Abstract
Arginine vasopressin (AVP) is a neuropeptide hormone and neurotransmitter that has peripheral functions in water regulation, and central functions in the stress response and social bonding in male rodents. In this study, we investigated the role of AVP in partner preference behavior in a monogamous primate, the coppery titi monkey (Callicebus cupreus). Seven titi males each received three intranasal treatments: saline, low AVP (40 IU), and high AVP (80 IU) in random order, one week apart. They experienced a series of stimulus exposures to their female partner, a female stranger, and an empty cage. Males were more likely to contact the stimulus and do so faster when either female stimulus was present. When pretreated with saline, males contacted the stranger more frequently than their partner; when pretreated with the high dosage of AVP, males contacted their partner more frequently than the stranger. We used microarray to measure peripheral changes in gene expression associated with intranasal AVP and found reduced expression of several genes coding for proinflammatory cytokines. The data presented here suggest that intranasally administered AVP has both central influences on social behavior and peripheral influences on inflammation in a non-human primate.
Keywords: Callicebus cupreus, arginine vasopressin, partner preference, inflammation, intranasal
Introduction
Arginine vasopressin (AVP) is a neuropeptide hormone with both central and peripheral functions (Shalts et al. 1992; Engelmann et al. 1996; Chickanza & Grossman 1998; Baker et al. 2003; Bielsky & Young 2004; Lim et al. 2004; Marler et al. 2005; Chassin et al. 2007; Knepper & Star 2008). Centrally, AVP plays a role in social behaviors such as initiating pair-bonding in male prairie voles (Microtus ochrogaster). AVP increases the tendency of male prairie voles to form a selective partner preference following cohabitation (Winslow et al. 1993a; Insel & Hulihan 1995; Insel et al. 1997; Lim & Young 2004). Additionally, AVP has been associated with social memory and social recognition (Bielsky et al. 2005; Dantzer et al. 1988; Engelmann & Landgraf 1994; Engelmann et al. 1996), affiliative behaviors (Young 2002), paternal behavior (Bamshad et al. 1994; Wang et al. 2000), and aggression (Bester-Meredith et al. 1999; Marler et al. 2005).
While the prairie vole has provided an excellent model to study the formation of rodent social bonds, we do not know whether the mechanisms for these bonds differ in primates. Coppery titi monkeys (Callicebus cupreus) are socially monogamous New World monkeys that show preferences for a specific partner, distress upon separation from that partner, and biparental care of offspring (Mason 1966). As such, they provide an ideal model for investigating the affects of AVP on social behavior and peripheral physiological changes in male primates. In order to assess the behavioral effects of centrally administered exogenous AVP, AVP was administered intranasally in order to bypass the blood brain barrier and ensure central uptake noninvasively. Intranasal administration has been shown to result in efficient absorption by the nasal epithelium reaching maximum uptake in approximately 60 minutes (Born et al. 2002) and has been used previously in non-human primate studies with the closely related peptide oxytocin (OT; Parker et al. 2005; Smith et al. 2010). We predicted that exogenous AVP would, in titi monkeys as in rodents, increase the preference a male had for his pair-mate relative to a female stranger.
Central AVP is also involved in the hypothalamic-pituitary-adrenal (HPA) axis stress response. HPA activation in primates begins with perception of a stressor, which results in the release of corticotropin releasing hormone (CRH) from the parvocellular neurons of the paraventricular nucleus (PVN) of the hypothalamus. These same neurons produce and secrete AVP, resulting in simultaneous release of both AVP and CRH. Activation of receptors in the anterior pituitary results in the release of adrenocorticotropic hormone (ACTH) into peripheral circulation. While CRH is capable of stimulating ACTH release independently, it is well established that maximal ACTH release is achieved following coactivation of pituitary receptors by both CRH and AVP (Rabadan-Diehl & Aguilera 1998; Tanoue et al. 2004; Lolait et al. 2007). Increasing doses of exogenous AVP result in correspondingly increasing ACTH responses (Gillies et al. 1982; Turkelson et al. 1982). ACTH activates receptors on the adrenal cortices resulting in the release of cortisol into circulation. A greater ACTH response has the potential for a more pronounced cortisol response. Therefore, central AVP concentrations can affect peripheral cortisol concentrations.
Glucocorticoids (GCs) have strong anti-inflammatory properties, and the cytokines that initiate the inflammatory response are influenced by GCs (Elenkov et al. 1996; Blotta et al. 1997; Dekruyff et al. 1998; reviewed in Sapolsky et al. 2000 and Calcagni & Elenkov 2006). Central administration of AVP may be expected to cause an anti-inflammatory state via amplified secretion of cortisol. CRH and GCs also modulate pair bonding in prairie voles. In male prairie voles, treatment with CRH or corticosterone facilitates pair bond formation (DeVries et al. 1996; DeVries et al. 2002). In female prairie voles the opposite pattern has been observed, with lower levels of corticosterone being associated with faster pair bond formation (DeVries et al. 1995). The sexually dimorphic nature of the findings in prairie voles may be linked to the neuropeptides associated with pair bonding in male and female voles: AVP and OT, respectively (Young & Wang 2004). As previously mentioned, AVP generally has a stimulatory effect on HPA activity, and OT has a suppressive effect (Gibbs 1986).
In an attempt to capture peripheral changes associated with changes in central AVP, we also investigated peripheral blood mononuclear cell (PBMC) gene expression profiles with and without AVP administration. We predicted that pharmacologically increasing central AVP would result in increased expression of the AVP receptor genes, and the OT receptor gene, which have been implicated in the social behavior of monogamous males (Winslow et al. 1993b). We further predicted that direct or indirect (e.g. via cortisol or ACTH activation) peripheral actions of AVP would change gene expression of stress-related loci.
Methods
Subjects
Seven captive-born male coppery titi monkeys (C. cupreus) housed at the California National Primate Research Center (CNPRC) were used in this study. All males were sexually mature and had been paired with a sexually mature female for at least one year. None of the males were caring for dependent offspring during the study. Males were 5.5 years of age on average (3.3 to 10.3 years) and were housed in cages (2.13m high × 1.27m wide × 1.27m deep) with their female pair-mate, identical to the housing described by Mendoza (1999). Twice daily (at 0830 and 1300 hrs) animals were fed a diet consisting of monkey chow, cottage cheese, marmoset jelly, apples, raisins, bananas, baby carrots, and vitamins.
AVP Treatment
Each animal received each of three treatments: saline (vehicle control, 300 μl), low dose AVP (40 IU) in 300 μl of saline, and high dose AVP (80 IU) in 300 μl of saline. Doses were based on work by Born and collaborators (2002) indicating what doses would lead to rises in AVP in cerebrospinal fluid. While being manually restrained, treatment was administered intranasally. Treatment solutions were dripped in 50 μl increments into alternate nostrils, and the nostril was then covered immediately to prevent the monkey from expelling the solution. Animals received treatment and were tested once per week for three consecutive weeks, with a single treatment administered on a given test day. Two animals were tested per day, and all seven animals experienced one test session per week. The order of treatments administered was approximately balanced across weeks and subjects.
Behavioral Assessment
Following AVP administration, males were returned to their home-cage with their mate for 20 minutes to allow sufficient uptake prior to beginning observation. During this uptake period animals were video recorded and behaviors involving social interaction with the mate were scored (Table 1a).
Table 1.
| TABLE 1a. AVP uptake period ethogram. | ||
|---|---|---|
| Behavior | Description | Measure |
| Contact | Male in physical contact with female | Duration |
| Tail twine | Male in contact with female and tails are entwined | Duration |
| Male approach | Male moves towards female and establishes contact | Frequency |
| Male leave | Male breaks contact with female | Frequency |
| Female approach | Female moves towards male and establishes contact | Frequency |
| Female leave | Female breaks contact with male | Frequency |
| Vocalize | Male engages in calling behavior, usually with female | Frequency |
| Chest rub | Male rubs chest or armpits with forearms | Frequency |
| Face rub/sneeze | Male rubs face with hands or on cage, or sneezes | Frequency |
| Groom | Male grooms female | Frequency |
| Autogroom | Male grooms himself | Frequency |
| Lipsmack | Male smacks lips toward female | Frequency |
| Arch | Male arches back while walking | Frequency |
| Tail lash | Male wags tail, usually a sign of agitation | Frequency |
| TABLE 1b. Stimulus testing behavioral categories | ||
|---|---|---|
| Behavior | Description | Measure |
| Proximity | Male comes within arm's reach of transport cage | Duration |
| Contact frequency | Male contacts transport cage | Frequency |
| Proximity latency | Time between start of trial and time when male comes into proximity with transport cage | Latency |
| Contact latency | Time between start of trail and time when male contacts transport cage | Latency |
Approximately 30 minutes post-administration stimulus presentations began. The partner and female stranger were placed in transport cages and served as the social stimuli, and an empty transport cage was used as a non-social control stimulus. The transport cage dimensions were approximately 0.3m high × 0.3m wide × 0.6m long, constructed of wire mesh that allowed visual, olfactory, auditory, and physical (e.g. fingers through the mesh) contact. Stimuli were presented into the home-cage consecutively for five minutes each, during which time behavioral scans were made every 15 sec. During pilot testing, inter-observer reliability was found to be 0.90. Duration of proximity was measured by the amount of time the male spent within arm's reach of the stimulus cage. Contact was scored as any time the male came into physical contact with the transport cage. All behaviors (Table 1b) were scored using Behavior Tracker 1.5 behavioral analysis software. Throughout the 60-min testing period, each of the three stimuli were presented three times, in random order with a given presentation lasting 5 min.
Blood Collection and Processing
A 3-mL blood sample was collected from the femoral vein after completion of stimuli presentations and two hours after treatment. Whole blood was collected into sterile vacuum-sealed tubes, and was immediately centrifuged at 4°C to separate and extract plasma. Plasma was stored at -80°C until assay. Prior to assay, samples were diluted 1:4 in PBS gel buffer. Plasma concentrations of cortisol were estimated in duplicate using commercial radioimmunoassay kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Assay procedures were modified with the addition of 0.5 and 2.5 μg/dl concentrations of standards along with the provided range of 1.0 to 50 μg/dl. Assay sensitivity has been determined to be 0.26069 μg/dl. Intra- and inter-assay coefficients of variation were 4.85% and 6.11%, respectively. OT and AVP were analyzed by enzyme immunoassay (Assay Designs, Ann Arbor, MI) in assays previously validated for titi monkeys (Bales et al. 2005). Intra-assay CV was 2.10% for OT and 3.23% for AVP.
Buffy layer was extracted to isolate PBMC, which were then lysed and RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA). RNA extraction followed protocols and procedures described in the Qiagen RNeasy Mini Handbook (www.qiagen.com).
Gene Expression
Two blood samples per male were used for microarray analysis on a subset of males (n = 4). From each of the four males two samples were assayed, one following saline treatment, and the second following high dose AVP treatment. Microarray analysis was performed with Rhesus Macaque Genome Array (Affymetrix, Santa Clara, CA). Isolated RNA was prepared and labelled following the protocols and procedures described in the Affymetrix Expression Analysis Technical Manual (www.affymetrix.com). Isolated RNA was used to create cDNA through reverse transcription. The cDNA was then used to synthesize biotin-labelled cRNA by in vitro transcription (IVT) using the GeneChip array IVT Labelling kit (Affymetrix, Santa Clara, CA). Amplified cRNA was fragmented and hybridized to the arrays according to the manufacturer's procedures (Affymetrix, Santa Clara, CA). The fluorescent signal from each probe on the GeneChip was read by Affymetrix GC3000 Scanner (Affymetrix, Santa Clara, CA). Microarray results were analyzed by robust multiarray analysis (RMA) using ArrayAssist (Iobion Informatics, La Jolla, CA). This software calculates a fold change value, which is a measure of the expression level of the treatment relative to that of the control. In addition, the expression levels of control and treatment were compared by t test.
Following microarray analysis several genes were confirmed in their expression levels by using quantitative polymerase chain reaction (qPCR). The genes that were investigated with qPCR are shown in Fig. 1. Manufacturer protocols were followed for qPCR technique (Applied Biosystems 7900HT Fast Real-Time PCR System and SDS Enterprise Database User Guide). The TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) contain forward and reverse primers, as well as probes, which are marked with a reporter and quencher dye. An increase in fluorescence signal is detected only if the target sequence is complementary to the probe and is amplified during PCR, thus minimizing nonspecific amplification to non-detectable levels. Cycle thresholds were determined and treatment samples were compared to the individual control sample to which they corresponded. Each additional cycle in the PCR reaction corresponds to a two-fold decrease in starting RNA quantity, so a treatment threshold cycle that is greater than the corresponding control by one cycle represents a two-fold down-regulation of the target gene. Microarray and qPCR expression profiles were significantly correlated across the five genes that were investigated with qPCR (r4 = 0.921, p = 0.026; Fig. 1).
FIGURE 1.
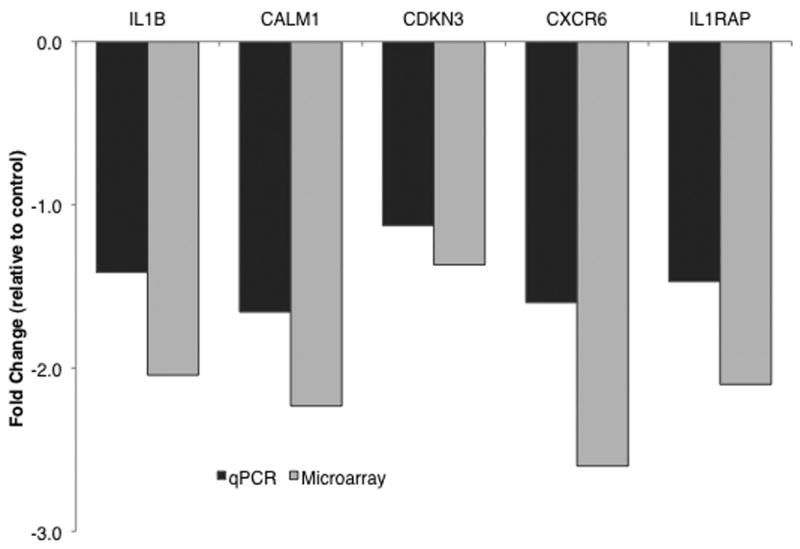
Gene expression as evaluated by microarray and qPCR. Fold change values represent difference in gene expression with AVP treatment compared to expression with saline treatment (r4=0.921, p=0.026). Negative values represent down-regulation of gene expression. IL1B = interleukin 1 beta, CALM1 = calmodulin 1, CDKN3 = cyclin-dependent kinase inhibitor 3, CXCR6 = C-X-C chemokine receptor type 6, IL1RAP = interleukin 1 receptor accessory protein.
Statistical Analysis
Male behavior during the AVP uptake period was analyzed with mixed models (PROC MIXED) using SAS v. 9.2 (SAS Institute, Cary, NC). The mixed effects model was chosen because the data set contains repeated measures for each animal, which violates the default assumption of identical and independent distribution inherent to traditional regression models (i.e. fixed effects models). By using mixed effects models we can describe the relationship between an outcome measure and treatment condition (i.e. fixed-effects) and variation between individuals (i.e. random effects), which is used to adjust the degrees of freedom. For example, we can determine what differences in tail twining are results of the treatments that animals received as opposed to differences that are a result of variation between individual titi males. The effect of AVP treatment during stimulus presentations was analyzed for two behavioral categories: proximity (latency and duration) and contact (latency, duration, frequency and likelihood (see below for description)).
Statistical analyses of stimulus testing were complicated by the fact that in addition to the treatment parameters and individual differences accounted for in the AVP uptake period models, these models had to incorporate variability due to the different stimuli, each of which were presented three times in random order. Therefore, for these analyses, the model incorporates seven animals, three treatment conditions, and 3 stimuli (presented 3 times each), resulting in 189 data points, or 189 total degrees of freedom. After subtracting the 11 degrees used on estimating fixed parameters, there are 178 degrees of freedom in the F-test. Post-hoc comparisons were made using t-tests and we used the Bonferonni correction to account for multiple comparisons.
To compare latency variables between treatment/stimulus conditions, we used proportional hazards mixed models for which we report a Chi-square and a hazard ratio (Xu 2004). The ‘coxme’ procedure of the Kinship library within R (R Foundation for Statistical Computing, Vienna, Austria) was used to fit the models. Since latency is defined as the amount of time from the beginning of a given trial to the occurrence of a behavior for our purposes, it is very similar to a survival time measuring the time to “death.” Because there were observations censored by the 5-minute trial time (i.e. behavior of interest was not observed before the end of the trial), standard linear regression modelling is difficult to apply. Therefore, a proportional hazard model for “survival times” was applied. Since multiple latency measures were collected on each animal, we added a random effect reflecting the variation between animals, and the proportional hazard model with mixed effects was used.
Proximity duration showed a normal distribution, and was therefore analyzed using a linear mixed model (PROC MIXED in SAS v. 9.2; SAS Institute, Cary, NC) to handle within-individual correlations (Littell et al. 1996).
Contact frequency had a zero-inflated distribution (Rodrigues 2006). That is, several of our trials ended without the male contacting the stimulus cage within the 5-minute time period. The possibility exists that had the trial been longer, fewer trials would have ended without contact. Therefore, these data were analyzed using a two-step model created and analyzed in R (R Foundation for Statistical Computing, Vienna, Austria). The first step determined whether or not the male contacted the stimulus. This binary variable was termed “contact likelihood” and is reported as the odds ratio ± 95% confidence interval. When the confidence interval does not include 1.0, it suggests that the estimate is significantly different from what would be expected by chance. A confidence interval less than 1.0 represents a reduction in likelihood, and a confidence interval greater than 1.0 represents an increase in likelihood. The second step of the model included only the subset of trials in which contact was made, and determined whether the contact frequency was predicted by the stimulus presented and/or the treatment administered to the male. This variable was termed “contact frequency” and is reported as median ± 95% confidence interval.
Results
AVP Uptake Observations
The only behavior that showed a significant response to AVP treatment during the uptake period (first 20 min post treatment) was rubbing the face (F2,12 = 4.19, p = 0.04), which increased when males received AVP (Mean ± SEM Saline: 2.86 ± 1.12 seconds (s); Low AVP: 8.14 ± 2.63 s; High AVP: 6.86 ± 1.04 s). Males administered the high dose of AVP showed a non-significant trend towards more time with their tails twined with their pair-mate (F2,12 = 3.04, p = 0.09; Mean ± SEM Saline: 108.29 ± 54.65 s; Low AVP: 112.14 ± 111.88 s; High AVP: 259.57 ± 133.08 s). Contact duration with partner, male and female approach and leave during the uptake period were not significantly affected by AVP treatment (all p > 0.25).
Stimulus Testing – Latency of proximity to stimulus cage
Female behavior in the stimulus cages was taken into account by recording the number of times she grabbed at the male when he approached. Females showed no trend for grabbing, either more or less, with respect to the treatment the males received or to the stimulus type they represented (partner or stranger).
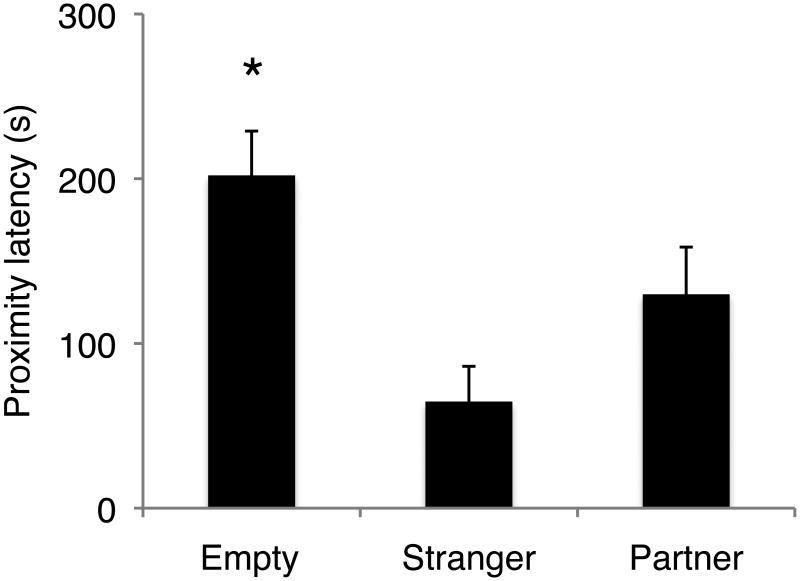
The latency to approach the stimulus cage was predicted by stimulus, but not by treatment (Fig. 2; stimulus X2=41.18, df=2, p<0.0001; treatment X2=3.00, df=2, p=0.22), and the interaction was not significant. Post-hoc analysis of fixed effects of stimulus revealed that males were faster to establish proximity with the stimulus cage when either the female partner or the stranger female were present than they were when the stimulus cage was empty (partner X2=9.10, df=1, p=0.0026; stranger X2 =38.96, df=1, p<0.0001).
FIGURE 2.
Stimulus type influences latency to approach in male titis. Stimulus X2=41.18, df=2, p<0.0001. Males approached their partner (X2=9.10, df=1, p=0.0026) and the stranger female (X2=38.96, df=1, p<0.0001) faster than the empty cage. Bars represent mean latency to approach ± SEM.
Stimulus Testing – Duration of proximity
Test of fixed effects showed both treatment and stimulus to be significant predictors of the duration of proximity to the stimulus cage (Fig. 3; treatment F2,178 = 6.74, p = 0.0015; stimulus F2,178 = 7.74, p = 0.0006). Post-hoc analysis of fixed effects of treatment showed that proximity duration was not significantly different when animals were given high dose AVP as compared to saline (t178 = -0.59, p > 0.5); however, proximity duration was significantly lower when males were administered low dose AVP (Fig. 3a; t178 = -3.43, p = 0.0007). Post-hoc analysis of fixed effects of stimulus showed that proximity duration was significantly lower when the empty cage was presented than when either the stranger or partner female was presented (Fig. 3b; t178 = -3.24, p = 0.0014). There was no difference in proximity duration between the partner and stranger female presentations (t178 = 0.31, p > 0.5).
FIGURE 3.
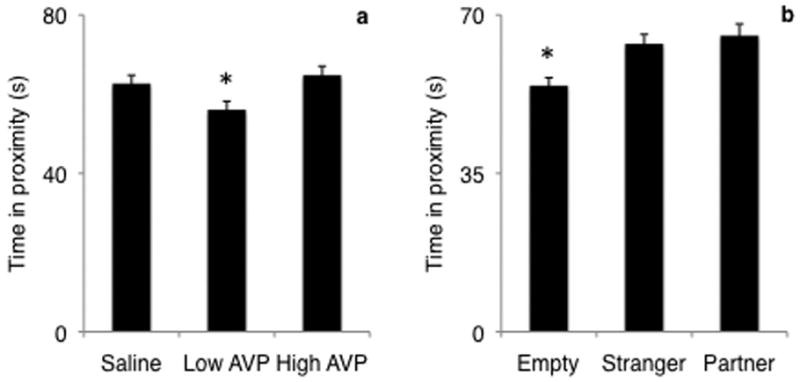
Effect of AVP treatment (a) and stimulus (b) on proximity duration. Male titis were administered varying doses of AVP and the amount of time that they spent in proximity to the stimulus cage was recorded. Both treatment (F2,178=6.74, p=0.0015) and stimulus (F2,178=7.74, p=0.0006) predicted proximity duration. Proximity was greater when low dose AVP was administered, but not when high dose AVP was administered. Proximity was greater when either the partner or the stranger female were presented. Bars represent mean proximity duration ± SEM. * = p < 0.01.
Stimulus Testing – Latency of contact with the stimulus
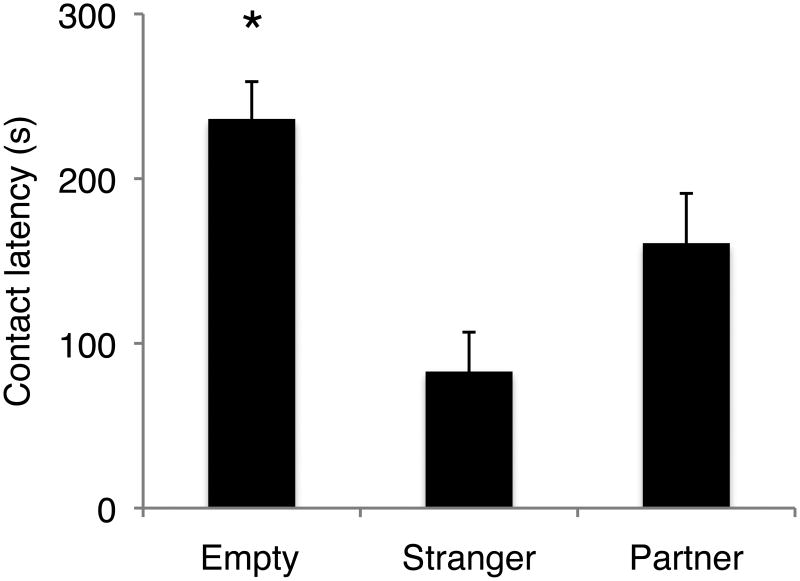
Contact latency was predicted by stimulus, but not by treatment (Fig. 4; stimulus X2=40.52, df=2, p<0.0001; treatment X2=2.57, df=2, p=0.28). Post-hoc analysis of fixed effects of stimulus showed that both partner and stranger presence significantly reduced the latency to contact the stimulus cage, when compared to the empty cage (partner X2=9.04, df=1, p=0.0026; stranger X2=38.39, df=1, p<0.0001).
FIGURE 4.
Effect of stimulus on latency to contact the stimulus cage. Males contacted the stimulus cage faster when either their partner or the stranger female were presented as compared to an empty stimulus cage. Bars represent mean ± SEM. * = p < 0.05.
Stimulus Testing – Likelihood to contact the stimulus cage
Likelihood to contact the stimulus cage was greater when the stranger female (Fig. 5a; OR=4.90, 95%CI: [1.57, 19.41]) was presented, as compared to an empty cage. There was no difference in contact likelihood between the partner and the empty cage (OR=1.10, 95%CI: [0.40, 3.09]). Treatment did not significantly affect contact likelihood (Fig. 5b).
FIGURE 5.
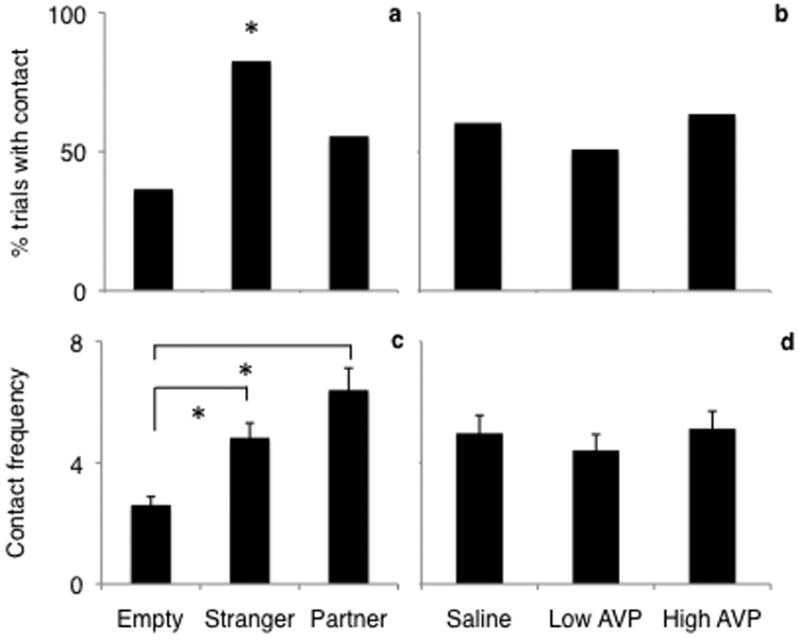
Effect of stimulus (a, c) and AVP treatment (b, d) on contact likelihood (a, b) and contact frequency (c, d). Likelihood to contact the stimulus cage was greater when the stranger female was presented. Males showed increased contact frequency towards either the partner or the female stranger when all treatment conditions were combined. Contact likelihood is presented as the percent of all trials during which contact occurred. Contact frequency is presented as the mean ± SEM. * = p < 0.05.
Stimulus Testing – Frequency of contact with the stimulus
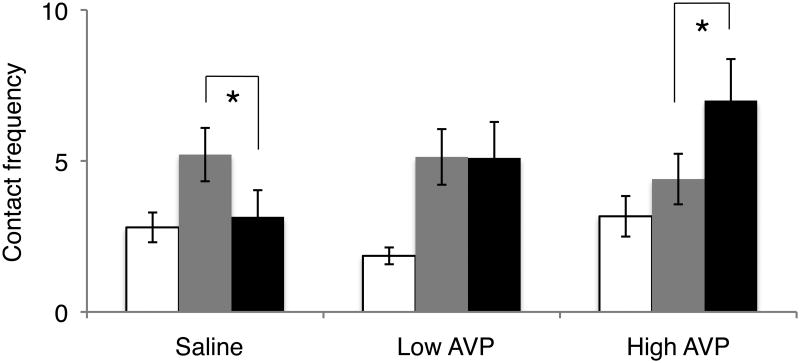
Males showed increased contact frequency toward either the partner (Fig. 5c; median difference=2.27, 95%CI: [1.39, 3.66]) or the female stranger (median difference=1.82, 95% CI: [1.15, 2.86]) as compared to an empty cage when all treatment conditions were combined. There was no overall effect of treatment (Fig. 5d). However, when saline was administered, males showed higher contact frequency towards the stranger as compared to the partner (Fig. 6; OR=0.221, 95%CI: [0.044, 0.889]), while with low dose AVP there was no difference in the frequency of contact between the partner or stranger (OR=0.331, 95%CI: [0.080, 0.889]), and with high dose AVP, increased contact frequency was directed toward the partner over the female stranger ([OR=15.625, 95%CI: [1.887, 468.604]).
FIGURE 6.
The combined effects of AVP treatment and stimulus on contact frequency. When males were treated with saline (control) they contacted the stranger female cage more frequently, however treatment with the high dose AVP was associated with more frequent contact of the partner cage. Bars represent mean ± SEM. * = p < 0.05.
Hormone Analysis
Cortisol did not show a differential response to treatment. Plasma cortisol concentrations when given saline, low AVP, and high AVP were 25.9 ± 3.7 μg/dl, 25.3 ± 2.1 μg/dl, and 30.8 ± 8.0 μg/dl, respectively (mean ± SE). Neither plasma OT nor AVP showed a response to AVP treatment at the time of blood collection. Plasma OT concentrations were 82.6 ± 42.4 pg/ml, 100.9 ± 59.2 pg/ml, and 94.6 ± 54.6 pg/ml when given saline, low AVP, and high AVP, respectively. Plasma AVP concentrations were 200.2 ± 54.7 pg/ml, 134.2 ± 23.7 pg/ml, and 162.9 ± 22.3 pg/ml when given saline, low AVP, and high AVP, respectively.
Gene Expression Analysis
Gene expression was analyzed first by pooling the results from all four individuals and analyzing by treatment (Table 2a). 372 out of 40,000 genes were identified as showing significantly different expression levels after the AVP treatment relative to the controls. Criteria for being significantly different due to treatment included a fold change of at least 1.5 (50% change in expression level), and fulfilling the 0.05 alpha level. Out of the 372 genes showing a change in expression levels, 267 were down-regulated and 105 were up-regulated. We did not see significant changes in any of the genes that we predicted to be affected by AVP administration; neither the AVP receptor genes nor the OT receptor gene showed changes in expression levels. Among the genes that showed significant changes in expression were 24 inflammatory response relevant genes (Table 2a). Inflammatory genes were significantly more likely to be down-regulated than up-regulated (X2=13.5, df=23, p<0.001).
Table 2.
| TABLE 2a. Genes showing significant changes in expression associated with AVP treatment. | ||
|---|---|---|
| Gene | Fold change | Function |
| CASP1 | -3.40 | Cleave and activate IL1 |
| CST7 | -2.50 | Immune response |
| LILRB1 | -2.25 | Immune response |
| SPA17 | -1.68 | Immune cell migration and metastasis |
| LTB | 1.56 | Immune response |
| IL13 | -1.43 | Interleukin 13 |
| IL7 | -1.42 | Interleukin 7 |
| CEACAM8 | -1.37 | Immune response |
| DEFA5 | -1.36 | Antimicrobial humoral response |
| CXCL16 | -1.36 | Chemotaxis, immune response |
| IL10B | -1.31 | Interleukin 10 beta |
| MHC2TA | -1.30 | Parasite perception, immune response |
| TABLE 2b. Genes showing consistent and significant changes in expression associated with AVP treatment in at least 3 of the 4 males. | ||
|---|---|---|
| Gene | Fold change | Function |
| IGJ | -2.77 | Immunoglobulin J chain, activation of B lymphocytes |
| IGK | -2.66 | Immunoglobulin K chain |
| IGHA1 | -2.51 | Immunoglobulin heavy constant alpha 1 |
| IL1B | -2.40 | Interleukin 1 beta |
| IL16 | -2.20 | Interleukin 16, chemoattractant factor |
| NFIL3 | -1.90 | Nuclear factor, interleukin 3 |
| CRHR1 | -1.86 | Corticotropin releasing hormone receptor 1 |
| CD94 | -1.86 | Antigen preferentially expressed on natural killer cells |
| TNFα-P6 | -1.64 | Tumor necrosis factor alpha-induced protein 6 |
| IL10RA | -1.64 | Interleukin 10 receptor activity |
| TNFSF13B | -1.62 | Tumor necrosis factor receptor binding, B cell activation and differentiation |
| IL6ST | -1.60 | Interleukin 6 signal transducer |
| IL15 | -1.46 | Interleukin 15, T and natural killer cell activation |
| A2M | -1.44 | Cytokine transporter |
Gene expression was then analyzed for consistency across individuals. Genes that showed consistent significant changes in expression in at least 3 of the 4 males are shown in Table 2b. Eight inflammatory response relevant genes showed consistent differences in regulation across individuals and were all down-regulated when AVP was administered (X2=8.00, df=7, p<0.005).
Discussion
Male titi monkeys showed an ability to distinguish between social and non-social stimuli in all of the behavioral measures we recorded: they contacted the transport cage more frequently, spent more time in proximity to the cage, and took less time to approach and contact the cage when social stimuli were present. Furthermore, they showed significantly different behavioral responses to the two social stimuli. When given saline, males contacted the stranger female more frequently than the partner, but when given high dose AVP they contacted their partner more frequently than the stranger, thus supporting our prediction that AVP would increase affiliative behavior directed to the partner. It is very common for monogamous male mammals (Williams et al. 1992; Smith et al. 2010), as it is for male mammals in general, to investigate an unfamiliar female for a longer period or prior to a familiar female (Bermant et al. 1968; Crawley 2007); so this result was not surprising. While we believe that central AVP is directly responsible for the observed behavioral changes, there remains the possibility that AVP augmented the peripheral stress response (although we did not observe a change at the time-point we chose, see below). This physiological response may have then altered brain functioning and behavior. Previous work in prairie voles has shown that acute stress, including administration of peripheral corticosterone, results in altered social bonding (DeVries et al. 1996). Future work will investigate the mechanisms driving the behavioral changes observed in this study.
An unexpected finding is that low dose (but not high dose) AVP resulted in decreased proximity to all stimuli, both social and non-social. While this contradicts our predictions, previous work has suggested that at some dosages AVP may be anxiogenic (Landgraf et al. 1995; Griebel et al. 2002; Bielsky et al. 2004). Some peptides exert their behavioral effects in an inverted u-shaped dose response fashion, and it is possible that AVP could result in increased anxiety and decreased willingness to approach the stimulus at one dose and not another.
The hormone analyses showed no difference in response to treatment. It is possible the stress response was activated by the handling and administration of AVP but returned to baseline by the time the blood samples were taken (2 hours post-administration). The lack of significant differences in OT and AVP two hours following administration is not surprising considering the short half-life of these peptide hormones (Share 1962; Rydén & Sjöholm 1969; Janáky et al. 1982). While cortisol has a longer half-life than OT and AVP (Kerrigan et al. 1993), it too would be expected to return to basal levels within two hours. The gene expression data provide evidence of a possible change in peripheral hormone concentrations that occurred before the blood samples were collected. The down-regulation of pro-inflammatory cytokines suggests a change in the inflammatory state. Cortisol is a known mediator of cytokine expression, however, the mechanism for the observed changes in gene expression in this study remains unclear.
While the blood collection time was not ideal for hormone analyses, two hours following a stressor is sufficient time to observe changes in inflammatory gene expression (Breen et al. 2000; Louis et al. 2007), and we found changes in the expression of several inflammatory genes following AVP administration. The proteins corresponding to the down-regulated genes include interleukin (IL)-1β, IL-7, IL-13, IL-15, and IL-16. IL-1β is well known for its role in inflammatory processes, and is suppressed by glucocorticoids (reviewed in Sapolsky et al. 2000). IL-7 is capable of expanding lymphocyte populations, leading to increases in both the B and T cell populations (Hofmeister et al. 1999), leading to humoral and cell-mediated immune response, respectively. IL-13 is secreted primarily by Th2 cells, and is an important mediator of allergic inflammation (Wills-Karp et al. 1998). IL-15 and IL-16 are critical to the induction of inflammatory processes (Center et al. 2000), and IL-15 is elevated during chronic inflammation. Taken together, reduced activity of these genes suggests a suppression of inflammatory processes. Therefore, manipulation of central AVP appears to have affected peripheral inflammatory state. One possible explanation is that intranasally administered exogenous AVP could have co-activated pituitary receptors with CRH and resulted in an increased adrenocortical response. Acutely increased peripheral cortisol concentrations would exert anti-inflammatory effects, resulting in decreased gene expression of genes coding for pro-inflammatory cytokines in any tissues containing glucocorticoid receptors. PBMC have glucocorticoid receptors (Lippman & Barr 1977), and are responsive to changes in glucocorticoid levels (Adcock et al. 1995).
An alternative explanation for the changes in gene expression is that they were caused by the altered social behavior. Growing evidence supports a role for social condition affecting inflammatory state (reviewed in Uchino et al. 1996; Kiecolt-Glaser 1999; Klein & Nelson 1999; DeVries et al. 2003). This idea predicts that individuals with better social support experience an anti-inflammatory state, while those without social support experience chronic inflammation. Chronic inflammation is currently known to be associated with several chronic health problems (e.g. cardiovascular disease, stroke, viral-mediated cancer). The results of this study, therefore, warrant further investigation of AVP as a possible mechanism mediating chronic inflammation and its downstream consequences.
There are a number of ways in which future studies, with different design parameters, could add to the information gained from this study. For example, in this study we administered treatments once per week. We chose this timing because we believed that it would allow more than enough time for the subjects to return to pre-treatment conditions. Based on the clearance rate of AVP, it is safe to assume that no exogenous AVP remained from a given treatment at the time of the second treatment a week later (Share 1962; Rydén & Sjöholm 1969; Janáky et al. 1982). However, we can not rule out the possibility that the physiological cascade of effects initiated by exogenous AVP administration remained beyond one week. A second choice we made with our behavioral assessment was to present stimuli sequentially rather than simultaneously. This allowed us to assess behavior in the subjects' home cages rather than in a novel apparatus, which had advantages because these animals are strongly neophobic (Fragaszy & Mason 1978; Hennessy et al. 1995). However, a simultaneous choice would have been closer to the model used in rodent research. A third design choice was that all three stimuli were presented during the same 60-minute period. While this was based on designs used in previous titi monkey studies (Mendoza & Mason 1986; Fernandez-Duque et al. 1997), it is possible that this testing method yielded different results than would be seen had each stimulus been presented on separate days.
In summary, intranasal AVP appeared to alter both central and peripheral physiology, resulting in both changed behavior and peripheral gene expression. Male titi social behavior changed in the predicted direction, as males contacted their female pair-mates more frequently when given high dose AVP. Pro-inflammatory cytokine gene expression was reduced in the periphery two hours after treatment with high dose AVP. Future work will further investigate the directionality of relationships between AVP, social behavior, and gene expression of pro-inflammatory cytokines.
Acknowledgments
We thank M. George and M. Rolston for assistance with microarray assay and data analysis and K. Abel and J. Lee for assistance with quantitative PCR assay and data analysis. We acknowledge our funding sources from the NIH (grant nos. HD053555 and RR00169) and the Good Nature Institute.
References
- Adcock IM, Brown CR, Gelder CM, Shirasaki H, Peters MJ, Barnes PJ. Effects of glucocorticoids on transcription factor activation in human peripheral blood mononuclear cells. Am J Physiol- Cell Physiol. 1995;268:C331–C338. doi: 10.1152/ajpcell.1995.268.2.C331. [DOI] [PubMed] [Google Scholar]
- Baker C, Richards LJ, Dayan CM, Jessop DS. Corticotropin-releasing hormone immunoreactivity in human T and B cells and macrophages: colocalization with arginine vasopressin. J Neuroendocrinol. 2003;15:1070–1074. doi: 10.1046/j.1365-2826.2003.01099.x. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Hostetler CM, Capitanio JP, Mendoza SP. Validation of oxytocin and vasopressin plasma assays for primates: what can blood tell us? Am J Primatol. 2005;66:73. [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Bermant G, Lott DF, Anderson L. Temporal characteristics of the Coolidge effect in male rat copulatory behavior. J Comp Physiol Psychol. 65:447–452. doi: 10.1037/h0025841. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Hormones and Behavior. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacol. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Breen EC, McDonald M, Fan J, Boscardin J, Fahey JL. Cytokine gene expression occurs more rapidly in stimulated peripheral blood mononuclear cells from human immunodeficiency virus-infected persons. Clin Diagn Lab Immunol. 2000;7:769–773. doi: 10.1128/cdli.7.5.769-773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann NY Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- Center DM, Kornfeld H, Ryan TC, Cruikshank WW. Interleukin 16: implications for CD4 functions and HIV-1 progression. Rev Immunol Today. 2000;21:273–280. doi: 10.1016/s0167-5699(00)01629-7. [DOI] [PubMed] [Google Scholar]
- Chassin C, Hornef MW, Bens M, Lotz M, Goujon JM, Vimont S, Arlet G, Hertig A, Rondeau E, Vandewalle A. Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J Exp Med. 2007;204:2837–2852. doi: 10.1084/jem.20071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chickanza IC, Grossman AC. Hypothalamic-mediated immunomodulation: arginine vasopressin is a neuroendocrine immune mediator. Br J Rheumatol. 1998;37:131–136. doi: 10.1093/rheumatology/37.2.131. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's wrong with my mouse: behavioral phenotyping of transgenic and knockout mice. John Wiley & Sons; Hoboken: 2007. [Google Scholar]
- Dantzer R, Koob GF, Bluthe RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Dekruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci USA. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad USA. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress response. Physiol and Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinol. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108:374–381. [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol and Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: Focus on learning and memory. Neuroscience Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch) Am J Primatol. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Mason WA. Response to novelty in Saimiri and Callicebus: Influence of social context. Primates. 1978;19:311–331. [Google Scholar]
- Gibbs DM. Vasopressin and oxytocin: hypothalamic modulators of the stress response: a review. Psychoneuroendocrinol. 1986;11:131–139. doi: 10.1016/0306-4530(86)90048-x. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR1499415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad USA. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Mendoza SP, Mason WA, Moburg GP. Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: Species differences in characteristic modes of responding to the environment. Physiol Behav. 1995;57:331–338. doi: 10.1016/0031-9384(94)00250-9. [DOI] [PubMed] [Google Scholar]
- Hofmeister R, Khaled AR, Benbemou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiology roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neuroscience. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ, Wang Z. Central oxytocin and reproductive behaviors. Rev Reproduction. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- Janáky T, László FA, Sirokmán F, Morgat JL. Biological half-life and organ distribution of [3H]8-arginine-vasopressin in the rat. J Endocrinol. 1982;93:295–303. doi: 10.1677/joe.0.0930295. [DOI] [PubMed] [Google Scholar]
- Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates for normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993;76:1505–1510. doi: 10.1210/jcem.76.6.8501158. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK. Stress, personal relationships, and immune function: health implications. Brain Behav Immunity. 1999;13:61–72. doi: 10.1006/brbi.1999.0552. [DOI] [PubMed] [Google Scholar]
- Klein SL, Nelson RJ. Influence of social factors on immune function and reproduction. Rev Reproduction. 1999;4:168–178. doi: 10.1530/ror.0.0040168. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Star RA. Vasopressin: friend or foe? Nature Med. 2008;14:14–16. doi: 10.1038/nm0108-14. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neuroscience. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Hammock EA, Young LJ. The role of vasopressin in the genetic and neural regulation of monogamy. J Neuroendocrinol. 2004;16:325–332. doi: 10.1111/j.0953-8194.2004.01162.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lippman M, Barr R. Glucocorticoid receptors in purified subpopulations of human peripheral blood lymphocytes. J Immunol. 1977;118:1977–1981. [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute Inc.; Cary, NC: 1996. p. 633. [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WS, 3rd, O'Carroll AM. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinol. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- Marler C, Trainor BC, Davis E. Paternal behavior and offspring aggression. Curr Dir Psychol Science. 2005;14:163–166. [Google Scholar]
- Mason WA. Social organization of the South American monkey, Callicebus moloch: A preliminary report. Tulane Stud Zool. 1966;13:23–28. [Google Scholar]
- Mendoza SP. Squirrel monkeys. In: Poole T, editor. The UFAW Handbook on the Care and Management of Laboratory Animals. 7th. Vol. 1. Blackwell Science Ltd; Oxford: 1999. pp. 591–600. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiol Behav. 1986;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinol. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Aguilera G. Glucocorticoids increase vasopressin V1b receptor coupling to phospholipase C. Endocrinol. 1998;139:3220–3226. doi: 10.1210/endo.139.7.6121. [DOI] [PubMed] [Google Scholar]
- Rodrigues J. Full Bayesian significance test for zero-inflated distributions. Comm Statistics—Theory and Methods. 2006;35:299–307. [Google Scholar]
- Rydén G, Sjöholm I. Half-life of oxytocin in blood of pregnant and non-pregnant women. Acta Endocrinologica. 1969;61:425–431. [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Shalts E, Feng YJ, Ferin M. Vasopressin mediates the interleukin-1 alpha-induced decrease in leuteinizing hormone secretion in the ovariectomized rhesus monkey. Endocrinol. 1992;131:153–158. doi: 10.1210/endo.131.1.1611995. [DOI] [PubMed] [Google Scholar]
- Share L. Rate of disappearance of arginine vasopressin from circulating blood in the dog. Am J Physiol. 1962;203:1179–1181. doi: 10.1152/ajplegacy.1962.203.6.1179. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu T, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkelson CM, Thomas CR, Arimura A, Chang D, Chang JK, Shimizu M. In vitro potentiation of the activity of synthetic ovine corticotropin-releasing factor by arginine vasopressin. Peptides. 1982;3:111–113. doi: 10.1016/0196-9781(82)90037-7. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preference in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin 13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CR, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993a;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro L, Carter CS, Insel TR. Oxytocin and complex social behavior: species comparisons. Psychopharmacol Bull. 1993b;29:409–414. [PubMed] [Google Scholar]
- Xu R. Proportional Hazards Mixed Models: A Review with Applications to Twin Models. Metodoloski Zvezki. 2004;1:205–212. [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]