Abstract
The role of centrioles changes as a function of the cell cycle. Centrioles promote formation of spindle poles in mitosis and act as basal bodies to assemble primary cilia in interphase. Stringent regulations govern conversion between these two states. Although the molecular mechanisms have not been fully elucidated, recent findings have begun to shed light on pathways that regulate the conversion of centrioles to basal bodies and vice versa. Emerging studies also provide insights into how defects in the balance between centrosome and cilia function could promote ciliopathies and cancer.
Introduction
Centrioles are barrel-shaped structures that play a central role in the formation of centrosomes, cilia, and flagella. The centrosome functions as a microtubule-organizing center. In cycling cells, centrosomes coordinate spindle pole formation during mitosis. In quiescent or interphase (G1 phase) cells, the centrosome migrates to the cell surface, whereupon the mother centriole forms a basal body that nucleates a primary cilium, an antenna-like organelle implicated in signal transduction and sensory functions. In this review, we summarize our understanding of the relationship between centrosomes and primary cilia, with a special emphasis on the transition between centriole and basal body assembly and the relationship between this switch and cell cycle control. We also discuss the need for an accurate switching mechanism, defects in which are expected to lead to several human pathologies, including ciliopathies and cancer. For a more general discussion of the biology of centrosomes and cilia, we direct the reader to several excellent recent reviews (Pedersen et al., 2008; Strnad and Gönczy, 2008; Gerdes et al., 2009; Azimzadeh and Marshall, 2010).
Centrosome structure and biogenesis
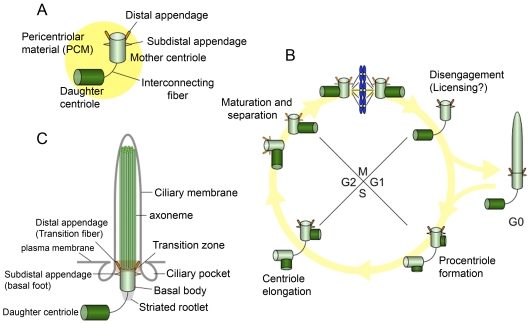
Centrosomes function as a microtubule-organizing center in animal cells and are a major organizer of spindle microtubules, although they are not essential for cell division in all cells. Centrosomes are comprised of two centrioles surrounded by material known as the pericentriolar matrix (Fig. 1 A). Typically, each centriole has nine triplets of microtubules and measures ∼0.5 µm in length with a diameter of ∼0.2 µm. In cycling cells, a pair of centrioles, termed the mother and the daughter centrioles, recruits the pericentriolar matrix, from which microtubules emanate. Mother and daughter centrioles inherited from a previous cell division are morphologically distinct: the mother centriole is the older of the two centrioles and contains subdistal and distal appendages (Fig. 1 A). Triplet microtubules transition to a doublet pattern at the distal ends of centrioles in the vicinity of the subdistal appendages (Paintrand et al., 1992). Subdistal appendages play a role in anchoring microtubules (De Brabander et al., 1982; Piel et al., 2000), whereas distal appendages are thought to be critical for the recruitment of basal bodies to the membrane during the assembly of cilia.
Figure 1.
Centrosomes, cilia, and the cell cycle. (A) The centrosome is composed of mother and daughter centrioles, and a protein matrix called pericentriolar material. The mother centriole has distal and subdistal appendages. Interconnecting fibers connect the two centrioles. (B) New centrioles (procentrioles) assemble in S phase and continue to elongate in G2. The two paired centrioles separate, and the original (oldest) daughter centriole acquires appendage proteins in late G2/early M, although these appendages are not visible at this stage (appendages are depicted as dotted lines). After mitosis, the paired centrioles become disengaged. In G0, the mother centriole migrates near the plasma membrane to become a basal body, and the primary cilium is formed. (C) The basal body localizes near the plasma membrane and nucleates a primary cilium. The mother centriole converts to the basal body, and structures that include the transition fibers/distal appendage, basal foot/subdistal appendage, and striated rootlet are observed. The transition fibers tether the basal body to the plasma membrane in the transition zone, in which triplet microtubules of the basal body transition to doublet microtubules in the axoneme. The axoneme is surrounded by a ciliary membrane. Ciliary pockets are observed near basal bodies.
Centrosomes duplicate once per cell cycle except in certain cell types or under conditions in which centrosomes are formed using a de novo pathway. In most mammalian cells, new centrioles, or procentrioles, are formed using preexisting centrioles (the mother and original daughter) as platforms in S phase and continue to elongate during passage through S and G2 phases, reaching a size approximating that of mother centrioles by M phase (Fig. 1 B). Although the original daughter centrioles acquire appendage proteins in G2 phase, structurally recognizable appendages are not yet present at this stage, and the mother centrioles lose their appendages during this time (Vorobjev and Chentsov, 1982). Thus, 1.5 cell cycles are required for the procentriole to become a mature centriole. Maintenance of an accurate complement of centrioles is thought to depend on cell cycle control and copy number control (Nigg, 2007). A cell cycle control mechanism dictates that centrioles duplicate only once per cell cycle. Cell fusion studies showed that centriole reduplication is intrinsically blocked by centrosomes during S and G2 phase (Wong and Stearns, 2003). Newly formed centrioles tightly associate with the parental centrioles until late mitosis, and the separation of the centrioles, termed disengagement, occurs in late mitosis (Tsou and Stearns, 2006a). Centriole disengagement is an essential event that requires the cysteine protease, separase, and Polo-like kinase (Plk1) and that “licenses” centriole duplication (Tsou and Stearns, 2006b; Tsou et al., 2009). Copy number controls specify that only one centriole is duplicated next to the parental centriole. Importantly, overexpression of Plk4 or Sas-6, proteins known to be essential for procentriole biogenesis, leads to the formation of extra centrioles, suggesting that the number of duplicated centrioles in S phase is limited by both Plk4 activation and by the amount of Sas-6 (Habedanck et al., 2005; Leidel et al., 2005; Kleylein-Sohn et al., 2007; Peel et al., 2007; Rodrigues-Martins et al., 2007; Strnad et al., 2007). These controls may, in turn, limit the number of primary cilia that can be formed in each cell.
Primary cilia and basal body assembly
Cilia are made up of a microtubule-based structure, termed the axoneme, and the surrounding ciliary membrane (Fig. 1 C). Motile cilia usually have a central pair of microtubules and nine outer doublet microtubules (9 + 2 structure), whereas nonmotile cilia, called primary cilia, lack the central pair (9 + 0 structure) and function in sensing and signal transduction. Primary cilia are involved in several signaling pathways essential for growth and differentiation, including the Hedgehog (Hh), Wnt, and PDGF pathways (Eggenschwiler and Anderson, 2007; Berbari et al., 2009). During its conversion to a basal body that nucleates the primary cilium, the mother centriole associates with vesicles (ciliary vesicles) that “cap” the distal end (Sorokin, 1962), migrating to the cell surface and subsequently docking at the plasma membrane. The transition zone, an intermediate region between the basal body and axoneme, is distinguished by a shift from triplet microtubules in the basal body to axonemal doublets and the presence of Y-shaped bridges connecting the outer doublet microtubules to the ciliary membrane. The association between the axonemal microtubules and membrane within this structure forms a barrier that allows the selective passage of materials into the cilium (Craige et al., 2010; Omran, 2010). Basal bodies possess accessory structures, including transition fibers, basal feet, and ciliary rootlets. Transition fibers and basal feet are ultrastructurally similar to distal and subdistal appendages, respectively. Transition fibers/distal appendages are believed to anchor the basal body to the membrane in the transition zone (Anderson, 1972). Transition fibers/distal appendages also appear to be required for the docking of intraflagellar transport (IFT) proteins before ciliogenesis (Deane et al., 2001). Striated rootlets extend from the proximal end of the basal body to the cytoplasm, providing structural support to the cilium (Tachi et al., 1974).
Because protein synthesis does not occur inside the cilium, proteins must be transported from the cytoplasm into cilia to build and maintain the organelle. Pioneering studies in Chlamydomonas reinhardtii and, subsequently, in Caenorhabditis elegans revealed an IFT pathway in which cargoes are conveyed from cytoplasm to ciliary tip by kinesin motors and back to the cytoplasm by dynein motors (Pedersen and Rosenbaum, 2008). In cooperation with these motors, multiprotein complexes termed IFT-A and -B particles are thought to connect the cargo and motors. Electron microscopy revealed that there are deep clefts within the membrane near the ciliary base, termed the ciliary pocket, and the Golgi network also localizes near the basal body (Pazour and Bloodgood, 2008; Rohatgi and Snell, 2010). Based on these observations, it is not surprising that proteins involved in vesicular transport play a critical role in ciliogenesis. For example, the small GTPase Rab8, which localizes to the Golgi, vesicles, and primary cilia, is required for ciliogenesis (Yoshimura et al., 2007). Rab8 interacts with a specific splice variant of the centrosomal distal/subdistal appendage protein ODF2 (outer dense fiber 2)/cenexin in vitro, and this interaction appears to be required for ciliogenesis (Yoshimura et al., 2007). Furthermore, Rab8 binds to Rabaptin-5, a regulator of endocytosis, and Rabaptin-5 also binds to an IFT protein in zebrafish, Elipsa (Dyf-11 in worms), suggesting a molecular link between membrane trafficking and the IFT machinery (Omori et al., 2008). Importantly, a protein complex (the so-called BBSome) consisting of seven proteins implicated in the ciliopathy Bardet-Biedl syndrome binds to Rabin-8, a guanine nucleotide exchange factor that controls Rab8 activity (Nachury et al., 2007). More recently, an ADP ribosylation factor family small GTPase, Arl6 (also known as BBS3), was reported to regulate BBSome-dependent cargo trafficking (Jin et al., 2010) and to act in ciliogenesis and Wnt signaling (Wiens et al., 2010). These findings suggest that vesicular transport pathways regulated by small GTPases are important for the assembly and function of cilia by delivering specific molecules from the cytoplasm into the primary cilium.
Links between the cell cycle and the basal body–centriole transition
The mother centriole is transformed into a basal body competent to nucleate a primary cilium in quiescent cells (see Introduction). Indeed, many centrosomal proteins identified by a proteomic approach are known to be required for ciliogenesis (Andersen et al., 2003; Graser et al., 2007). Clearly, the presence of a cilium is incompatible with cell division, presumably because the basal body must be released from the cell surface to function in mitosis. When cells reenter the cell cycle, primary cilia are disassembled, and basal bodies migrate to a position near the nucleus to act as a mitotic apparatus, indicating that the conversion between centrioles and basal bodies/primary cilia is reversible and depends on the cell cycle. Although our understanding of the conversion between centriolar and basal body function is still quite rudimentary, several studies have begun to provide mechanistic insights into the proteins involved in this switching mechanism.
Mother centriole to basal body transition and ciliogenesis
The role of CP110, Ofd1, and centriole length.
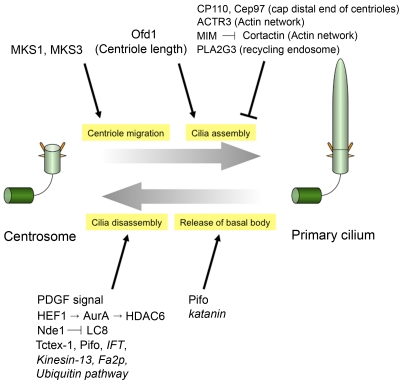
Given that basal bodies are assembled from centrioles, it stands to reason (a) that a strong set of controls must exist to suppress the inappropriate assembly of basal bodies and primary cilia in cycling cells and (b) that these controls must depend on cell cycle cues. The ability to suppress the expression of genes underlying the control of this switching mechanism, thus leading to the aberrant assembly of primary cilia in proliferating cells (and, reciprocally, the inability to disassemble the organelle), has begun to shed light on the molecular mechanisms associated with basal body formation. Two distal centriolar proteins, CP110 and its binding partner Cep97, were shown to suppress primary cilia assembly in cycling cells (Spektor et al., 2007). Depletion of CP110 or Cep97 leads to inappropriate cilia formation in growing retinal pigment epithelial (RPE-1) and 3T3 cells, two in vitro models for ciliogenesis, whereas ectopic expression of these proteins suppresses cilia formation in quiescent cells. Furthermore, CP110 levels drastically decrease in quiescent cells, and the protein completely disappears from mother centrioles. These data suggest that CP110 and Cep97 may prevent the conversion of mother centrioles to basal bodies in cycling cells (Fig. 2). Cep97 loss triggers the disappearance of CP110, suggesting that the former protein might be required to stabilize the latter. CP110 encodes a protein without an obvious enzymatic activity (Chen et al., 2002; Spektor et al., 2007), suggesting that CP110 could function structurally to limit microtubule growth and centriole length. Indeed, an elegant ultrastructural study suggests that CP110 localizes to the distal ends of centrioles, forming a cap above the growing microtubules (Kleylein-Sohn et al., 2007). However, it is also possible that an associated protein with enzymatic activity is needed to carry out an inhibitory role to suppress inappropriate cilia assembly.
Figure 2.
Modulating the balance between centriolar and ciliary fates. The events involved in the conversion of the centriole to the basal body and vice versa are shown. Proteins that impact these pathways are listed. Functional links to proteins in italics were shown exclusively in C. reinhardtii.
The transition zone is a poorly defined structure, but given that it is positioned between the distal region of the basal body and the ciliary axoneme and that it assembles exclusively during ciliogenesis, proteins associated with this region will undoubtedly play a pivotal role in the early events associated with basal body function and assembly of primary cilia. Interestingly, the Cep290 protein (the human homologue of which is mutated in ciliopathies) was shown to localize to the transition zone in C. reinhardtii, and Cep290 mutants exhibit defects in the connectors that link transition zone microtubules to the overlying membrane, resulting in abnormal transport of materials into flagella (Craige et al., 2010). Cep290 in mammalian cells localizes to the distal region of basal bodies near the transition zone and to centriolar satellites, granules that surround basal bodies and centrosomes (Kim et al., 2008; Tsang et al., 2008). Because CP110 binds to Cep290 in mammalian cells, and the ability of CP110 to suppress ciliogenesis depends on the integrity of its Cep290-binding domain (Tsang et al., 2008), it is tempting to speculate that CP110 might prevent inappropriate cilia formation in cycling cells by antagonizing the ability of Cep290 to tether mother centrioles to the membrane or by influencing the targeting of proteins to the organelle. However, such models await further testing.
Although recruitment of appendage proteins and formation of appendages occur independently of ciliogenesis, these proteins are required for ciliogenesis (Ishikawa et al., 2005; Graser et al., 2007). ODF2 was shown to be essential for the assembly of distal and subdistal appendages and for ciliogenesis in mouse cells (Ishikawa et al., 2005). Furthermore, immunoelectron microscopy indicated that the centriolar protein Cep164 localizes to the distal appendages and that this protein is also required for cilia formation (Graser et al., 2007). Depletion of ninein, a subdistal appendage protein, also impaired cilia formation (Graser et al., 2007). These findings suggest that the appendage-specific proteins function in basal body formation. However, it is important to note that mechanistic roles for these proteins, if any, beyond their association with discrete structures are not yet known. High resolution structural and biochemical studies aimed at understanding the proteins that associate with appendages and populate the transition zone will play a critical role in elucidating the centriole to basal body transition.
It is also notable that several proteins that regulate centriole length exhibit intimate connections to cilia assembly. For example, mammalian Ofd1 (oral–facial–digital syndrome 1) was recently reported to control centriole length (Singla et al., 2010). Remarkably, removal of this distal centriole protein resulted in abnormally long centrioles and defects in cilia formation. This decreased ciliogenesis was correlated with both abnormally long and short centrioles, suggesting that proper formation of distal ends and, perhaps, correct centriolar length regulation are important for efficient cilia assembly. A lissencephaly type-1–like homology (LisH) domain found in Ofd1 has been previously implicated in the binding and regulation of microtubule dynamics, and this may offer further clues for the function of this protein (Singla et al., 2010). LisH domain mutations are found in human patients with Ofd1, and these defects lead to loss of cilia. Interestingly, these mutations also compromised regulation of centriole length, recruitment of Cep164, and localization of IFT88 but not other IFT proteins (Singla et al., 2010). In addition to regulation of ciliogenesis, CP110 and Cep97 also control the length of centriolar microtubules because depletion results in abnormal structures that resemble extra long centrioles in nonciliated cells (Kohlmaier et al., 2009; Schmidt et al., 2009). Moreover, ectopic expression of centrosomal proteins Poc1 or CPAP induced abnormally long centrioles in nonciliated cells (Keller et al., 2009; Kohlmaier et al., 2009; Schmidt et al., 2009; Tang et al., 2009). However, the role of CPAP and Poc1 in ciliated cells is currently unknown. Despite the recent identification of this cohort of proteins that regulate centriole length, the functional relationships between them, if any, and the detailed mechanisms that underlie length regulation have not been elucidated.
The role of the actin cytoskeleton and other proteins.
Several studies have illustrated a pivotal role for the actin cytoskeletal network in assembling primary cilia. Recently, Kim et al. (2010) performed a comprehensive siRNA screen and showed that silencing of an actin-related protein, ACTR3, or treatment with an inhibitor of actin polymerization (cytochalasin D) induced cilia formation in cycling RPE-1 cells. MIM (missing in metastasis) was also reported to be required for ciliogenesis in mesenchymal cells (Bershteyn et al., 2010). MIM antagonizes Src-dependent phosphorylation of cortactin, an activator of actin branching and polymerization, resulting in primary cilia formation. These data suggest that formation of the actin network negatively modulates ciliogenesis. Interestingly, a mutation in another gene, Talpid3, causes defects in ciliogenesis and actin organization in chickens (Yin et al., 2009). In Talpid3 mutants, docking of basal bodies to the apical cell membrane is abrogated, although mature basal bodies appear to form. These results suggest that formation of the apical actin network affects the attachment of basal bodies to the cell membrane.
Other proteins have also been associated with the formation of basal bodies. Kim et al. (2010) also found that depletion of PLA2G3, a secreted phospholipase, results in cilia formation in cycling cells. PLA2G3 localized to centrioles, and knockdown of this protein altered the dynamics of recycling endosomes, leading to their enhanced concentration around centrosomes. Thus, it will be interesting to examine the relationship between recycling endosomes and ciliary vesicle transport and assembly. Two proteins, MKS1 and MKS3, implicated in the ciliopathy Meckel–Gruber syndrome were shown to be required for ciliogenesis and for migration of centrosomes to the plasma membrane (Dawe et al., 2007). Given that centrosomes can localize near the membrane in IFT88-depleted cells, these data suggest that proteins like MKS1 and MKS3 are necessary for the translocation of centrioles to the cell surface during basal body formation but IFT is not. Although Meckel syndrome and several candidate proteins have been proposed to play a role in the conversion from centrioles to basal bodies, the biochemical mechanisms through which these proteins function are not clear. It will be essential to dissect their roles not only in the assembly and structure of centrioles (especially distal regions) but also their connections to relevant intracellular events, such as centriole migration, vesicular trafficking, and IFT. It will also be interesting to determine how the abundance and localization of these proteins are controlled during cell cycle exit and reentry.
Cilia disassembly and the basal body to centriole transition
Mitogen stimulation of quiescent cells promotes both cell cycle reentry and primary cilia disassembly, which was reported to occur in two waves (Pugacheva et al., 2007). The first wave of disassembly occurs in G1 phase, shortly after mitogen stimulation, and the second wave occurs 18–24 h after mitogen stimulation (Pugacheva et al., 2007). Examination of the kinetics of cilia shortening and cell cycle progression in serum-stimulated cells led to the identification of proteins involved in a cilia disassembly program. Tucker et al. (1979) found that PDGF induced cilia disassembly without DNA synthesis, and furthermore, Ca2+ or FGF could substitute for PDGF in 3T3 cells. Interestingly, a PDGF receptor, PDGFαα, localizes to primary cilia in NIH3T3 cells and mouse embryonic fibroblasts, and ligand-dependent activation of PDGFαα is followed by activation of Akt and the Mek1/2–Erk1/2 pathways (Schneider et al., 2005), raising the possibility that these pathways mediate the signal to induce cilia disassembly. Activation of these pathways may also be relevant to ciliary loss in cancer cells (see Diseases related to centrosome and cilia dysfunction).
Events downstream of the receptor have received greater attention. It has been proposed that Aurora A kinase (AurA) in concert with a scaffold protein, HEF1, activates HDAC6, a deacetylase for tubulin, and HDAC6, in turn, deacetylates axonemal microtubules, resulting in cilia disassembly in mammalian cells (Fig. 2; Pugacheva et al., 2007). It was shown that AurA interacts with HEF1 at centrosomes and is activated by serum stimulation during cilia resorption, and AurA phosphorylates HDAC6 to activate its cilia disassembly activity. The inhibition of HEF1, AurA, or HDAC6 prevented cilia disassembly, and conversely, microinjection of activated AurA triggers rapid cilia resorption. These data suggest that a HEF1–AurA–HDAC6 pathway mediates cilia disassembly after serum stimulation in mammalian cells. Moreover, the C. reinhardtii CALK protein, which is distantly related to mammalian AurA, plays a role in flagellar disassembly, suggesting that AurA may be universally required for cilia resorption (Pan et al., 2004). However, hdac6 mouse knockouts did not reveal phenotypes that would be expected to arise from compromised cilia formation (Zhang et al., 2008). A possible explanation is that AurA might mediate a cilia disassembly pathway in the majority of ciliated cells, whereas HDAC6 might act only in specific cells. Recently, an enzyme that acetylates α-tubulin on lysine 40 (α-tubulin acetyltransferase [α-TAT]) was identified (Akella et al., 2010; Shida et al., 2010), and it was shown that α-TAT loss provoked a delay in cilia formation in mammalian cells, although cilia were, nevertheless, able to assemble. These data suggest that α-TAT positively regulates ciliogenesis and that acetylation of axoneme microtubules is required for normal kinetics of cilia assembly in mammalian cells. It should now be possible to dissect this pathway further, to examine cell cycle control and the balance between acetylation and deacetylation of tubulin as it impinges upon cilia assembly/disassembly, and to identify other proteins downstream of AurA to fully elucidate the mechanisms through which AurA mediates cilia disassembly.
Recently, a centrosomal protein, Nde1, was shown to negatively regulate ciliary length, and the abnormally long cilia resulting from Nde1 depletion induced a delay in cell cycle reentry (Kim et al., 2011). Nde1 was shown to regulate ciliary length via interaction with a dynein light chain protein, LC8 (Kim et al., 2011). Tctex-1, another protein originally identified as a light chain subunit of cytoplasmic dynein, was also reported to be phosphorylated and recruited to the transition zone before S-phase entry and to control cilia disassembly and cell cycle progression (Li et al., 2011). These studies suggest that dynein-dependent control of primary cilia length and resorption mediates cell cycle reentry.
Several proteins have been shown to promote the release of basal bodies from cilia, thereby reversing the constraints imposed by tethering basal bodies to the cell surface and freeing up centrioles to function during the mitosis after cilia retraction. In C. reinhardtii, katanin, a microtubule-severing ATPase, appears to dissolve the link between basal bodies and the transition zone when flagella are resorbed (Parker et al., 2010). Another protein, Pitchfork (Pifo), was shown to be essential for releasing basal bodies from cilia and for cilia retraction in mouse cells (Kinzel et al., 2010). Pifo localizes to basal bodies, and Pifo mutant mice exhibit nodal cilia-related phenotypes, such as altered left–right patterning and heart defects. Interestingly, Pifo activates AurA, and this activation is required for cilia disassembly, raising the possibility that Pifo functions in cilia resorption through an AurA-mediated cilia disassembly pathway.
Several studies in C. reinhardtii have identified proteins that function in flagella resorption. Although IFT is known to be indispensable for assembling and maintaining cilia in nearly all ciliated cells, IFT also mediates flagella disassembly (Pan and Snell, 2005). C. reinhardtii kinesin-13, a member of the Kif-13 family, which possesses microtubule-depolymerizing activity, functions in both flagella assembly and disassembly and is transported into flagella by IFT when flagella are shortening (Piao et al., 2009). A NIMA family kinase, Fa2p, is also essential for normal flagella resorption and the G2/M transition (Mahjoub et al., 2002). Intriguingly, the kinase activity of Fa2p is required for deflagellation but not for cell cycle progression (Mahjoub et al., 2004). These data suggest that the cell cycle defect in Fa2 mutants is independent of deflagellation and that Fa2p may function to link the two events. The ubiquitin conjugation system was also reported to be involved in flagellar disassembly (Huang et al., 2009). It will be interesting to clarify whether each of these proteins also mediates cilia disassembly in mammalian cells.
A central question regarding the role of cell cycle progression in the assembly and disassembly of cilia pertains to the issue of cause versus consequence. Clearly, the links between cellular quiescence and cilia assembly are robust, and given the associated kinetics observed in cultured cells, it is tempting to suggest that cilia assembly triggers the quiescent state, whereas mitogen stimulation triggers its reversal. However, it is extremely challenging to make an unequivocal argument for causality in either direction, given their clear interdependence. Unraveling this complex yet fundamental problem will require the development of genetic tools or chemical inhibitors that can uncouple the two processes as well as high resolution imaging (in time and space) to visualize the dynamic events associated with them.
Transcriptional control
The Foxj1 (Forkhead box J1) and RFX (regulatory factor X) family transcription factors have been shown to modulate ciliogenesis by controlling the expression of cilia-specific proteins (Thomas et al., 2010). Several studies indicate that Foxj1 is necessary for motile cilia formation in zebrafish, Xenopus laevis, and mice (Chen et al., 1998; Brody et al., 2000; Stubbs et al., 2008; Yu et al., 2008; Jacquet et al., 2009). Foxj1 regulates many genes involved in cilia motility, such as axonemal dyneins (Dnahc9, Dnaic1, and Dnali1), a central pair complex component (Spag6), and a radial spoke protein (Rsph4a; Thomas et al., 2010). The RFX transcription factor DAF-19 was shown to be required for sensory cilia assembly through direct regulation of expression of ciliary genes in C. elegans (Swoboda et al., 2000; Blacque et al., 2005; Efimenko et al., 2005; Chen et al., 2006). RFX proteins are also necessary for assembly of both motile and primary cilia in metazoans (Dubruille et al., 2002; Bonnafe et al., 2004; Ait-Lounis et al., 2007; Ashique et al., 2009; El Zein et al., 2009). RFX proteins regulate expression of IFT-related proteins (Ift88 and Ift172), Bardet-Biedl syndrome proteins (Bbs-1, -3, -5, and -8), and components of the basal body (B9d1 and B9d2; Thomas et al., 2010). Whether these transcriptional controls and/or analogous ones are essential for cell cycle–dependent assembly/disassembly of primary cilia is unknown, and it will be very interesting to determine the nature of the transcriptional regulatory networks that govern the centriole–basal body switch in mammalian cells as they enter or exit from a quiescent state.
The connection between centriole age and ciliogenesis
Remarkably, centriole age appears to influence cilia formation. Anderson and Stearns (2009) found that, after mitosis, cells receiving older mother centrioles tend to form primary cilia first and that the mother centriole–specific protein ODF2 asymmetrically localizes to older mother centrioles. Given that ODF2 is essential for basal body formation, these data suggest that centriole age determines the amount of ODF2 on mother centrioles and, in turn, basal body conversion from mother centrioles. Because centriole length and/or distal end formation could influence ciliogenesis, it will be interesting to precisely correlate length with cilia assembly and to examine mother centrioles of different ages at the ultrastructural level.
Diseases related to centrosome and cilia dysfunction
Genetic diseases caused by defects in the function and/or formation of cilia are termed ciliopathies, and an ever-increasing number of genes have been linked to them. As cilia are found on most human cells, an array of diverse pathologies has emerged, including polydactyly, mental retardation, situs inversus, retinal degeneration, obesity, diabetes, cyst development in the kidney, liver, and pancreas, and cancer. This subject has been covered extensively in other reviews (Badano et al., 2006; Fliegauf et al., 2007; Sharma et al., 2008), and here, we use two examples (cystic kidney disease and pancreatic cancer) to illustrate how proper controls during primary cilia formation are needed to maintain normal tissue homeostasis. Both cancer and cystic kidney disease are characterized by cellular overproliferation. Thus, a major outstanding question that is relevant to the cilia and cancer biology fields pertains to how ciliogenesis is deregulated in these cells and how these ciliary defects adversely affect growth regulation.
Kidney cysts.
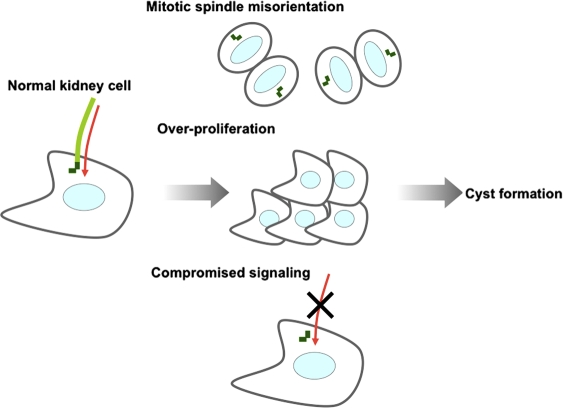
Polycystic kidney disease is one of the best-studied ciliopathies, characterized by numerous fluid-filled cysts that result in massive enlargement of the kidneys (Chapin and Caplan, 2010). Interestingly, knocking out IFT20, a component of IFT-B, in mouse kidney ductal cells prevented cilia formation and promoted rapid postnatal cyst formation (Jonassen et al., 2008). The kidney ductal cells in IFT20 knockouts show defects in centrosome positioning and misorientation of the mitotic spindles at an early stage (P5) and abnormal enhanced proliferation and aberrant Wnt signaling at later stages (after P15; Jonassen et al., 2008). Although IFT20 has also been shown to play a role in membrane trafficking (Finetti et al., 2009), this work suggests that loss of cilia in kidney cells causes the release of basal bodies, provoking aberrant centrosome positioning and inappropriate cell division and, later, overproliferation of kidney cells as well as aberrant Wnt signaling. On the other hand, ablation of the ift88 or kif3a gene in the adult mouse did not cause rapid cyst formation in the kidney despite the absence of cilia, and the cystic kidney pathology in these mutants depends on when cilia are lost, suggesting that cyst formation is not simply caused by lack of cilia (Davenport et al., 2007). Adult-specific inactivation of Kif3a did not lead to overproliferation in precystic cells or rapid cyst development; however, defects in planar cell polarity were induced in response to abnormal orientation of the mitotic spindle in precystic ductal cells (Patel et al., 2008). Furthermore, acute kidney injury, which stimulates proliferation, induced rapid cyst formation in this adult kif3a mutant mouse (Patel et al., 2008), suggesting that both aberrant planar cell polarity caused by lack of cilia and increased cell proliferation are required for rapid cyst formation in the adult mouse. Collectively, these studies suggest that ciliary loss contributes to kidney cyst formation by inducing aberrant cell divisions, although it is also possible that loss of cilia, in some settings, promotes overproliferation and abnormal signaling in a time-dependent manner (Fig. 3).
Figure 3.
Model for cyst formation caused by loss of primary cilia in kidney cells. In normal kidney cells, mother centrioles function as basal bodies upon which primary cilia are assembled. Several signaling pathways are mediated through primary cilia (red arrows). Loss of cilia may promote defects in centrosome positioning, resulting in mitotic spindle misorientation and aberrant cell division; release of basal bodies, permitting increased proliferation; and defects in signaling pathways orchestrated by primary cilia. These abnormalities could lead to cyst formation in kidney cells.
Another example is von Hippel-Lindau (VHL) syndrome, an inherited disease characterized by the development of tumors in adrenal glands, blood vessels, and the kidney. The VHL tumor suppressor protein (pVHL) is an E3 ubiquitin ligase that promotes degradation of hypoxia-inducible factors, and up-regulation of hypoxia-inducible factors in VHL patients is a key step in tumor formation. Several groups showed that pVHL localizes to primary cilia and is required for cilia assembly (Esteban et al., 2006; Lutz and Burk, 2006; Schermer et al., 2006), suggesting that abnormal cilia assembly in kidney cells of VHL patients causes cyst formation. However, later studies showed that loss of pVHL can suppress cilia assembly only in combination with depletion of the kinase GSK3β or the phosphatase and tensin homologue tumor suppressor (PTEN), and loss of either of these proteins can result in aberrant cell proliferation (Thoma et al., 2007; Frew et al., 2008). More recently, Thoma et al. (2009) showed that pVHL also localizes to spindle poles, and pVHL loss causes spindle misorientation linked to unstable astral microtubules and chromosome instability. Thus, additional studies will be needed to determine whether ciliary defects caused by mutations in pVHL directly lead to cyst formation. Interestingly, roscovitine, an inhibitor of Cdk’s, which are known to drive cell cycle progression, effectively blocks cyst formation in cultured cells and in mouse models of polycystic kidney disease, although the ability to restore cilia was not investigated (Bukanov et al., 2006).
Pancreatic cancer.
Like kidney cells that develop into cysts, transformed cells generally lack cilia. Importantly, Seeley et al. (2009) recently showed that normal duct, islet, and centroacinar cells assemble primary cilia in the pancreas, whereas pancreatic ductal adenocarcinoma cells and precursor lesions (pancreatic intraepithelial neoplasia), in which K-ras is frequently activated, lacked cilia. Intriguingly, these cells lacked cilia in the absence of active proliferation. Furthermore, inhibitors of two Ras effectors, phosphatidylinositol 3-kinase and mitogen-activated protein kinase, restored cilia formation in pancreatic cancer cells, but serum starvation, which caused cell cycle arrest, did not. These data argue that ongoing proliferation cannot fully explain the lack of cilia in pancreatic tumors and neoplasia and indicate that activated K-ras signaling can actively suppresses primary cilia formation independently from proliferation. These findings may have important implications for the etiology of pancreatic (and perhaps other) cancers and suggest the need to identify direct targets of the Ras pathway involved in cilia assembly/disassembly. At least two advantages of ciliary loss could be envisioned that would promote tumorigenesis or polycystic kidney disease: (1) ciliary loss could release centrioles, which can assemble mitotic spindles, thereby promoting overproliferation and/or aberrant cell division via misoriented spindle poles, and (2) normal signaling pathways orchestrated by cilia would be lost. Another important question that emerges is the extent to which oncogenic signaling—beyond K-ras activation—will contribute to the loss of cilia. If proliferative stimuli are, in some cases, sufficient to promote disassembly of cilia, activation of a multitude of oncogenic signaling pathways (or loss of tumor suppressors) may be sufficient to cause disassembly of this organelle, and this issue will need to be addressed in the context of studies of human cancer. On the other hand, if aberrant proliferation is not a sine qua non, alternate mechanisms and downstream effectors of a given oncogene will need to be investigated. Animal models will be useful for testing these possibilities.
Hh signaling depends on the assembly of cilia, and aberrant Hh signaling is associated with human cancer. Surprisingly, two studies using mouse models showed that disruption of cilia can either mediate or suppress Hh signaling, leading to both inhibition and acceleration of tumorigenesis (Han et al., 2009; Wong et al., 2009). These results suggest that cilia are important for balancing wide-ranging Hh signaling.
Several findings also indicate that AurA is up-regulated in many cancer cells, and overexpression can cause tumors. Because AurA was shown to be required for cilia disassembly (Pan et al., 2004; Pugacheva et al., 2007) and overexpression of AurA promotes centrosome amplification and chromosome instability (Meraldi et al., 2004), AurA might control the balance between cilia formation and centrosome duplication. However, because AurA is involved in many aspects of mitosis, it will be important to dissect the specific contributions of AurA in cilia disassembly to overall growth deregulation.
Perspectives
Studies of the primary cilium, once viewed as a vestigial organelle, have recently undergone a virtual renaissance with the recognition of its pivotal importance in development and disease. Nevertheless, we will need to develop additional chemical and genetic tools to dissect the relationship between cilia assembly and entry into a quiescent state on one hand and cell cycle exit and cilia disassembly on the other. Our understanding of mechanisms that convert centrioles to basal bodies will be enhanced by additional proteomic approaches, and understanding in detail the origin of asymmetries and differences between daughter and mother centrioles will be essential. The use of high resolution techniques to visualize, both in space and in time, the assembly of proteins that interact at the distal ends of centrioles, at the appendages of mother centrioles, and within the transition zone will be especially fruitful. Clearly, one interesting possibility for treatment of both ciliopathies and human cancer could involve finding therapeutic avenues that restore the balance between basal body and centrosome function, and this possibility should further serve to fuel the discovery process.
Acknowledgments
The authors thank three anonymous reviewers for their constructive and encouraging advice.
B.D. Dynlacht wishes to express his gratitude to the March of Dimes for supporting work in his laboratory.
Footnotes
Abbreviations used in this paper:
- α-TAT
- α-tubulin acetyltransferase
- AurA
- Aurora A kinase
- Hh
- Hedgehog
- IFT
- intraflagellar transport
- Pifo
- Pitchfork
- VHL
- von Hippel-Lindau
References
- Ait-Lounis A., Baas D., Barras E., Benadiba C., Charollais A., Nlend Nlend R., Liègeois D., Meda P., Durand B., Reith W. 2007. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes. 56:950–959 10.2337/db06-1187 [DOI] [PubMed] [Google Scholar]
- Akella J.S., Wloga D., Kim J., Starostina N.G., Lyons-Abbott S., Morrissette N.S., Dougan S.T., Kipreos E.T., Gaertig J. 2010. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 467:218–222 10.1038/nature09324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.T., Stearns T. 2009. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr. Biol. 19:1498–1502 10.1016/j.cub.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J.S., Wilkinson C.J., Mayor T., Mortensen P., Nigg E.A., Mann M. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 426:570–574 10.1038/nature02166 [DOI] [PubMed] [Google Scholar]
- Anderson R.G. 1972. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J. Cell Biol. 54:246–265 10.1083/jcb.54.2.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique A.M., Choe Y., Karlen M., May S.R., Phamluong K., Solloway M.J., Ericson J., Peterson A.S. 2009. The Rfx4 transcription factor modulates Shh signaling by regional control of ciliogenesis. Sci. Signal. 2:ra70 10.1126/scisignal.2000602 [DOI] [PubMed] [Google Scholar]
- Azimzadeh J., Marshall W.F. 2010. Building the centriole. Curr. Biol. 20:R816–R825 10.1016/j.cub.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano J.L., Mitsuma N., Beales P.L., Katsanis N. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148 10.1146/annurev.genom.7.080505.115610 [DOI] [PubMed] [Google Scholar]
- Berbari N.F., O’Connor A.K., Haycraft C.J., Yoder B.K. 2009. The primary cilium as a complex signaling center. Curr. Biol. 19:R526–R535 10.1016/j.cub.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn M., Atwood S.X., Woo W.M., Li M., Oro A.E. 2010. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev. Cell. 19:270–283 10.1016/j.devcel.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque O.E., Perens E.A., Boroevich K.A., Inglis P.N., Li C., Warner A., Khattra J., Holt R.A., Ou G., Mah A.K., et al. 2005. Functional genomics of the cilium, a sensory organelle. Curr. Biol. 15:935–941 10.1016/j.cub.2005.04.059 [DOI] [PubMed] [Google Scholar]
- Bonnafe E., Touka M., Ait-Lounis A., Baas D., Barras E., Ucla C., Moreau A., Flamant F., Dubruille R., Couble P., et al. 2004. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol. Cell. Biol. 24:4417–4427 10.1128/MCB.24.10.4417-4427.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S.L., Yan X.H., Wuerffel M.K., Song S.K., Shapiro S.D. 2000. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell Mol. Biol. 23:45–51 [DOI] [PubMed] [Google Scholar]
- Bukanov N.O., Smith L.A., Klinger K.W., Ledbetter S.R., Ibraghimov-Beskrovnaya O. 2006. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 444:949–952 10.1038/nature05348 [DOI] [PubMed] [Google Scholar]
- Chapin H.C., Caplan M.J. 2010. The cell biology of polycystic kidney disease. J. Cell Biol. 191:701–710 10.1083/jcb.201006173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Knowles H.J., Hebert J.L., Hackett B.P. 1998. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J. Clin. Invest. 102:1077–1082 10.1172/JCI4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Mah A., Blacque O.E., Chu J., Phgora K., Bakhoum M.W., Newbury C.R., Khattra J., Chan S., Go A., et al. 2006. Identification of ciliary and ciliopathy genes in Caenorhabditis elegans through comparative genomics. Genome Biol. 7:R126 10.1186/gb-2006-7-12-r126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Indjeian V.B., McManus M., Wang L., Dynlacht B.D. 2002. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell. 3:339–350 10.1016/S1534-5807(02)00258-7 [DOI] [PubMed] [Google Scholar]
- Craige B., Tsao C.C., Diener D.R., Hou Y., Lechtreck K.F., Rosenbaum J.L., Witman G.B. 2010. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190:927–940 10.1083/jcb.201006105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J.R., Watts A.J., Roper V.C., Croyle M.J., van Groen T., Wyss J.M., Nagy T.R., Kesterson R.A., Yoder B.K. 2007. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17:1586–1594 10.1016/j.cub.2007.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe H.R., Smith U.M., Cullinane A.R., Gerrelli D., Cox P., Badano J.L., Blair-Reid S., Sriram N., Katsanis N., Attie-Bitach T., et al. 2007. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum. Mol. Genet. 16:173–186 10.1093/hmg/ddl459 [DOI] [PubMed] [Google Scholar]
- Deane J.A., Cole D.G., Seeley E.S., Diener D.R., Rosenbaum J.L. 2001. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11:1586–1590 10.1016/S0960-9822(01)00484-5 [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. 1982. Microtubule stability and assembly in living cells: the influence of metabolic inhibitors, taxol and pH. Cold Spring Harb. Symp. Quant. Biol. 46:227–240 [DOI] [PubMed] [Google Scholar]
- Dubruille R., Laurençon A., Vandaele C., Shishido E., Coulon-Bublex M., Swoboda P., Couble P., Kernan M., Durand B. 2002. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development. 129:5487–5498 10.1242/dev.00148 [DOI] [PubMed] [Google Scholar]
- Efimenko E., Bubb K., Mak H.Y., Holzman T., Leroux M.R., Ruvkun G., Thomas J.H., Swoboda P. 2005. Analysis of xbx genes in C. elegans. Development. 132:1923–1934 10.1242/dev.01775 [DOI] [PubMed] [Google Scholar]
- Eggenschwiler J.T., Anderson K.V. 2007. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23:345–373 10.1146/annurev.cellbio.23.090506.123249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zein L., Ait-Lounis A., Morlé L., Thomas J., Chhin B., Spassky N., Reith W., Durand B. 2009. RFX3 governs growth and beating efficiency of motile cilia in mouse and controls the expression of genes involved in human ciliopathies. J. Cell Sci. 122:3180–3189 10.1242/jcs.048348 [DOI] [PubMed] [Google Scholar]
- Esteban M.A., Harten S.K., Tran M.G., Maxwell P.H. 2006. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J. Am. Soc. Nephrol. 17:1801–1806 10.1681/ASN.2006020181 [DOI] [PubMed] [Google Scholar]
- Finetti F., Paccani S.R., Riparbelli M.G., Giacomello E., Perinetti G., Pazour G.J., Rosenbaum J.L., Baldari C.T. 2009. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 11:1332–1339 10.1038/ncb1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M., Benzing T., Omran H. 2007. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8:880–893 10.1038/nrm2278 [DOI] [PubMed] [Google Scholar]
- Frew I.J., Thoma C.R., Georgiev S., Minola A., Hitz M., Montani M., Moch H., Krek W. 2008. pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J. 27:1747–1757 10.1038/emboj.2008.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J.M., Davis E.E., Katsanis N. 2009. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 137:32–45 10.1016/j.cell.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S., Stierhof Y.D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M., Nigg E.A. 2007. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179:321–330 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R., Stierhof Y.D., Wilkinson C.J., Nigg E.A. 2005. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7:1140–1146 10.1038/ncb1320 [DOI] [PubMed] [Google Scholar]
- Han Y.G., Kim H.J., Dlugosz A.A., Ellison D.W., Gilbertson R.J., Alvarez-Buylla A. 2009. Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 15:1062–1065 10.1038/nm.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Diener D.R., Rosenbaum J.L. 2009. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J. Cell Biol. 186:601–613 10.1083/jcb.200903066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Kubo A., Tsukita S., Tsukita S. 2005. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7:517–524 10.1038/ncb1251 [DOI] [PubMed] [Google Scholar]
- Jacquet B.V., Salinas-Mondragon R., Liang H., Therit B., Buie J.D., Dykstra M., Campbell K., Ostrowski L.E., Brody S.L., Ghashghaei H.T. 2009. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 136:4021–4031 10.1242/dev.041129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., White S.R., Shida T., Schulz S., Aguiar M., Gygi S.P., Bazan J.F., Nachury M.V. 2010. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 141:1208–1219 10.1016/j.cell.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen J.A., San Agustin J., Follit J.A., Pazour G.J. 2008. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 183:377–384 10.1083/jcb.200808137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L.C., Geimer S., Romijn E., Yates J., III, Zamora I., Marshall W.F. 2009. Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol. Biol. Cell. 20:1150–1166 10.1091/mbc.E08-06-0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Krishnaswami S.R., Gleeson J.G. 2008. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 17:3796–3805 10.1093/hmg/ddn277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee J.E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J.G. 2010. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 464:1048–1051 10.1038/nature08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Zaghloul N.A., Bubenshchikova E., Oh E.C., Rankin S., Katsanis N., Obara T., Tsiokas L. 2011. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 13:351–360 10.1038/ncb2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzel D., Boldt K., Davis E.E., Burtscher I., Trümbach D., Diplas B., Attié-Bitach T., Wurst W., Katsanis N., Ueffing M., Lickert H. 2010. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev. Cell. 19:66–77 10.1016/j.devcel.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y.D., Nigg E.A. 2007. Plk4-induced centriole biogenesis in human cells. Dev. Cell. 13:190–202 10.1016/j.devcel.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Kohlmaier G., Loncarek J., Meng X., McEwen B.F., Mogensen M.M., Spektor A., Dynlacht B.D., Khodjakov A., Gönczy P. 2009. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 19:1012–1018 10.1016/j.cub.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S., Delattre M., Cerutti L., Baumer K., Gönczy P. 2005. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7:115–125 10.1038/ncb1220 [DOI] [PubMed] [Google Scholar]
- Li A., Saito M., Chuang J.Z., Tseng Y.Y., Dedesma C., Tomizawa K., Kaitsuka T., Sung C.H. 2011. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat. Cell Biol. 13:402–411 10.1038/ncb2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M.S., Burk R.D. 2006. Primary cilium formation requires von hippel-lindau gene function in renal-derived cells. Cancer Res. 66:6903–6907 10.1158/0008-5472.CAN-06-0501 [DOI] [PubMed] [Google Scholar]
- Mahjoub M.R., Montpetit B., Zhao L., Finst R.J., Goh B., Kim A.C., Quarmby L.M. 2002. The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. J. Cell Sci. 115:1759–1768 [DOI] [PubMed] [Google Scholar]
- Mahjoub M.R., Qasim Rasi M., Quarmby L.M. 2004. A NIMA-related kinase, Fa2p, localizes to a novel site in the proximal cilia of Chlamydomonas and mouse kidney cells. Mol. Biol. Cell. 15:5172–5186 10.1091/mbc.E04-07-0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P., Honda R., Nigg E.A. 2004. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr. Opin. Genet. Dev. 14:29–36 10.1016/j.gde.2003.11.006 [DOI] [PubMed] [Google Scholar]
- Nachury M.V., Loktev A.V., Zhang Q., Westlake C.J., Peränen J., Merdes A., Slusarski D.C., Scheller R.H., Bazan J.F., Sheffield V.C., Jackson P.K. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 129:1201–1213 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- Nigg E.A. 2007. Centrosome duplication: of rules and licenses. Trends Cell Biol. 17:215–221 10.1016/j.tcb.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Omori Y., Zhao C., Saras A., Mukhopadhyay S., Kim W., Furukawa T., Sengupta P., Veraksa A., Malicki J. 2008. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10:437–444 10.1038/ncb1706 [DOI] [PubMed] [Google Scholar]
- Omran H. 2010. NPHP proteins: gatekeepers of the ciliary compartment. J. Cell Biol. 190:715–717 10.1083/jcb.201008080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintrand M., Moudjou M., Delacroix H., Bornens M. 1992. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J. Struct. Biol. 108:107–128 10.1016/1047-8477(92)90011-X [DOI] [PubMed] [Google Scholar]
- Pan J., Snell W.J. 2005. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev. Cell. 9:431–438 10.1016/j.devcel.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Pan J., Wang Q., Snell W.J. 2004. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev. Cell. 6:445–451 10.1016/S1534-5807(04)00064-4 [DOI] [PubMed] [Google Scholar]
- Parker J.D., Hilton L.K., Diener D.R., Rasi M.Q., Mahjoub M.R., Rosenbaum J.L., Quarmby L.M. 2010. Centrioles are freed from cilia by severing prior to mitosis. Cytoskeleton (Hoboken). 67:425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. 2008. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17:1578–1590 10.1093/hmg/ddn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Bloodgood R.A. 2008. Targeting proteins to the ciliary membrane. Curr. Top. Dev. Biol. 85:115–149 10.1016/S0070-2153(08)00805-3 [DOI] [PubMed] [Google Scholar]
- Pedersen L.B., Rosenbaum J.L. 2008. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85:23–61 10.1016/S0070-2153(08)00802-8 [DOI] [PubMed] [Google Scholar]
- Pedersen L.B., Veland I.R., Schrøder J.M., Christensen S.T. 2008. Assembly of primary cilia. Dev. Dyn. 237:1993–2006 10.1002/dvdy.21521 [DOI] [PubMed] [Google Scholar]
- Peel N., Stevens N.R., Basto R., Raff J.W. 2007. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 17:834–843 10.1016/j.cub.2007.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao T., Luo M., Wang L., Guo Y., Li D., Li P., Snell W.J., Pan J. 2009. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc. Natl. Acad. Sci. USA. 106:4713–4718 10.1073/pnas.0808671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel M., Meyer P., Khodjakov A., Rieder C.L., Bornens M. 2000. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149:317–330 10.1083/jcb.149.2.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P., Golemis E.A. 2007. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 129:1351–1363 10.1016/j.cell.2007.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D.M., Bettencourt-Dias M. 2007. Revisiting the role of the mother centriole in centriole biogenesis. Science. 316:1046–1050 10.1126/science.1142950 [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Snell W.J. 2010. The ciliary membrane. Curr. Opin. Cell Biol. 22:541–546 10.1016/j.ceb.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer B., Ghenoiu C., Bartram M., Müller R.U., Kotsis F., Höhne M., Kühn W., Rapka M., Nitschke R., Zentgraf H., et al. 2006. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 175:547–554 10.1083/jcb.200605092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T.I., Kleylein-Sohn J., Westendorf J., Le Clech M., Lavoie S.B., Stierhof Y.D., Nigg E.A. 2009. Control of centriole length by CPAP and CP110. Curr. Biol. 19:1005–1011 10.1016/j.cub.2009.05.016 [DOI] [PubMed] [Google Scholar]
- Schneider L., Clement C.A., Teilmann S.C., Pazour G.J., Hoffmann E.K., Satir P., Christensen S.T. 2005. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15:1861–1866 10.1016/j.cub.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Seeley E.S., Carrière C., Goetze T., Longnecker D.S., Korc M. 2009. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 69:422–430 10.1158/0008-5472.CAN-08-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Berbari N.F., Yoder B.K. 2008. Ciliary dysfunction in developmental abnormalities and diseases. Curr. Top. Dev. Biol. 85:371–427 10.1016/S0070-2153(08)00813-2 [DOI] [PubMed] [Google Scholar]
- Shida T., Cueva J.G., Xu Z., Goodman M.B., Nachury M.V. 2010. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA. 107:21517–21522 10.1073/pnas.1013728107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V., Romaguera-Ros M., Garcia-Verdugo J.M., Reiter J.F. 2010. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell. 18:410–424 10.1016/j.devcel.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. 1962. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15:363–377 10.1083/jcb.15.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor A., Tsang W.Y., Khoo D., Dynlacht B.D. 2007. Cep97 and CP110 suppress a cilia assembly program. Cell. 130:678–690 10.1016/j.cell.2007.06.027 [DOI] [PubMed] [Google Scholar]
- Strnad P., Gönczy P. 2008. Mechanisms of procentriole formation. Trends Cell Biol. 18:389–396 10.1016/j.tcb.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Strnad P., Leidel S., Vinogradova T., Euteneuer U., Khodjakov A., Gönczy P. 2007. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell. 13:203–213 10.1016/j.devcel.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J.L., Oishi I., Izpisúa Belmonte J.C., Kintner C. 2008. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat. Genet. 40:1454–1460 10.1038/ng.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda P., Adler H.T., Thomas J.H. 2000. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol. Cell. 5:411–421 10.1016/S1097-2765(00)80436-0 [DOI] [PubMed] [Google Scholar]
- Tachi S., Tachi C., Lindner H.R. 1974. Influence of ovarian hormones on formation of solitary cilia and behavior of the centrioles in uterine epithelial cells of the rat. Biol. Reprod. 10:391–403 10.1095/biolreprod10.4.391 [DOI] [PubMed] [Google Scholar]
- Tang C.J., Fu R.H., Wu K.S., Hsu W.B., Tang T.K. 2009. CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 11:825–831 10.1038/ncb1889 [DOI] [PubMed] [Google Scholar]
- Thoma C.R., Frew I.J., Hoerner C.R., Montani M., Moch H., Krek W. 2007. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat. Cell Biol. 9:588–595 10.1038/ncb1579 [DOI] [PubMed] [Google Scholar]
- Thoma C.R., Toso A., Gutbrodt K.L., Reggi S.P., Frew I.J., Schraml P., Hergovich A., Moch H., Meraldi P., Krek W. 2009. VHL loss causes spindle misorientation and chromosome instability. Nat. Cell Biol. 11:994–1001 10.1038/ncb1912 [DOI] [PubMed] [Google Scholar]
- Thomas J., Morlé L., Soulavie F., Laurençon A., Sagnol S., Durand B. 2010. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol. Cell. 102:499–513 10.1042/BC20100035 [DOI] [PubMed] [Google Scholar]
- Tsang W.Y., Bossard C., Khanna H., Peränen J., Swaroop A., Malhotra V., Dynlacht B.D. 2008. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell. 15:187–197 10.1016/j.devcel.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M.F., Stearns T. 2006a. Controlling centrosome number: licenses and blocks. Curr. Opin. Cell Biol. 18:74–78 10.1016/j.ceb.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Tsou M.F., Stearns T. 2006b. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 442:947–951 10.1038/nature04985 [DOI] [PubMed] [Google Scholar]
- Tsou M.F., Wang W.J., George K.A., Uryu K., Stearns T., Jallepalli P.V. 2009. Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev. Cell. 17:344–354 10.1016/j.devcel.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R.W., Scher C.D., Stiles C.D. 1979. Centriole deciliation associated with the early response of 3T3 cells to growth factors but not to SV40. Cell. 18:1065–1072 10.1016/0092-8674(79)90219-8 [DOI] [PubMed] [Google Scholar]
- Vorobjev I.A., Chentsov Y.S. 1982. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 93:938–949 10.1083/jcb.93.3.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens C.J., Tong Y., Esmail M.A., Oh E., Gerdes J.M., Wang J., Tempel W., Rattner J.B., Katsanis N., Park H.W., Leroux M.R. 2010. Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signaling. J. Biol. Chem. 285:16218–16230 10.1074/jbc.M109.070953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Stearns T. 2003. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 5:539–544 10.1038/ncb993 [DOI] [PubMed] [Google Scholar]
- Wong S.Y., Seol A.D., So P.L., Ermilov A.N., Bichakjian C.K., Epstein E.H., Jr, Dlugosz A.A., Reiter J.F. 2009. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 15:1055–1061 10.1038/nm.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Bangs F., Paton I.R., Prescott A., James J., Davey M.G., Whitley P., Genikhovich G., Technau U., Burt D.W., Tickle C. 2009. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 136:655–664 10.1242/dev.028464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Egerer J., Fuchs E., Haas A.K., Barr F.A. 2007. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178:363–369 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Ng C.P., Habacher H., Roy S. 2008. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat. Genet. 40:1445–1453 10.1038/ng.263 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kwon S., Yamaguchi T., Cubizolles F., Rousseaux S., Kneissel M., Cao C., Li N., Cheng H.L., Chua K., et al. 2008. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell. Biol. 28:1688–1701 10.1128/MCB.01154-06 [DOI] [PMC free article] [PubMed] [Google Scholar]