000; Schupp et al., 2000; Schupp et al., 2003a; 2004b; Hajcak et al., 2007; Hajcak & Olvet, 2008; Foti et al., 2009). These ERPs capture different stages within emotional processing; specifically, the EPN reflects early selective attentional processing, while the LPP reflects continued processing of motivationally significant stimuli. Also, evidence suggests that the LPP is further comprised of both early (reflecting initial attention capture; similar to the P300) and later parietal components (reflecting sustained processing and encoding; Foti et al., 2009).
Several studies have reported that larger LPPs are elicited by drug-related compared to neutral pictures in alcohol (Namkoong et al., 2004), heroin (Franken et al., 2003), and cocaine addicted individuals (Franken et al., 2004; van de Laar et al., 2004); studies have not similarly examined the EPN. The extant LPP data have been interpreted as increased allocation of neural resources to addiction-related stimuli among addicts. However, more nuanced interpretations have been complicated given the inconsistent use of control groups, comparison stimuli, and variability in recency of drug use in the addicted participants. Specifically, only a few ERP studies directly compared drug-related to other emotional stimuli in both drug addicted and control individuals (Lubman et al., 2008; Lubman et al., 2009). This is a crucial comparison considering that emotion-related abnormalities have been implicated among substance dependent individuals (Aguilar de Arcos et al., 2005; Verdejo-Garcia et al., 2006). In addition, recency of drug use may relate to aberrant neural reactivity to drug cues among addicted individuals (Wilson et al., 2004). Therefore, the current sample of individuals with cocaine use disorders (CUD) included those who tested positive (CUD+) versus negative (CUD−) for recent cocaine use.
Specifically, we examined the EPN and LPP in 55 CUD (28 CUD+ and 27 CUD−) and 29 matched healthy controls while subjects passively viewed pleasant, unpleasant, neutral, and cocaine-related pictures. We hypothesized that in both CUD groups, LPPs elicited by cocaine pictures would be larger than neutral LPPs and similar to other emotional picture LPPs in the early time window, suggestive of enhanced attention to drug-related cues. We further hypothesized cocaine-related LPPs to be most pronounced in CUD+, consistent with suggestions for enhanced drug cue cortical reactivity with perceived drug use opportunity (Wilson et al., 2004) and enhanced cortical glucose metabolism among cocaine abusers during earlier compared to later abstinence (see Goldstein & Volkow, 2002). Analyses of the EPN were more exploratory.
Materials and Methods
Participants
Fifty-five individuals with CUD and 29 healthy control subjects participated in the current study. All subjects were medically healthy right-handed native English-speakers (exclusionary criteria are described in Supporting Information). Based on an extensive psychiatric interview (see Supporting Information), all CUD met DSM-IV criteria for current cocaine dependence. A positive urine screen indicated cocaine use within 72 hours (maximal resolution of the urine test) of study day in 28 participants (CUD+), while 27 participants tested negative (CUD−). The three groups (CUD+, CUD−, Controls) did not differ in distributions of sex and race, or in mean age, socio-economic status, years of education, and non-verbal intellectual functioning (Table 1; also see Supporting Information for treatment seeking status and analyses). However, CUD scored higher on depression and reported greater history of cigarette smoking than the controls (Table 1), and these variables’ possible confounding effects were examined as described in Analyses and Results. Additionally, compared to CUD−, CUD+ had increased frequency of cocaine use over the past 30 days, shorter duration of current abstinence, and higher craving but less self-reported severity of cocaine dependence (these variables did not correlate with LPPs). Subjects were fully informed of all study procedures and provided written consent for their involvement in this study in accordance with the local Institutional Review Board.
Table 1.
Demographic Characteristics and Drug Use by Study Subjects
| CUD+ (N=28) | CUD− (N=27) | Controls (N=29) | |
|---|---|---|---|
| Gender (male/female) | 27/1 | 26/1 | 25/4 |
| Ethnicity (African-American/Caucasian/Hispanic) | 20/5/3 | 19/5/3 | 20/8/1 |
| History of cigarette smoking (current or past/never or tried)1 | 21/7c | 21/6c | 6/23a,b |
| Education (years) | 13.09 (1.76) | 12.69 (1.82) | 13.81 (2.02) |
| Age (years) | 45.55 (4.81) | 42.47 (8.82) | 41.17 (7.32) |
| Socio-economic status (Hollingshead, 1975) | 32.32 (11.03) | 33.06 (11.63) | 32.90 (13.56) |
| Non-verbal intellectual functioning: Wechsler Abbreviated | 9.43 (3.72) | 9.92 (3.06) | 11.03 (2.72) |
| Scale of Intelligence: Matrix Reasoning scaled score (Wechsler, 1999) | |||
| Self-reported state depression (Beck et al., 1996)2 | 7.57 (7.56)c | 7.63 (6.38)c | 1.48 (2.87)a,b |
| Age at onset of cocaine use (years) | 26.68 (5.23) | 25.04 (6.47) | -- |
| Duration of use (years) | 16.56 (7.09) | 14.46 (8.80) | -- |
| Frequency of use (days/week) last 30 days3 | 4.23 (2.40)b | 1.53 (2.29)a | -- |
| Current use in $ per use (min – max, median) last 30 days | 62.36 (2–200, 50) | 109.23 (0–300, 80) | -- |
| Duration of current abstinence (days) (min – max, median)4 | 2.25 (0–14, 2)b | 214.85 (2–2555, 25)a | -- |
| Length of longest abstinence (days) (min – max, median) | 869.89 (19–3650, 365) | 818.63 (25–2555, 560) | -- |
| Total score on the Cocaine Selective Severity Assessment | 19.11 (10.84) | 15.74 (10.36) | -- |
| Scale (measure of withdrawal symptoms; 0–126) | |||
| Severity of Dependence Scale (0–15)5 | 6.61 (3.40)b | 8.44 (3.15)a | -- |
| Cocaine Craving Questionnaire (0–45)6 | 22.18 (10.66)b | 10.96 (9.58)a | -- |
Note. Values in parentheses are standard deviations unless otherwise indicated.
χ2(2)=23.79, p<.001 (Kruskal-Wallis);
χ2(2)=25.07, p<.001 (Kruskal-Wallis);
Z=−4.00, p<.001 (Mann-Whitney);
Z=−5.71, p<.001 (Mann-Whitney);
t(53)=−2.08, p<.05;
t(53)=4.10, p<.001. Superscript letters designate group differences
significantly different from CUD+;
significantly different from CUD−;
significantly different from control group.
Stimuli
Ninety pictures were selected from the International Affective Picture System (IAPS; Lang et al., 2005) based on normative ratings of valence and arousal, such that pleasant and unpleasant pictures would be more arousing than neutral pictures, and that each category of pictures would differ in their respective valence scores (see Supporting Information for analyses). Of these 90 pictures, there were 30 pleasant (e.g., smiling faces, nudes), 30 unpleasant (e.g., sad faces, violent images), and 30 neutral scenes (e.g., neutral faces, household objects).
We created a fourth category that included 30 pictures of cocaine and individuals preparing or using cocaine (e.g., snorting or smoking), collected from freely available online sources and adapted (as still images) from a cocaine video used previously in our laboratory (Volkow et al., 2006). Cocaine pictures were matched to the IAPS pictures on size of presentation and ratio of human to non-human content.
One of 10 sequences of completely randomized pictures (across all four picture categories) was randomly assigned to each subject. Within each sequence, four blocks of 30 randomized pictures were presented during the task. All 120 pictures were presented for 2000 ms, with a 2500 ms inter-trial interval; each picture was viewed only once.
Procedure
After a brief description of the experiment, electroencephalograph (EEG) sensors were attached and participants were given more detailed task instructions. Participants were told that they would be viewing pictures depicting a wide range of scenes; some pleasant, unpleasant, neutral, or drug-related. Participants were asked to focus on the screen and simply watch all of the pictures as they were displayed. Following the EEG task, subjects then rated each picture on valence (“rate how pleasant or unpleasant you felt about this picture”), arousal (“rate how strong of an emotional response you had to this picture”), like cocaine (“rate how much you like (or do not like) cocaine in response to this picture”), and want cocaine (“rate how much you want (or do not want) cocaine in response to this picture”). Subjects responded using a computerized version of the Self-Assessment Manikin (SAM; Bradley & Lang, 1994). The SAM depicted five characters that ranged on valence (happy to unhappy) or arousal (strong visceral response to no response); these same SAM scales were used to assess liking and wanting of cocaine. Subjects chose the numbers ‘1’ through ‘9’ (‘1’ corresponded to happy/liking/wanting/high visceral response, ‘9’ corresponded to unhappy/not liking/not wanting/no response) that appeared below the SAM characters. Thus, low numerical ratings correspond to higher levels of pleasantness, arousal, and liking and wanting cocaine. Consistent with previous work, we hypothesized that, of these four ratings, the arousal ratings would most parallel the LPP results (Cuthbert et al., 2000; Olofsson et al., 2008), at least in healthy control subjects (note self-report/awareness difficulties in CUD as we previously reported; Moeller et al., 2010). Analyses and results pertaining to these ratings are reported in the Supporting Information.
Psychophysiological Recording and Data Reduction
Continuous recordings of the EEG (Neuroscan Inc., Sterling USA) and electro-oculogram were obtained throughout passive picture viewing using a 64 silver-silver chloride electrode cap positioned according to the International 10/20 System (Klem et al., 1999). All recordings were performed using a fronto-central electrode as ground, and electronically linked mastoid electrodes as reference. Electrodes were placed above and below the left eye to record vertical eye movements, and placed on the outer canthi of both eyes to record horizontal eye movements. The EEG was digitized at a rate of 500 Hz and amplified with a gain of 250, and band-pass filtered from 0 to 70 Hz. Amplifiers were calibrated prior to each recording. Electrode impedances were at or below 10 kΩ for all electrodes used in the analysis.
Analyses
All bioelectric signals were analyzed off-line using Brain Vision Analyzer (Brain Products, Germany). Offline, all data were re-referenced to the numeric mean of the mastoids and band-pass filtered between 0.1 and 30 Hz; the EEG was corrected for blinks and eye movements using a previously developed method (Gratton et al., 1983). In addition, a semiautomated procedure was used to identify and reject physiological artifacts according to the following criteria: a voltage step of more than 50.0 μV between sample points, a voltage difference of more than 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100 ms intervals; additional artifacts were identified and rejected through visual inspection following the semi-automated procedure.
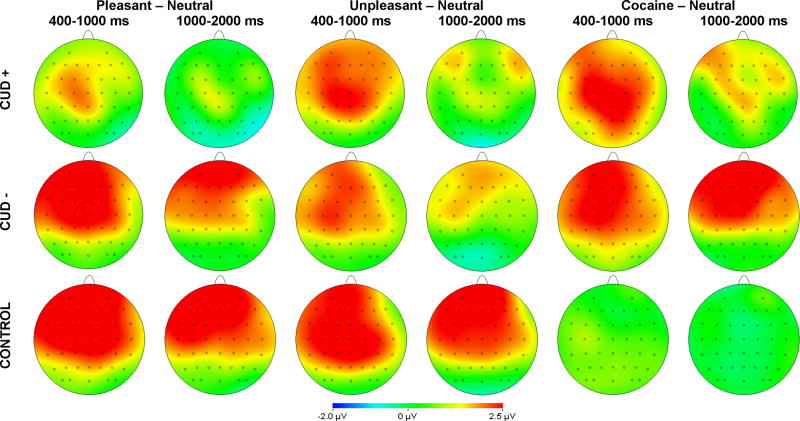
The ERPs were constructed by separately averaging trials based on picture type: pleasant, unpleasant, neutral, and cocaine-related pictures. For each ERP averaged waveform, the average activity in the 200 ms window prior to picture onset served as the baseline. Previous research indicates that the EPN is maximal at temporo-occipital electrode sites during a 200–300 ms window after stimulus onset (Schupp et al., 2003a; b; 2004b); therefore, we defined the EPN as the average activity at the Oz, POz, O1, and O2 electrode sites in a 200–300 ms time window after picture onset. For the LPP, previous research among both non-addicted (Cuthbert et al., 2000; Hajcak et al., 2007; Hajcak & Olvet, 2008; Foti et al., 2009) and addicted (see Franken et al., 2003; Franken et al., 2004; van de Laar et al., 2004; Franken et al., 2008) samples has assessed multiple time windows. In fact, a recent study utilizing PCA to investigate emotional picture processing (Foti et al., 2009) provided insight on how to best conceptualize the LPP. Results indicated that an initial parietal positivity in the LPP, which occurred in the range of 300–600 ms, was highly consistent with the P3, likely indicative of initial attention capture. A later sustained portion of the LPP appeared distinct from the earlier P3-like component and likely was indicative of additional and sustained processes relevant for emotional processing (Foti et al., 2009). Therefore, in the present study, we defined the LPP as the average activity in an early (400–1000 ms) and late (1000–2000 ms) time windows after picture onset. Previous investigations also indicate that drug-specific LPP modulation is maximal at fronto-central recording sites (Littel & Franken, 2007; Franken et al., 2008); scalp topographies in the present study corroborated this effect (Figure 1). Therefore, the LPP was scored as the average activity at the Cz, FCz, FC1, FC2, and Fz electrodes.
Figure 1.
Scalp topography of pleasant minus neutral (left columns), unpleasant minus neutral (middle columns), and cocaine minus neutral (right columns) differences in both the early (400–1000 ms) and late (1000–2000 ms) windows during passive viewing in CUD+ (top row), CUD− (middle row), and controls (bottom row).
For each time window, the averaged LPP was analyzed with a 4 (Picture Type: pleasant, unpleasant, neutral, cocaine-related) x 3 (Group: CUD+, CUD−, controls) mixed-model analysis of variance (ANOVA). The EPN was similarly analyzed. If significant interaction effects were present in the normally distributed EPN and LPP data, paired samples t-tests were used to assess within-group differences. Interaction effects were further explored through ERP difference scores, computed by subtracting neutral from all other picture types (pleasant, unpleasant, cocaine); these difference scores were then used to examine between-groups differences via independent t-tests. This approach controls for general differences in ERPs across participants, examining the degree of emotional modulation of the EPN and LPP across groups. All averaged ERP amplitudes are presented in Table 2.
Table 2.
Averaged ERP (EPN and LPP) Amplitudes to Each Picture Type in CUD+, CUD−, and Controls
| EPN (200–300 ms) | LPP (400–1000 ms) | LPP (1000–2000 ms) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CUD+ | CUD− | Controls | CUD+ | CUD− | Controls | CUD+ | CUD− | Controls | |
| Pleasant | 3.50 (2.16) | 4.16 (3.15) | 3.68 (3.01) | −1.70 (2.31) | −0.81 (3.04) | −0.85 (3.30) | −2.17 (1.97) | −1.30 (2.34) | −0.96 (2.57) |
| Unpleasant | 3.07 (2.27) | 4.05 (2.76) | 3.53 (3.15) | −1.34 (3.24) | −1.86 (2.83) | −1.38 (3.91) | −2.06 (2.64) | −2.02 (2.27) | −0.95 (3.52 |
| Neutral | 4.17 (2.19) | 4.54 (2.76) | 4.47 (3.60) | −3.23 (2.92) | −3.63 (3.27) | −3.78 (3.32) | −2.64 (2.79) | −3.30 (2.97) | −3.40 (3.08) |
| Cocaine | 3.11 (2.47) | 3.72 (2.73) | 3.72 (3.20) | −1.10 (4.18) | −1.16 (3.87) | −3.43 (3.63) | −1.55 (3.04) | −0.63 (3.14) | −3.64 (3.51) |
Note. Mean (standard deviation). Averaged ERP amplitudes are in microvolts (μV).
Correlations
Correlations were conducted between ERPs and selected drug use variables (in CUD only: whole sample and both subgroups separately; see Table 3). Since drug use variables were distributed non-normally, non-parametric Spearman correlations were used. All correlations were corrected for a family-wise error rate (p<0.01).
Table 3.
Correlations between cocaine-related LPPs and drug use variables
| CUD+ (N=28) | CUD− (N=27) | CUD Combined (N=55) | ||||
|---|---|---|---|---|---|---|
| Early LPP | Late LPP | Early LPP | Late LPP | Early LPP | Late LPP | |
| Age at onset of cocaine use (years) | −.21 | −.06 | .14 | .03 | −.04 | −.10 |
| Duration of use (years) | .32 | −.08 | .18 | .29 | .28* | .17 |
| Frequency of use (days/week) last 30 days | .23 | .10 | −.10 | −.15 | .09 | −.02 |
| Current use in $ per use, last 30 days | .16 | .68** | .09 | −.05 | .11 | .30 |
| Duration of current abstinence (days) | −.17 | −.09 | .02 | .15 | −.05 | .14 |
| Length of longest abstinence (days) | .13 | .15 | .19 | .30 | .18 | .24 |
| Total score on the Cocaine Selective Severity Assessment Scale | −.23 | .03 | −.45* | −.42* | −.32* | −.22 |
| Severity of Dependence Scale | −.24 | −.12 | .27 | .15 | .04 | .09 |
| Cocaine Craving Questionnaire | .14 | .23 | −.25 | −.19 | −.05 | −.05 |
Note.
p < .05;
p < .01.
All values are Spearman correlation coefficients, reflecting the relationships between cocaine-related early and late LPP amplitudes and the respective cocaine use variables.
All Analyses and Effects of Possible Covariates
SPSS (Version 16.0) was used for all analyses. For ANOVAs, the General Linear Model was used and Greenhouse-Geisser correction was applied for violations of sphericity. In all analyses, p<0.05 was considered statistically significant. To control for the effects of possible covariates, we conducted correlations between dependent variables (ERPs) with depression (drug use variables that differed between CUD subgroups were similarly treated). For history of cigarette smoking, differences in the dependent variables were inspected with t-tests. If significantly associated with the dependent variables across all study subjects (p<0.05), these variables were entered as covariates in the relevant ANOVA (Stevens, 1992).
Results
EPN
The EPN varied as a function of Picture Type (F(3,243)=14.80, p<0.001), but not group (F(2,81)=.41, p>0.65); the interaction between Picture Type and Group was also not significant (F(6,243)=.86, p>0.50). The main effect was driven by more negative EPNs for pleasant, unpleasant, and cocaine pictures than neutral pictures (all significant ts>|3.60|, ps<0.001); the emotional pictures did not differ from each other (all ts<|1.76|, ps>0.05). Hence, affective (including cocaine) compared to neutral pictures elicited increased EPNs across all study groups.
Early LPP (400–1000 ms)
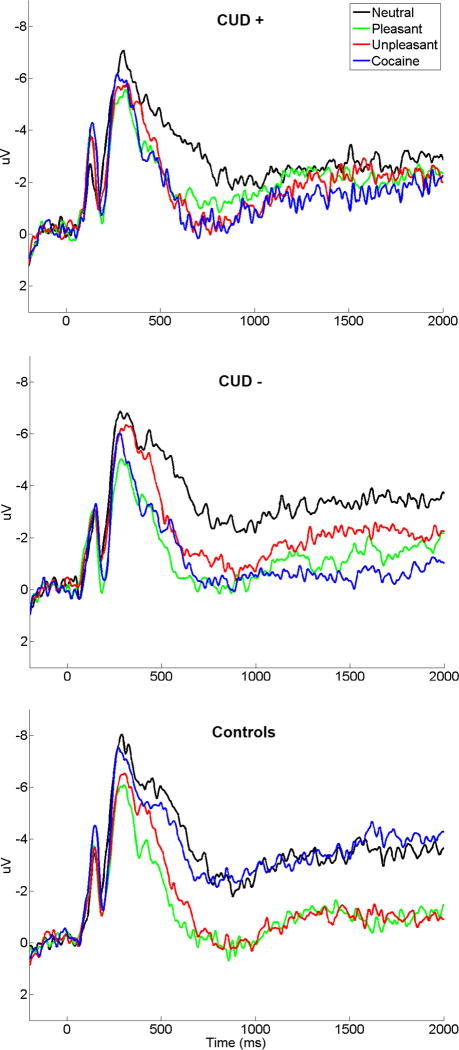
The LPP varied as a function of Picture Type (F(3,243)=26.57, p<0.001) and was qualified by a significant interaction between Picture Type and Group (F(6,243)=4.84, p<0.001). Groups did not differ overall (F(2,81)=.28, p>0.70). The interaction was driven by significantly larger cocaine-related compared to neutral LPPs in both CUD subgroups (CUD+: t(27)=−3.25, p<0.005; CUD−: t(26)=−5.05, p<0.001) but not control subjects (t(28)=−.79, p>0.40) (Figures 1 and 2). Also, only in the CUD, cocaine LPPs did not differ from either pleasant (CUD+: t(27)=−.86, p>0.35; CUD−: t(26)=.58, p>0.55) or unpleasant (CUD+: t(27)=−.38, p>0.70; CUD−: t(26)=−1.27, p>0.20) LPPs, while in controls cocaine LPPs were significantly smaller than both pleasant (t(28)=6.23, p<0.001) and unpleasant (t(28)=4.62, p<0.001) LPPs. Furthermore, pleasant and unpleasant pictures elicited larger LPPs than neutral pictures (t(28)=6.17, p<0.001 and t(28)=5.80, p<0.001, respectively), but did not differ from each other (t(28)=1.22, p>0.20) in the healthy subjects (Figure 1, bottom; Figure 2, bottom). The same pattern was observed in the CUD+ (pleasant and unpleasant>neutral: t(27)=3.86, p<0.001 and t(27)=4.12, p<0.001, respectively; pleasant=unpleasant: t(27)=−.69, p>0.45). In CUD−, pleasant and unpleasant LPPs were similarly larger than neutral LPPs (t(26)=6.15, p<0.001 and t(26)=3.99, p<0.001, respectively), also showing enhanced pleasant than unpleasant LPPs (t(26)=2.26, p<0.05) (Figure 1, middle; and Figure 2, middle).
Figure 2.
Grand averaged late positive potentials (at the average of sites Cz, FCz, FC1, FC2, and Fz) elicited by neutral, pleasant, unpleasant, and cocaine-related pictures for CUD+ (top), CUD− (middle), and control subjects (bottom). Stimulus onset occurred at 0 ms.
In examining LPP difference scores, between-group analyses for each picture category revealed that both CUD groups had larger cocaine LPPs than controls (all significant ts>|2.24|, ps<0.05), CUD− and controls had larger pleasant LPPs than CUD+ (all significant ts>|2.25|, ps<0.05), and there were no group differences for the unpleasant LPPs (all ts<|1.04|, ps>0.30).
Thus, for all CUD, the magnitude of LPPs elicited by cocaine, pleasant, and unpleasant pictures was larger than the LPP elicited by neutral pictures; further, the cocaine and other emotional picture LPPs did not differ from each other. In controls by contrast, LPPs elicited by cocaine and neutral pictures were comparable in magnitude and both significantly smaller than LPPs elicited by the pleasant and unpleasant pictures. Direct group comparisons showed that compared to controls, both CUD groups exhibited increased response to cocaine pictures. Interestingly, the CUD+ group also displayed reduced processing of pleasant pictures in the early LPP window.
Late LPP (1000–2000 ms)
In the late window, the LPP again varied as a function of Picture Type (F(3,243)=10.40, p<0.001) and was qualified by a significant interaction between Picture Type and Group (F(6,243)=32.49, p<0.001); a main effect of group was not significant (F(2,81)=.26, p>0.75). The interaction was driven by a similar pattern of results as for the early LPP in controls (all significant ts>|4.15|, ps<0.001; Figure 1, bottom; Figure 2, bottom) and largely in the CUD− (all significant ts>|2.55|, ps<0.05; the only change included a significant difference between cocaine-related and unpleasant LPPs, t(26)=−2.52, p<0.05; Figure 1, middle; Figure 2, middle) but not in CUD+ where no statistically significant differences emerged between any of the LPPs (all ts<|1.83|, ps>0.05; Figure 1, top; Figure 2, top).
In comparing difference scores across groups, results were similar to the earlier window, except that in this later window, enhanced processing of the cocaine pictures was only discernible in the CUD− group (CUD−>Controls, t(54)=3.16, p<0.005; CUD+=Controls, t(55)=1.42, p>0.15; CUD−=CUD+, t(53)=−1.91, p>0.05). Further, CUD+ displayed decreased processing of both pleasant (CUD+<Controls, t(55)=−2.7, p<0.01) and unpleasant (CUD+<Controls, t(55)=−2.60, p<0.05) pictures.
Thus, in the late LPP window, CUD− maintained enhanced processing of cocaine pictures; both CUD− and controls continued to show an increased LPP in response to both pleasant and unpleasant compared to neutral pictures. Of note, the CUD+ group demonstrated an attenuated late LPP to pleasant, unpleasant, and cocaine stimuli (the former two in between-subjects analyses and as compared to controls, the latter in within-subjects analyses and as compared to neutral pictures).
Correlations
The more money spent per each cocaine use in the last 30 days, the larger (more positive) the cocaine-related LPPs in the late window (rs=0.676, p<0.001) for CUD+ (Supporting Information Figure S1). However, this was the only correlation reaching family-wise correction level (see Table 3 for all correlations between LPPs and drug use variables); hence, because this correlation could be spurious, we reserve its interpretation until replicated in an independent study.
Control for depression and cigarette smoking as possible covariates
There were no significant correlations between the LPPs and depression scores (all rs<|.26|, ps>0.15) in either time window. Hence, it is unlikely that between-groups differences in depression could have accounted for the differential LPP patterns reported in the current study. In regard to smoking history, non-smokers elicited larger pleasant, neutral, and cocaine LPPs than smokers (all significant ts>|2.16|, ps<0.05). In the 400–1000 ms window, smoking history interacted with study group and could not be entered as a covariate; therefore, we examined LPPs as a function of smoking history (as an independent variable) across all study subjects. Results revealed no main effect of smoking history (F(1,82)<1) nor an interaction between Picture Type and smoking history (F(3,246)<1). In the 1000–2000 ms window, we were able to enter smoking history as a covariate in the ANOVA for LPPs; both our main effect of Picture Type (F(3,240)=5.80, p<0.001) and interaction between Picture Type and Group (F(6,240)=5.75, p<0.001) remained highly significant.
Discussion
In the present study, we examined the EPN and early and late windows of the LPP, neurophysiological measures of increased attention to motivationally relevant stimuli, to study processing of drug-related versus other emotional stimuli in drug addicted individuals. This study includes several important novel elements: (1) this study is the first to comprehensively include cocaine and emotional pictures in both cocaine addicted individuals and a matched control group, (2) processing of cocaine and emotional cues are examined as a function of recent cocaine use, hence the unique inclusion of both CUD+ and CUD− groups, and (3) we have examined the EPN (which has not been investigated in previous cocaine-related studies) in addition to multiple windows of the LPP in an effort to elaborate on the time-course of emotional and drug cue processing among all experimental groups.
Given their excellent temporal resolution, ERPs are ideally suited to investigate the time-course of emotional processing and drug cue reactivity. Importantly, neural generators of the LPP invoke cortical regions that play an integral part in the cycle of drug addiction which is characterized by Impairments in Response Inhibition and Salience Attribution (I-RISA), such that drug and drug-related reinforcers overpower all other non-drug related reinforcers and with a concomitant decrease in the ability to control behavior (Goldstein & Volkow, 2002). For example, stimulation of the dorsolateral prefrontal cortex, which is hyperactive in a drug-related context but hypoactive when performing neutral cognitive tasks in drug addicted individuals, modulates the LPP (Hajcak et al., in press).
The current EPN results suggested that very early attentional allocation to emotional stimuli, including cocaine images, was similar across all groups. In addition, and in line with previous reports, all subjects displayed increased motivated attention to pleasant and unpleasant compared to neutral pictures in the early LPP time window (Cuthbert et al., 2000; Schupp et al., 2000; Schupp et al., 2003b; Schupp et al., 2004a; Hajcak et al., 2007; Hajcak & Olvet, 2008; Foti et al., 2009). However, for both CUD groups compared to controls in the early LPP window, cocaine pictures elicited electrocortical activity on par with highly appetitive and aversive images and greater than neutral picture activity. These results support our first a priori hypothesis and extend results by others (Franken et al., 2004; van de Laar et al., 2004; Franken et al., 2008), and suggest that cocaine stimuli are similar to other emotional stimuli in increasing motivated attention in CUD – and this effect is first evident in the time window of the early LPP. In line with previous research in healthy controls (Schupp et al., 2003a; 2004b), this suggests that early processing of emotional stimuli also remains intact in CUD.
Even during the early LPP response to emotional stimuli, however, CUD subgroups began to differ from one another: compared to CUD− (and controls), CUD+ showed attenuated responses to pleasant pictures. Abnormalities in the LPP were even more pronounced among CUD+ in the later window, which typically reflects sustained attentional engagement and elaborative processing and encoding of motivationally significant stimuli. Here, CUD+ did not differentiate any emotional pictures (including cocaine) from neutral, and compared to controls, the CUD+ displayed significant impairments when processing both the pleasant and unpleasant pictures. These results are broadly consistent with previous work in addicted populations. Specifically, Lubman et al. (2009) demonstrated lack of the typical startle-elicited P300 attenuation during pleasant versus neutral (or drug) pictures in opiate dependent individuals compared to controls. Also, the bilateral dorsolateral prefrontal cortex was activated to pleasant pictures in 18 healthy controls but not in 16 inpatient (abstinent 1–24 weeks) male heroin addicted individuals (Zijlstra et al., 2009). This emotional processing impairment, which was most evident during the late LPP window in the CUD+, cannot be attributed to a cognitive deficit, which we showed to be less pronounced in the CUD+ than CUD− subgroups (Woicik et al., 2009). Results instead suggest a difficulty in sustaining motivated attention to emotional stimuli that may predispose CUD to compulsive drug use as a compensatory mechanism. Indeed, habituated, or blunted, response to emotional stimuli has also been reported in other psychopathologies impacting affective processing, such as major depressive disorder (Rottenberg et al., 2009; Foti et al., 2010). Future replications could determine whether this impairment reliably generalizes to other motivational stimuli (e.g., monetary reward).
In contrast to CUD+, a processing bias to cocaine images remained significant for CUD−: the LPP was larger for cocaine than neutral (and unpleasant) images even during the late window. Thus, results did not support our second hypothesis that LPPs to cocaine stimuli would be most pronounced in CUD+. Nevertheless, we caution against drawing firm conclusions about the strength of the electrophysiological signature of attention bias to drug related cues in CUD+ vs. CUD− because differences between these subgroups – for the cocaine related LPPs – were not significant in a direct group comparison. In general, these unexpected results remain to be studied in larger and more heterogeneous samples of CUD, including subjects with longer abstinence/remission periods. For example, consistent with results where glucose metabolism was higher in the orbitofrontal cortex in cocaine users within one week of last use than in healthy controls, while during more protracted withdrawal (1–6 weeks since last use) brain metabolism was lower in the cocaine abusers than controls (summarized in Goldstein & Volkow, 2002), we would predict eventual attenuation of LPPs to the cocaine-related stimuli in the CUD−.
Limitations of the study include: 1) one could question our choice of dividing the CUD into two subgroups based on cocaine in urine. We therefore report in the Supporting Information results where the CUD are treated as a single group further exploring correlations with time since last cocaine use; results were largely unchanged from those reported here; 2) results pertain mainly to males and remain to be further substantiated in women. Results in males only (excluding females) are reported in the Supporting Information, again with no major change to current conclusions. For purposes of generalizability, we included females in the current report; 3) depression and history of cigarette smoking differed between the groups. However, depression did not correlate with LPPs, and all original effects remained significant after controlling for cigarette smoking; 4) correlations between the LPPs with drug use were mostly not significant, which we attribute to the high nominal statistical threshold we used to protect against Type I error. In this vein, of note is a recent meta-analysis that demonstrated a modest overall correlation (r=0.19) between various measures of attentional bias and drug craving across several addiction types. In our study, inspection of similar raw correlations was consistent with the meta-analysis results (r=|.14 – .25|, Table 3); and 5) the motivational relevance of the LPP among CUD needs further examination in future studies. For example, preliminary results suggest that LPPs predict actual choice behavior (as measured with a newly designed drug choice task; Moeller et al., 2009; Moeller et al., 2010) and longer-term abstinence in CUD (results to be reported separately).
In conclusion, we found that cocaine pictures capture motivated attention in ways similar to affectively pleasant and unpleasant pictures in abstinent and current users of cocaine but not in matched healthy controls. Further, deficient electrophysiological response to pleasant and unpleasant images was most evident during later processing and in current users of cocaine. This deficit may be indicative of impairments in sustaining non-drug-related goal-oriented motivation, predisposing addicted individuals to drug use as a mechanism compensating for the reduced response to other reinforcement (including reward).
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (to RZG: 1R01DA023579 and R21DA02062) and the General Clinical Research Center (5-MO1-RR-10710).
Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Abbreviations
- ANOVA
Analysis of variance
- CUD
Cocaine use disorder
- CUD+
Individuals with cocaine use disorders that tested positive for recent cocaine use
- CUD−
Individuals with cocaine use disorders that tested negative for recent cocaine use
- EEG
Electroencephalograph
- EPN
Early posterior negativity
- ERP
Event-related potential
- IAPS
International Affective Picture System
- LPP
Late positive potential
- SAM
Self-Assessment Manikin
References
- Aguilar de Arcos F, Verdejo-Garcia A, Peralta-Ramirez MI, Sanchez-Barrera M, Perez-Garcia M. Experience of emotions in substance abusers exposed to images containing neutral, positive, and negative affective stimuli. Drug and Alcohol Dependence. 2005;78:159–167. doi: 10.1016/j.drugalcdep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Dietvorst RC, Hesselmans M, Franzek EJ, van de Wetering BJM, Van Strien JW. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addiction Biology. 2008;13:386–392. doi: 10.1111/j.1369-1600.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Hulstijn KP, Stam CJ, Hendriks VM, Van den Brink W. Two new neurophysiological indices of cocaine craving: Evoked brain potentials and cue moderated startle reflex. Journal of Psychopharmacology. 2004;18:544–552. doi: 10.1177/0269881104047282. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Stam CJ, Hendriks VM, van den Brink W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology. 2003:170. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Anderson B, Arana A, Borckardt J, Takacs I, George MS, Nahas Z. Dorsolateral prefrontal cortex stimulation modulates electrocortical measures of visual attention: Evidence from direct bilateral epidural cortical stimulation in treatment-resistant mood disorder. Neuroscience. doi: 10.1016/j.neuroscience.2010.04.069. (in press) [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Neural response to emotional pictures in unaffected by concurrent task difficulty: An event-related potential study. Behavioral Neuroscience. 2007;121:1156–1162. doi: 10.1037/0735-7044.121.6.1156. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1999;52:3–6. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Hillsdale, NJ: 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; Gainesville, FL: 2005. International affective picture system (IAPS): Affective ratings of pictures and instructional manual. [Google Scholar]
- Littel M, Franken IHA. The effects of prolonged abstinence on the processing of smoking cues: an ERP study among smokers, ex-smokers and never-smokers. Journal of Psychopharmacology. 2007;21:873–882. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JFW. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychopharmacology. 2008;22:836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later heroin use. Archives in General Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang G, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: Laboratory evidence and effects on cocaine-seeking behavior. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang G, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biological Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong K, Lee E, Lee CH, Lee BO, An SK. Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28:1317–1323. doi: 10.1097/01.alc.0000139828.78099.69. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2009;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 2004a;18:593–611. [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003a;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological Science. 2003b;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004b;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, Hamm AO. Selective visual attention to emotion. The Journal of Neuroscience. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. Erlbaum; Mahwah, NJ: 1992. [Google Scholar]
- van de Laar MC, Licht R, Franken IHA, Hendriks VM. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology. 2004;177:121–129. doi: 10.1007/s00213-004-1928-1. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive, and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Telang F, Fowler JS, Logan J, Childress A, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: Mechanisms of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang G, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: Recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IHA. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug and Alcohol Dependence. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.